Long-Term Impact of COVID-19 Pandemic in Sleep Quality and Lifestyle in Young Adults
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Statistical Analysis
3. Results
3.1. Demographic Characteristics
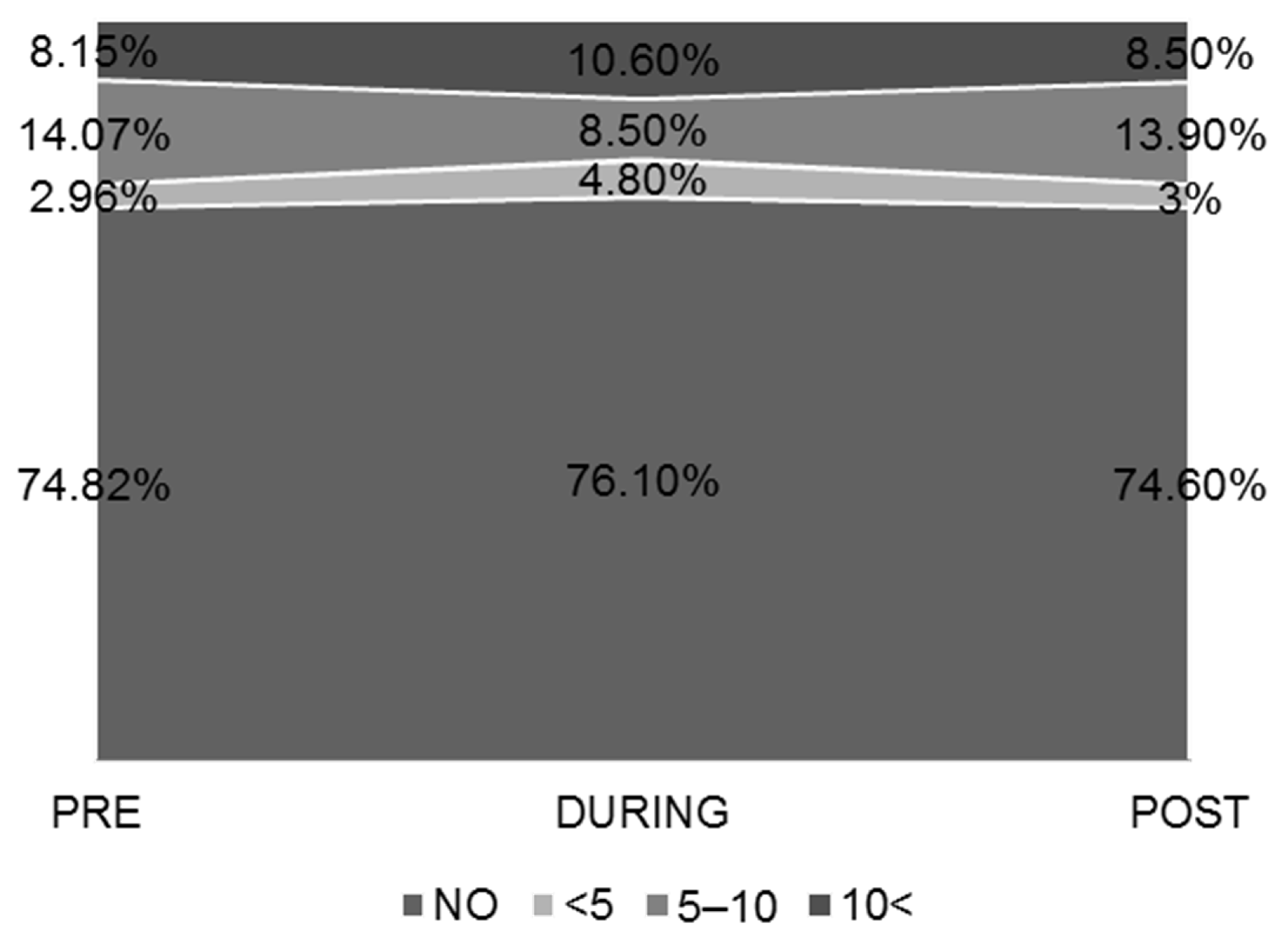
3.2. Sleep–Wake Habits
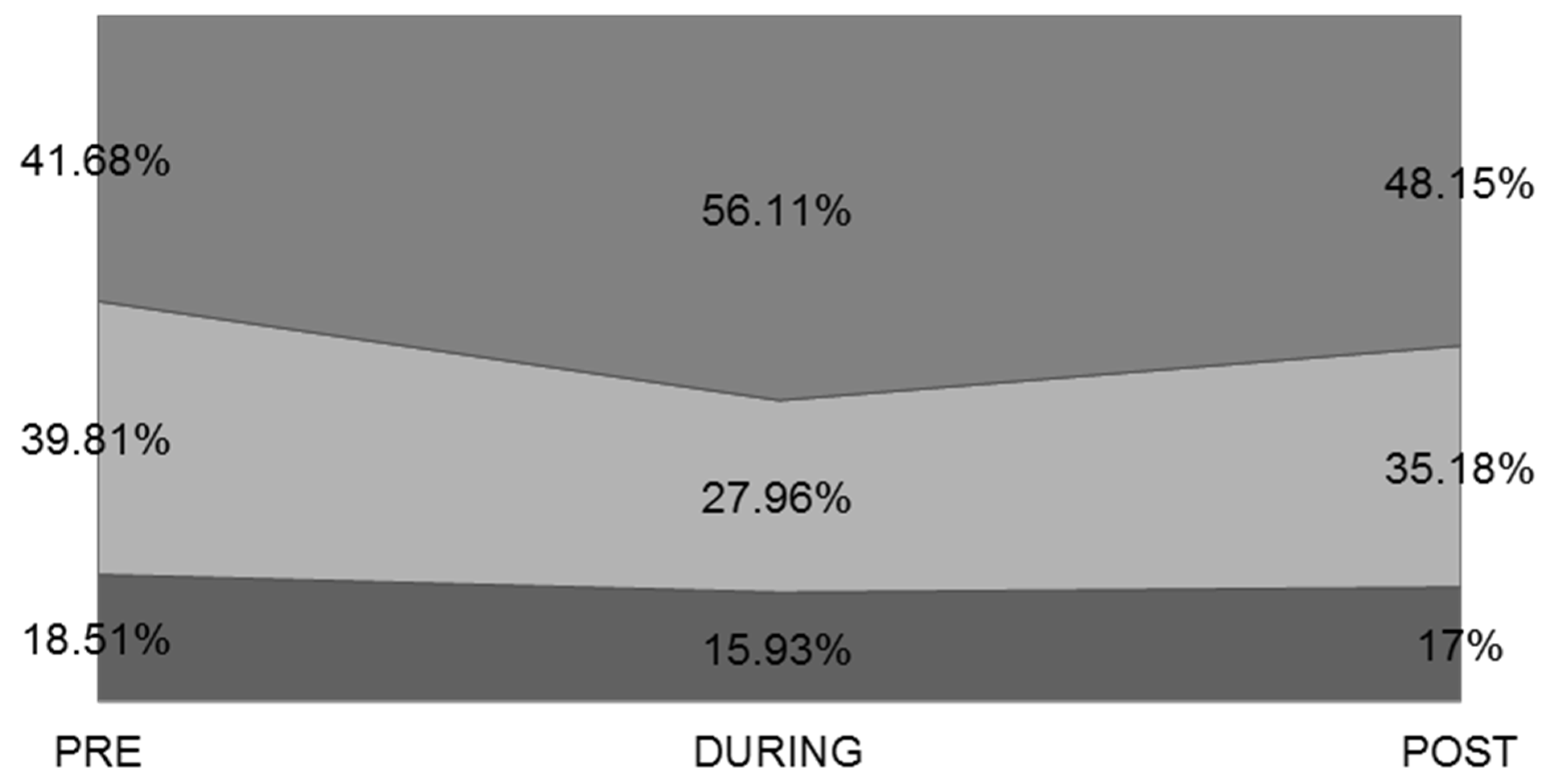
