Effects of the Ketogenic Diet on Muscle Hypertrophy in Resistance-Trained Men and Women: A Systematic Review and Meta-Analysis
Abstract
:1. Introduction
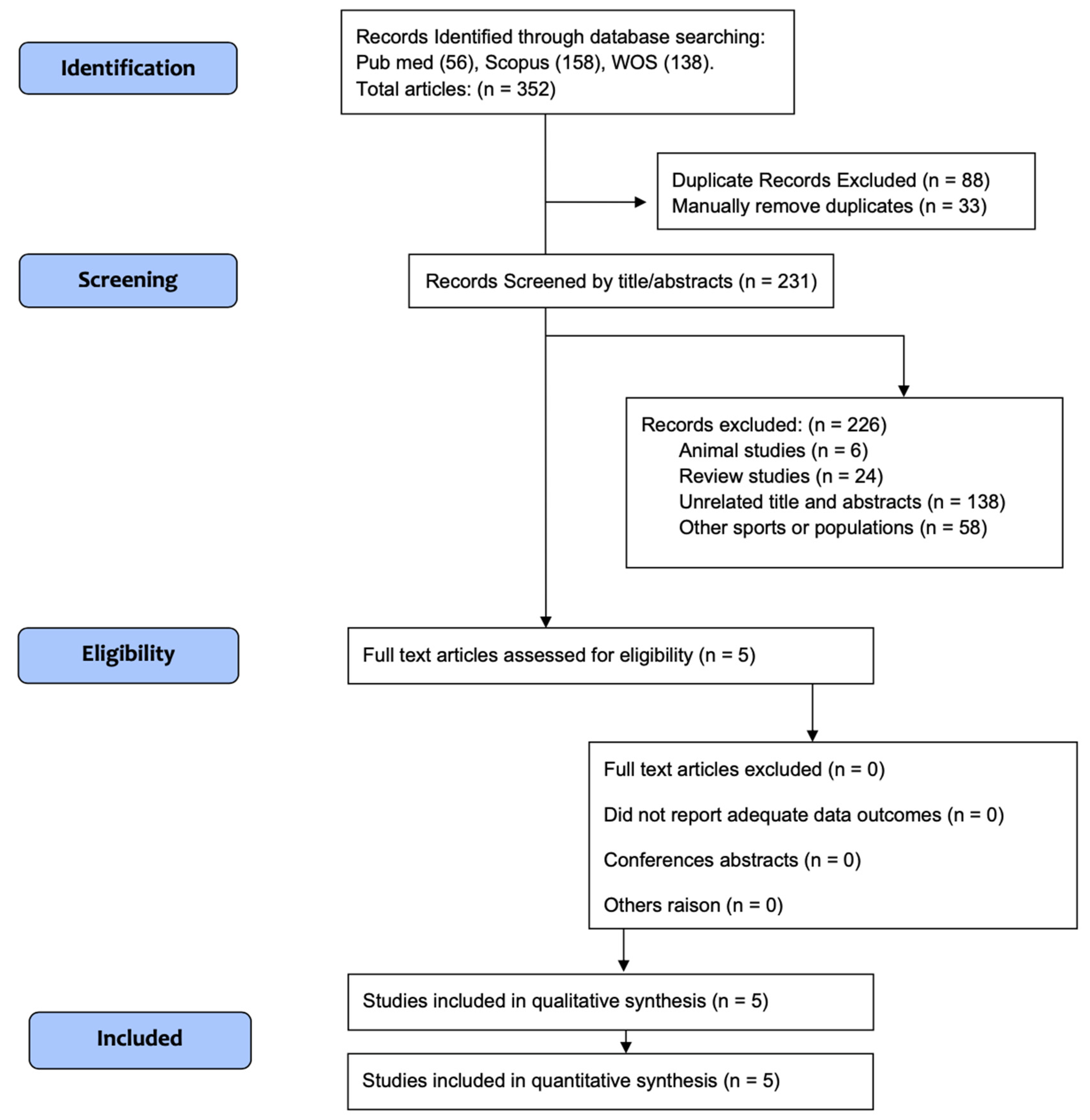
2. Materials and Methods
2.1. Search Strategy
2.2. Study Selection
2.3. Data Extraction
2.4. Quality Assessment
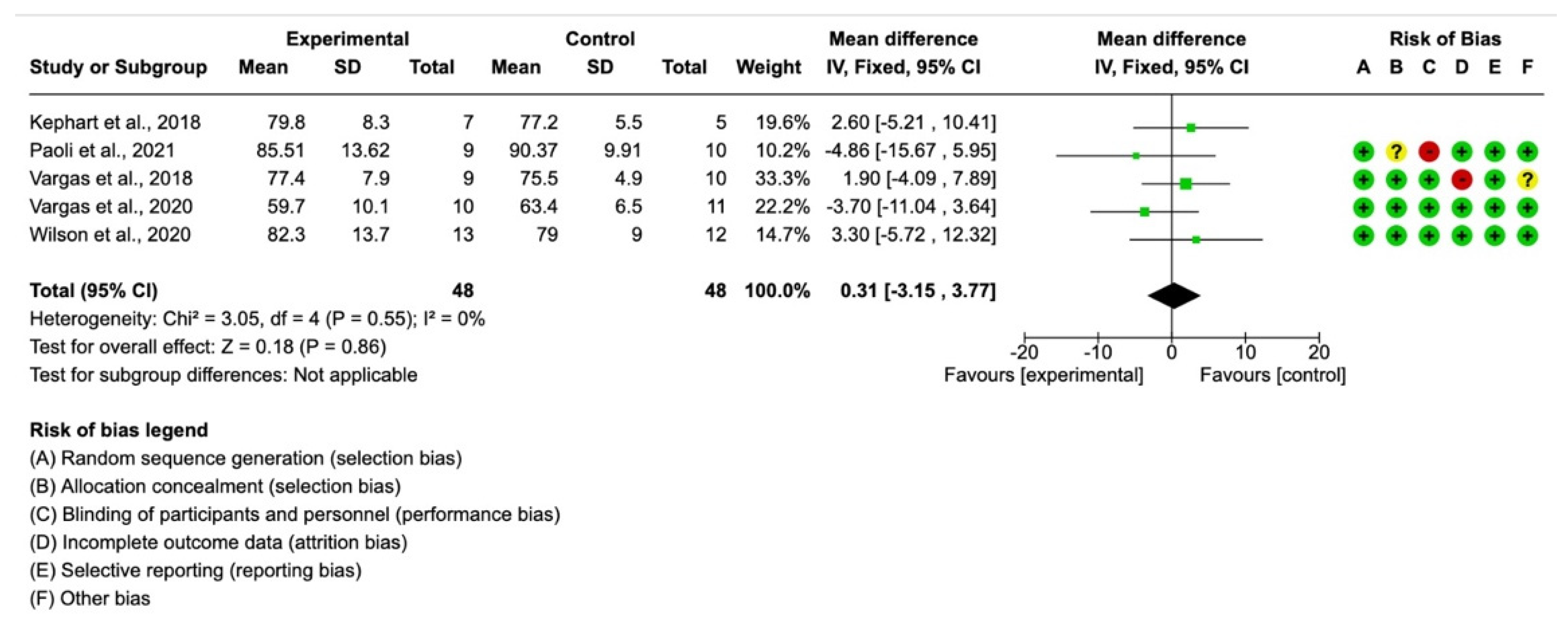
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aragon, A.A.; Schoenfeld, B.J.; Wildman, R.; Kleiner, S.; VanDusseldorp, T.; Taylor, L.; Earnest, C.P.; Arciero, P.J.; Wilborn, C.; Kalman, D.S.; et al. International society of sports nutrition position stand: Diets and body composition. J. Int. Soc. Sports Nutr. 2017, 14, 16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, S.; Suzuki, K. Keto-Adaptation and Endurance Exercise Capacity, Fatigue Recovery, and Exercise-Induced Muscle and Organ Damage Prevention: A Narrative Review. Sports 2019, 7, 40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, J.; Ratamess, N.A.; Faigenbaum, A.D.; Bush, J.A. Ergogenic Properties of Ketogenic Diets in Normal-Weight Individuals: A Systematic Review. J. Am. Coll. Nutr. 2020, 39, 665–675. [Google Scholar] [CrossRef] [PubMed]
- Murphy, N.E.; Carrigan, C.T.; Margolis, L.M. High-Fat Ketogenic Diets and Physical Performance: A Systematic Review. Adv. Nutr. 2021, 12, 223–233. [Google Scholar] [CrossRef] [PubMed]
- Husari, K.S.; Cervenka, M.C. The ketogenic diet all grown up-Ketogenic diet therapies for adults. Epilepsy Res. 2020, 162, 106319. [Google Scholar] [CrossRef]
- Vargas-Molina, S.; Carbone, L.; Romance, R.; Petro, J.L.; Schoenfeld, B.J.; Kreider, R.B.; Bonilla, D.A.; Benitez-Porres, J. Effects of a low-carbohydrate ketogenic diet on health parameters in resistance-trained women. Eur. J. Appl. Physiol. 2021, 121, 2349–2359. [Google Scholar] [CrossRef]
- Martín-Moraleda, E.; Delisle, C.; Collado Mateo, D.; Aznar-Lain, S. Weight loss and body composition changes through ketogenic diet and physical activity: A methodological and systematic review. Nutr. Hosp. 2019, 36, 1196–1204. [Google Scholar]
- Ashtary-Larky, D.; Bagheri, R.; Asbaghi, O.; Tinsley, G.M.; Kooti, W.; Abbasnezhad, A.; Afrisham, R.; Wong, A. Effects of resistance training combined with a ketogenic diet on body composition: A systematic review and meta-analysis. Crit. Rev. Food Sci. Nutr. 2021, 62, 1–16. [Google Scholar]
