Continuous Glucose Monitoring and Physical Activity
Abstract
1. Physical Activity and Diabetes
2. Benefits and Effects of CGM in the Context of Physical Activity—Which Measurement Parameters Can Be Presented in an Optimized Way?
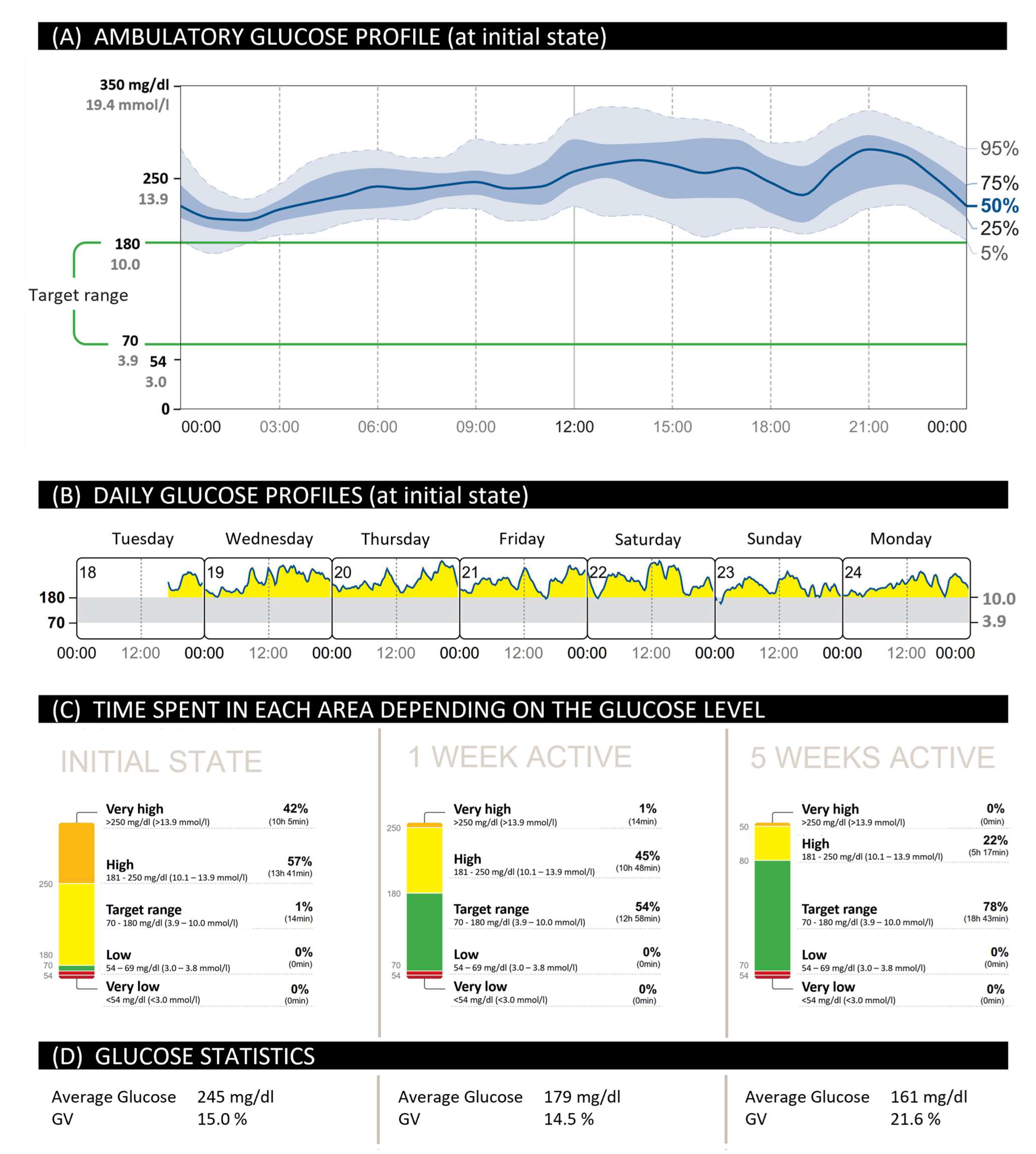
2.1. Increase in TIR for People with T2D during Training through the Use of CGM
2.2. Optimized Therapy and Documentation through the Use of CGM
2.3. Relevance of CGM for Prevention of Hypoglycemia
3. Improved Compliance and Motivation for Physical Activity after CGM Use
4. Importance of Exercise in Everyday Life in Prediabetes and Diabetes
4.1. Influence on Glycemic Control
4.2. Influence on Glycemic Variability
4.3. Influence on Postprandial Glucose Levels
4.4. Night Course of Glucose Level after Exercise
4.5. Different Effects Depending on the Time of Day When Training Was Executed
4.6. Reduction of IR
4.7. Long-Term Effect of Exercise on Glycemic Control
4.8. Effects of Extreme Conditions as Low Temperature and High Altitude on CGM Measured Physical Activity
4.9. Exercise in Everyday Life for Prediabetes
5. International Recommendations on Physical Activity of People with Diabetes in Interaction with CGM
5.1. ADA and ACSM Recommendations
5.1.1. Generally Applicable for T1D and T2D
5.1.2. Type 2 Diabetes
5.1.3. Recommendations to Prevent Adverse Events due to Physical Activity of People with Diabetes
5.2. German National Health Care Guidelines and Practice Recommendations of the DDG
5.3. How Many Steps Can Be Recommended?
5.4. Risk for Complications Depending on the Number of Steps per Day
6. Exercise on Prescription
7. Different Moderate Forms and Types of Physical Activity Which Can Be Easily Integrated into Everyday Life and Strategies for Better Integration
7.1. Anaerobic/Untrained und Aerobic Sports
7.2. Integration of the BORG and OMNI Scale for Intensity Determination
- Stroll/Walking
- E-Bike
8. The Importance of Physical Activity for People with Pre-Existing Conditions—What Should Be Considered in the Case of Pre-Existing Conditions and Which Preventive Examinations Are Necessary? When Should a Sports Physician Be Consulted?
9. Summary—Key Messages for the Person with Diabetes in Relation to Exercise and CGM
10. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pelliccia, A.; Sharma, S.; Gati, S.; Bäck, M.; Börjesson, M.; Caselli, S.; Collet, J.-P.; Corrado, D.; Drezner, J.A.; Halle, M.; et al. 2020 ESC Guidelines on sports cardiology and exercise in patients with cardiovascular disease: The Task Force on sports cardiology and exercise in patients with cardiovascular disease of the European Society of Cardiology (ESC). Eur. Heart J. 2020, 42, 17–96. [Google Scholar] [CrossRef] [PubMed]
- Meinolf Behrens, P.B.; Kress, S. (Eds.) Gemeinsam Bewegen; Deutsche Diabetes Gesellschaft (DDG) und Diabetes DE—Deutsche Diabetes-Hilfe: Mainz, Germany, 2020. [Google Scholar]
