A Systematic Review of the Literature Examining the Effects of Cigarette Smoke and e-Cigarette Vapor on the Virulence of Human Pathogenic Bacteria
Abstract
:1. Introduction
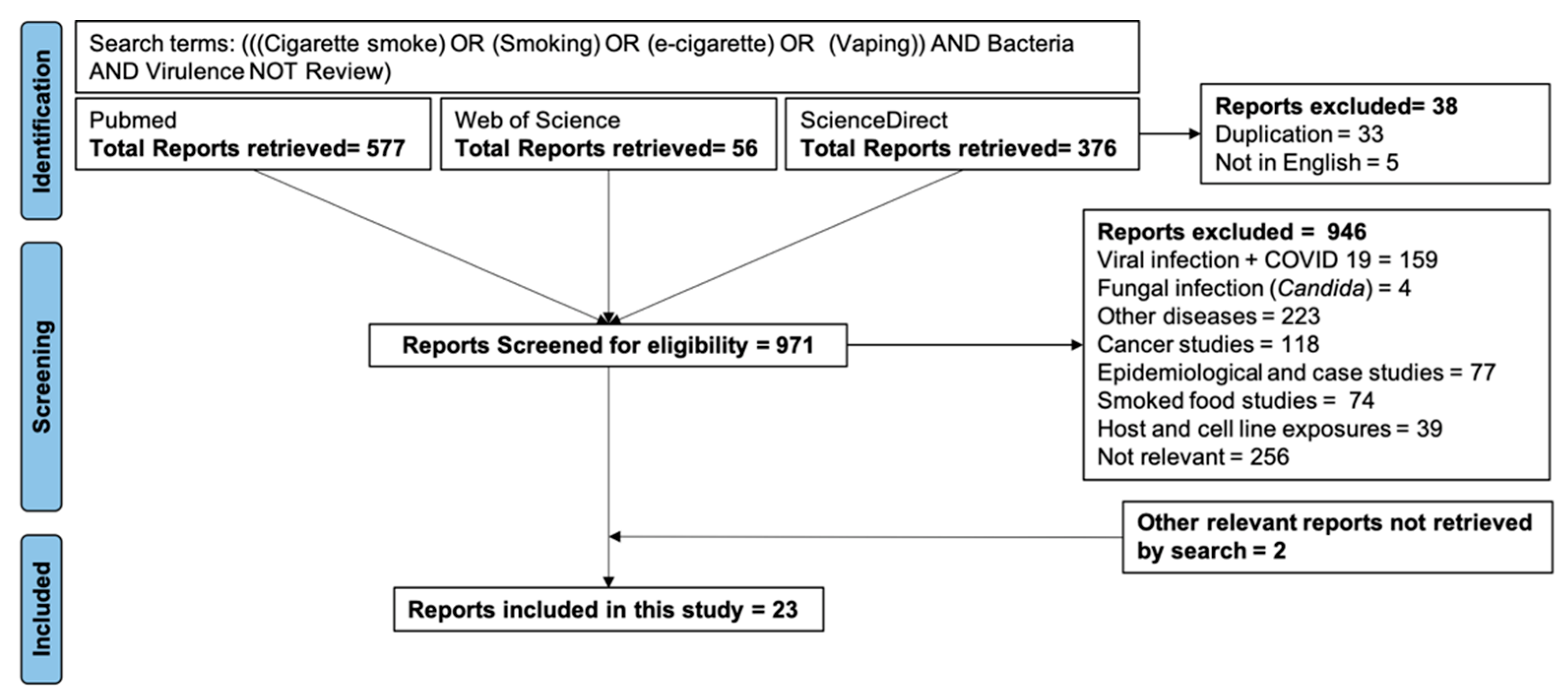
2. Materials and Methods
3. Results
3.1. The Effects of CS and EV Exposure on the Bacterial Growth
3.2. The Effects of CS and EV Exposure on Bacterial Biofilm Formation
3.3. The Effects of CS and EV Exposure on Bacterial Adherence
3.4. The Effects of CS and EV on Other Virulence Characteristics
3.5. The Effects of CS and EV Exposure on Bacterial Gene Expression
3.6. Examining the Effects of CS/EV on Bacterial Virulence in an In Vivo Mouse Model
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alexander, L.E.C.; Shin, S.; Hwang, J.H. Inflammatory Diseases of the Lung Induced by Conventional Cigarette Smoke: A Review. Chest 2015, 148, 1307–1322. [Google Scholar] [CrossRef] [PubMed]
- Leite, F.R.M.; Nascimento, G.G.; Scheutz, F.; López, R. Effect of Smoking on Periodontitis: A Systematic Review and Meta-regression. Am. J. Prev. Med. 2018, 54, 831–841. [Google Scholar] [CrossRef] [PubMed]
- Talhout, R.; Schulz, T.; Florek, E.; van Benthem, J.; Wester, P.; Opperhuizen, A. Hazardous compounds in tobacco smoke. Int. J. Environ. Res. Public Health 2011, 8, 613–628. [Google Scholar] [CrossRef] [PubMed]
- U.S. Department of Health and Human Services. Smoking Cessation. A Report of the Surgeon General; U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health: Atlanta, GA, USA, 2020.
