Cultural Adaptation and Validation of the Premature Infant Pain Profile-Revised (PIPP-R) Pain Measurement Scale: Research Protocol
Abstract
:1. Introduction
2. Materials and Methods
2.1. Aims
- To culturally adapt the scale and its instructions for use in the Spanish healthcare context.
- To explore the validity of this adaptation.
- To measure its reliability.
2.2. Design
2.2.1. Translation and Back-Translation
2.2.2. Content Validity
2.2.3. Multicenter Study
2.2.4. Study Setting
- Intermediate or Basic Neonatal Care: care of NBs of gestational age above 32 weeks or weighing more than 1500 grams with a mild condition requiring special intermediate care techniques.
- Neonatal Intensive Care: care of NBs with a life-threatening medical/surgical condition requiring special treatment and care on a continuous basis.
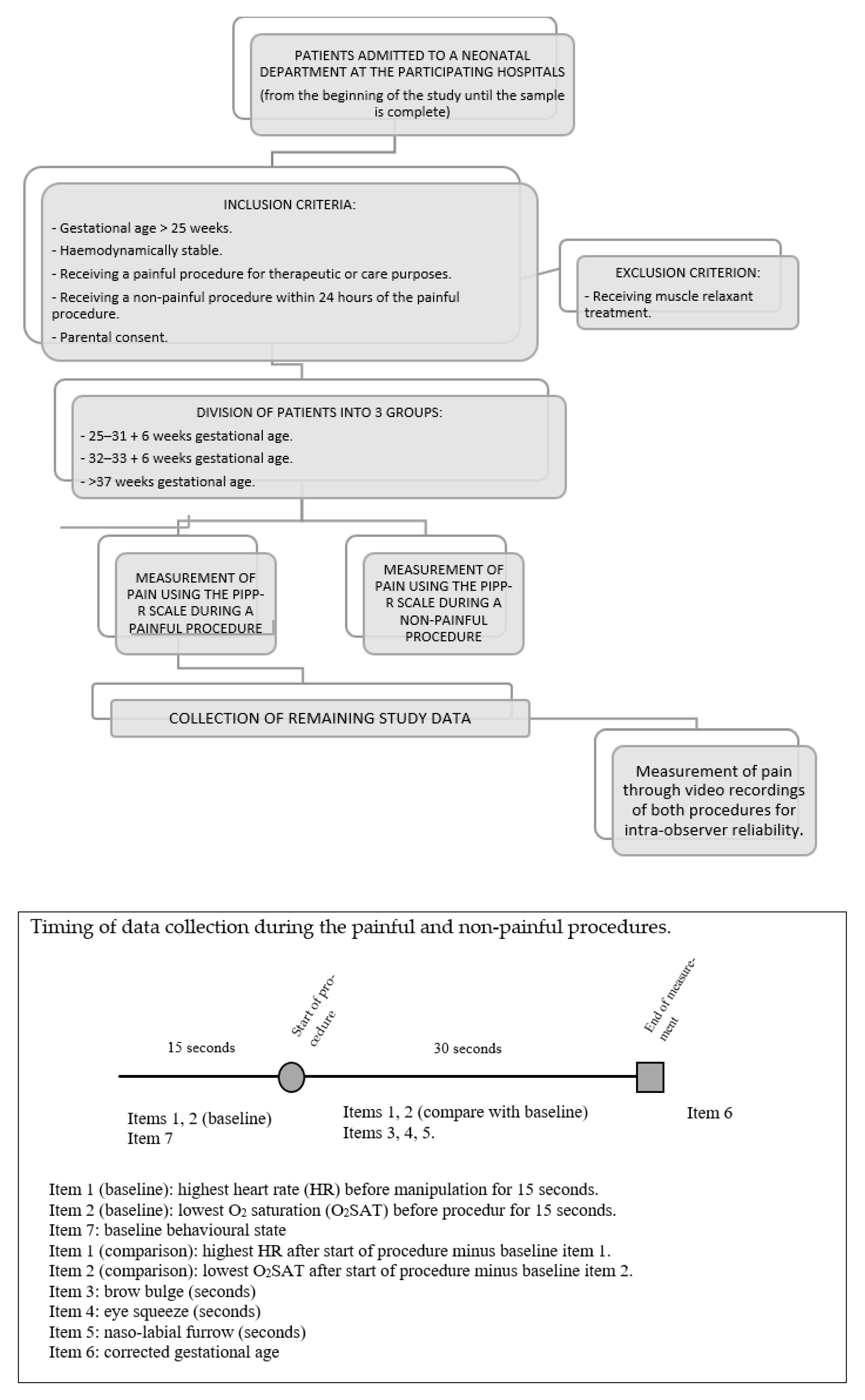
2.2.5. Participants
2.2.6. Sample
- Very and extremely premature infant: 25–31 + 6 weeks gestational age.