- Cummiskey, J.; Lollgen, H.; Zupet, P.; Borjesson, M.; Natsis, K.; Cummiskey, A.; Stafrace, K.M.; Debruyne, A.; Bachl, N. The four “E” pillars of exercise prescription for health: The EFSMA program. Eur. J. Sports Med. 2016, 4, 15–32. [Google Scholar]
- Krug, S.; Jordan, S.; Lampert, T. (Eds.) Körperliche Aktivität: Wie Aktiv Sind Die Deutschen? DEGS—Studie zur Gesundheit Erwachsener in Deutschland: Berlin, Germany, 2012. [Google Scholar]
- Tudor-Locke, C.; Craig, C.L.; Brown, W.J.; Clemes, S.A.; De Cocker, K.; Giles-Corti, B.; Hatano, Y.; Inoue, S.; Matsudo, S.M.; Mutrie, N.; et al. How many steps/day are enough for adults. Int. J. Behav. Nutr. Phys. Act. 2011, 8, 79. [Google Scholar] [CrossRef] [PubMed]
- Aberle, J. (Ed.) Adipositas aus Sicht der Diabetologie—Stellenwert der Konservativen und Bariatrischen Therapie; Deutsche Diabetes Gesellschaft (DDG) und Diabetes DE—Deutsche Diabetes-Hilfe: Mainz, Germany, 2020. [Google Scholar]
- Tönnies, T.; Röckl, S.; Hoyer, A.; Heidemann, C.; Baumert, J.; Du, Y.; Scheidt-Nave, C.; Brinks, R. Projected number of people with diagnosed Type 2 diabetes in Germany in 2040. Diabet. Med. 2019, 36, 1217–1225. [Google Scholar] [CrossRef]
- Rosenbauer, J.; Neu, A.; Rothe, U.; Seufert, J.; Holl, R.W. Diabetestypen sind nicht auf Altersgruppen beschränkt: Typ-1-Diabetes bei Erwachsenen und Typ-2-Diabetes bei Kindern und Jugendlichen. J. Health Monit. 2019, 4, 31–53. [Google Scholar] [CrossRef]
- Colberg, S. Physical activity: The forgotten tool for type 2 diabetes management. Front. Endocrinol. 2012, 3, 70. [Google Scholar] [CrossRef]
- Najafipour, F.; Mobasseri, M.; Yavari, A.; Nadrian, H.; Aliasgarzadeh, A.; Mashinchi Abbasi, N.; Niafar, M.; Houshyar Gharamaleki, J.; Sadra, V. Effect of regular exercise training on changes in HbA1c, BMI and VO(2)max among patients with type 2 diabetes mellitus: An 8-year trial. BMJ Open Diabetes Res. Care 2017, 5, e000414. [Google Scholar] [CrossRef]
- Fabris, C.; Ozaslan, B.; Breton, M.D. Continuous Glucose Monitors and Activity Trackers to Inform Insulin Dosing in Type 1 Diabetes: The University of Virginia Contribution. Sensor 2019, 19, 5386. [Google Scholar] [CrossRef]
- Lakerveld, J.; Palmeira, A.L.; van Duinkerken, E.; Whitelock, V.; Peyrot, M.; Nouwen, A. Motivation: Key to a healthy lifestyle in people with diabetes? Current and emerging knowledge and applications. Diabet. Med. 2020, 37, 464–472. [Google Scholar] [CrossRef]
- Brazeau, A.-S.; Rabasa-Lhoret, R.; Strychar, I.; Mircescu, H. Barriers to physical activity among patients with type 1 diabetes. Diabetes Care 2008, 31, 2108–2109. [Google Scholar] [CrossRef]
- Löllgen, H.; Zupet, P.; Wismach, J.; Bachl, N.; Predel, G. Körperliche Aktivität und gesundes Leben: Das Rezept für Bewegung. Herzmedizin 2017, 33, 27–32. [Google Scholar]