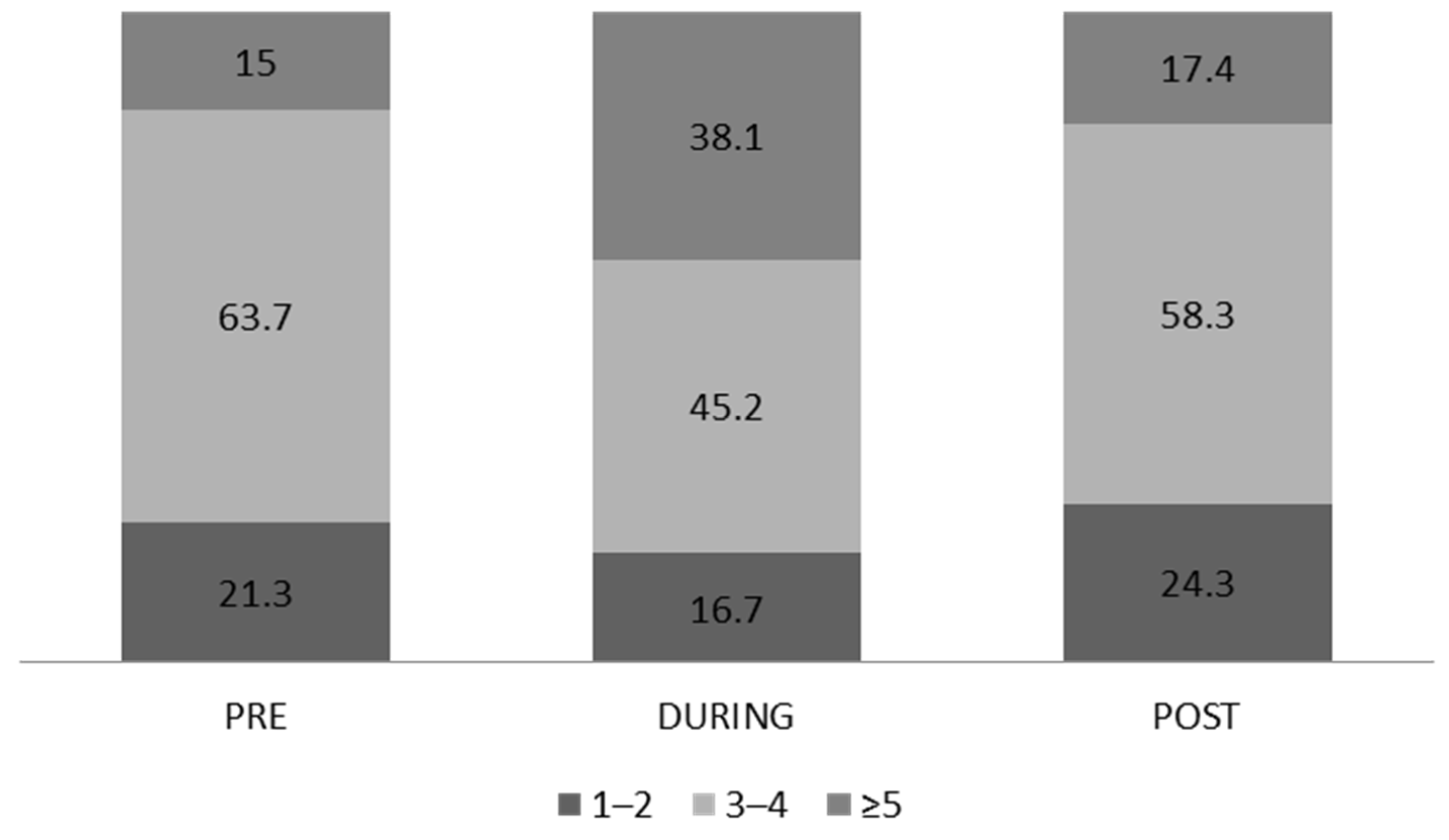
3.3. Dietary Habits and Consumption of Alcoholic Beverages
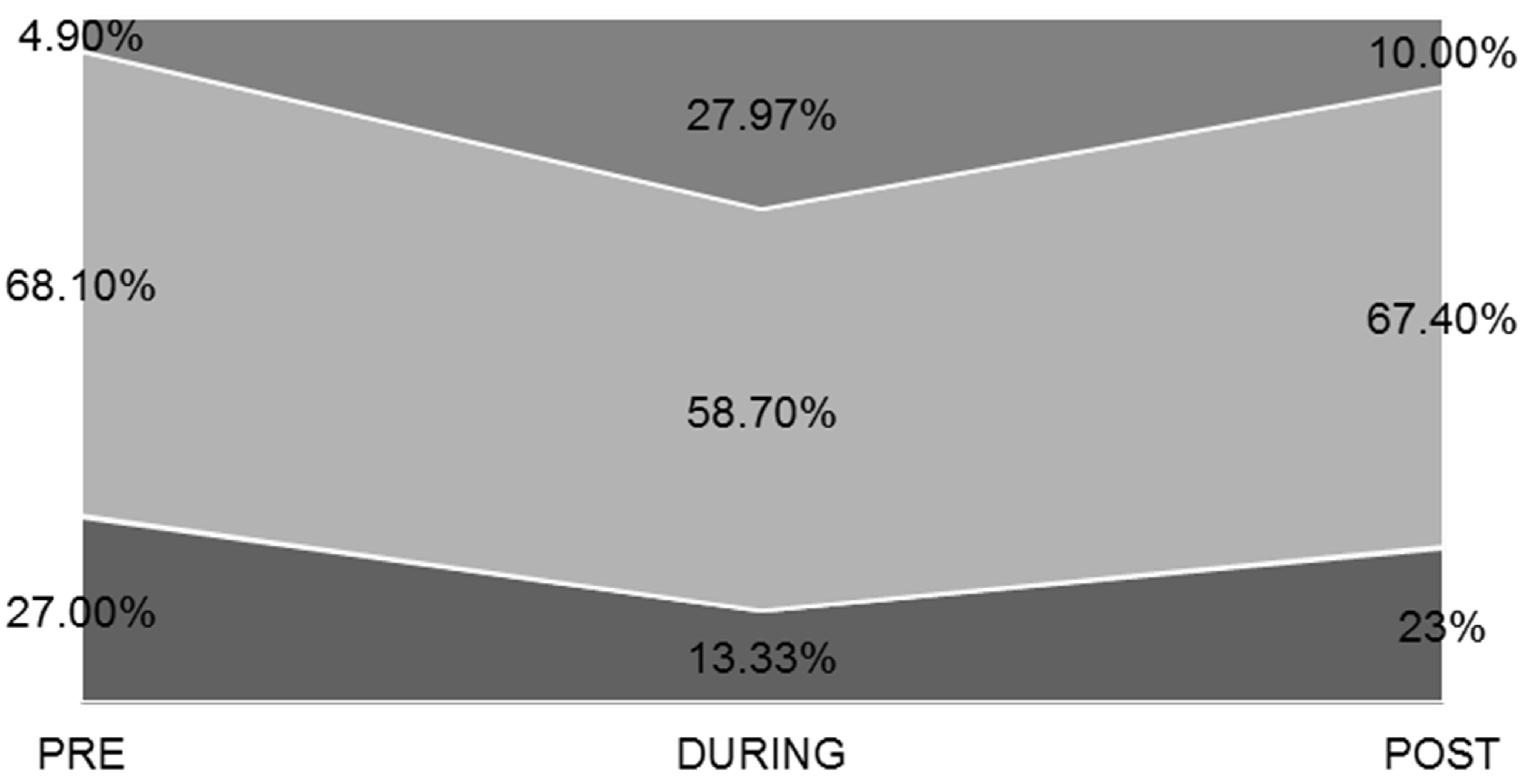
3.4. Physical Activity-Exercise
3.5. Bivariate Correlations
3.6. Predictors of POST Lockdown Variables
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Parasher, A. COVID-19: Current understanding of its pathophysiology, clinical presentation and treatment. Postgrad. Med. J. 2021, 97, 312–320. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. Basics of COVID-19. Available online: https://www.cdc.gov/coronavirus/2019-ncov/your-health/about-covid-19/basics-covid-19.html (accessed on 11 July 2021).