- Castellana, M.; Conte, E.; Cignarelli, A.; Perrini, S.; Giustina, A.; Giovanella, L.; Giorgino, F.; Trimboli, P. Efficacy and safety of very low calorie ketogenic diet (VLCKD) in patients with overweight and obesity: A systematic review and meta-analysis. Rev. Endocr. Metab. Disord 2020, 21, 5–16. [Google Scholar] [CrossRef]
- Volek, J.S.; Sharman, M.J.; Love, D.M.; Avery, N.G.; Gomez, A.L.; Scheett, T.P.; Kraemer, W.J. Body composition and hormonal responses to a carbohydrate-restricted diet. Metabolism 2002, 51, 864–870. [Google Scholar] [CrossRef]
- Urbain, P.; Strom, L.; Morawski, L.; Wehrle, A.; Deibert, P.; Bertz, H. Impact of a 6-week non-energy-restricted ketogenic diet on physical fitness, body composition and biochemical parameters in healthy adults. Nutr. Metab. 2017, 14, 17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vargas, S.; Romance, R.; Petro, J.L.; Bonilla, D.A.; Galancho, I.; Espinar, S.; Kreider, R.B.; Benítez-Porres, J. Efficacy of ketogenic diet on body composition during resistance training in trained men: A randomized controlled trial. J. Int. Soc. Sports Nutr. 2018, 15, 31. [Google Scholar] [CrossRef]
- Greene, D.A.; Varley, B.J.; Hartwig, T.B.; Chapman, P.; Rigney, M. A Low-Carbohydrate Ketogenic Diet Reduces Body Mass Without Compromising Performance in Powerlifting and Olympic Weightlifting Athletes. J. Strength Cond. Res. 2018, 32, 3373–3382. [Google Scholar] [CrossRef] [PubMed]
- Kephart, W.C.; Pledge, C.D.; Roberson, P.A.; Mumford, P.W.; Romero, M.A.; Mobley, C.B.; Martin, J.S.; Young, K.C.; Lowery, R.P.; Wilson, J.M.; et al. The Three-Month Effects of a Ketogenic Diet on Body Composition, Blood Parameters, and Performance Metrics in CrossFit Trainees: A Pilot Study. Sports 2018, 6, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schoenfeld, B.J. The mechanisms of muscle hypertrophy and their application to resistance training. J. Strength Cond. Res. 2010, 24, 2857–2872. [Google Scholar] [CrossRef] [Green Version]
- Figueiredo, V.C.; de Salles, B.F.; Trajano, G.S. Volume for Muscle Hypertrophy and Health Outcomes: The Most Effective Variable in Resistance Training. Sports Med. 2018, 48, 499–505. [Google Scholar] [CrossRef]
- Helms, E.R.; Cronin, J.; Storey, A.; Zourdos, M.C. Application of the Repetitions in Reserve-Based Rating of Perceived Exertion Scale for Resistance Training. Strength Cond. J. 2016, 38, 42–49. [Google Scholar] [CrossRef] [Green Version]
- Vargas, S.; Petro, J.L.; Romance, R.; Bonilla, D.A.; Florido, M.; Kreider, R.B.; Schoenfeld, B.J.; Benítez-Porres, J. Comparison of changes in lean body mass with a strength-versus muscle endurance-based resistance training program. Eur. J. Appl. Physiol. 2019, 119, 933–940. [Google Scholar] [CrossRef]
