The Lived Experience of Patients Utilizing Second-Generation Direct-Acting Antiviral for Treatment of Chronic Hepatitis C Virus Infection: A Phenomenological Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Data Collection
2.3. Data Analysis
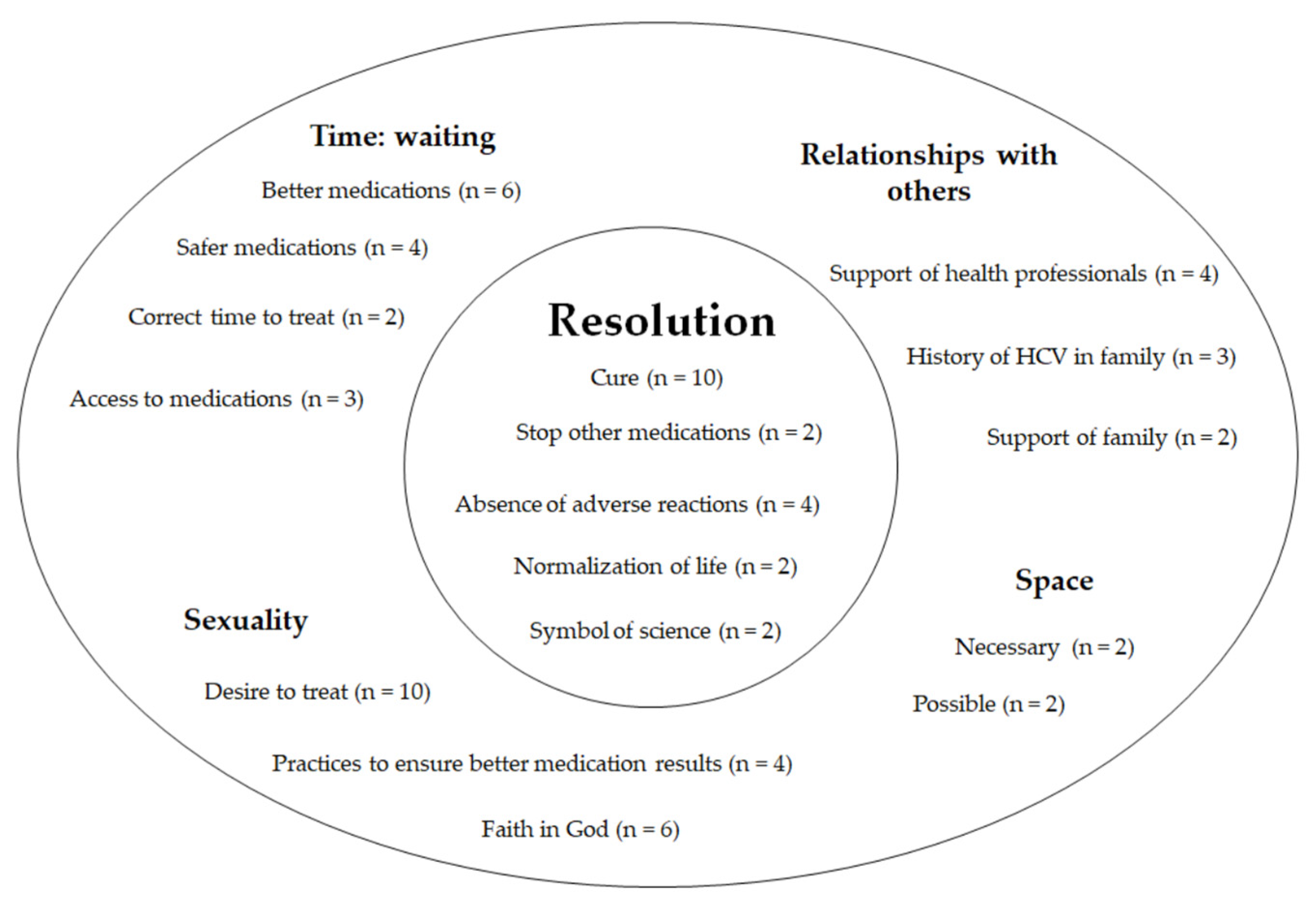
3. Results
3.1. The Use of DAAs by Patients with Chronic Hepatitis C: The Experience of Resolution
“Worried about passing it on to others. Afraid of cutting myself. I have to think of everything, right. I’m worried about contaminating others.”(Ilma)
“Actually, the hepatitis medication is everything to me. Because it’s going to heal me.”(Rui)
“The cure means not having the bug anymore. The virus. Not having it pestering me anymore.”(Rui)
“Because the hepatitis medication will stop as soon as the hepatitis is cured. I’ll stop taking the ascites ones. Because if I don’t have it, right… Automatically maybe even the glucose medication. Because my glucose is altered because of hepatitis… So that’s it, I’m waiting, and the cure will be everything to me.”(Rui)
“Then I’ll take care of my life, my family, my business. And not be so dependent.”(Rui)
“Girl, that last one … I almost died with the last one. They made me very agitated, and all those blisters started popping up. I was in a real state!”(Ilma)
“I’m not feeling a thing. This one is the same thing as the other one I was already on [for other diseases], I felt no difference at all.”(Flora)
“I’m still holding on well. My platelets dropped at first, but now they’ve gone up a bit. Because platelets are supposed to go down, right?”(Laís)
“I read the leaflet. I know it doesn’t harm the heart. But it also doesn’t… So, I don’t know, but I think it has some side effects, but… I don’t think it’s in my best interest to know because I only want to get better. I just want to think about how great it is!”(Rui)
“I think about how science has advanced. That it has made such a blessed medication. And it keeps on trying and trying until it finds one that works!”(Ilma)
3.2. The Experience of Time: The Wait
“I did six months of treatment and felt very sick… My viral load went to zero. But less than two years later, the virus had relapsed. The doctor already told me about this medication that was going to arrive. She kept monitoring me, to see if we could get to where we are now. But there were times I was afraid. I was worried. But it worked out, thank God! And now I’m on this medication.”(Flora)