- Pollard, C.A.; Morran, M.P.; Kalinoski, A.L. The COVID-19 pandemic: A global health crisis. Physiol. Genom. 2020, 52, 549–557. [Google Scholar] [CrossRef] [PubMed]
- Kotsiou, O.S.; Papagiannis, D.; Fradelos, E.C.; Perlepe, G.; Miziou, A.; Siachpazidou, D.S.; Gourgoulianis, K.I. Understanding COVID-19 epidemiology and implications for control: The experience from a Greek semi-closed community. J. Clin. Med. 2021, 10, 2765. [Google Scholar] [CrossRef] [PubMed]
- Iliopoulos, D.C. COVID-19: A Greek perspective. J. Card. Surg. 2021, 36, 1637–1640. [Google Scholar] [CrossRef]
- Kalaitzaki, A.; Rovithis, M. Secondary traumatic stress and vicarious posttraumatic growth in healthcare workers during the first COVID-19 lockdown in Greece: The role of resilience and coping strategies. Psychiatriki 2021, 32, 19–25. [Google Scholar] [CrossRef]
- Clemente-Suárez, V.J.; Dalamitros, A.A.; Beltran-Velasco, A.I.; Mielgo-Ayuso, J.; Tornero-Aguilera, J.F. Social and psychophysiological consequences of the COVID-19 pandemic: An extensive literature review. Front. Psychol. 2020, 11, 580225. [Google Scholar] [CrossRef]
- Banerjee, D.; Rai, M. Social isolation in COVID-19: The impact of loneliness. Int. J. Soc. Psychiatry 2020, 66, 525–527. [Google Scholar] [CrossRef]
- Sivakanthan, S.; Pan, J.; Kim, L.; Ellenbogen, R.; Saigal, R. Economic impact of COVID-19 on a high-volume academic neurosurgical practice. World Neurosurg. 2020, 143, e561–e566. [Google Scholar] [CrossRef]
- Sepúlveda-Loyola, W.; Rodríguez-Sánchez, I.; Pérez-Rodríguez, P.; Ganz, F.; Torralba, R.; Oliveira, D.V.; Rodríguez-Mañas, L. Impact of social isolation due to COVID-19 on health in older people: Mental and physical effects and recommendations. J. Nutr. Health Aging 2020, 24, 938–947. [Google Scholar] [CrossRef]
- Haleem, A.; Javaid, M.; Vaishya, R. Effects of COVID-19 pandemic in daily life. Curr. Med. Res. Pract. 2020, 10, 78–79. [Google Scholar] [CrossRef]
- Peteet, J.R. COVID-19 anxiety. J. Relig. Health 2020, 59, 2203–2204. [Google Scholar] [CrossRef] [PubMed]
- Blustein, D.L.; Duffy, R.; Ferreira, J.A.; Cohen-Scali, V.; Cinamon, R.G.; Allan, B.A. Unemployment in the time of COVID-19: A research agenda. J. Vocat. Behav. 2020, 119, 103436. [Google Scholar] [CrossRef] [PubMed]
- Hwang, T.J.; Rabheru, K.; Peisah, C.; Reichman, W.; Ikeda, M. Loneliness and social isolation during the COVID-19 pandemic. Int. Psychogeriatr. 2020, 32, 1217–1220. [Google Scholar] [CrossRef]
- Morrissette, M. School closures and social anxiety during the COVID-19 pandemic. J. Am. Acad. Child Adolesc. Psychiatr. 2021, 60, 6–7. [Google Scholar] [CrossRef] [PubMed]
- Machado, C.L.F.; Pinto, R.S.; Brusco, C.M.; Cadore, E.L.; Radaelli, R. COVID-19 pandemic is an urgent time for older people to practice resistance exercise at home. Exp. Gerontol. 2020, 141, 111101. [Google Scholar] [CrossRef]
- Sidor, A.; Rzymski, P. Dietary choices and habits during COVID-19 lockdown: Experience from Poland. Nutrients 2020, 12, 1657. [Google Scholar] [CrossRef]
- Schwendinger, F.; Pocecco, E. Counteracting physical inactivity during the COVID-19 pandemic: Evidence-based recommendations for home-based exercise. Int. J. Environ. Res. Public Health 2020, 17, 3909. [Google Scholar] [CrossRef]
- Grogan, S.; Walker, L.; McChesney, G.; Gee, I.; Gough, B.; Cordero, M.I. How has COVID-19 lockdown impacted smoking? A thematic analysis of written accounts from UK smokers. Psychol. Health 2020, 37, 17–33. [Google Scholar] [CrossRef]
- Jackson, S.E.; Garnett, C.; Shahab, L.; Oldham, M.; Brown, J. Association of the COVID-19 lockdown with smoking, drinking and attempts to quit in England: An analysis of 2019–2020 data. Addiction 2021, 116, 1233–1244. [Google Scholar] [CrossRef]
- Wheaton, A.E.; Chapman, D.P.; Croft, J.B. School start times, sleep, behavioral, health, and academic outcomes: A review of the literature. J. Sch. Health 2016, 86, 363–381. [Google Scholar] [CrossRef] [Green Version]
- Silva, E.S.M.; Ono, B.H.V.S.; Souza, J.C. Sleep and immunity in times of COVID-19. Rev. Assoc. Med. Bras. 2020, 66, 143–147. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Chen, F.; Li, W.A.; Geng, X.; Li, C.; Meng, X.; Feng, Y.; Liu, W.; Yu, F. A review of sleep disorders and melatonin. Neurol. Res. 2017, 39, 559–565. [Google Scholar] [CrossRef]
- Mattioli, A.V.; Sciomer, S.; Cocchi, C.; Maffei, S.; Gallina, S. Quarantine during COVID-19 outbreak: Changes in diet and physical activity increase the risk of cardiovascular disease. Nutr. Metab. Cardiovasc. Dis. 2020, 28, 1409–1417. [Google Scholar] [CrossRef] [PubMed]
- Palmer, K.; Monaco, A.; Kivipelto, M.; Onder, G.; Maggi, S.; Michel, J.P.; Prieto, R.; Sykara, G.; Donde, S. The potential long-term impact of the COVID-19 outbreak on patients with non-communicable diseases in Europe: Consequences for healthy ageing. Aging. Clin. Exp. Res. 2020, 32, 1189–1194. [Google Scholar] [CrossRef] [PubMed]
- Al-Rahamneh, H.; Arafa, L.; Al Orani, A.; Baqleh, R. Long-term psychological effects of COVID-19 pandemic on children in Jordan. Int. J. Environ. Res. Public Health 2021, 18, 7795. [Google Scholar] [CrossRef] [PubMed]
- Siachpazidou, D.I.; Kotsiou, O.S.; Chatziparasidis, G.; Papagiannis, D.; Vavougios, G.D.; Gogou, E.; Stavrou, V.T.; Konstantinos, I.; Gourgoulianis, K.I. Action and reaction of pre-primary and primary school-age children to restrictions during COVID-19 Pandemic in Greece. J. Pers. Med. 2021, 11, 451. [Google Scholar] [CrossRef] [PubMed]
- Tsimtsiou, Z.; Haidich, A.B.; Kokkali, S.; Dardavesis, T.; Young, K.S.; Arvanitidou, M. Greek version of the Internet Addiction Test: A validation study. Psychiatr. Q. 2014, 85, 187–195. [Google Scholar] [CrossRef]
- McHugh, M.L. The chi-square test of independence. Biochem. Med. 2013, 23, 143–149. [Google Scholar] [CrossRef]
- Di Renzo, L.; Gualtieri, P.; Pivari, F.; Soldati, L.; Attinà, A.; Cinelli, G.; Leggeri, C.; Caparello, G.; Barrea, L.; Scerbo, F.; et al. Eating habits and lifestyle changes during COVID-19 lockdown: An Italian survey. J. Transl. Med. 2020, 18, 229–244. [Google Scholar] [CrossRef]
- Hellenic Statistical Authority. Available online: https://www.statistics.gr/documents/20181/5856c8ff-59cd-aef0-2883-f90e4b265706 (accessed on 11 July 2021).
