Concentrations, Speciation, and Potential Release of Hazardous Heavy Metals from the Solid Combustion Residues of Coal-Fired Power Plants
Abstract
1. Introduction
2. Materials and Methods
2.1. Sequential Extraction of Various Fractions of Heavy Metals
2.1.1. CFPPs and Sample Collection
2.1.2. Determination of Pb, Cd, and Cr in Solid Samples
2.1.3. Sequential Extraction Experiment
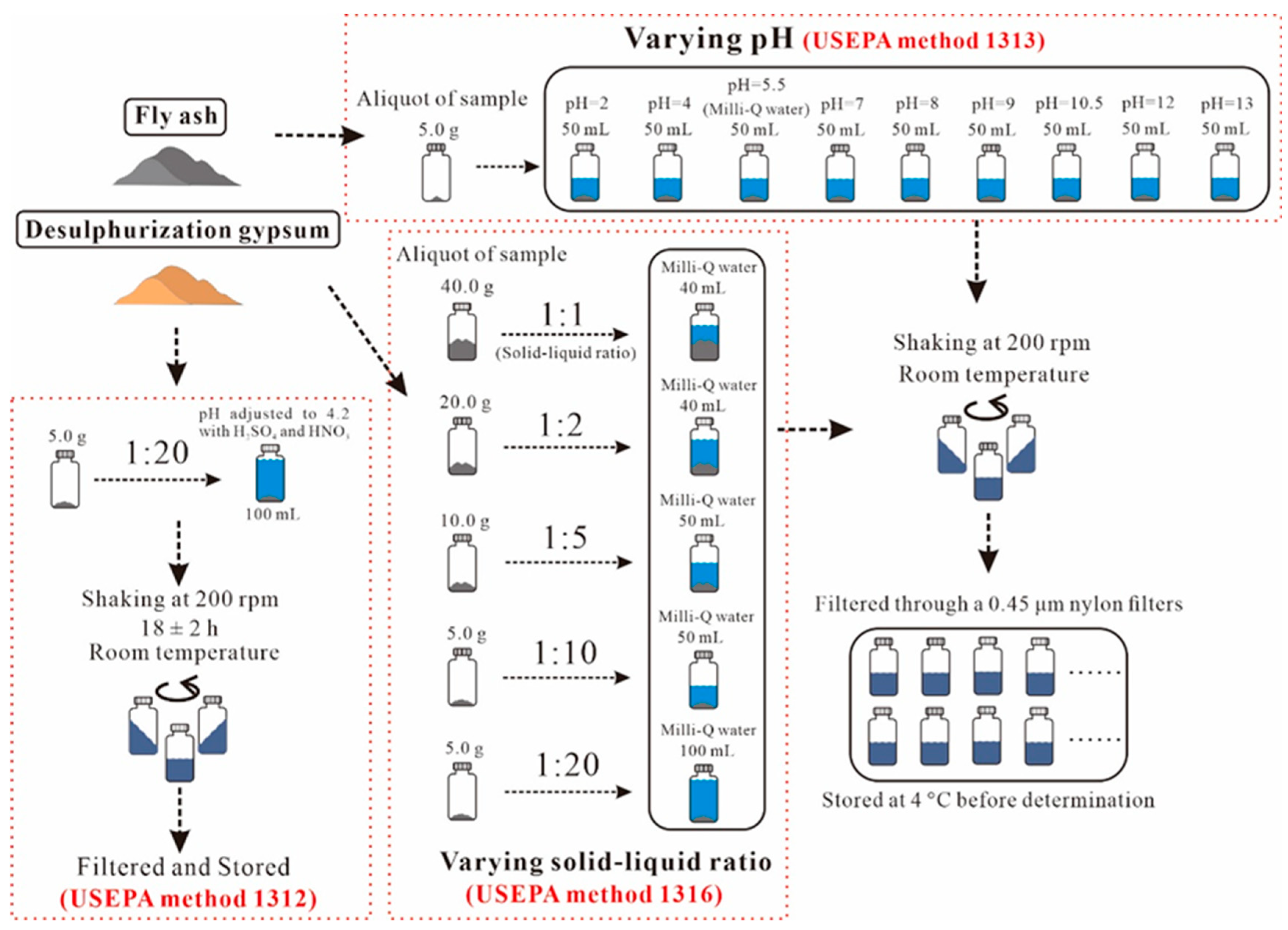
2.2. Heavy-Metal Leaching Experiment
2.3. Field Sample Collection
2.4. Quality Assurance and Quality Control
3. Results and Discussion
3.1. Concentrations of Heavy Metals in FA and FGD Gypsum
3.2. Heavy-Metal Migration Potential of FA and FGD Gypsum
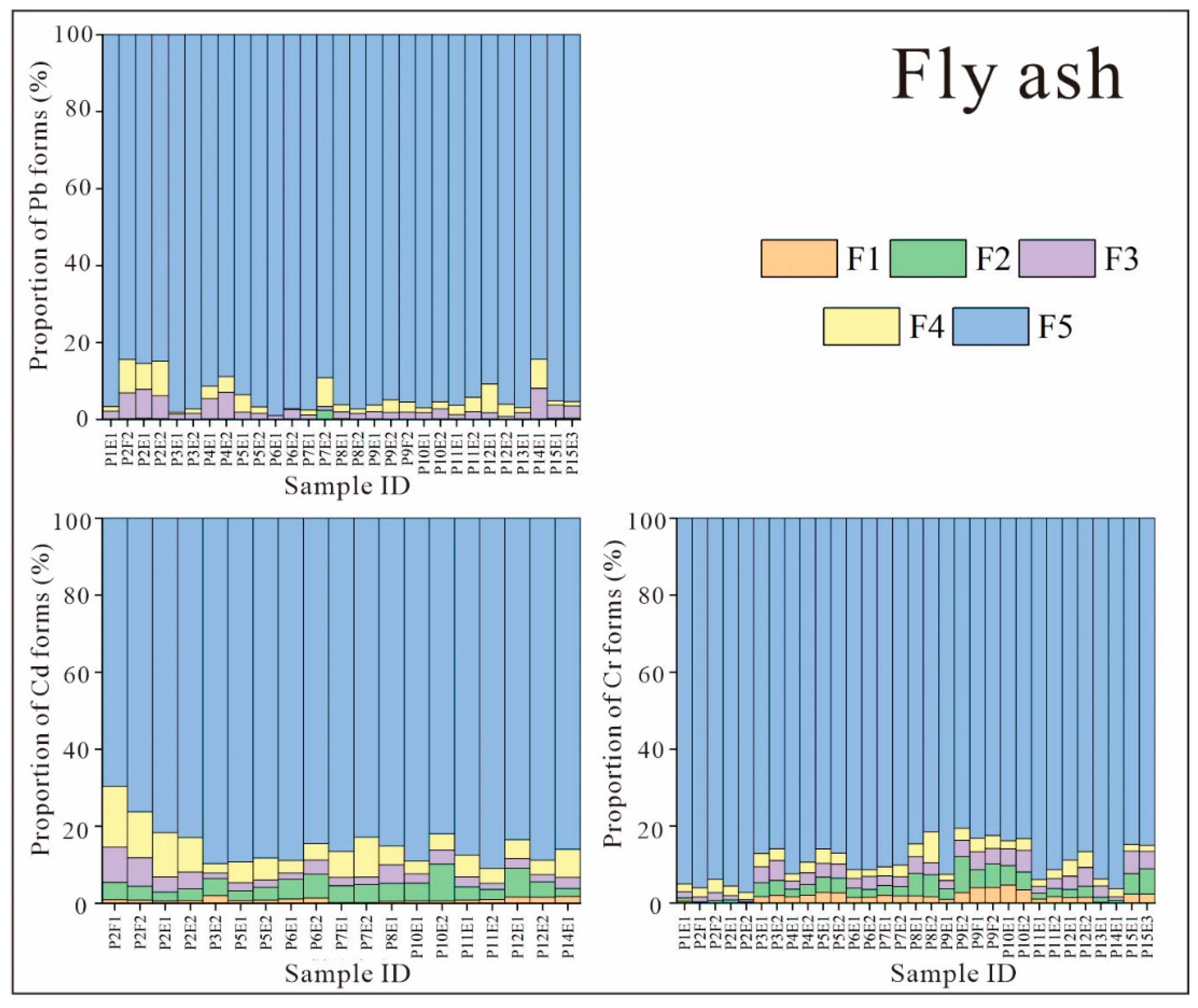
3.2.1. Fractional Distribution of Heavy Metals in FA
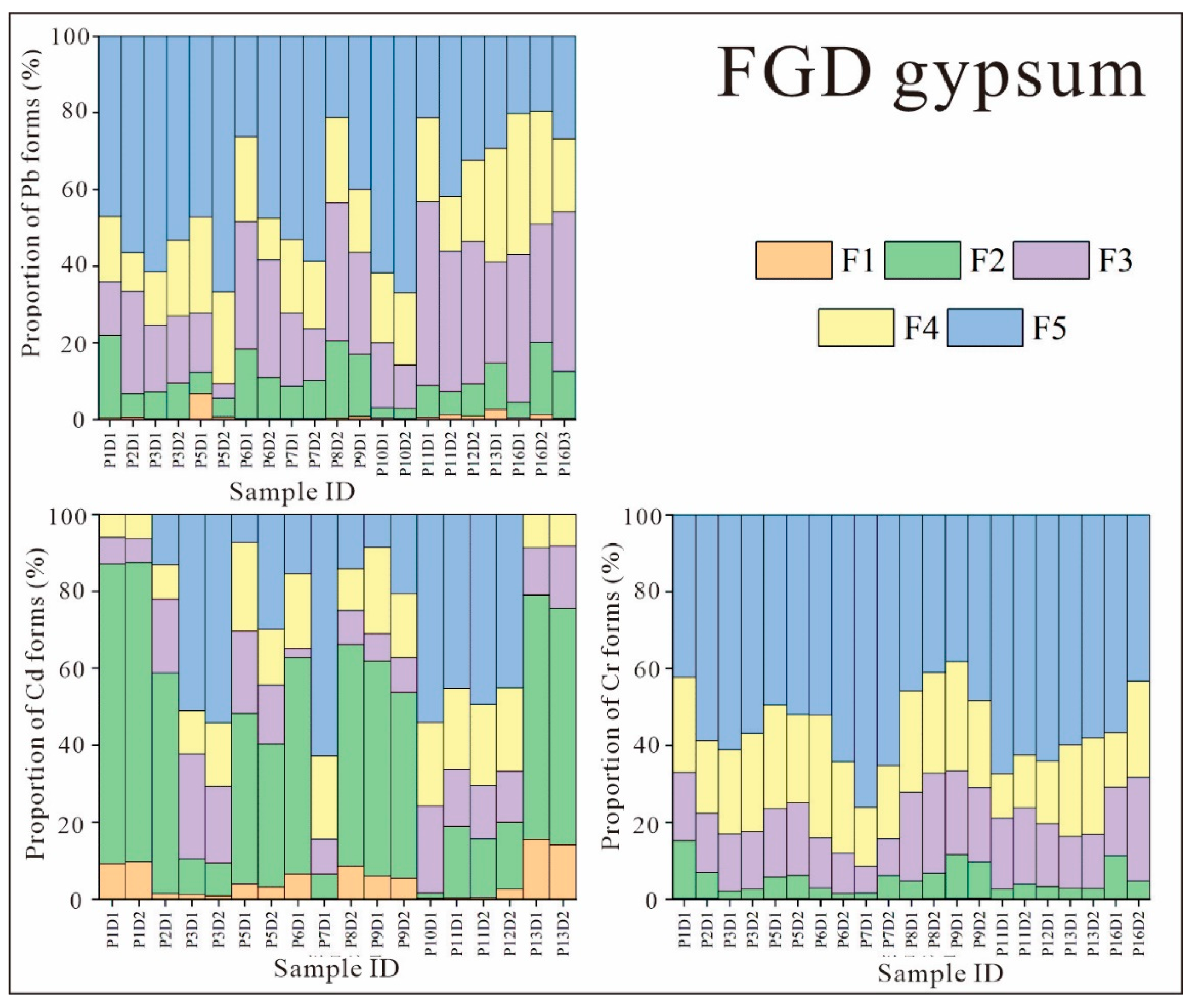