“I couldn’t take the first one, because my platelets were so low, and I could die. There were too many side effects!”(Rui)
“The doctor said: The medication is here, but now that you’re on this other treatment, you can’t take it.”(Clara)
“I didn’t have money to file a lawsuit, so I had to wait. Then the drugs arrived, but the Health Surveillance Agency blocked them. For a year… Then they released them again. But the government blocked them. Another year…”
“This is a gift. You understand? Because if it was available to everyone, cool. But it’s not! You know it’s not!”(João)
“The doctor talked about follow-up. I didn’t start treatment right then because my mother still was completely dependent on me. My mother passed away a year and a half later. But when I got here, the team thought my platelets were too low. So, the process took two years, waiting for this new medication.”
“It’s three months [treatment]. Twelve weeks. I’m in the fifth week, so halfway there, right?”(Laís)
“When treatment stopped, I had to stop too, but always going after it.”(Ilma)
“I want to know what they’re going to do, because as soon as the virus is killed…then they’ll have to treat liver cirrhosis, right? The only thing on my mind before was treating the…virus. I was under the impression that when the virus was killed, everything would be over, the liver would regenerate. Then on second thought…cirrhosis cannot be regenerated… So, it’ll take even more time. They will have to wait, see if the liver reacts and if they will have to do something.”(Laís)
3.3. The Experience of Space—Possible Spaces and Necessary Spaces
“Then I will take care of my life, my family, my business.”(Rui)
“I’m afraid of doing nothing, of not being able to work. Being stuck in the house, it’s really bad.”(Ilma)
“This routine is stressful, you don’t have time to take care of yourself (…). Because I have to get here at five in the morning! And I hate it. I wasn’t used to going to the doctor like this. For example, when it’s my day to come here, I am anxious the entire week! Just knowing that I will be stuck here, and I lose a whole day!”(Laís)
“Because you used to have a healthy life. But now you have a controlled life. By you, the doctors, by the situation.”