- World Health Organization. Regional Office for Europe. Available online: https://www.euro.who.int/en/health-topics/disease-prevention/tobacco/data-and-statistics (accessed on 11 July 2021).
- Znazen, H.; Slimari, M.; Bragazzi, N.L.; Tod, D. The relationship between cognitive function, lifestyle behaviours and perception of stress during the COVID-19 induced confinement: Insights from correlational and mediation analyses. Int. J. Environ. Res. Public Health 2021, 18, 3194. [Google Scholar] [CrossRef]
- Koyama, S.; Tabushi, T.; Okawa, S.; Kadobayashi, T.; Shirai, H.; Nakatani, T.; Miyashiro, I. Changes in smoking behavior since the declaration of the COVID-19 state of emergency in Japan: A cross-sectional study from the Osaka health app. J. Epidemiol. 2021, 31, 378–386. [Google Scholar] [CrossRef]
- Koopmann, A.; Georgiadou, E.; Reinhard, I.; Muller, A.; Lemenager, T.; Kiefer, F.; Hillemacher, T. The effects of the lockdown during the COVID-19 pandemic on alcohol and tobacco consumption behavior in Germany. Eur. Addict. Res. 2021, 27, 242–256. [Google Scholar] [CrossRef] [PubMed]
- Salman, A.; Sigodo, K.O.; Al-Ghadban, F.; Al-Lahou, B.; Alnashmi, M.; Hermassi, S.; Chun, S. Effects of COVID-19 lockdown on physical activity and dietary behaviors in Kuwait: A cross-sectional study. Nutrients 2021, 13, 2252. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Li, J.; Xia, T.; Matthews, T.A.; Tseng, T.S.; Shi, L.; Zhang, D.; Chen, Z.; Han, X.; Li, H.; et al. Changes of exercise, screen time, fast food consumption, alcohol, and cigarette smoking during the COVID-19. Pandemic among adults in the United States. Nutrients 2021, 13, 3359. [Google Scholar] [CrossRef] [PubMed]
- Bourdas, D.I.; Zacharakis, E.D.; Travlos, A.K.; Souglis, A.; Georgali, T.I.; Gofas, D.C.; Ktistakis, I.E.; Deltsidou, A. Impact of lockdown on smoking and sleeping in the early COVID-19 presence: Datasets of Greek adults sample. Data Brief 2021, 39, 107480. [Google Scholar] [CrossRef]
- Tavolacci, M.P.; Wouters, E.; van de Velde, S.; Buffel, V.; Dechelotte, P.; Van Hal, G.; Ladner, J. The impact of COVID-19 lockdown on health behaviors among students of a French university. Int. J. Environ. Res. Public Health 2021, 18, 4346. [Google Scholar] [CrossRef]
- Ekstrom, S.; Andersson, N.; Lovquist, A.; Lauber, A.; Georgelis, A.; Kull, I.; Melin, E.; Bergstrom, A. COVID-19 among young adults in Sweden: Self-reported long-term symptoms and associated factors. Scand. J. Public Health 2022, 50, 85–93. [Google Scholar] [CrossRef]
- Niedzwiedz, C.L.; Green, M.J.; Benzeval, M.; Campell, D.; Craig, P.; Demou, E.; Leyland, A.; Pearce, A.; Thomson, R.; Whitley, E.; et al. Mental health and health behaviours before and during the initial phase of the COVID-19 lockdown: Longitudinal analyses of the UK Household Longitudinal Study. J. Epidemiol. Community Health 2021, 75, 224–231. [Google Scholar] [CrossRef]
- Steffen, J.; Schlichtiger, J.; Brunner, S.; Huber, B.C. Health promoting behaviour of medical versus non-medical students during COVID-19 pandemic: Results from the COLA cross-sectional study. J. Transl. Med. 2021, 19, 242. [Google Scholar] [CrossRef]
- Van Hooijdonk, K.J.M.; Rubio, M.; Simons, S.H.; Van Noorden, T.H.J. Student-, study- and COVID-19-related predictors of students’ smoking, binge drinking and cannabis use before and during the initial COVID-19. Lockdown in The Netherlands. Int. J. Environ. Res. Public Health 2022, 19, 812. [Google Scholar] [CrossRef]