- Giovacchini, C.X.; Crotty Alexander, L.E.; Que, L.G. Electronic Cigarettes: A Pro-Con Review of the Current Literature. J. Allergy Clin. Immunol. Pract. 2022. online ahead of print. [Google Scholar] [CrossRef]
- Allen, J.G.; Flanigan, S.S.; LeBlanc, M.; Vallarino, J.; MacNaughton, P.; Stewart, J.H.; Christiani, D.C. Flavoring Chemicals in E-Cigarettes: Diacetyl, 2,3-Pentanedione, and Acetoin in a Sample of 51 Products, Including Fruit-, Candy-, and Cocktail-Flavored E-Cigarettes. Environ. Health Perspect. 2016, 124, 733–739. [Google Scholar] [CrossRef]
- Cahn, Z.; Siegel, M. Electronic cigarettes as a harm reduction strategy for tobacco control: A step forward or a repeat of past mistakes? J. Public Health Policy 2011, 32, 16–31. [Google Scholar] [CrossRef]
- Hilty, M.; Wüthrich, T.M.; Godel, A.; Adelfio, R.; Aebi, S.; Burgener, S.S.; Illgen-Wilcke, B.; Benarafa, C. Chronic cigarette smoke exposure and pneumococcal infection induce oropharyngeal microbiota dysbiosis and contribute to long-lasting lung damage in mice. Microb. Genom. 2020, 6, mgen000485. [Google Scholar] [CrossRef]
- Yang, I.; Sandeep, S.; Rodriguez, J. The oral health impact of electronic cigarette use: A systematic review. Crit. Rev. Toxicol. 2020, 50, 97–127. [Google Scholar] [CrossRef]
- Shi, L.; Wu, Y.; Yang, C.; Ma, Y.; Zhaang, Q.Z.; Huang, W.; Zhu, X.Y.; Yan, Y.J.; Wang, J.X.; Zhu, T.; et al. Effect of nicotine on Staphylococcus aureus biofilm formation and virulence factors. Sci. Rep. 2019, 9, 20243. [Google Scholar] [CrossRef]
- Lacoma, A.; Edwards, A.M.; Young, B.C.; Dominguez, J.; Prat, C.; Laabei, M. Cigarette smoke exposure redirects Staphylococcus aureus to a virulence profile associated with persistent infection. Sci. Rep. 2019, 9, 10798. [Google Scholar] [CrossRef] [Green Version]
- Gilpin, D.F.; McGown, K.A.; Gallagher, K.; Bengoechea, J.; Dumigan, A.; Einarsson, G.; Elborn, J.S.; Tunney, M.M. Electronic cigarette vapour increases virulence and inflammatory potential of respiratory pathogens. Respir. Res. 2019, 20, 267. [Google Scholar] [CrossRef]
- Kulkarni, R.; Caskey, J.; Singh, S.K.; Paudel, S.; Baral, P.; Schexnayder, M.; Kim, J.; Kim, N.; Kosmider, B.; Ratner, A.J.; et al. Cigarette Smoke Extract-Exposed Methicillin-Resistant Staphylococcus aureus Regulates Leukocyte Function for Pulmonary Persistence. Am. J. Respir. Cell Mol. Biol. 2016, 55, 586–601. [Google Scholar] [CrossRef]
- Hwang, J.H.; Lyes, M.; Sladewski, K.; Enany, S.; McEachern, E.; Mathew, D.P.; Das, S.; Moshensky, A.; Bapat, S.; Pride, D.T.; et al. Electronic cigarette inhalation alters innate immunity and airway cytokines while increasing the virulence of colonizing bacteria. J. Mol. Med. 2016, 94, 667–679. [Google Scholar] [CrossRef]
- McEachern, E.K.; Hwang, J.H.; Sladewski, K.M.; Nicatia, S.; Dewitz, C.; Mathew, D.P.; Nizet, V.; Crotty Alexander, L.E. Analysis of the effects of cigarette smoke on staphylococcal virulence phenotypes. Infect. Immun. 2015, 83, 2443–2452. [Google Scholar] [CrossRef]