3.2.2. Fractional Distribution of Heavy Metals in FGD Gypsum
3.3. Liquid-Phase Transport Capacity of Heavy Metals
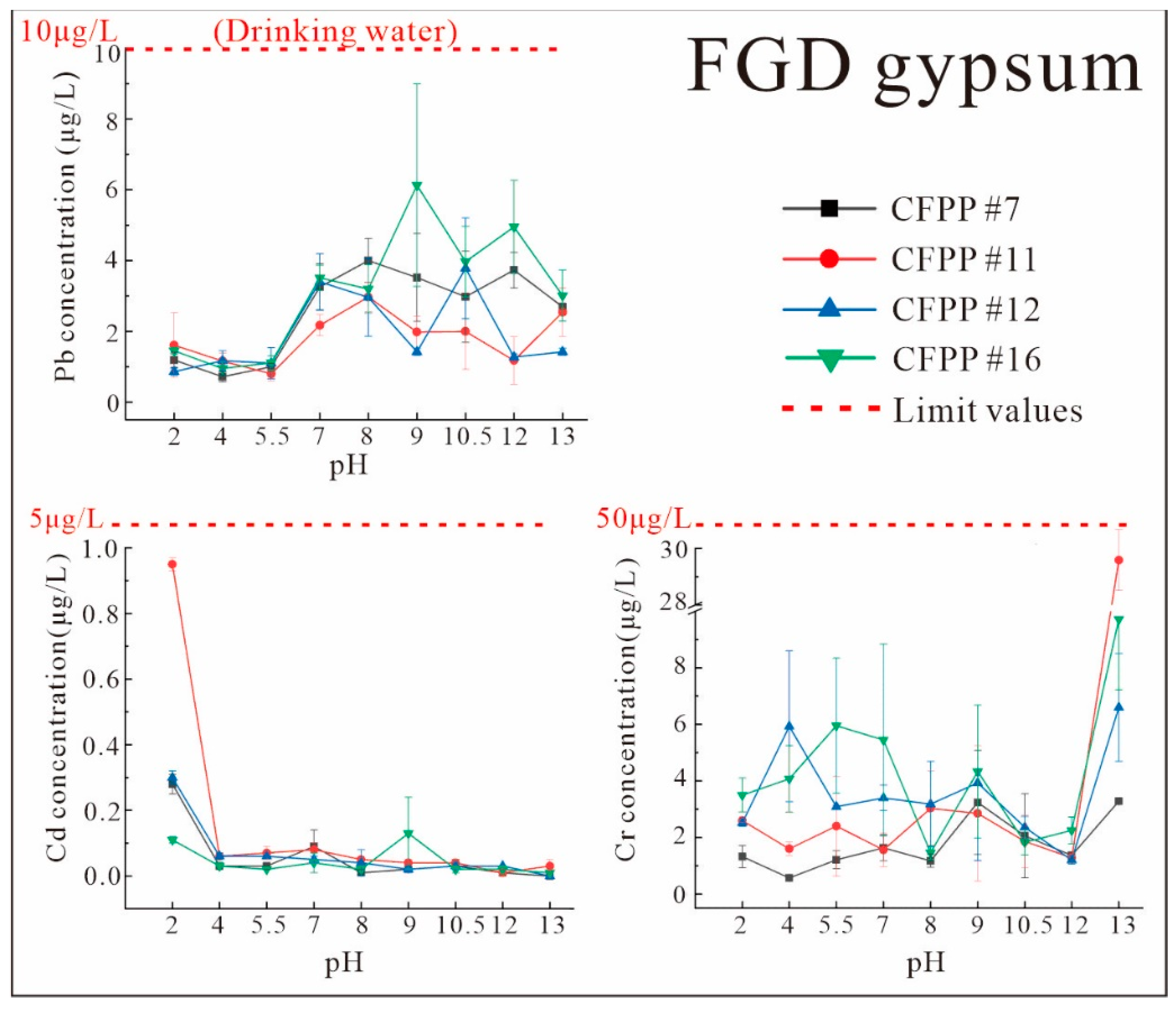
3.3.1. Characteristics of Leaching under Different pH Values
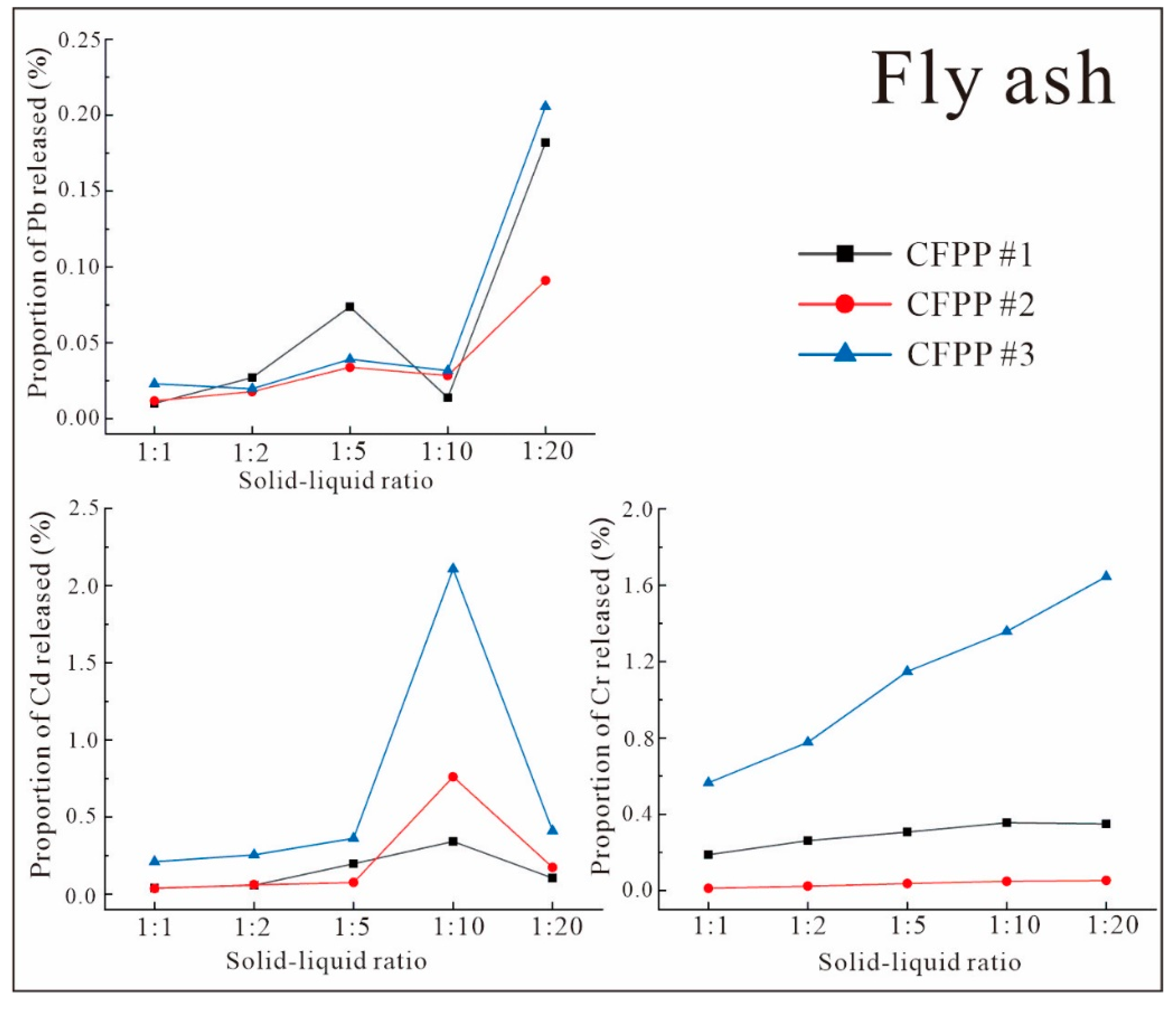
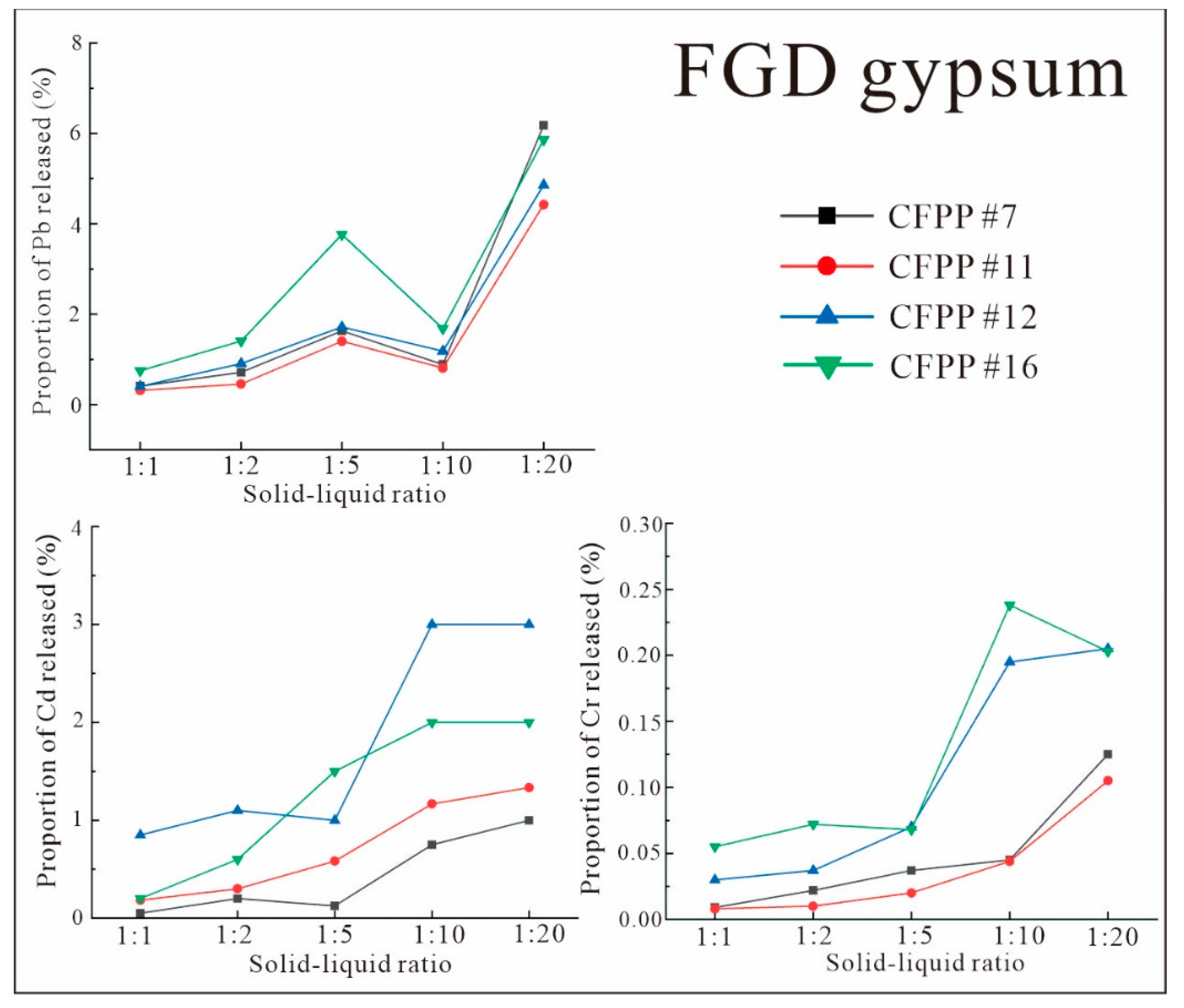
3.3.2. Characteristics of Leaching under Different Solid–Liquid Ratios
3.4. Heavy-Metal Release under Indoor Simulated Rainfall and Field Sampling
4. Conclusions
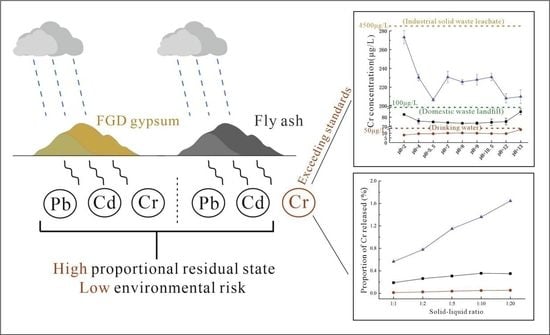
- Pb, Cd, and Cr were all predominantly in the residual state in FA, indicating that there is little release of these elements from FA. The forms of Pb, Cd, and Cr in FGD gypsum did not show a clear pattern, and their total contents were extremely low, posing low threats to the environment.