- Schoenfeld, B.J.; Grgic, J.; Van Every, D.W.; Plotkin, D.L. Loading Recommendations for Muscle Strength, Hypertrophy, and Local Endurance: A Re-Examination of the Repetition Continuum. Sports 2021, 9, 32. [Google Scholar] [CrossRef]
- Iraki, J.; Fitschen, P.; Espinar, S.; Helms, E. Nutrition Recommendations for Bodybuilders in the Off-Season: A Narrative Review. Sports 2019, 7, 154. [Google Scholar] [CrossRef] [Green Version]
- Phillips, S.M.; Van Loon, L.J. Dietary protein for athletes: From requirements to optimum adaptation. J. Sports Sci. 2011, 29 (Suppl. 1), S29–S38. [Google Scholar] [CrossRef] [PubMed]
- Helms, E.R.; Aragon, A.A.; Fitschen, P.J. Evidence-based recommendations for natural bodybuilding contest preparation: Nutrition and supplementation. J. Int. Soc. Sports Nutr. 2014, 11, 20. [Google Scholar] [CrossRef] [PubMed]
- Escobar, K.A.; VanDusseldorp, T.A.; Kerksick, C.M. Carbohydrate intake and resistance-based exercise: Are current recommendations reflective of actual need? Br. J. Nutr. 2016, 116, 2053–2065. [Google Scholar] [CrossRef] [PubMed]
- Slater, G.; Phillips, S.M. Nutrition guidelines for strength sports: Sprinting, weightlifting, throwing events, and bodybuilding. J. Sports Sci. 2011, 29 (Suppl. 1), S67–S77. [Google Scholar] [CrossRef] [Green Version]
- Moher, D.; Shamseer, L.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A.; Group, P.-P. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst. Rev. 2015, 4, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Higgins, J.P.; Altman, D.G.; Gotzsche, P.C.; Juni, P.; Moher, D.; Oxman, A.D.; Savovic, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A.; et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef] [Green Version]
- Paoli, A.; Cenci, L.; Pompei, P.; Sahin, N.; Bianco, A.; Neri, M.; Caprio, M.; Moro, T. Effects of Two Months of Very Low Carbohydrate Ketogenic Diet on Body Composition, Muscle Strength, Muscle Area, and Blood Parameters in Competitive Natural Body Builders. Nutrients 2021, 13, 374. [Google Scholar] [CrossRef]
- Wilson, J.M.; Lowery, R.P.; Roberts, M.D.; Sharp, M.H.; Joy, J.M.; Shields, K.A.; Partl, J.M.; Volek, J.S.; D’Agostino, D.P. Effects of Ketogenic Dieting on Body Composition, Strength, Power, and Hormonal Profiles in Resistance Training Men. J. Strength Cond. Res. 2020, 34, 3463–3474. [Google Scholar] [CrossRef]