- Kulkarni, R.; Antala, S.; Wang, A.; Amaral, F.E.; Rampersaud, R.; Larussa, S.J.; Planet, P.J.; Ratner, A.J. Cigarette smoke increases Staphylococcus aureus biofilm formation via oxidative stress. Infect. Immun. 2012, 80, 3804–3811. [Google Scholar] [CrossRef]
- Manna, S.; Waring, A.; Papanicolaou, A.; Hall, N.E.; Bozinovski, S.; Dunne, E.M.; Satzke, C. The transcriptomic response of Streptococcus pneumoniae following exposure to cigarette smoke extract. Sci. Rep. 2018, 8, 15716. [Google Scholar] [CrossRef]
- Bagale, K.; Paudel, S.; Cagle, H.; Sigel, E.; Kulkarni, R. Electronic Cigarette (E-Cigarette) Vapor Exposure Alters the Streptococcus pneumoniae Transcriptome in a Nicotine-Dependent Manner without Affecting Pneumococcal Virulence. Appl. Environ. Microbiol. 2020, 86, e02125-19. [Google Scholar] [CrossRef]
- Cockeran, R.; Dix-Peek, T.; Dickens, C.; Steel, H.C.; Anderson, R.; Feldman, C. Biofilm formation and induction of stress response genes is a common response of several serotypes of the pneumococcus to cigarette smoke condensate. J. Infect. 2020, 80, 204–209. [Google Scholar] [CrossRef]
- Mutepe, N.D.; Cockeran, R.; Steel, H.C.; Theron, A.J.; Mitchell, T.J.; Feldman, C.; Anderson, R. Effects of cigarette smoke condensate on pneumococcal biofilm formation and pneumolysin. Eur. Respir. J. 2013, 41, 392–395. [Google Scholar] [CrossRef]
- Han, Y. Effects of cigarette smoking on the growth of Streptococcus mutans biofilms: An in vitro study. PLoS ONE 2021, 16, e0259895. [Google Scholar] [CrossRef]
- Rouabhia, M.; Semlali, A. Electronic cigarette vapor increases Streptococcus mutans growth, adhesion, biofilm formation, and expression of the biofilm-associated genes. Oral. Dis. 2021, 27, 639–647. [Google Scholar] [CrossRef]
- Chien, J.; Hwang, J.H.; Nilaad, S.; Masso-Silva, J.A.; Jeong Ahn, S.; McEachern, E.K.; Moshensky, A.; Byun, M.K.; Crotty Alexander, L.E. Cigarette Smoke Exposure Promotes Virulence of Pseudomonas aeruginosa and Induces Resistance to Neutrophil Killing. Infect. Immun. 2020, 88, e00527-20. [Google Scholar] [CrossRef]
- Bagaitkar, J.; Williams, L.R.; Renaud, D.E.; Bemakanakere, M.R.; Martin, M.; Scott, D.A.; Demuth, D.R. Tobacco-induced alterations to Porphyromonas gingivalis-host interactions. Environ. Microbiol. 2009, 11, 1242–1253. [Google Scholar] [CrossRef] [PubMed]
- Bagaitkar, J.; Demuth, D.R.; Daep, C.A.; Renaud, D.E.; Pierce, D.L.; Scott, D.A. Tobacco upregulates P. gingivalis fimbrial proteins which induce TLR2 hyposensitivity. PLoS ONE 2010, 5, e9323. [Google Scholar] [CrossRef]
- Baek, O.; Zhu, W.; Kim, H.C.; Lee, S.W. Effects of nicotine on the growth and protein expression of Porphyromonas gingivalis. J. Microbiol. 2012, 50, 143–148. [Google Scholar] [CrossRef]