- The release rates of Pb, Cd, and Cr from FA and FGD gypsum into leachate varied according to pH. The release rates of Pb and Cd from FA and from FGD gypsum were very low in all experiments. However, the release of Cr from FA and FGD may have been greater due to the high proportion of water-soluble states observed in some samples; this was confirmed by both indoor simulations and field observations.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tian, H.Z.; Zhu, C.Y.; Gao, J.J.; Cheng, K.; Hao, J.M.; Wang, K.; Hua, S.B.; Wang, Y.; Zhou, J.R. Quantitative assessment of atmospheric emissions of toxic heavy metals from anthropogenic sources in China: Historical trend, spatial distribution, uncertainties, and control policies. Atmos. Chem. Phys. 2015, 15, 10127–10147. [Google Scholar] [CrossRef]
- Ribeiro, J.; Taffarel, S.R.; Sampaio, C.H.; Flores, D.; Silva, L.F.O. Mineral speciation and fate of some hazardous contaminants in coal waste pile from anthracite mining in Portugal. Int. J. Coal Geol. 2013, 109–110, 15–23. [Google Scholar] [CrossRef]
- Oliveira, M.L.S.; Ward, C.R.; Izquierdo, M.; Sampaio, C.H.; Brum, I.A.S.; Kautzmann, R.M.; Sabedot, S.; Querol, X.; Silva, L.F.O. Chemical composition and minerals in pyrite ash of an abandoned sulphuric acid production plant. Sci. Total Environ. 2012, 430, 34–47. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Li, H.; Wu, S.; Wu, C.Y.; Wang, J.; Li, L. SCR atmosphere induced reduction ofoxidized mercury over CuO−CeO2/TiO2 catalyst. Environ. Sci. Technol. 2015, 49, 7373–7379. [Google Scholar]
- Meij, R. Trace elements behavior in coal-fired power plants. Fuel Process. Technol. 1994, 39, 199–217. [Google Scholar] [CrossRef]
- Yang, Y.F.; Huang, Q.F.; Wang, Q. Ignoring emissions of Hg from coal ash and desulfurized gypsum will lead to ineffective mercury control in coal-fired power plants in China. Environ. Sci. Technol. 2012, 46, 3058–3059. [Google Scholar] [CrossRef]
- Li, X.Y.; Li, Z.G.; Fu, C.C.; Tang, L.; Chen, J.; Wu, T.T.; Lin, C.J.; Feng, X.B.; Fu, X.W. Fate of mercury in two CFB utility boilers with different fueled coals and air pollution control devices. Fuel 2019, 251, 651–659. [Google Scholar] [CrossRef]
- Poykio, R.; Makela, M.; Watkins, G.; Nurmesniemi, H.; Dahl, O. Heavy metals leaching in bottom ash and fly ash fractions from industrial-scale BFB-boiler for environmental risks assessment. Trans. Nonferrous Met. Soc. China 2016, 26, 256–264. [Google Scholar] [CrossRef]
- National Development, and Reform Commission (NDRC). Annual Report on Comprehensive Utilization of Resources in China. 2014. Available online: https://www.ndrc.gov.cn/xwdt/xwfb/201410/W020190905399746121257.pdf (accessed on 10 January 2022). (In Chinese)
- Tang, Y.X.; Chen, W.E. Comparative research on Sino-Japanese fly ash comprehensive utilization. J. Anhui Agric. Sci. 2011, 38, 20852–20854. (In Chinese) [Google Scholar]
- Liu, X.L.; Wang, S.X.; Zhang, L.; Wu, Y.; Duan, L.; Hao, J.M. Speciation of mercury in FGD gypsum and mercury emission during the wallboard production in China. Fuel 2013, 111, 621–627. [Google Scholar] [CrossRef]
- Zhao, S.L.; Duan, Y.F.; Lu, J.C.; Liu, S.; Pudasainee, D.; Gupta, R.; Liu, M.; Lu, J.H. Enrichment characteristics, thermal stability and volatility of hazardous trace elements in fly ash from a coal-fired power plant. Fuel 2018, 225, 490–498. [Google Scholar] [CrossRef]
- Zheng, C.H.; Xu, L.J.; Liu, S.J.; Wang, L.; Liang, C.S.; Zhao, H.T.; Zhang, Y.X.; Du, X.S.; Gao, X. Speciation and thermal stability of mercury in solid products from ultralow emission air pollution control devices. Energy Fuels 2018, 32, 12655–12664. [Google Scholar] [CrossRef]
- Lopez-Anton, M.A.; Perry, R.; Abad-Valle, P.; Díaz-Somoano, M.; Martínez-Tarazona, M.R.; Maroto-Valer, M.M. Speciation of mercury in fly ashes by temperature programmed decomposition. Fuel Process. Technol. 2011, 92, 707–711. [Google Scholar] [CrossRef]
- Xin, M.; Gustin, M.S.; Ladwig, K. Laboratory study of air–water–coal combustion product (fly ash and FGD solid) mercury exchange. Fuel 2006, 85, 2260–2267. [Google Scholar] [CrossRef]
- Wang, W.F.; Qin, Y.; Song, D.Y.; Wang, K.X. Column leaching of coal and its combustion residues, Shizuishan, China. Int. J. Coal Geol. 2008, 75, 81–87. [Google Scholar] [CrossRef]
- Skodras, G.; Grammelis, P.; Prokopidou, M.; Kakaras, E.; Sakellaropoulos, G. Chemical, leaching and toxicity characteristics of CFB combustion residues. Fuel 2009, 88, 1201–1209. [Google Scholar] [CrossRef]
- Świetlik, R.; Trojanowska, M.; Jóźwiak, M.A. Evaluation of the distribution of heavy metals and their chemical forms in ESP-fractions of fly ash. Fuel Process. Technol. 2012, 95, 109–118. [Google Scholar] [CrossRef]
- Quispe, D.; Pérez-López, R.; Silva, L.F.; Nieto, J.M. Changes in the mobility of hazardous elements during coal combustion in Santa Catarina power plant (Brazil). Fuel 2012, 94, 495–503. [Google Scholar] [CrossRef]
- Zhao, S.L.; Duan, Y.F.; Lu, J.C.; Gupta, R.; Pudasainee, D.; Liu, S.; Liu, M.; Lu, J.H. Thermal stability, chemical speciation and leaching characteristics of hazardous trace elements in FGD gypsum from coal-fired power plants. Fuel 2018, 231, 94–100. [Google Scholar] [CrossRef]