- Jodczyk, A.M.; Kasiak, P.S.; Adamczyk, N.; Gebarowska, J.; Sikora, Z.; Gruba, G.; Mamcarz, A.; Sliz, D. PaLS Study: Tobacco, alcohol and drugs usage among Polish University students in the context of stress caused by the COVID-19 pandemic. Int. J. Environ. Res. Public Health 2022, 19, 1261. [Google Scholar] [CrossRef] [PubMed]
- Abouzid, M.; El-Sherif, D.M.; Eltewacy, N.K.; Dahman, N.B.H.D.; Okasha, S.A.; Ghozy, S.; Islam, S.M.S.I. Influence of COVID-19 on lifestyle behaviors in the Middle East and North Africa Region: A survey of 5896 individuals. J. Transl. Med. 2021, 19, 129. [Google Scholar] [CrossRef]
- Ismail, L.C.; Osaili, T.M.; Mohamad, M.N.; Marzouqi, A.A.L.; Jarrar, A.H.; Jamous, D.O.; Magriplis, E.; Ali, H.I.; Sabbah, H.A.; Hasan, H.; et al. Eating habits and lifestyle during COVID-19 lockdown in the United Arab Emirates: A cross-sectional study. Nutrients 2020, 12, 3314. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.; Huang, W.Y.; Sheridan, S.; Sit, C.H.-P.; Chen, X.-K.; Wong, S.H.-S. COVID-19 pandemic brings a sedentary lifestyle in young adults: A cross-sectional and longitudinal study. Int. J. Environ. Res. Public Health 2020, 17, 6035. [Google Scholar] [CrossRef]
- Trakada, A.; Nikolaidis, P.T.; Andrade, M.D.S.; Puccinelli, P.J.; Economou, N.-T.; Steiropoulos, P.; Knechtle, B.; Trakada, G. Sleep during "Lockdown" in the COVID-19 pandemic. Int. J. Environ. Res. Public Health 2020, 17, 9094. [Google Scholar] [CrossRef] [PubMed]
- Androutsos, O.; Perperidi, M.; Georgiou, C.; Chouliaras, G. Lifestyle changes and determinants of children’s and adolescents’ body weight increase during the first COVID-19. Lockdown in Greece: The COV-EAT study. Nutrients 2021, 13, 930. [Google Scholar] [CrossRef]
- Bottary, R.; Fields, E.C.; Kensinger, E.A.; Cunningham, T.J. Age and chronotype influenced sleep timing changes during the first wave of the COVID-19 pandemic. J. Sleep. Res. 2021, 31, e13495. [Google Scholar] [CrossRef]
- López-Moreno, M.; López, M.T.I.; Miguel, M.; Garcés-Rimón, M. Physical and psychological effects related to food habits and lifestyle changes derived from COVID-19 home confinement in the Spanish population. Nutrients 2020, 12, 3445. [Google Scholar] [CrossRef]
- Florea, C.; Topalidis, P.; Hauser, T.; Angerer, M.; Kurapov, A.; Leon, C.A.B.; Brandao, D.S.; Schabus, M. Sleep during COVID-19 lockdown: A cross-cultural study investigating job system relevance. Biochem. Pharmacol. 2021, 191, 114463. [Google Scholar] [CrossRef]
- Marelli, S.; Castelnuovo, A.; Somma, A.; Castronovo, V.; Mombelli, S.; Bottoni, D.; Leither, C.; Fossati, A.; Ferini-Strambi, L. Impact of COVID-19 lockdown on sleep quality in university students and administration staff. J. Neurol. 2021, 268, 8–15. [Google Scholar] [CrossRef]
- Trabelshi, K.; Ammar, A.; Masmoudi, L.; Boukhris, O.; Chtourou, H.; Bouaziz, B.; Brach, M.; Bentlage, E.; How, D.; Mueller, P.; et al. Globally altered sleep patterns and physical activity levels by confinement in 5056 individuals: ECLB COVID-19 international online survey. Biol. Sport 2021, 38, 495–506. [Google Scholar] [CrossRef] [PubMed]
- Robertson, M.; Duffy, F.; Newman, E.; Bravo, C.P.; Ates, H.H.; Sharpe, H. Exploring changes in body image, eating and exercise during the COVID-19 lockdown: A UK survey. Appetite 2021, 159, 105062. [Google Scholar] [CrossRef] [PubMed]
- Blaszczyk-Bebenek, E.; Jagielski, P.; Boleslawska, I.; Jagielska, A.; Nitsch-Osuch, A.; Kawalec, P. Nutrition behaviors in Polish adults before and during COVID-19 lockdown. Nutrients 2020, 12, 3084. [Google Scholar] [CrossRef]