- Beck, R.W.R.; Tonya, D.; Ruedy, K.; Ahmann, A. Continuous Glucose Monitoring Versus Usual Care in Patients with Type 2 Diabetes Receiving Multiple Daily Insulin Injections. Ann. Intern. Med. 2017, 167, 365–374. [Google Scholar] [CrossRef] [PubMed]
- Vigersky, R.; Shrivastav, M. Role of continuous glucose monitoring for type 2 in diabetes management and research. J. Diabetes Its Complicat. 2017, 31, 280–287. [Google Scholar] [CrossRef] [PubMed]
- Ceriello, A.; Esposito, K.; Piconi, L.; Ihnat, M.A.; Thorpe, J.E.; Testa, R.; Boemi, M.; Giugliano, D. Oscillating Glucose Is More Deleterious to Endothelial Function and Oxidative Stress Than Mean Glucose in Normal and Type 2 Diabetic Patients. Diabetes 2008, 57, 1349–1354. [Google Scholar] [CrossRef]
- Moser, O.; Riddell, M.C.; Eckstein, M.L.; Adolfsson, P.; Rabasa-Lhoret, R.; van den Boom, L.; Gillard, P.; Nørgaard, K.; Oliver, N.S.; Zaharieva, D.P.; et al. Glucose management for exercise using continuous glucose monitoring (CGM) and intermittently scanned CGM (isCGM) systems in type 1 diabetes: Position statement of the European Association for the Study of Diabetes (EASD) and of the International Society for Pediatric and Adolescent Diabetes (ISPAD) endorsed by JDRF and supported by the American Diabetes Association (ADA). Diabetologia 2020, 63, 2501–2520. [Google Scholar] [CrossRef]
- Yoo, H.J.; An, H.G.; Park, S.Y.; Ryu, O.H.; Kim, H.Y.; Seo, J.A.; Hong, E.G.; Shin, D.H.; Kim, Y.H.; Kim, S.G.; et al. Use of a real time continuous glucose monitoring system as a motivational device for poorly controlled type 2 diabetes. Diabetes Res. Clin. Pract. 2008, 82, 73–79. [Google Scholar] [CrossRef]
- Allen, N.A.; Fain, J.A.; Braun, B.; Chipkin, S.R. Continuous glucose monitoring counseling improves physical activity behaviors of individuals with type 2 diabetes: A randomized clinical trial. Diabetes Res. Clin. Pract. 2008, 80, 371–379. [Google Scholar] [CrossRef]
- Colberg, S.R.; Sigal, R.J.; Yardley, J.E.; Riddell, M.C.; Dunstan, D.W.; Dempsey, P.C.; Horton, E.S.; Castorino, K.; Tate, D.F. Physical Activity/Exercise and Diabetes: A Position Statement of the American Diabetes Association. Diabetes Care 2016, 39, 2065–2079. [Google Scholar] [CrossRef]
- Kudva, Y.C.; Ahmann, A.J.; Bergenstal, R.M.; Gavin, J.R., 3rd; Kruger, D.F.; Midyett, L.K..; Miller, E.; Harris, D.R. Approach to Using Trend Arrows in the FreeStyle Libre Flash Glucose Monitoring Systems in Adults. J. Endocr. Soc. 2018, 2, 1320–1337. [Google Scholar] [CrossRef]
- Younk, L.M.; Mikeladze, M.; Tate, D.; Davis, S.N. Exercise-related hypoglycemia in diabetes mellitus. Expert Rev. Endocrinol. Metab. 2011, 6, 93–108. [Google Scholar] [CrossRef]