3.4. Relationships with Others—Partnerships along the Way
“You never discouraged us; you always said a better medication would come along! Because I used to think that there was no solution and that you would discharge me, not care at all. But God blessed me, and you guys kept going. You walked beside us, right? Walking…”(Ilma)
“He got cancer and it was all so fast. He was the kind of person who loved life, so I thought, man, he didn’t get the opportunity I’m being given now. I believe that if he had had the time, he would have gone after it! So, if I have this opportunity, I’d better grab it (crying)”(Flora)
3.5. Sexuality—“This Is a Medication I Want”
“This is a medication I want. I want it, because God forbid, we should get to the point where I’ve seen others in, which is very difficult.”(Zoé)
“I wasn’t usually strict with times, but now I think I’m better. Now I’ve created a routine, the alarm clock on my phone goes off and I’ll take the medication right away. And it’s not getting in my way.”(Flora)
“It’s very hard for me to take medication. I’m taking these now without fail, asking God not to fail, dying to get rid of this…”(Zoé)
“I’m the type of person who really loves living. I love life very much, I always joke and say, watch out, I’m going to live to be two hundred! And with this will to live, I have been dealing with this virus for twenty years now.”(João)
“I don’t want to feel guilty if at the end they say, “It didn’t go down at all.” I will be aware that I did everything they prescribed, the way they told me to.”(Nice)
“I saw God’ hands in this on that first day. I said, He will take me to the end… And I worried no more! Positive thinking is very good for you, right?”(Laís)
“The medication is the complement of what God already gave us… I think God has already cured me. You are godsent. The doctor of doctors is God.”(Rui)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Passos, A.D.C. Hepatite C: Aspectos críticos de uma epidemia silenciosa. Cad. Saude Publica 2006, 22, 1764–1765. [Google Scholar] [CrossRef]
- Webster, D.P.; Klenerman, P.; Dusheiko, G.M. Hepatitis C. Lancet 2015, 385, 1124–1135. [Google Scholar] [CrossRef] [Green Version]
- Alter, M.J. The detection, transmission, and outcome of hepatitis C virus infection. Infect. Agents Dis. 1993, 2, 155–166. [Google Scholar] [PubMed]
- Thrift, A.P.; El-Seag, H.B.; Kanwal, F. Global epidemiology and burden of HCV infection and HCV-related disease. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 122–132. [Google Scholar] [CrossRef]
- Dultz, G.; Graubard, B.I.; Martin, P.; Welker, M.W.; Vermehren, J.; Zeuzem, S.; McGlynn, K.A.; Welzel, T.M. Liver transplantation for chronic hepatitis C virus infection in the United States 2002–2014: An analysis of the UNOS/OPTN registry. PLoS ONE 2017, 12, e0186898. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization. Global Hepatitis Report; World Health Organization: Geneva, Switzerland, 2017; p. 83. ISBN 978-92-4-156545-5. [Google Scholar]
- Welch, N.M.; Jensen, D.M. Pegylated interferon-based therapy with second-wave direct-acting antivirals in genotype 1 chronic hepatitis C. Liver Int. 2015, 35, 11–17. [Google Scholar] [CrossRef]
- Ghany, M.G.; Nelson, D.R.; Strader, D.B.; Thomas, D.L.; Seeff, L.B. AASLD Practice Guideline. An Update on Treatment of Genotype 1 Chronic Hepatitis C Virus Infection: 2011 Practice Guideline by the American Association for the Study of Liver Diseases. Hepatology 2011, 54, 1433–1444. [Google Scholar] [CrossRef] [Green Version]
- The European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatitis C vírus infection. J. Hepatol. 2011, 55, 245–264. [Google Scholar] [CrossRef]
- Sarrazin, C.; Hézode, C.; Zeuzem, S.; Pawlotsky, J.M. Antiviral strategies in hepatitis C virus infection. J. Hepatol. 2012, 56, S88–S100. [Google Scholar] [CrossRef]
- Koh, C.; Zhao, X.; Samala, S.; Sakiani, S.; Liang, T.J.; Talwalkar, J.A. AASLD clinical practice guidelines: A critical review of scientific evidence and evolving recommendations. Hepatology 2013, 58, 2142–2152. [Google Scholar] [CrossRef] [Green Version]
- The European Association for the Study of the Liver. EASL clinical practice guidelines. Management of hepatitis C virus infection. J. Hepatol. 2014, 60, 392–420. [Google Scholar] [CrossRef]
- Wedemeyer, H.; Dore, G.J.; Ward, J.W. Estimates on HCV disease burden worldwide—Filling the gaps. J. Viral Hepat. 2015, 22, 1–5. [Google Scholar] [CrossRef]
- Brasil. Protocolo Clínico e Diretrizes Terapêuticas para Hepatite C e Coinfecções/—Brasília: Ministério da Saúde, Secretaria de Vigilância em Saúde, Departamento de Vigilância, Prevenção e Controle das Infecções Sexualmente Transmissíveis, do HIV/Aids e das Hepatites Virais. Brasilia, 2019. Available online: http://www.vs.saude.ms.gov.br/wp-content/uploads/2019/06/pcdt_hepatite_c_03_2019_web.pdf (accessed on 1 June 2022).
- Brasil. Nota Informativa nº 13/2019-COVIG/CGVP/MINDIAHV/SVS/MS. Atualizada em 17 de Outubro de 2019, Retificada em 31 de outubro de 2019. Available online: https://www.gov.br/aids/pt-br/centrais-de-conteudo/copy_of_notas-informativas/2019/sei_ms_-_0011998370_-_despacho.pdf/view (accessed on 1 June 2022).