- Moderately premature infant: 32–36 + 6 weeks gestational age.
- Term infant: >37 weeks gestational age.
2.2.7. Study Measures
Outcome Variables: Pain Score as Measured by the PIPP-R scale in Spanish
2.2.8. Validation of the Scale
Feasibility of the Scale: Survey of the Nurses Collaborating in the Study
2.2.9. Recruitment and Data Collection
- Observe infant for 15 s at rest (without manipulation) and assess vital sign indicators (highest heart rate and lowest oxygen saturation) and baseline behavioral state.
- Observe infant for 30 s after procedure and assess change in vital sign indicators and duration of facial actions observed.
- If the sub-total is >0, score for corrected gestational age and baseline behavioral state and calculate the total score by adding all sub-scores.
2.2.10. Data Analysis
2.2.11. Ethical Considerations
3. Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Goksan, S.; Hartley, C.; Emery, F.; Cockrill, N.; Poorun, R.; Moultrie, F.; Rogers, R.; Campbell, J.; Sanders, M.; Adams, E.; et al. fMRI reveals neural activity overlap between adult and infant pain. eLife 2015, 4, e06356. [Google Scholar] [CrossRef] [PubMed]
- Committee on Fetus and Newborn and Section on Anesthesiology and Pain Medicine; Keels, E.; Sethna, N.; Watterberg, K.L.; Cummings, J.J.; Benitz, W.E.; Eichenwald, E.C.; Poindexter, B.B.; Stewart, D.L.; Aucott, S.W.; et al. Prevention and Management of Procedural Pain in the Neonate: An Update. Pediatrics 2016, 137, e20154271. [Google Scholar] [CrossRef]
- Carbajal, R.; Eriksson, M.; Courtois, E.; Boyle, E.; Avila-Alvarez, A.; Andersen, R.D.; Sarafidis, K.; Polkki, T.; Matos, C.; Lago, P.; et al. Sedation and analgesia practices in neonatal intensive care units (EUROPAIN): Results from a prospective cohort study. Lancet Respir. Med. 2015, 3, 796–812. [Google Scholar] [CrossRef]
- Avila-Alvarez, A.; Carbajal, R.; Courtois, E.; Pertega-Diaz, S.; Anand, K.J.; Muñiz-Garcia, J.; Grupo Español del Proyecto Europain. Valoración clínica del dolor en unidades de cuidados intensivos neonatales españolas [Clinical assessment of pain in Spanish Neonatal Intensive Care Units]. An. Pediatr. 2016, 85, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Giordano, V.; Edobor, J.; Deindl, P.; Wildner, B.; Goeral, K.; Steinbauer, P.; Werther, T.; Berger, A.; Olischar, M. Pain and Sedation Scales for Neonatal and Pediatric Patients in a Preverbal Stage of Development: A Systematic Review. JAMA Pediatr. 2019, 173, 1186–1197. [Google Scholar] [CrossRef] [PubMed]
- Anand, K.; Hickey, P. Pain and Its Effects in the Human Neonate and Fetus. N. Engl. J. Med. 1987, 317, 1321–1329. [Google Scholar] [CrossRef]
- Brummelte, S.; Grunau, R.E.; Chau, V.; Poskitt, K.J.; Brant, R.; Ba, J.V.; Gover, A.; Synnes, A.R.; Miller, S. Procedural pain and brain development in premature newborns. Ann. Neurol. 2012, 71, 385–396. [Google Scholar] [CrossRef] [PubMed]
- Walker, S.M.; Tochiki, K.K.; Fitzgerald, M. Hindpaw incision in early life increases the hyperalgesic response to repeat surgical injury: Critical period and dependence on initial afferent activity. Pain 2009, 147, 99–106. [Google Scholar] [CrossRef] [PubMed]
- McPherson, C.; Miller, S.; El-Dib, M.; Massaro, A.N.; Inder, T.E. The influence of pain, agitation, and their management on the immature brain. Pediatr. Res. 2020, 88, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Carbajal, R.; Rousset, A.; Danan, C.; Coquery, S.; Nolent, P.; Ducrocq, S.; Saizou, C.; Lapillonne, A.; Granier, M.; Durand, P.; et al. Epidemiology and Treatment of Painful Procedures in Neonates in Intensive Care Units. JAMA 2008, 300, 60–70. [Google Scholar] [CrossRef] [Green Version]
- Cruz, D.; Fernandes, A.; Oliveira, C.R. Epidemiology of painful procedures performed in neonates: A systematic review of observational studies. Eur. J. Pain 2016, 20, 489–498. [Google Scholar] [CrossRef] [PubMed]