- Cogo, K.; de Andrade, A.; Labate, C.A.; Bergamaschi, C.C.; Berto, L.A.; Franco, G.C.; Goncalves, R.B.; Groppo, F.C. Proteomic analysis of Porphyromonas gingivalis exposed to nicotine and cotinine. J. Periodontal. Res. 2012, 47, 766–775. [Google Scholar] [CrossRef]
- Donlan, R.M. Biofilms: Microbial life on surfaces. Emerg. Infect. Dis. 2002, 8, 881–890. [Google Scholar] [CrossRef]
- Parsek, M.R.; Singh, P.K. Bacterial biofilms: An emerging link to disease pathogenesis. Annu. Rev. Microbiol. 2003, 57, 677–701. [Google Scholar] [CrossRef] [PubMed]
- Crouzet, M.; Le Senechal, C.; Brozel, V.S.; Costaglioli, P.; Barthe, C.; Bonneu, M.; Garbay, B.; Vilain, S. Exploring early steps in biofilm formation: Set-up of an experimental system for molecular studies. BMC Microbiol. 2014, 14, 253. [Google Scholar] [CrossRef] [PubMed]
- Boisvert, A.A.; Cheng, M.P.; Sheppard, D.C.; Nguyen, D. Microbial Biofilms in Pulmonary and Critical Care Diseases. Ann. Am. Thorac. Soc. 2016, 13, 1615–1623. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goldstein-Daruech, N.; Cope, E.K.; Zhao, K.Q.; Vukovic, K.; Kofonow, J.M.; Doghramji, L.; González, B.; Chiu, A.G.; Kennedy, D.W.; Palmer, J.N.; et al. Tobacco smoke mediated induction of sinonasal microbial biofilms. PLoS ONE 2011, 6, e15700. [Google Scholar] [CrossRef]
- Cockeran, R.; Herbert, J.A.; Mitchell, T.J.; Dix-Peek, T.; Dickens, C.; Anderson, R.; Feldman, C. Exposure of a 23F serotype strain of Streptococcus pneumoniae to cigarette smoke condensate is associated with selective upregulation of genes encoding the two-component regulatory system 11 (TCS11). Biomed. Res. Int. 2014, 2014, 976347. [Google Scholar] [CrossRef]
- Novick, R.P.; Geisinger, E. Quorum sensing in staphylococci. Annu. Rev. Genet. 2008, 42, 541–564. [Google Scholar] [CrossRef]
- Boles, B.R.; Horswill, A.R. Agr-mediated dispersal of Staphylococcus aureus biofilms. PLoS Pathog. 2008, 4, e1000052. [Google Scholar] [CrossRef]
- Luong, T.T.; Lei, M.G.; Lee, C.Y. Staphylococcus aureus Rbf activates biofilm formation in vitro and promotes virulence in a murine foreign body infection model. Infect. Immun. 2009, 77, 335–340. [Google Scholar] [CrossRef]
- Cogo, K.; Calvi, B.M.; Mariano, F.S.; Franco, G.C.; Gonçalves, R.B.; Groppo, F.C. The effects of nicotine and cotinine on Porphyromonas gingivalis colonisation of epithelial cells. Arch. Oral. Biol. 2009, 54, 1061–1067. [Google Scholar] [CrossRef]
- Xu, M.; Zhang, H.; Yu, N.; Dong, Y.; Wang, W.; Chen, Y.; Kang, J. Cigarette smoke extract induces the Pseudomonas aeruginosa nfxC drug-resistant phenotype. J. Infect. Chemother. 2020, 26, 1278–1282. [Google Scholar] [CrossRef]
- Abdelmalek, S.M.A.; Alhadad, S.; Abu-Omar, O.; Afaneh, M.; Abu-Qatouseh, L.; Collier, P.J. The effect of cigarette smoke condensate (CSC) on Pseudomonas aeruginosa virulence and antibiotic sensitivity. J. Appl. Microbiol. 2022, 132, 3951–3958. [Google Scholar] [CrossRef]