- Clark, J.A.; Gifford, A.L. Resolute efforts to cure hepatitis C: Understanding patients’ reasons for completing antiviral treatment. Health 2014, 19, 473–489. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hill, R.; Pfeil, M.; Moore, J.; Richardson, B. Living with hepatitis C: A phenomenological study. J. Clin. Nurs. 2014, 24, 428–438. [Google Scholar] [CrossRef] [PubMed]
- North, C.S.; Devereaux, R.; Pollio, D.E.; Hong, B.A.; Jain, M.K. Patient perspectives on hepatitis C and its treatment. Eur. J. Gastroenterol. Hepatol. 2014, 26, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Teston, E.F.; Silva, R.L.D.T.; Marcon, S.S. Convivendo com hepatite: Repercussões no cotidiano do indivíduo infectado. Rev. Esc. Enferm. USP 2013, 47, 860–868. [Google Scholar] [CrossRef] [Green Version]
- Kinder, M. The Lived Experience of Treatment for Hepatitis C. Gastroenterol. Nurs. 2009, 32, 401–408. [Google Scholar] [CrossRef]
- Harris, M. Managing expense and expectation in a treatment revolution: Problematizing prioritisation through an exploration of hepatitis C treatment ‘benefit’. Int. J. Drug Policy 2017, 47, 161–168. [Google Scholar] [CrossRef]
- Rasi, M.; Kunzler-Heule, P.; Schmid, P.; Semela, D.; Bruggmann, P.; Fehr, J.; Saxer, S.; Nicca, D. “Fighting an uphill battle”: Experience with the HCV triple therapy: A qualitative thematic analysis. BMC Infect. Dis. 2014, 14, 507. [Google Scholar] [CrossRef] [Green Version]
- Sublette, V.A.; Smith, S.K.; George, J.; McCaffery, K.; Douglas, M.W. The Hepatitis C treatment experience: Patients’ perceptions of the facilitators of and barriers to uptake, adherence and completion. Psychol. Health 2015, 30, 987–1004. [Google Scholar] [CrossRef]
- Richamond, J.A.; Ellard, J.; Wallace, J.; Thorpe, R.; Higgs, P.; Hellard, M.; Thompson, A. Achieving a hepatitis C cure: A qualitative exploration of the experiences and meanings of achieving a hepatitis C cure using the direct acting antivirals in Australia. Hepatol. Med. Policy 2018, 3, 8. [Google Scholar] [CrossRef]
- Whiteley, D.; Whittaker, A.; Elliott, L.; Cunningham-Burley, S. The lived experience of interferon-free treatments for hepatites C: A thematic analysis. Int. J. Drug Policy 2016, 38, 21–28. [Google Scholar] [CrossRef] [Green Version]
- Whiteley, D.; Whittaker, A.; Elliott, L.; Cunningham-Burley, S. Hepatitis C in a new therapeutic era: Recontextualizing the lived experience J. Clin. Nurs. 2018, 27, 2729–2739. [Google Scholar] [CrossRef]
- Shoemaker, S.J.; Ramalho de Oliveira, D. Understanding the meaning of medications for patients: The medication experience. Pharm. World Sci. 2008, 30, 86–91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilde, M.H. Why Embodiment Now? Adv. Nurs. Sci. 1999, 22, 25–38. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, Y.A.; Ramalho de Oliveira, D. The Subjective Experience of Using Medications: What We Know and the Paths Forward. Pharmacy 2021, 9, 50. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, Y.A.; Silva, L.D.; Ramalho de Oliveira, D. Experiences with the daily use of medications among chronic hepatitis C patients. Res. Social Adm. Pharm. 2020, 16, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, Y.A.; Filardi, A.F.R.; Abath, A.J.; Silva, L.D.; Ramalho de Oliveira, D. A fenomenologia de Merleau-Ponty nas investigações sobre o uso de medicamentos: Construção de uma cascata metodológica. Rev. Esc Enferm USP 2017, 51, 1–8. [Google Scholar] [CrossRef]
- Carel, H. Phenomenology of Illness; Oxford Press: Oxford, UK, 2016; p. 247. ISBN 978-0-19-966965-3. [Google Scholar]
- Dahlberg, K.; Drew, N.; Nyström, M. Reflective Lifeworld Research; Studentlitteratur: Lund, Sweden, 2001; p. 259. ISBN 978-9144016955. [Google Scholar]
- Englander, M. The interview: Data collection in descriptive phenomenological human scientific research. J. Phenomenol. Psychol. 2012, 43, 13–35. [Google Scholar] [CrossRef] [Green Version]
- Morrow, S.L. Quality and trustworthiness in qualitative research in counseling psychology. J. Couns. Psychol. 2005, 52, 250–260. [Google Scholar] [CrossRef] [Green Version]