- Correa, C.R.; Costa, B.G.G.; Dezanetti, T.; Filipini, R.E.; Nunes, E.A. Changes in eating habits, sleep, and physical activity during coronavirus disease (COVID-19) pandemic: A longitudinal study in young Brazilian adult males. Nutr. Health 2022, 2, 2601060221081653. [Google Scholar] [CrossRef] [PubMed]
- Jaffe, A.E.; Kumar, S.A.; Ramirez, J.; DiLillo, D. Is the COVID-19 pandemic a high-risk period for college student alcohol use? A comparison of three spring semesters. Alcohol Clin. Exp. Res. 2021, 45, 854–863. [Google Scholar] [CrossRef]
- Tison, G.H.; Avram, R.; Kuhar, P.; Abreau, S.; Marcus, G.M.; Pletcher, M.J.; Olgin, J.E. Worldwide effect of COVID-19 on physical activity: A descriptive study. Ann. Intern. Med. 2020, 173, 767–770. [Google Scholar] [CrossRef]
- Sylvia, L.G.; Bernstein, E.E.; Hubbard, J.L.; Anderson, E.J. Practical guide to measuring physical activity. J. Acad. Nutr. Diet. 2014, 114, 199–208. [Google Scholar] [CrossRef] [Green Version]

| Variable | Total N = 540 | Males N = 201 (37.2%) | Females N = 339 (62.8%) | p-Value a |
|---|---|---|---|---|
| Age, (years) | 21.2 ± 2.3 | 21.44 ± 2.32 | 21.09 ± 2.29 | 0.089 |
| Weight, (kgr) | 69.9 ± 15.8 | 79.45 ± 15.11 | 64.37 ± 3.43 | <0.001 |
| Height, (cm) | 171 ± 9.3 | 179.61 ± 7.17 | 165.98 ± 6.33 | <0.001 |
| BMI, (kg/m2) | 23.8 ± 4.5 | 24.58 ± 4.15 | 23.35 ± 4.66 | <0.001 |
| Underweight | 38 (7%) | 7 (18.4%) | 31 (81.6%) | |
| Normal | 336 (62.2%) | 122 (36.3%) | 214 (63.7%) | |
| Overweight | 114 (21.1%) | 51 (44.7%) | 63 (55.3%) | |
| Obese | 52 (9.6%) | 21 (40.4%) | 31 (59.6%) |
| Total N = 540 | Males N = 201 (37.2%) | Females N = 339 (62.8%) | p-Value | |
|---|---|---|---|---|
| Variable | ||||
| Smoker | 138 (25.5%) | 66 (47.8%) | 72 (52.2%) | |
| Years of smoking | 5.07 ± 2.49 | 5.32 ± 2.71 | 4.84 ± 2.26 * | 0.296 a |
| Pack-Years | 0.73 ± 1.76 | 1.18 ± 2.33 | 0.46 ± 1.24 | 0.001 a |
| Vaping | 12 (2.2%) | 8 (66.7%) | 4 (33.3%) | |
| Years of vaping | 2.63 ± 1.4 | 2.2 ± 1.3 | 3.33 ± 1.52 | 0.3 b |
| Smoker + Vaping | 8 (1.5%) | 1 (12.5%) | 7 (87.5%) | |
| Years of smoking | 2.53 ± 1.5 | 3 ± 1.41 | 2.38 ± 1.55 | 0.492 b |
| Years of vaping | 2.11 ± 1.05 | 2 ± 1 | 2.25 ± 1.25 | 0.749 b |
| Former smokeror /and vaping | 12 (2.2%) | 5 (41.7%) | 7 (58.3%) | |
| NOΝ SMOKERS | 370 (68.4%) | 121 (32.7) | 249 (67.3%) |
| Total | Males | Females | p-Value a | |
|---|---|---|---|---|
| PRE | 10.10 ± 5.84 | 12.18 ± 6.11 | 8.19 ± 4.88 | <0.001 |
| DURING | 10.76 ± 8.5 | 12.67 ± 8.96 | 9.01 ± 7.7 | 0.009 |
| POST | 11.25 ± 6.72 | 12.89 ± 6.8 | 9.74 ± 6.32 | 0.005 |
| Total N = 540 | Males N = 201 (37.2%) | Females N = 339 (62.8%) | |
|---|---|---|---|
| Restless sleep | |||
| PRE | 154 (28.51%) | 52 (25.9% a) | 102 (30.1% b) |
| DURING | 235 (43.51%) | 67 (33.3% a) | 168 (49.6% b) |
| POST | 266 (49.26%) | 80 (39.8% a) | 186 (54.9% b) |
| Difficulty in getting up | |||
| PRE | 278 (51.4%) | 91 (45.3% a) | 187 (55.2% b) |
| DURING | 292 (54%) | 90 (44.8% a) | 202 (59.6% b) |
| POST | 295 (54.5%) | 97 (48.3% a) | 198 (58.4% b) |
| Morning headache | |||
| PRE | 54 (10%) | 13 (6.5% a) | 41 (12.1% b) |
| DURING | 139 (25.75%) | 31 (15.4% a) | 108 (31.9% b) |
| POST | 133 (24.6%) | 32 (15.9% a) | 101 (29.8% b) |
| Morning irritability | |||
| PRE | 127 (23.52%) | 37 (18.4% a) | 90 (26.5% b) |
| DURING | 228 (42.22%) | 71 (35.3% a) | 157 (46.3% b) |
| POST | 184 (34.07%) | 63 (31.3% a) | 121 (35.7% b) |
| Total N = 540 | Males N = 201 (37.2%) | Females N = 339 (62.8%) | |