- Bai, X.F.; Ding, H.; Lian, J.J.; Ma, D.; Yang, X.Y.; Sun, N.X.; Xue, W.L.; Chang, Y.J. Coal production in China: Past, present, and future projections. Int. Geol. Rev. 2018, 60, 535–547. [Google Scholar] [CrossRef]
- Chen, C.L.; Fang, K.H.; Zhao, Z.H.; Ye, W. Study on quality and comprehensive utilization of fly ash in Guizhou Province. Fly Ash Compr. Utiization 2012, 4, 22–26. (In Chinese) [Google Scholar] [CrossRef]
- Chen, H.F.; Zhang, J.G. Study on comprehensive utilization and countermeasures of solid waste in regional thermal power enterprises in Guizhou. Guizhou Electr. Power Technol. 2017, 20, 84–86. (In Chinese) [Google Scholar] [CrossRef]
- Li, Z.F. Karst landform zoning in Guizhou. Geol. Guizhou 2011, 28, 177–181. (In Chinese) [Google Scholar] [CrossRef]
- Lv, P.; Han, G.L.; Wu, Q.X. Chemical characteristics of rainwater in karst rural areas, Guizhou Province, Southwest China. Acta Geochim. 2017, 36, 572–576. [Google Scholar] [CrossRef]
- Jiang, Z.; Lian, Y.; Qin, X. Rocky desertification in Southwest China: Impacts, causes, and restoration. Earth-Sci. Rev. 2014, 132, 1–12. [Google Scholar] [CrossRef]
- Li, W.H.; Zhai, J. Ash, sulfur and calorific value of Chinese thermal coal. Coal Convers. 1994, 1, 15–25. (In Chinese) [Google Scholar]
- Liang, Q.; Grégoire, D.C. Determination of trace elements in twenty-six Chinese geochemistry reference materials by inductively coupled plasma-mass spectrometry. Geostand. Geoanal. Res. 2000, 24, 51–63. [Google Scholar] [CrossRef]
- Ure, A.M.; Quevauviller, P.; Muntau, H.; Griepink, H. Speciation of heavy metals in soils and sediments. An account of the improvement and harmonization of extraction techniques undertaken under the auspices of the BCR of the commission of the European communities. Int. J. Environ. Anal. Chem. 1993, 51, 135–151. [Google Scholar] [CrossRef]
- State Environmental Protection Agency of the People’s Republic of China. Background Values of Soil Elements in China; China Environmental Science Press: Beijing, China, 1990; pp. 354–476. (In Chinese)
- Zhao, S.L.; Duan, Y.F.; Lu, J.C.; Gupta, R.; Pudasainee, D.; Liu, S.; Liu, M.; Lu, J.H. Chemical speciation and leaching characteristics of hazardous trace elements in coal and fly ash from coal-fired power plants. Fuel 2018, 232, 463–469. [Google Scholar] [CrossRef]
- GB 15618-2018; Soil Environmental Quality Risk Control Standard for Soil Contamination of Agricultural Land. State Administration for Market Regulation of Ministry of Ecology and Environment of the People’s Republic of China: Beijing, China, 2018. Available online: https://www.mee.gov.cn/ywgz/fgbz/bz/bzwb/trhj/201807/W020190626595212456114.pdf (accessed on 27 March 2022). (In Chinese)
- Aunela-Tapola, L.; Hatanpaa, E.; Hoffren, H.; Laitinen, T.; Larjava, K.; Rasila, P.; Tolvanen, M. A study of trace element behaviour in two modern coal-fired power plants: II. Trace element balances in two plants equipped with semi-dry flue gas desulphurisation facilities. Fuel Process. Technol. 1998, 55, 13–34. [Google Scholar] [CrossRef]
- Suzuki, K.; Ono, Y. Leaching characteristics of stabilized/solidified fly ash generated from ash-melting plant. Chemosphere 2008, 71, 922–932. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yang, J.P.; Yu, X.H.; Sun, P.; Zhao, Y.C.; Zhang, J.Y.; Gang, C.; Yao, H.; Zheng, C.G. Migration and emission characteristics of Hg in coal-fired power plant of China with ultra low emission air pollution control devices. Fuel Process. Technol. 2017, 158, 272–280. [Google Scholar] [CrossRef]
- Hao, Y.; Wu, S.M.; Pan, Y.; Li, Q.; Zhou, J.Z.; Xu, Y.B.; Qian, G.R. Characterization and leaching toxicities of mercury in flue gas desulfurization gypsum from coal-fired power plants in China. Fuel 2016, 177, 157–163. [Google Scholar] [CrossRef]
- GB 5749-2006; Sanitary Standard for Drinking Water. Environmental Protection Agency of China: Beijing, China, 2006. Available online: http://c.gb688.cn/bzgk/gb/showGb?type=online&hcno=73D81F4F3615DDB2C5B1DD6BFC9DEC86 (accessed on 27 March 2022). (In Chinese)
- Gong, X. Leaching Characteristics of Heavy Metal in the Coal Ash from West China. Ph.D. Thesis, Huazhong University of Science and Technology, Wuhan, China, 2010. (In Chinese). [Google Scholar]
- GB 16889-2008; Standard for Pollution Control on the Landfill Site of Municipal Solid Waste. State Administration for Market Regulation of Ministry of Ecology and Environment of the People’s Republic of China: Beijing, China, 2008. Available online: https://www.mee.gov.cn/ywgz/fgbz/bz/bzwb/gthw/gtfwwrkzbz/200804/W020120719581734247724.pdf (accessed on 27 March 2022). (In Chinese)
- Department of Ecology and Environment of Guizhou Province. 2018 Guizhou Province Ecological Environment Status Bulletin; Department of Ecology and Environment of Guizhou Province: Guiyang, China, 2019. Available online: https://sthj.guizhou.gov.cn/hjsj/hjzlsjzx_5802731/hjzkgb_5802732/201906/W020201104507232615760.pdf (accessed on 22 May 2022).