- El Ahmer, O.R.; Essery, S.D.; Saadi, A.T.; Raza, M.W.; Ogilvie, M.M.; Weir, D.M.; Blackwell, C.C. The effect of cigarette smoke on adherence of respiratory pathogens to buccal epithelial cells. FEMS Immunol. Med. Microbiol. 1999, 23, 27–36. [Google Scholar] [CrossRef]
- Grigg, J.; Walters, H.; Sohal, S.S.; Wood-Baker, R.; Reid, D.W.; Xu, C.B.; Edvinsson, L.; Morissette, M.C.; Stämpfli, M.R.; Kirwan, M.; et al. Cigarette smoke and platelet-activating factor receptor dependent adhesion of Streptococcus pneumoniae to lower airway cells. Thorax 2012, 67, 908–913. [Google Scholar] [CrossRef] [Green Version]
- Miyashita, L.; Suri, R.; Dearing, E.; MMudway, I.; Dove, R.E.; Neill, D.R.; Van Zyl-Smit, R.; Kadioglu, A.; Grigg, J. E-cigarette vapour enhances pneumococcal adherence to airway epithelial cells. Eur. Respir. J. 2018, 51, 1701592. [Google Scholar] [CrossRef] [PubMed]
- Lewis, B.W.; Sultana, R.; Sharma, R.; Noel, A.; Langohr, I.; Patial, S.; Penn, A.L.; Saini, Y. Early Postnatal Secondhand Smoke Exposure Disrupts Bacterial Clearance and Abolishes Immune Responses in Muco-Obstructive Lung Disease. J. Immunol. 2017, 199, 1170–1183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Exposure a | Growth | Virulence b | Mouse Infection c | Comments d | Ref. | ||||
|---|---|---|---|---|---|---|---|---|---|
| BF | Adh | Inv | Strain | CFU | Survival | ||||
| Staphylococcus aureus (including MRSA) | |||||||||
| Nicotine |  | ↑ | ↑ * | ↓ $ | ND | ND | ND | * Adherence to polyethylene $ Invasion of A549 cells | [10] |
| CSE | ↓ | ↑ | ND | ↑ $ | ND | ND | ND | $ Invasion of bronchial epithelial cells | [11] |
| CSE |  |  | ND | ND | ND | ND | ↓ # | #Galleria mellonella infection model | [12] |
| EVENIC+ |  | ↑ | ND | ND | ND | ND | ↓ # | ||
| CSE | ND | ND | ND | ND | C57bl6 BALB/c A/J | ↑ ↑ ↑ | ND ND ND | [13] | |
| EVENIC+ | ↓ | ↑ | ↑ * | ↑ * | CD1 | ↑ | ↓ | * Adherence to, * invasion of HaCaT | [14] |
| CSE | ↓ | ↑ * | ↑ * | CD1 | ↑ | ↓ | * Adherence to, * invasion of HaCaT | [15] | |
| CSE | ND | ↑ | ↑ * | ND | ND | ND | ND | * Adherence to A549 cells and to human fibronectin | [16] |
| Streptococcus pneumoniae | |||||||||
| CSE |  | ND |  * * | ND | ND | ND | ND | * Adherence to A549 cells | [17] |
| CSE | ND | ↑ | ND | ND | ND |  | ND | [18] | |
| EVENIC+ | ND | ↑ | ND | ND | ND |  | ND | ||
| EVENIC− | ND |  | ND | ND | ND |  | ND | ||
| CSC | ND | ↑ | ND | ND | ND | ND | ND | [19] | |
| CSE |  | ↑ | ND | ND | ND | ND | ↓ # | #Galleria mellonella infection model | [12] |
| EVENIC+ |  |  | ND | ND | ND | ND | ↓ # | ||
| CSC |  | ↑ | ND | ND | ND | ND | ND | [20] | |
| Streptococcus mutans | |||||||||
| CS | ND | ↓ ^ | ND | ND | ND | ND | ND | ^ Biofilms pre-formed on hydroxyapatite discs showed reduced biomass upon CS exposure | [21] |
| CS | ND | ↑ | ↑ * | ND | ND | ND | ND | * Adherence of pre-exposed S. mutans to human teeth was examined in vitro | [22] |