- Vargas-Molina, S.; Petro, J.L.; Romance, R.; Kreider, R.B.; Schoenfeld, B.J.; Bonilla, D.A.; Benítez-Porres, J. Effects of a ketogenic diet on body composition and strength in trained women. J. Int. Soc. Sports Nutr. 2020, 17, 19. [Google Scholar] [CrossRef] [Green Version]
- Koerich, A.C.C.; Borszcz, F.K.; Thives Mello, A.; de Lucas, R.D.; Hansen, F. Effects of the ketogenic diet on performance and body composition in athletes and trained adults: A systematic review and Bayesian multivariate multilevel meta-analysis and meta-regression. Crit. Rev. Food Sci. Nutr. 2022, 27, 1–26. [Google Scholar]
- Amini, M.R.; Aminianfar, A.; Naghshi, S.; Larijani, B.; Esmaillzadeh, A. The effect of ketogenic diet on body composition and anthropometric measures: A systematic review and meta-analysis of randomized controlled trials. Crit. Rev. Food Sci. Nutr. 2021, 62, 3644–3657. [Google Scholar] [CrossRef] [PubMed]
- Jabekk, P.T.; Moe, I.A.; Meen, H.D.; Tomten, S.E.; Hostmark, A.T. Resistance training in overweight women on a ketogenic diet conserved lean body mass while reducing body fat. Nutr. Metab. 2010, 7, 17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wood, R.J.; Gregory, S.M.; Sawyer, J.; Milch, C.M.; Matthews, T.D.; Headley, S.A. Preservation of fat-free mass after two distinct weight loss diets with and without progressive resistance exercise. Metab. Syndr. Relat. Disord 2012, 10, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Paoli, A.; Grimaldi, K.; D’Agostino, D.; Cenci, L.; Moro, T.; Bianco, A.; Palma, A. Ketogenic diet does not affect strength performance in elite artistic gymnasts. J. Int. Soc. Sports Nutr. 2012, 9, 34. [Google Scholar] [CrossRef] [Green Version]
- Gregory, R.; Hamdan, H.; Torisky, D.; Akers., J. A lowcarbohydrate ketogenic diet combined with 6-weeks of crossfit training improves body composition and performance. Int. J. Sport. Exerc. Med. 2017, 3, 54. [Google Scholar] [CrossRef]
- LaFountain, R.A.; Miller, V.J.; Barnhart, E.C.; Hyde, P.N.; Crabtree, C.D.; McSwiney, F.T.; Beeler, M.K.; Buga, A.; Sapper, T.N.; Short, J.A.; et al. Extended Ketogenic Diet and Physical Training Intervention in Military Personnel. Mil. Med. 2019, 184, e538–e547. [Google Scholar] [CrossRef]
- Hadizadeh, M.; Gan, W.Y.; Mohafez, H.; Sugajima, Y. Impact of ketogenic diet on body composition during resistance training among untrained individuals the open sports. Open Sport. Sci. J. 2020, 13, 114–119. [Google Scholar] [CrossRef]
- Rhyu, H.S.; Cho, S.Y. The effect of weight loss by ketogenic diet on the body composition, performance-related physical fitness factors and cytokines of Taekwondo athletes. J. Exerc. Rehabil. 2014, 10, 326–331. [Google Scholar] [CrossRef]