- Department of Ecology and Environment of Guizhou Province. 2019 Guizhou Province Ecological Environment Status Bulletin; Department of Ecology and Environment of Guizhou Province: Guiyang, China, 2020. Available online: https://sthj.guizhou.gov.cn/hjsj/hjzlsjzx_5802731/hjzkgb_5802732/202006/P020201104766918362433.pdf (accessed on 22 May 2022).
- Department of Ecology and Environment of Guizhou Province. 2020 Guizhou Province Ecological Environment Status Bulletin; Department of Ecology and Environment of Guizhou Province: Guiyang, China, 2021. Available online: https://sthj.guizhou.gov.cn/hjsj/hjzlsjzx_5802731/hjzkgb_5802732/202106/P020210608399555630441.pdf (accessed on 22 May 2022).
- Department of Ecology and Environment of Guizhou Province. 2021 Guizhou Province Ecological Environment Status Bulletin; Department of Ecology and Environment of Guizhou Province: Guiyang, China, 2022. Available online: https://sthj.guizhou.gov.cn/hjsj/hjzlsjzx_5802731/hjzkgb_5802732/202206/P020220602309696994066.pdf (accessed on 22 May 2022).
- Huang, Y.M.; Liu, J.L.; Wang, G.; Wang, Q.F.; Zeng, B.P.; Xiao, Z.J.; Sun, G.Y.; Li, Z.G. Leachability of mercury in coal fly ash from coal-fired power plants in southwest China. Front. Environ. Sci. 2022, 10, 887837. [Google Scholar] [CrossRef]
- US EPA. National Primary Drinking Water Regulations, Safe Drinking Water Act (SDWA). 2022. Available online: https://www.epa.gov/ground-water-and-drinking-water/national-primary-drinking-water-regulations (accessed on 22 May 2022).


| CFPP ID | Location in Guizhou | Boiler Horsepower | Boiler Type 1 | Pollutant Control Facilities 2 | Sample Type | Sample ID 3 |
|---|---|---|---|---|---|---|
| #1 | Central | 3 × 200 MW | PC | SCR + ESP + WFGD | FA from ESP | P1E1, P1E2 |
| FGD gypsum | P1D1, P1D2 | |||||
| #2 | West | 1 × 300 MW | CFB | SNCR + ESP-FF + WFGD | FA from ESP and FF | P2E1, P2E2, P2F1, P2F2 |
| FGD gypsum | P2D1 | |||||
| #3 | Central | 4 × 300 MW | PC | SCR + ESP + WFGD | FA from ESP | P3E1, P3E2 |
| FGD gypsum | P3D1, P3D2 | |||||
| #4 | Northwest | 2 × 150 MW | CFB | ESP | FA from ESP | P4E1, P4E2 |
| #5 | Northwest | 4 × 300 MW | PC | SCR + ESP + WFGD | FA from ESP | P5E1, P5E2 |
| FGD gypsum | P5D1, P5D2 | |||||
| #6 | East | 2 × 300 MW | PC | SCR + ESP + WFGD | FA from ESP | P6E1, P6E2 |
| FGD gypsum | P6D1, P6D2 | |||||
| #7 | North | 2 × 660 MW | PC | SCR + ESP + WFGD | FA from ESP | P7E1, P7E2 |
| FGD gypsum | P7D1, P7D2 | |||||
| #8 | West | 2 × 600 MW | PC | SCR + ESP + WFGD | FA from ESP | P8E1, P8E2 |
| FGD gypsum | P8D1, P8D2 | |||||
| #9 | West | 2 × 660 MW | PC | SCR + ESP-FF + WFGD | FA from ESP and FF | P9E1, P9E2, P9F1, P9F2 |
| FGD gypsum | P9D1, P9D2 | |||||
| #10 | Northwest | 4 × 300 MW | PC | SCR + ESP-FF + WFGD | FA from ESP | P10E1, P10E2 |
| FGD gypsum | P10D1, P10D2 | |||||
| #11 | Northwest | 8 × 300 MW | PC | SCR + ESP-FF + WFGD | FA from ESP | P11E1, P11E2 |
| FGD gypsum | P11D1, P11D2 | |||||
| #12 | Central | 2 × 600 MW | PC | SCR + ESP + WFGD | FA from ESP | P12E1, P12E2 |
| FGD gypsum | P12D1, P12D2 | |||||
| #13 | West | 4 × 600 MW | PC | SCR + ESP + WFGD | FA from ESP | P13E1, P13E2 |
| FGD gypsum | P13D1, P13D2 | |||||
| #14 | Central | 2 × 150 MW | CFB | ESP | FA from ESP | P14E1 |
| #15 | North | 4 × 300 MW | PC | SCR + ESP-FF + WFGD | FA from ESP | P15E1, P15E2, P15E3 |
| #16 | Northwest | 4 × 300 MW | PC | SCR + ESP-FF + WFGD | FGD gypsum | P16D1, P16D2, P16D3, P16D4 |
| Step | Extraction Reagent | Experimental Conditions | Description |
|---|---|---|---|
| 1 | Deionized water | Room temperature, 16 h | Water soluble |
| 2 | 0.5 M CH3COOH | Room temperature, 16 h | Acid soluble |
| 3 | 0.5 M HONH2HCl | Room temperature, 16 h | Reducible |