| EVNIC+ | ND | ↑ | ↑ * | ND | ND | ND | ND | ||
| EVNIC- | ND |  |  * * | ND | ND | ND | ND | ||
| Pseudomonas aeruginosa | |||||||||
| CSE | ↓ | ↑ | ND | ND | CD1 | ND | ↓ | [23] | |
| CSE |  | ↑ | ND | ND | ND | ND | ↓ # | #Galleria mellonella infection model | [12] |
| EVENIC+ |  | ↑ | ND | ND | ND | ND | ↓ # | ||
| Haemophilus influenzae | |||||||||
| CSE |  |  | ND | ND | ND | ND | ↓ # | #Galleria mellonella infection model | [12] |
| EVENIC+ |  |  | ND | ND | ND | ND | ↓ # | ||
| Porphyromonas gingivalis | |||||||||
| CSE |  | ND | ND | ND | ND | ND | ND | [24] | |
| CSE | ND | ↑ | ↑ * | ND | ND | ND | ND | * Increased adherence to fibronectin | [25] |
 indicates no change, ↑ increase, ↓ decrease, and ND indicates not done. Comments d also define the cell lines or biotic materials used in adherence * assays and the cell lines used in invasion $ assays.
indicates no change, ↑ increase, ↓ decrease, and ND indicates not done. Comments d also define the cell lines or biotic materials used in adherence * assays and the cell lines used in invasion $ assays.| Pathogen | Exposure a | Technique | DEG b | Ref. | ||||
|---|---|---|---|---|---|---|---|---|
| Med | Form | Time | Total | Up | Down | |||
| S. pneumoniae | THY c | CSE | 120 | RNASeq | 264 | 188 | 76 | [18] |
| EVENIC+ | 982 | 500 | 482 | |||||
| EVENIC− | 14 | 14 | 0 | |||||
| S. pneumoniae | THY c | CSE | 45 | RNASeq | 122 | 59 | 63 | [17] |
| S. aureus | TS d | CSE | 360 | RNASeq | 344 | 204 | 140 | [13] |
| P. gingivalis | GAM e | CSE | NA | Microarray | 104 | 58 | 46 | [24] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bagale, K.; Kulkarni, R. A Systematic Review of the Literature Examining the Effects of Cigarette Smoke and e-Cigarette Vapor on the Virulence of Human Pathogenic Bacteria. Int. J. Environ. Res. Public Health 2022, 19, 12518. https://doi.org/10.3390/ijerph191912518
Bagale K, Kulkarni R. A Systematic Review of the Literature Examining the Effects of Cigarette Smoke and e-Cigarette Vapor on the Virulence of Human Pathogenic Bacteria. International Journal of Environmental Research and Public Health. 2022; 19(19):12518. https://doi.org/10.3390/ijerph191912518
Chicago/Turabian StyleBagale, Kamal, and Ritwij Kulkarni. 2022. "A Systematic Review of the Literature Examining the Effects of Cigarette Smoke and e-Cigarette Vapor on the Virulence of Human Pathogenic Bacteria" International Journal of Environmental Research and Public Health 19, no. 19: 12518. https://doi.org/10.3390/ijerph191912518
APA StyleBagale, K., & Kulkarni, R. (2022). A Systematic Review of the Literature Examining the Effects of Cigarette Smoke and e-Cigarette Vapor on the Virulence of Human Pathogenic Bacteria. International Journal of Environmental Research and Public Health, 19(19), 12518. https://doi.org/10.3390/ijerph191912518