- Taleb, N.; Rabasa-Lhoret, R. Can somatostatin antagonism prevent hypoglycaemia during exercise in type 1 diabetes? Diabetologia 2016, 59, 1632–1635. [Google Scholar] [CrossRef] [PubMed]
- Riddell, M.C.; Zaharieva, D.P.; Yavelberg, L.; Cinar, A.; Jamnik, V.K. Exercise and the Development of the Artificial Pancreas: One of the More Difficult Series of Hurdles. J. Diabetes Sci. Technol. 2015, 9, 1217–1226. [Google Scholar] [CrossRef] [PubMed]
- Tagougui, S.; Taleb, N.; Rabasa-Lhoret, R. The Benefits and Limits of Technological Advances in Glucose Management Around Physical Activity in Patients Type 1 Diabetes. Front. Endocrinol. 2019, 9, 818. [Google Scholar] [CrossRef]
- Yardley, J.E.; Kenny, G.P.; Perkins, B.A.; Riddell, M.C.; Malcolm, J.; Boulay, P.; Khandwala, F.; Sigal, R.J. Effects of performing resistance exercise before versus after aerobic exercise on glycemia in type 1 diabetes. Diabetes Care 2012, 35, 669–675. [Google Scholar] [CrossRef]
- Turner, D.; Gray, B.J.; Luzio, S.; Dunseath, G.; Bain, S.C.; Hanley, S.; Richards, A.; Rhydderch, D.C.; Ayles, M.; Kilduff, L.P.; et al. Similar magnitude of post-exercise hyperglycemia despite manipulating resistance exercise intensity in type 1 diabetes individuals. Scand. J. Med. Sci. Sports 2016, 26, 404–412. [Google Scholar] [CrossRef]
- Fahey, A.J.; Paramalingam, N.; Davey, R.J.; Davis, E.A.; Jones, T.W.; Fournier, P.A. The Effect of a Short Sprint on Postexercise Whole-Body Glucose Production and Utilization Rates in Individuals with Type 1 Diabetes Mellitus. J. Clin. Endocrinol. Metab. 2012, 97, 4193–4200. [Google Scholar] [CrossRef] [PubMed]
- Guelfi, K.J.; Jones, T.W.; Fournier, P.A. Intermittent High-Intensity Exercise Does Not Increase the Risk of Early Postexercise Hypoglycemia in Individuals with Type 1 Diabetes. Diabetes Care 2005, 28, 416–418. [Google Scholar] [CrossRef]
- Maran, A.; Pavan, P.; Bonsembiante, B.; Brugin, E.; Ermolao, A.; Avogaro, A.; Zaccaria, M. Continuous glucose monitoring reveals delayed nocturnal hypoglycemia after intermittent high-intensity exercise in nontrained patients with type 1 diabetes. Diabetes Technol. Ther. 2010, 12, 763–768. [Google Scholar] [CrossRef]
- Riddell, M.C.; Milliken, J. Preventing Exercise-Induced Hypoglycemia in Type 1 Diabetes Using Real-Time Continuous Glucose Monitoring and a New Carbohydrate Intake Algorithm: An Observational Field Study. Diabetes Technol. Ther. 2011, 13, 819–825. [Google Scholar] [CrossRef]
- Brinkmann, C.; Kröger, J.; Siegmund, T.; Thurm, U.; Halle, M.; Schubert-Olesen, O. AGP-Fibel Bewegung—Mit CGM Glukoseverläufe bei Bewegung analysieren; Kirchheim Verlag: Mainz, Germany, 2021. [Google Scholar]
- Paing, A.C.; Kirk, A.F.; Collier, A.; Kubiak, T.; Chastin, S.F.M. Are glucose profiles well-controlled within the targets recommended by the International diabetes Federation in type 2 diabetes? A meta-analysis of results from continuous glucose monitoring based studies. Diabetes Res. Clin. Pract. 2018, 146, 289–299. [Google Scholar] [CrossRef]