|---|---|---|---|
| High | |||
| PRE | 91 (16.85%) | 33 (16.4%) | 58 (17.1%) |
| DURING | 64 (11.85%) | 22 (10.9%) | 42 (12.4%) |
| POST | 97 (17.96%) | 31 (15.4%) | 66 (19.5%) |
| Medium | |||
| PRE | 347 (64.29%) | 133 (66.2%) | 214 (63.1%) |
| DURING | 221 (40.93%) | 97 (48.3%) | 124 (36.6%) |
| POST | 317 (58.70%) | 124 (61.7%) | 193 (56.9%) |
| Low | |||
| PRE | 102 (18.86%) | 35 (17.4%) | 67 (19.8%) |
| DURING | 255 (47.22%) | 82 (40.8%) | 173 (51%) |
| POST | 126 (23.34%) | 46 (22.9%) | 80 (23.6%) |
| Total N = 540 | Males N = 201 (37.2%) | Females N = 339 (62.8%) | |
|---|---|---|---|
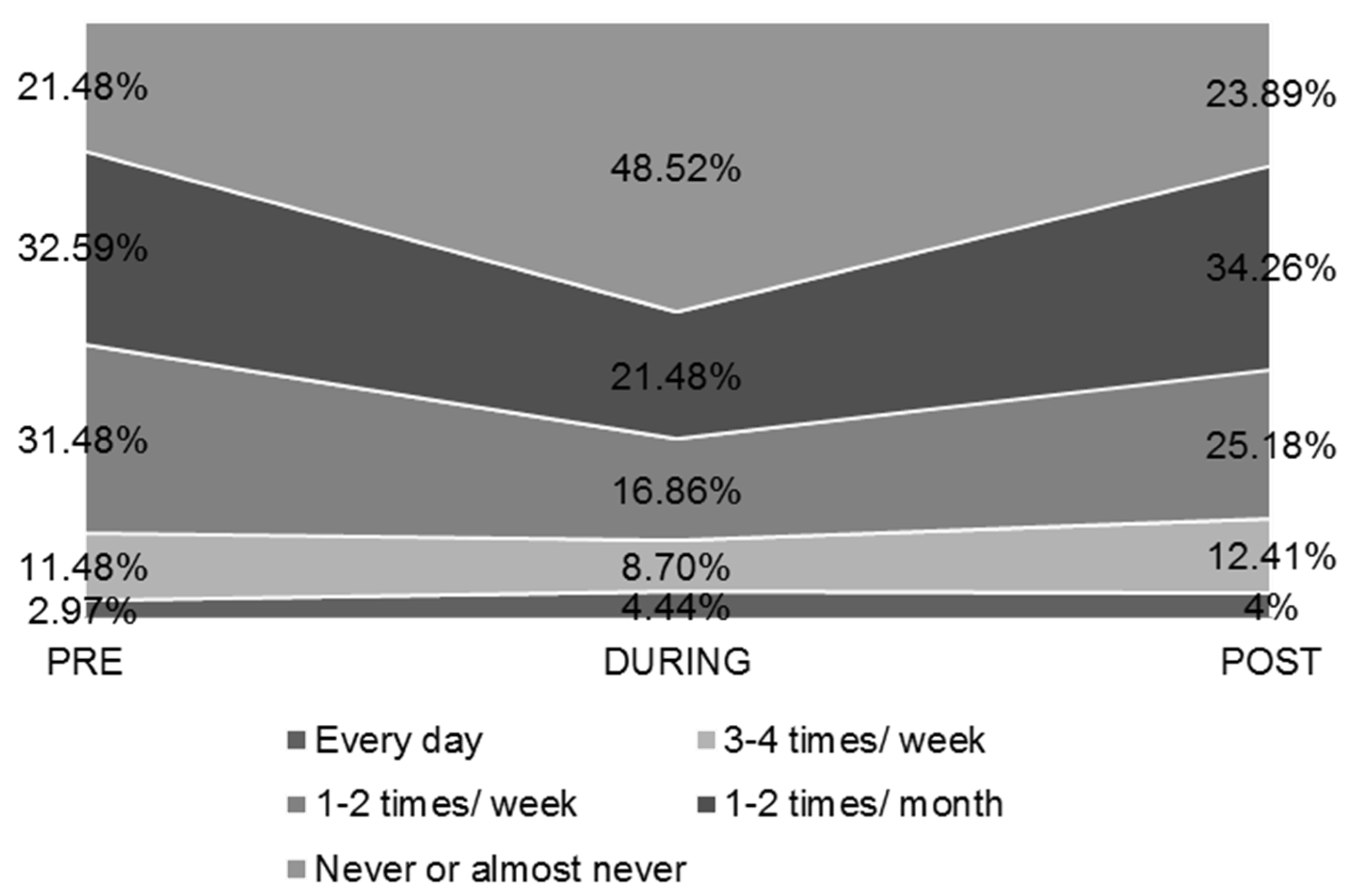
| Every day | |||
| PRE | 16 (2.97%) | 6 (3%) | 10 (2.9%) |
| DURING | 24 (4.44%) | 8 (10.9%) | 16 (4.7%) |
| POST | 23 (4.26%) | 12 (6%) | 11 (3.2%) |
| 3–4 times/week | |||
| PRE | 62 (11.48%) | 28 (13.9%) | 34 (10%) |
| DURING | 47 (8.70%) | 25 (12.4%) | 22 (6.5%) |
| POST | 67 (12.41%) | 27 (13.4%) | 40 (11.8%) |
| 1–2 times/week | |||
| PRE | 170 (31.48%) | 63 (31.3%) | 107 (31.6%) |
| DURING | 91 (16.86%) | 35 (17.4%) | 56 (16.5%) |
| POST | 136 (25.18%) | 46 (22.9%) | 90 (26.5%) |
| 1–2 times/month | |||
| PRE | 176 (32.59%) | 57 (28.4%) | 119 (35.1%) |
| DURING | 116 (21.48%) | 45 (22.4%) | 71 (20.9%) |
| POST | 185 (34.26%) | 67 (33.3%) | 118 (34.8%) |
| Never or almost never | |||
| PRE | 116 (21.48%) | 47 (23.4%) | 69 (20.4%) |
| DURING | 262 (48.52%) | 88 (43.8%) | 174 (51.3%) |
| POST | 129 (23.89%) | 49 (24.4%) | 80 (23.6%) |
| Total N = 433 | Males N = 164 (37.87%) | Females N = 269 (62.13%) | p-Value | |
|---|---|---|---|---|
| PRE | 3.11 ± 1.61 | 3.25 ± 1.66 | 2.98 ± 1.56 | 0.37 |
| DURING | 3.18 ± 2.11 | 3.47 ± 2.32 | 2.92 ± 1.88 | 0.22 |
| POST | 3.08 ± 1.61 | 3.33 ± 1.61 | 2.85 ± 1.60 | 0.06 |
| Total N = 540 | Males N = 201 (37.2%) | Females N = 339 (62.8%) | |
|---|---|---|---|
| Kind | |||
| Fitness center | 100 (18.51%) | 49 (24.4%) | 51 (15%) |
| Biking/cycling | 30 (5.56%) | 13 (6.5%) | 17 (5%) |
| Walking | 188 (34.81%) | 57 (28.4%) | 131 (38.6%) |
| Other | 115 (21.3%) | 45 (22.4%) | 70 (20.6%) |
| No activity/exercise | 107 (19.82%) | 37 (18.4%) | 70 (20.6%) |
| Type | |||
| Free | 305 (56.48%) | 108 (53.7%) | 197 (58.1%) |
| Instructed | 128 (23.7%) | 56 (27.9%) | 72 (21.2%) |
| No activity/exercise | 107 (19.82%) | 37 (18.4%) | 70 (20.6%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barbouzas, A.E.; Malli, F.; Daniil, Z.; Gourgoulianis, K. Long-Term Impact of COVID-19 Pandemic in Sleep Quality and Lifestyle in Young Adults. Int. J. Environ. Res. Public Health 2022, 19, 12333. https://doi.org/10.3390/ijerph191912333
Barbouzas AE, Malli F, Daniil Z, Gourgoulianis K. Long-Term Impact of COVID-19 Pandemic in Sleep Quality and Lifestyle in Young Adults. International Journal of Environmental Research and Public Health. 2022; 19(19):12333. https://doi.org/10.3390/ijerph191912333
Chicago/Turabian StyleBarbouzas, Argyrios Eleftherios, Foteini Malli, Zoe Daniil, and Konstantinos Gourgoulianis. 2022. "Long-Term Impact of COVID-19 Pandemic in Sleep Quality and Lifestyle in Young Adults" International Journal of Environmental Research and Public Health 19, no. 19: 12333. https://doi.org/10.3390/ijerph191912333
APA StyleBarbouzas, A. E., Malli, F., Daniil, Z., & Gourgoulianis, K. (2022). Long-Term Impact of COVID-19 Pandemic in Sleep Quality and Lifestyle in Young Adults. International Journal of Environmental Research and Public Health, 19(19), 12333. https://doi.org/10.3390/ijerph191912333