- Stevens, B.; Johnston, C.; Petryshen, P.; Taddio, A. Premature Infant Pain Profile: Development and Initial Validation. Clin. J. Pain 1996, 12, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Gibbins, S.; Stevens, B.J.; Yamada, J.; Dionne, K.; Campbell-Yeo, M.; Lee, G.; Caddell, K.; Johnston, C.; Taddio, A. Validation of the Premature Infant Pain Profile-Revised (PIPP-R). Early Hum. Dev. 2014, 90, 189–193. [Google Scholar] [CrossRef]
- Perry, M.; Tan, Z.; Chen, J.; Weidig, T.; Xu, W.; Cong, X.S. Neonatal Pain: Perceptions and Current Practice. Crit. Care Nurs. Clin. N. Am. 2018, 30, 549–561. [Google Scholar] [CrossRef]
- Chan, A.-W.; Tetzlaff, J.M.; Gøtzsche, P.C.; Altman, D.G.; Mann, H.; A Berlin, J.; Dickersin, K.; Hróbjartsson, A.; Schulz, K.F.; Parulekar, W.R.; et al. SPIRIT 2013 explanation and elaboration: Guidance for protocols of clinical trials. BMJ 2013, 346, e7586. [Google Scholar] [CrossRef]
- Wild, D.; Grove, A.; Martin, M.; Eremenco, S.; McElroy, S.; Verjee-Lorenz, A.; Erikson, P. Principles of Good Practice for the Translation and Cultural Adaptation Process for Patient-Reported Outcomes (PRO) Measures: Report of the ISPOR Task Force for Translation and Cultural Adaptation. Value Health 2005, 8, 94–104. [Google Scholar] [CrossRef] [PubMed]
- Quatrini Carvalho Passos Guimarães, H.C.; Pena, S.B.; Lopes, J.D.L.; Lopes, C.T.; Barros, A. Experts for Validation Studies in Nursing: New Proposal and Selection Criteria. Int. J. Nurs. Knowl. 2016, 27, 130–135. [Google Scholar] [CrossRef]
- Lynn, M.R. Determination and quantification of content validity. Nurs. Res. 1986, 35, 382–385. [Google Scholar] [CrossRef]
- Yaghmale, F. Content validity and its estimation. J. Med. Educ. 2003, 3, 25–27. [Google Scholar]
- Polit, D.F.; Beck, C.T. The content validity index: Are you sure you know what’s being reported? critique and recommendations. Res. Nurs. Health 2006, 29, 489–497. [Google Scholar] [CrossRef]
- Rite, S.; Fernández, J.R.; Echániz, I.; Botet Musson, F.; Herranz, G.; Moreno, J.; Salguero, E.; Sánchez, M.; Comité de Estándares y la Junta Directiva de la Sociedad Española de Neonatología. Niveles asistenciales y recomendaciones de mínimos para la atención neonatal [Health care levels and minimum recommendations for neonatal care]. An. Pediatr. 2013, 79, 51.e1–51.e11. [Google Scholar] [CrossRef]
- Streiner, D.L.; Kottner, J. Recommendations for reporting the results of studies of instrument and scale development and testing. J. Adv. Nurs. 2014, 70, 1970–1979. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Villegas, A.; Razquin, C.; Martínez-González, M.Á. Análisis factorial [Factor Analysis]. In Bioestadística Amigable [Friendly Biostatistics], 4th ed.; Martínez-González, M.A., Ed.; Elsevier: Madrid, Spain, 2020; pp. e31–e55. [Google Scholar]
- Campbell-Yeo, M.; Eriksson, M.; Benoit, B. Assessment and Management of Pain in Preterm Infants: A Practice Update. Children 2022, 9, 244. [Google Scholar] [CrossRef] [PubMed]
- Speyer, R.; Cordier, R.; Bouix, C.; Gallois, Y.; Woisard, V. Using Classical Test Theory to Determine the Psychometric Properties of the Deglutition Handicap Index. Dysphagia 2022, 37, 65–73. [Google Scholar] [CrossRef]
- Streiner, D.L.; Norman, G.R.; Cairney, J. Health Measurement Scales: A Practical Guide to Their Development and Use; Oxford University Press: Oxford, UK, 2003; Volume 37, pp. 65–73. [Google Scholar]
- Argimon, J.M.; Jiménez, J. Métodos de Investigación Clínica y Epidemiología, 5th ed.; Elsevier: Madrid, Spain, 2020. [Google Scholar]
- Schiller, C.J. Clinical Utility of Two Neonatal Pain Assessment Measures. Ph.D. Thesis, University of Toronto, Toronto, ON, Canada, 1999. Available online: https://tspace.library.utoronto.ca/handle/1807/13107 (accessed on 22 June 2022).