- Biswas, A.; Oh, P.I.; Faulkner, G.E.; Bajaj, R.R.; Silver, M.A.; Mitchell, M.S.; Alter, D.A. Sedentary Time and Its Association with Risk for Disease Incidence, Mortality, and Hospitalization in Adults. Ann. Intern. Med. 2015, 162, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Swartz, A.M.; Squires, L.; Strath, S.J. Energy expenditure of interruptions to sedentary behavior. Int. J. Behav. Nutr. Phys. Act. 2011, 8, 69. [Google Scholar] [CrossRef] [PubMed]
- Colberg, S.R.; Zarrabi, L.; Bennington, L.; Nakave, A.; Thomas Somma, C.; Swain, D.P.; Sechrist, S.R. Postprandial Walking is Better for Lowering the Glycemic Effect of Dinner than Pre-Dinner Exercise in Type 2 Diabetic Individuals. J. Am. Med. Dir. Assoc. 2009, 10, 394–397. [Google Scholar] [CrossRef] [PubMed]
- Heath, G.W.; Gavin, J.R., 3rd; Hinderliter, J.M.; Hagberg, J.M.; Bloomfield, S.A.; Holloszy, J.O. Effects of exercise and lack of exercise on glucose tolerance and insulin sensitivity. J. Appl. Physiol. Respir. Environ. Exerc. Physiol. 1983, 55, 512–517. [Google Scholar] [CrossRef]
- Balducci, S.; Zanuso, S.; Massarini, M.; Corigliano, G.; Nicolucci, A.; Missori, S.; Cavallo, S.; Cardelli, P.; Alessi, E.; Pugliese, G.; et al. The Italian Diabetes and Exercise Study (IDES): Design and methods for a prospective Italian multicentre trial of intensive lifestyle intervention in people with type 2 diabetes and the metabolic syndrome. Nutr. Metab. Cardiovasc. Dis. 2008, 18, 585–595. [Google Scholar] [CrossRef] [PubMed]
- Umpierre, D.; Ribeiro, P.A.B.; Kramer, C.K.; Leitão, C.B.; Zucatti, A.T.N.; Azevedo, M.J.; Gross, J.L.; Ribeiro, J.P.; Schaan, B.D. Physical Activity Advice Only or Structured Exercise Training and Association with HbA1c Levels in Type 2 Diabetes: A Systematic Review and Meta-analysis. JAMA 2011, 305, 1790–1799. [Google Scholar] [CrossRef]
- Boniol, M.; Dragomir, M.; Autier, P.; Boyle, P. Physical activity and change in fasting glucose and HbA1c: A quantitative meta-analysis of randomized trials. Acta Diabetol. 2017, 54, 983–991. [Google Scholar] [CrossRef]
- Sigal, R.J.; Kenny, G.P.; Boulé, N.G.; Wells, G.A.; Prud’homme, D.; Fortier, M.; Reid, R.D.; Tulloch, H.; Coyle, D.; Phillips, P.; et al. Effects of aerobic training, resistance training, or both on glycemic control in type 2 diabetes: A randomized trial. Ann. Intern. Med. 2007, 147, 357–369. [Google Scholar] [CrossRef]
- Shenoy, S.; Arora, E.; Jaspal, S.S. Effects of progressive resistance training and aerobic exercise on type 2 diabetics in Indian population. Int. J. Diabetes Metab. 2019, 17, 27–30. [Google Scholar]