| 4 | H2O2; 1 M NH4C2H3O2 | 85 °C; room temperature, 20 h | Oxidizable |
| 5 | HCl, HNO3, HF, HClO4 | 195 °C | Residual |
| CFPP ID | Pb | Cd | Cr | |||
|---|---|---|---|---|---|---|
| FA | FGD Gypsum | FA | FGD Gypsum | FA | FGD Gypsum | |
| #1 | 31 | 0.55 | 0.38 | 0.33 | 211 | 33 |
| #2 | 37 (38) 1 | 0.50 | 0.47 (0.46) 1 | 0.31 | 199 (193) 1 | 40 |
| #3 | 35 | 0.03 | 0.73 | 0.03 | 152 | 50 |
| #4 | 55 | / 2 | 1.00 | / 2 | 104 | / 2 |
| #5 | 64 | 0.43 | 1.12 | 0.11 | 162 | 62 |
| #6 | 37 | 2.37 | 0.87 | 0.05 | 180 | 36 |
| #7 | 57 | 1.13 | 3.52 | 0.04 | 186 | 27 |
| #8 | 52 | 1.25 | 0.49 | 0.16 | 108 | 42 |
| #9 | 58 (80) 1 | 0.66 | 0.65 (1.07) 1 | 0.22 | 131 (146) 1 | 28 |
| #10 | 73 | 1.52 | 1.99 | 0.03 | 125 | 11 |
| #11 | 37 | 0.99 | 0.60 | 0.06 | 164 | 55 |
| #12 | 41 | 0.93 | 0.79 | 0.02 | 170 | 35 |
| #13 | 32 | 0.31 | 0.44 | 0.27 | 156 | 35 |
| #14 | 44 | / 2 | 2.28 | / 2 | 136 | / 2 |
| #15 | 53 | / 2 | 2.33 | / 2 | 163 | / 2 |
| #16 | / 2 | 0.66 | / 2 | 0.01 | / 2 | 25 |
| CFPP ID | Sample Type | Concentrations of Heavy Metals (μg/L) | ||
|---|---|---|---|---|
| Pb | Cd | Cr | ||
| CFPP #1 | Fly ash | 2.731 | 0.029 | 33.778 |
| CFPP #2 | Fly ash | 2.207 | 0.030 | 4.836 |
| CFPP #3 | Fly ash | 3.365 | 0.136 | 97.123 |
| CFPP #7 | Desulfurization gypsum | 3.325 | 0.027 | 1.856 |
| CFPP #11 | Desulfurization gypsum | 2.006 | 0.034 | 1.588 |
| CFPP #12 | Desulfurization gypsum | 2.304 | 0.049 | 1.334 |
| CFPP #16 | Desulfurization gypsum | 3.238 | 0.010 | 2.047 |
| Drinking water limits in China (μg/L) [39] | 10 | 5 | 50 | |
| Drinking water limits in the US (μg/L) [45] | 15 | 5 | 100 | |
| Emission limits for water pollutants from landfills in China (μg/L) | 100 | 10 | 100 | |
| Water Quality Parameters | Water Sample #1 | Water Sample #2 | Water Sample #3 | Water Sample #4 | Drinking Water Limit in China |
|---|---|---|---|---|---|
| pH | 7.47 | 7.48 | 8.85 | 9.39 | 6.5~8.5 |
| Salinity (psu) | 4.35 | 34.26 | 1.26 | 1.24 | / |
| Electrical conductivity (mS) | 8.21 | 55.8 | 2.57 | 2.55 | 2 |
| Total dissolved solids (mg/L) | 3990 | 14,000 | 1254 | 1238 | 1000 |
| Pb content (μg/L) | 4.25 | 3.91 | 0.41 | 0.35 | 10 |
| Cd content (μg/L) | 0.57 | 1.98 | 1.30 | 1.31 | 5 |
| Cr content (μg/L) | 19.72 | 64.07 | 42.39 | 41.78 | 50 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, Y.; Liu, J.; Wang, G.; Bi, X.; Sun, G.; Wu, X.; Wang, Q.; Li, Z. Concentrations, Speciation, and Potential Release of Hazardous Heavy Metals from the Solid Combustion Residues of Coal-Fired Power Plants. Int. J. Environ. Res. Public Health 2022, 19, 12617. https://doi.org/10.3390/ijerph191912617
Huang Y, Liu J, Wang G, Bi X, Sun G, Wu X, Wang Q, Li Z. Concentrations, Speciation, and Potential Release of Hazardous Heavy Metals from the Solid Combustion Residues of Coal-Fired Power Plants. International Journal of Environmental Research and Public Health. 2022; 19(19):12617. https://doi.org/10.3390/ijerph191912617
Chicago/Turabian StyleHuang, Yiming, Jinling Liu, Guan Wang, Xiangyang Bi, Guangyi Sun, Xian Wu, Qingfeng Wang, and Zhonggen Li. 2022. "Concentrations, Speciation, and Potential Release of Hazardous Heavy Metals from the Solid Combustion Residues of Coal-Fired Power Plants" International Journal of Environmental Research and Public Health 19, no. 19: 12617. https://doi.org/10.3390/ijerph191912617
APA StyleHuang, Y., Liu, J., Wang, G., Bi, X., Sun, G., Wu, X., Wang, Q., & Li, Z. (2022). Concentrations, Speciation, and Potential Release of Hazardous Heavy Metals from the Solid Combustion Residues of Coal-Fired Power Plants. International Journal of Environmental Research and Public Health, 19(19), 12617. https://doi.org/10.3390/ijerph191912617