- Westman, E.C.; Feinman, R.D.; Mavropoulos, J.C.; Vernon, M.C.; Volek, J.S.; Wortman, J.A.; Yancy, W.S.; Phinney, S.D. Low-carbohydrate nutrition and metabolism. Am. J. Clin. Nutr. 2007, 86, 276–284. [Google Scholar] [CrossRef] [Green Version]
- Gibson, A.A.; Seimon, R.V.; Lee, C.M.; Ayre, J.; Franklin, J.; Markovic, T.P.; Caterson, I.D.; Sainsbury, A. Do ketogenic diets really suppress appetite? A systematic review and meta-analysis. Obes. Rev. 2015, 16, 64–76. [Google Scholar] [CrossRef] [Green Version]
- Hall, K.D.; Chen, K.Y.; Guo, J.; Lam, Y.Y.; Leibel, R.L.; Mayer, L.E.; Reitman, M.L.; Rosenbaum, M.; Smith, S.R.; Walsh, B.T.; et al. Energy expenditure and body composition changes after an isocaloric ketogenic diet in overweight and obese men. Am. J. Clin. Nutr. 2016, 104, 324–333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tinsley, G.M.; Willoughby, D.S. Fat-Free Mass Changes During Ketogenic Diets and the Potential Role of Resistance Training. Int. J. Sport Nutr. Exerc. Metab. 2016, 26, 78–92. [Google Scholar] [CrossRef] [PubMed]
- Abe, T.; Dankel, S.J.; Loenneke, J.P. Body Fat Loss Automatically Reduces Lean Mass by Changing the Fat-Free Component of Adipose Tissue. Obesity 2019, 27, 357–358. [Google Scholar] [CrossRef] [PubMed]
- Heymsfield, S.B.; Gallagher, D.; Kotler, D.P.; Wang, Z.; Allison, D.B.; Heshka, S. Body-size dependence of resting energy expenditure can be attributed to nonenergetic homogeneity of fat-free mass. Am. J. Physiol. Endocrinol. Metab. 2002, 282, E132–E138. [Google Scholar] [CrossRef] [PubMed]
- Franchi, M.V.; Longo, S.; Mallinson, J.; Quinlan, J.I.; Taylor, T.; Greenhaff, P.L.; Narici, M.V. Muscle thickness correlates to muscle cross-sectional area in the assessment of strength training-induced hypertrophy. Scand. J. Med. Sci. Sports 2018, 28, 846–853. [Google Scholar] [CrossRef] [Green Version]
- Schoenfeld, B.J.; Ogborn, D.; Krieger, J.W. Dose-response relationship between weekly resistance training volume and increases in muscle mass: A systematic review and meta-analysis. J. Sports Sci. 2017, 35, 1073–1082. [Google Scholar] [CrossRef]
- Schoenfeld, B.J.; Ogborn, D.; Krieger, J.W. Effects of Resistance Training Frequency on Measures of Muscle Hypertrophy: A Systematic Review and Meta-Analysis. Sports Med. 2016, 46, 1689–1697. [Google Scholar] [CrossRef]