- Winding, K.M.; Munch, G.W.; Iepsen, U.W.; Van Hall, G.; Pedersen, B.K.; Mortensen, S.P. The effect on glycaemic control of low-volume high-intensity interval training versus endurance training in individuals with type 2 diabetes. Diabetes Obes. Metab. 2018, 20, 1131–1139. [Google Scholar] [CrossRef]
- Ceriello, A.; Piconi, L.; Quagliaro, L.; Wang, Y.; Schnabel, C.A.; Ruggles, J.A.; Gloster, M.A.; Maggs, D.G.; Weyer, C. Effects of Pramlintide on Postprandial Glucose Excursions and Measures of Oxidative Stress in Patients with Type 1 Diabetes. Diabetes Care 2005, 28, 632–637. [Google Scholar] [CrossRef] [PubMed]
- Monnier, L.; Lapinski, H.; Colette, C. Contributions of Fasting and Postprandial Plasma Glucose Increments to the Overall Diurnal Hyperglycemia of Type 2 Diabetic Patients—Variations with increasing levels of HbA. Diabetes Care 2003, 26, 881–885. [Google Scholar] [CrossRef] [PubMed]
- Solomon, T.P.J.; Tarry, E.; Hudson, C.O.; Fitt, A.I.; Laye, M.J. Immediate post-breakfast physical activity improves interstitial postprandial glycemia: A comparison of different activity-meal timings. Pflug. Arch. Eur. J. Physiol. 2020, 472, 271–280. [Google Scholar] [CrossRef]
- Li, Z.; Hu, Y.; Yan, R.; Li, H.; Zhang, D.; Li, F.; Su, X.; Ma, J. Twenty Minute Moderate-Intensity Post-Dinner Exercise Reduces the Postprandial Glucose Response in Chinese Patients with Type 2 Diabetes. Med. Sci. Monit. 2018, 24, 7170–7177. [Google Scholar] [CrossRef] [PubMed]
- Metcalf, K.M.; Singhvi, A.; Tsalikian, E.; Tansey, M.J.; Zimmerman, M.B.; Esliger, D.W.; Janz, K.F. Effects of Moderate-to-Vigorous Intensity Physical Activity on Overnight and Next-Day Hypoglycemia in Active Adolescents with Type 1 Diabetes. Diabetes Care 2014, 37, 1272–1278. [Google Scholar] [CrossRef] [PubMed]
- Yardley, J.E.; Kenny, G.P.; Perkins, B.A.; Riddell, M.C.; Balaa, N.; Malcolm, J.; Boulay, P.; Khandwala, F.; Sigal, R.J. Resistance versus aerobic exercise: Acute effects on glycemia in type 1 diabetes. Diabetes Care 2013, 36, 537–542. [Google Scholar] [CrossRef] [PubMed]
- McMahon, S.K.; Ferreira, L.D.; Ratnam, N.; Davey, R.J.; Youngs, L.M.; Davis, E.A.; Fournier, P.A.; Jones, T.W. Glucose requirements to maintain euglycemia after moderate-intensity afternoon exercise in adolescents with type 1 diabetes are increased in a biphasic manner. J. Clin. Endocrinol. Metab. 2007, 92, 963–968. [Google Scholar] [CrossRef]
- Zaharieva, D.P.; Riddell, M.C. Prevention of exercise-associated dysglycemia: A case study-based approach. Diabetes Spectr. 2015, 28, 55–62. [Google Scholar] [CrossRef][Green Version]
- Yamanouchi, K.; Abe, R.; Takeda, A.; Atsumi, Y.; Shichiri, M.; Sato, Y. The effect of walking before and after breakfast on blood glucose levels in patients with type 1 diabetes treated with intensive insulin therapy. Diabetes Res. Clin. Pract. 2002, 58, 11–18. [Google Scholar] [CrossRef]