- Fitri, S.Y.R.; Lusmilasari, L.; Juffrie, M. The Indonesian version of the Premature Infant Pain Profile–Revised: Translation and adaptation of a neonatal pain assessment. Int. J. Nurs. Sci. 2019, 6, 439–444. [Google Scholar] [CrossRef]
- Olsson, E.; Anderzén-Carlsson, A.; Atladóttir, S.M.; Axelin, A.; Campbell-Yeo, M.; Eriksson, M.; Kristjánsdóttir, G.; Peltonen, E.; Stevens, B.; Vederhus, B.; et al. Cultural adaptation and harmonization of four Nordic translations of the revised Premature Infant Pain Profile (PIPP-R). BMC Pediatr. 2018, 18, 349. [Google Scholar] [CrossRef]
- Bueno, M.; Ramos, M.C.M.; Forni, E.; Kimura, A.F. Adaptation and Initial Validation of the Premature Infant Pain Profile–Revised (PIPP-R) in Brazil. Pain Manag. Nurs. 2019, 20, 512–515. [Google Scholar] [CrossRef]
- Taplak, A.; Bayat, M. Psychometric Testing of the Turkish Version of the Premature Infant Pain Profile Revised-PIPP-R. J. Pediatr. Nurs. 2019, 48, e49–e55. [Google Scholar] [CrossRef]
| Scale´s Name | Characteristics of Scale | Use to Gestational Age | |||
|---|---|---|---|---|---|
| Multidimensional | Behavioural | Number of Variable | Preterm | Term | |
| ABC pain scale | no | yes | univariable | yes | yes |
| Acute Pain in Newborns | no | yes | univariable | yes | yes |
| Adapted COMFORT | yes | no | multivariable | yes | no |
| Behavioral Indicators of Infant Pain | no | yes | multivariable | yes | no |
| COMFORT-Behavior Scale | no | yes | multivariable | yes | yes |
| COVERS neonatal pain scale | yes | no | multivariable | yes | yes |
| CRIES Scale | yes | no | multivariable | yes | yes |
| Faceless Acute Neonatal Pain Scale | yes | no | multivariable | yes | no |
| Harrison | yes | no | multivariable | yes | yes |
| Infant Body Coding System | no | yes | multivariable | yes | yes |
| Neonatal Acute Pain Assessment Scale | yes | no | multivariable | yes | yes |
| Neonatal Infant Pain Scale | no | yes | multivariable | yes | yes |
| Neonatal Pain, Agitation and Sedation Scale | yes | no | multivariable | yes | no |
| Nepean Neonatal Intensive Care Unit Pain Assessment Tool | yes | no | multivariable | yes | no |
| Observational visual analog scale | no | yes | univariable | yes | yes |
| Pain assessment scale for preterm infants | yes | no | multivariable | yes | no |
| Premature Infant Pain Profile | yes | no | multivariable | yes | yes |
| Premature Infant Pain Profile Revised | yes | no | multivariable | yes | yes |
| Scale for Use in Newborns | yes | no | multivariable | yes | yes |
| Criterion | Score | Expert Rating |
|---|---|---|
| Doctorate | 4 points | 2.69 |
| Doctoral thesis on neonatal pain | 1 point | 0.22 |
| Clinical experience with neonates | 1 point per year | 9.22 |
| Research projects in neonatal pain | 1 point | 0.67 |
| Publications in neonatal pain | 1 point | 0.44 |
| Specific training in neonatal pain | 2 points | 1.56 |
| Participation in a working group on neonatal pain | 1 point | 0.78 |
| Delivery of specific training in neonatal pain | 2 points | 0.67 |
| Total score | ||
| Activity/Measurement | Team Member | Estimated Completion Time | Before Assessment of Procedural Pain | During Assessment of Procedural Pain | After Assessment of Procedural Pain | |
|---|---|---|---|---|---|---|
| Intervention | Measurement of painful procedure | Coordinator and trained professional | 1 min | x | ||
| Measurement of non-painful procedure | Coordinator and trained professional | 1 min | x | |||
| Pain assessment from video recordings | Coordinator and trained professional | 4 min | x | |||