- Dankel, S.J.; Mattocks, K.T.; Jessee, M.B.; Buckner, S.L.; Mouser, J.G.; Counts, B.R.; Laurentino, G.C.; Loenneke, J.P. Frequency: The Overlooked Resistance Training Variable for Inducing Muscle Hypertrophy? Sports Med. 2017, 47, 799–805. [Google Scholar] [CrossRef]
- Israetel, M.; Feather, J.; Tiago, V.F.; Juneau, C.-E. Mesocycle Progression in Hypertrophy: Volume Versus Intensity. Natl. Strength Cond. Assoc. 2019, 42, 2–6. [Google Scholar] [CrossRef]
- Henselmans, M.; Bjornsen, T.; Hedderman, R.; Varvik, F.T. The Effect of Carbohydrate Intake on Strength and Resistance Training Performance: A Systematic Review. Nutrients 2022, 14, 856. [Google Scholar] [CrossRef]
- Bostock, E.C.S.; Kirkby, K.C.; Taylor, B.V.; Hawrelak, J.A. Consumer Reports of “Keto Flu” Associated With the Ketogenic Diet. Front. Nutr. 2020, 7, 20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Katsanos, C.S.; Kobayashi, H.; Sheffield-Moore, M.; Aarsland, A.; Wolfe, R.R. A high proportion of leucine is required for optimal stimulation of the rate of muscle protein synthesis by essential amino acids in the elderly. Am. J. Physiol. Endocrinol. Metab. 2006, 291, E381–E387. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crozier, S.J.; Kimball, S.R.; Emmert, S.W.; Anthony, J.C.; Jefferson, L.S. Oral Leucine Administration Stimulates Protein Synthesis in Rat Skeletal Muscle. J. Nutr. 2005, 135, 376–382. [Google Scholar] [CrossRef] [PubMed]
- Koopman, R.; Verdijk, L.B.; Beelen, M.; Gorselink, M.; Kruseman, A.N.; Wagenmakers, A.J.M.; Kuipers, H.; van Loon, L.J.C. Co-ingestion of leucine with protein does not further augment post-exercise muscle protein synthesis rates in elderly men. Br. J. Nutr. 2008, 99, 571–580. [Google Scholar] [CrossRef] [PubMed]
| Reference | Sample | Duration | Ketogenic Diet Intervention | Control Intervention | Main Results | Country | Measurement of FFM |
|---|---|---|---|---|---|---|---|
| Paoli et al. (2021) [27] | Body builder males (KD; n = 9, NKD; n = 10) 27.42 ± 10.54. years BMI; 26.80 ± 1.91 kg/m2 | 8 weeks | 45 kcal∙kg-FFM−1∙d−1 Distribution 2.5 g∙kg−1·d−1 PRO, <50 g∙d−1 CHO, remaining calories FAT; | 45 kcal∙kg-FFM−1∙d−1 Distribution: 2.5 g∙kg−1·d−1 PRO, 55% CHO, remaining calories FAT) | No significant changes in FFM in KD (>0.05). Significant changes in NKD (<0.05). | Italy | BIA |
| Vargas-Molina et al. (2020) [29] | Resistance-trained women (n = 21) 27.6 ± 4. years 62.3 ± 7.8 kg 162 ± 6.6 cm | 8 weeks | 40–45 kcal∙kg-FFM−1∙d−1 Distribution: 1.7 g∙kg−1·d−1 PRO, 30–40 g∙kg∙d−1 CHO, remaining calories FAT. | 40–45 kcal∙kg-FFM−1∙d−1 Distribution: 1.7 g∙kg−1·d−1 PRO, 1 g∙kg−1·d−1 FAT, remaining calories CHO | No significant changes in FFM in KD (−0.7 ± 1.7 kg; p = 0.202; d = −0.1) | Spain | DXA |