- Esefeld, K.; Heinicke, V.; Kress, S.; Behrens, M.; Zimmer, P.; Stumvoll, M.; Brinkmann, C.; Halle, M. Diabetes, Sport und Bewegung. Der Diabetol. 2020, 16, 292–299. [Google Scholar] [CrossRef]
- Myers, J.; Kokkinos, P.; Nyelin, E. Physical Activity, Cardiorespiratory Fitness, and the Metabolic Syndrome. Nutrients 2019, 11, 1652. [Google Scholar] [CrossRef] [PubMed]
- Cheng, F.; Yuan, G.; He, J.; Shao, Y.; Zhang, J.; Guo, X. Aberrant expression of miR-214 is associated with obesity-induced insulin resistance as a biomarker and therapeutic. Diagn. Pathol. 2020, 15, 18. [Google Scholar] [CrossRef] [PubMed]
- de Andrade, M.I.S.; Oliveira, J.S.; Leal, V.S.; da Silva Lima, N.M.; Bezerra, P.B.; Santiago, E.R.C.; de Lira, P.I.C. Prevalence of Insulin Resistance and Association with Metabolic Risk Factors and Food Consumption in Adolescents—Recife/Brazil. Rev. Paul. De Pediatr. 2020, 38, 18. [Google Scholar] [CrossRef] [PubMed]
- Roberts, C.K.; Hevener, A.L.; Barnard, R.J. Metabolic syndrome and insulin resistance: Underlying causes and modification by exercise training. Compr. Physiol. 2013, 3, 158. [Google Scholar] [CrossRef]
- Muniyappa, R.; Lee, S.; Chen, H.; Quon, M.J. Current approaches for assessing insulin sensitivity and resistance in vivo: Advantages, limitations, and appropriate usage. Am. J. Physiol. Endocrinol. Metab. 2008, 294, E15–E26. [Google Scholar] [CrossRef]
- Chimen, M.; Kennedy, A.; Nirantharakumar, K.; Pang, T.T.; Andrews, R.; Narendran, P. What are the health benefits of physical activity in type 1 diabetes mellitus? A literature review. Diabetologia 2012, 55, 542–551. [Google Scholar] [CrossRef]
- Oshida, Y.; Yamanouchi, K.; Hayamizu, S.; Sato, Y. Long-term mild jogging increases insulin action despite no influence on body mass index or VO2 max. J. Appl. Physiol. 1989, 66, 2206–2210. [Google Scholar] [CrossRef]
- Nassis, G.P.; Papantakou, K.; Skenderi, K.; Triandafillopoulou, M.; Kavouras, S.A.; Yannakoulia, M.; Chrousos, G.P.; Sidossis, L.S. Aerobic exercise training improves insulin sensitivity without changes in body weight, body fat, adiponectin, and inflammatory markers in overweight and obese girls. Metab. Clin. Exp. 2005, 54, 1472–1479. [Google Scholar] [CrossRef]
- Duncan, G.E.; Perri, M.G.; Theriaque, D.W.; Hutson, A.D.; Eckel, R.H.; Stacpoole, P.W. Exercise training, without weight loss, increases insulin sensitivity and postheparin plasma lipase activity in previously sedentary adults. Diabetes Care 2003, 26, 557–562. [Google Scholar] [CrossRef]
- Karstoft, K.; Clark, M.A.; Jakobsen, I.; Müller, I.A.; Pedersen, B.K.; Solomon, T.P.J.; Ried-Larsen, M. The effects of 2 weeks of interval vs. continuous walking training on glycaemic control and whole-body oxidative stress in individuals with type 2 diabetes: A controlled, randomised, crossover trial. Diabetologia 2017, 60, 508–517. [Google Scholar] [CrossRef]