| Measurements | Highest baseline HR | Coordinator and trained professional | 15 s | x | ||
| Lowest baseline O2SAT | Coordinator and trained professional | 15 s | x | |||
| Baseline behaviour | Coordinator and trained professional | 15 s | x | |||
| Highest HR during procedure | Coordinator and trained professional | 30 s | x | |||
| Lowest O2SAT during procedure | Coordinator and trained professional | 30 s | x | |||
| HR comparison (proc-baseline) | Coordinator and trained professional | 10 s | x | |||
| O2SAT comparison (proc-baseline) | Coordinator and trained professional | 10 s | x | |||
| Intensity of brow bulge (sec) | Coordinator and trained professional | 30 s | x | |||
| Intensity of eye squeeze (sec) | Coordinator and trained professional | 30 s | x | |||
| Intensity of naso-labial furrow (sec) | Coordinator and trained professional | 30 s | x | |||
| Sub-total score | Coordinator and trained professional | 10 s | x | |||
| Corrected gestational age | Coordinator and trained professional | 1 min | x | |||
| Total score | Coordinator and trained professional | 10 s | x | |||
| Patient data | Birth weight | Coordinator | 1 min | x | ||
| Corrected gestational age | Coordinator | 1 min | x | |||
| Sex | Coordinator | 1 min | x | |||
| Diagnosis on admission | Coordinator | 1 min | x | |||
| Days of life | Coordinator | 1 min | x | |||
| Pain relief | Sucrose | Coordinator | 1 min | x | ||
| Breastfeeding | Coordinator | 1 min | x | |||
| Expressed breast milk | Coordinator | 1 min | x | |||
| Restraints | Coordinator | 1 min | x | |||
| Non-nutritive sucking | Coordinator | 1 min | x | |||
| Intermittent pharmacological analgesia 2 hours prior | Coordinator | 1 min | x | |||
| Continuous pharmacological analgesia 2 hours prior | Coordinator | 1 min | x | |||
| Drug used for pharmacological analgesia | Coordinator | 1 min | x | |||
| Trained professional data | Years of experience in neonatal care | Coordinator | 1 min | x | ||
| Age | Coordinator | 1 min | x | |||
| Sex | Coordinator | 1 min | x | |||
| Hospital | Coordinator | 1 min | x |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Núñez-López, I.; Collados-Gómez, L.; Abalo, R.; Martínez-Pérez, P.; Moreno-Vicente, Á.; Cid-Expósito, M.-G. Cultural Adaptation and Validation of the Premature Infant Pain Profile-Revised (PIPP-R) Pain Measurement Scale: Research Protocol. Int. J. Environ. Res. Public Health 2022, 19, 12338. https://doi.org/10.3390/ijerph191912338
Núñez-López I, Collados-Gómez L, Abalo R, Martínez-Pérez P, Moreno-Vicente Á, Cid-Expósito M-G. Cultural Adaptation and Validation of the Premature Infant Pain Profile-Revised (PIPP-R) Pain Measurement Scale: Research Protocol. International Journal of Environmental Research and Public Health. 2022; 19(19):12338. https://doi.org/10.3390/ijerph191912338
Chicago/Turabian StyleNúñez-López, Irene, Laura Collados-Gómez, Raquel Abalo, Patricia Martínez-Pérez, Álvaro Moreno-Vicente, and María-Gema Cid-Expósito. 2022. "Cultural Adaptation and Validation of the Premature Infant Pain Profile-Revised (PIPP-R) Pain Measurement Scale: Research Protocol" International Journal of Environmental Research and Public Health 19, no. 19: 12338. https://doi.org/10.3390/ijerph191912338
APA StyleNúñez-López, I., Collados-Gómez, L., Abalo, R., Martínez-Pérez, P., Moreno-Vicente, Á., & Cid-Expósito, M.-G. (2022). Cultural Adaptation and Validation of the Premature Infant Pain Profile-Revised (PIPP-R) Pain Measurement Scale: Research Protocol. International Journal of Environmental Research and Public Health, 19(19), 12338. https://doi.org/10.3390/ijerph191912338