| Wilson et al. (2020) [28] | Resistance-trained males. (n = 25) KD: 23.0 ± 4.5 and NKD: 21.3 ± 3.7 years | 11 weeks | Distribution: 5% CHO 20% PRO 75% FAT; | Distribution: 55% CHO, 20% PRO, 25% FAT | FFM increased (2.4% and 4.4%; KD and WD) at week 10. FFM, only increased KD (4.8%) between weeks 10 and 11. | United States | DXA Ultrasonography |
| Vargas et al. (2018) [12] | Resistance-trained males, (n = 24) 30 ± 4.7 years; 76.7 ± 8.2 kg 174.16 ± 7 cm | 8 weeks | 39 kcal∙kg-FFM−1∙d−1 Distribution: <10% CHO, 20% PRO, 70% FAT | 39 kcal∙kg-FFM−1∙d−1 Distribution: 55% CHO, 20% PRO, 25% FAT | FFM in KD (p > 0.05). No increase. NKD (p < 0.05) showed increased FFM. | Spain | DXA |
| Kephart et al. (2018) [14] | Resistance-trained males/women (n = 12; 9 M, 3 W) 31 ± 2 years; 80.3 ± 5.1 kg | 12 weeks | Not reported Ad libitum | Not reported Ad libitum | FFM no significant changes between groups. Leg FFM decreased in KD (1.4%-p = 0.068), | United States | DXA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vargas-Molina, S.; Gómez-Urquiza, J.L.; García-Romero, J.; Benítez-Porres, J. Effects of the Ketogenic Diet on Muscle Hypertrophy in Resistance-Trained Men and Women: A Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2022, 19, 12629. https://doi.org/10.3390/ijerph191912629
Vargas-Molina S, Gómez-Urquiza JL, García-Romero J, Benítez-Porres J. Effects of the Ketogenic Diet on Muscle Hypertrophy in Resistance-Trained Men and Women: A Systematic Review and Meta-Analysis. International Journal of Environmental Research and Public Health. 2022; 19(19):12629. https://doi.org/10.3390/ijerph191912629
Chicago/Turabian StyleVargas-Molina, Salvador, José L. Gómez-Urquiza, Jerónimo García-Romero, and Javier Benítez-Porres. 2022. "Effects of the Ketogenic Diet on Muscle Hypertrophy in Resistance-Trained Men and Women: A Systematic Review and Meta-Analysis" International Journal of Environmental Research and Public Health 19, no. 19: 12629. https://doi.org/10.3390/ijerph191912629
APA StyleVargas-Molina, S., Gómez-Urquiza, J. L., García-Romero, J., & Benítez-Porres, J. (2022). Effects of the Ketogenic Diet on Muscle Hypertrophy in Resistance-Trained Men and Women: A Systematic Review and Meta-Analysis. International Journal of Environmental Research and Public Health, 19(19), 12629. https://doi.org/10.3390/ijerph191912629