- Kvigne, K.; Kirkevold, M. Living with Bodily Strangeness: Women’s Experiences of Their Changing and Unpredictable Body Following a Stroke. Qual. Health Res. 2003, 13, 1291–1310. [Google Scholar] [CrossRef]
- Merleau-Ponty, M. Fenomenologia da Percepção, 4th ed.; WMF Martins Fontes: São Paulo, Brazil, 2011; p. 662. ISBN 978-85-7287-116-9. [Google Scholar]
- Stelter, R. The transformation of body experience into language. J. Phenomenol. Psychol. 2000, 31, 63–77. [Google Scholar] [CrossRef]
- Wilde, M.H. Embodied knowledge in chronic illness and injury. Nurs. Inq. 2003, 10, 170–176. [Google Scholar] [CrossRef] [PubMed]
- Dowling, M. From Husserl to van Manen. A review of different phenomenological approaches. Int. J. Nurs. Stud. 2007, 44, 131–142. [Google Scholar] [CrossRef] [PubMed]
- Dowsett, L.E.; Coward, S.; Lorenzetti, D.L.; MacKean, G.; Clement, F. Living with Hepatitis C Virus: A Systematic Review and Narrative Synthesis of Qualitative Literature. Can. J. Gastroenterol. Hepatol. 2017, 17, 3268650. [Google Scholar] [CrossRef] [PubMed]
- Miller, E.R.; McNally, S.; Wallace, J.; Schlichthorst, M. The ongoing impacts of hepatitis c—A systematic narrative review of the literature. BMC Public Health 2012, 12, 672. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chung, R.T.; Baumert, T.F. Curing chronic hepatitis C- the arc of a medical triumph. N. Engl. J. Med. 2014, 370, 1576–1578. [Google Scholar] [CrossRef]
- Nahon, P.; Bourcier, V.; Layese, R.; Audureau, E.; Cagnot, C.; Marcellin, P.; Guyader, D.; Fontaine, H.; Larrey, D.; Lédinghen, V.; et al. Eradication of hepatitis C virus infection in patients with cirrhosis reduces risk of liver and non-liver complications. Gastroenterology 2017, 152, 142–156. [Google Scholar] [CrossRef] [Green Version]
- Calvaruso, V.; Petta, S.; Caxì, A. Is global elimination of HCV realistic? Liver Int. 2018, 38, 40. [Google Scholar] [CrossRef] [Green Version]
- Paterson, B.L.; Butt, G.; Mcguinness, L.; Moffat, B. The Construction of Hepatitis C as a Chronic Illness. Clin. Nurs. Res. 2006, 5, 209–224. [Google Scholar] [CrossRef]
| Patient | Year of Diagnosis | Previous Treatment | HCV Medication History | HCV Complications |
|---|---|---|---|---|
| Rui | 2011 | No | Contraindication to previously available treatments | Yes |
| Nice | 2011 | No | Contraindication to previously available treatments | Yes |
| Laís | 2001 | Yes | Failure with two previous treatments | Yes |
| João | 1994 | Yes | Failure with one previous treatment | No |
| Zoé | 2014 | No | Recently diagnosed | Yes |
| Ilma | 2006 | Yes | Failure with two previous treatments | Yes |
| Flora | 1997 | Yes | Failure with one previous treatment | No |
| Bruna | 2000 | Yes | Failure with one previous treatment | No |
| Sara | 2012 | No | Recently diagnosed | No |
| Clara | 2011 | Yes | Failure with one previous treatment | No |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nascimento, Y.d.A.; Silva, L.D.; Ramalho de Oliveira, D. The Lived Experience of Patients Utilizing Second-Generation Direct-Acting Antiviral for Treatment of Chronic Hepatitis C Virus Infection: A Phenomenological Analysis. Int. J. Environ. Res. Public Health 2022, 19, 12540. https://doi.org/10.3390/ijerph191912540
Nascimento YdA, Silva LD, Ramalho de Oliveira D. The Lived Experience of Patients Utilizing Second-Generation Direct-Acting Antiviral for Treatment of Chronic Hepatitis C Virus Infection: A Phenomenological Analysis. International Journal of Environmental Research and Public Health. 2022; 19(19):12540. https://doi.org/10.3390/ijerph191912540
Chicago/Turabian StyleNascimento, Yone de Almeida, Luciana Diniz Silva, and Djenane Ramalho de Oliveira. 2022. "The Lived Experience of Patients Utilizing Second-Generation Direct-Acting Antiviral for Treatment of Chronic Hepatitis C Virus Infection: A Phenomenological Analysis" International Journal of Environmental Research and Public Health 19, no. 19: 12540. https://doi.org/10.3390/ijerph191912540