- De Mol, P.; De Vries, S.T.; De Koning, E.J.; Gans, R.O.; Tack, C.J.; Bilo, H.J. Increased insulin requirements during exercise at very high altitude in type 1 diabetes. Diabetes Care 2011, 34, 591–595. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Larsen, J.J.; Hansen, J.M.; Olsen, N.V.; Galbo, H.; Dela, F. The effect of altitude hypoxia on glucose homeostasis in men. J. Physiol. 1997, 504 Pt 1, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Mohajeri, S.; Perkins, B.A.; Brubaker, P.L.; Riddell, M.C. Diabetes, trekking and high altitude: Recognizing and preparing for the risks. Diabet. Med. A J. Br. Diabet. Assoc. 2015, 32, 1425–1437. [Google Scholar] [CrossRef] [PubMed]
- Brubaker, P.L. Adventure Travel and Type 1 Diabetes. Complicat. Eff. High Alt. 2005, 28, 2563–2572. [Google Scholar] [CrossRef] [PubMed]
- Matejko, B.; Gawrecki, A.; Wróbel, M.; Hohendorff, J.; Benbenek-Klupa, T.; Malecki, M.T.; Zozulińska-Ziółkiewicz, D.; Klupa, T. Type 1 Diabetes at High Altitude: Performance of Personal Insulin Pumps and Patient Metabolic Control. Diabetes Technol. Ther. 2017, 19, 600–602. [Google Scholar] [CrossRef]
- International Diabetes Federation. IDF Diabetes Atlas, 9th ed.; International Diabetes Federation: Brussels, Belgium, 2019. [Google Scholar]
- Braga, T.; Kraemer-Aguiar, L.G.; Docherty, N.G.; Le Roux, C.W. Treating prediabetes: Why and how should we do it? Minerva Med. 2019, 110, 52–61. [Google Scholar] [CrossRef]
- Knowler, W.C.; Barrett-Connor, E.; Fowler, S.E.; Hamman, R.F.; Lachin, J.M.; Walker, E.A.; Nathan, D.M.; Watson, P.G.; Mendoza, J.T.; Smith, K.A.; et al. Reduction in the Incidence of Type 2 Diabetes with Lifestyle Intervention or Metformin. New Engl. J. Med. 2002, 346, 393–403. [Google Scholar] [CrossRef]
- Pan, X.R.; Li, G.W.; Hu, Y.H.; Wang, J.X.; Yang, W.Y.; An, Z.X.; Hu, Z.X.; Lin, J.; Xiao, J.Z.; Cao, H.B.; et al. Effects of diet and exercise in preventing NIDDM in people with impaired glucose tolerance. The Da Qing IGT and Diabetes Study. Diabetes Care 1997, 20, 537–544. [Google Scholar] [CrossRef]
- Hou, L.; Ge, L.; Li, Y.; Chen, Y.; Li, H.; He, J.; Cao, C.; Li, R.; Tian, J.; Chen, Y.; et al. Physical activity recommendations for patients with type 2 diabetes: A cross-sectional survey. Acta Diabetol. 2020, 57, 765–777. [Google Scholar] [CrossRef]
- Colberg, S.R.; Sigal, R.J.; Fernhall, B.; Regensteiner, J.G.; Blissmer, B.J.; Rubin, R.R.; Chasan-Taber, L.; Albright, A.L.; Braun, B. Exercise and Type 2 Diabetes. Am. Coll. Sports Med. Am. Diabetes Assoc. Jt. Position Statement Exec. Summ. 2010, 33, 2692–2696. [Google Scholar] [CrossRef]
- Moser, O.; Riddell, M.C.; Eckstein, M.L.; Adolfsson, P.; Rabasa-Lhoret, R.; van den Boom, L.; Gillard, P.; Nørgaard, K.; Oliver, N.S.; Zaharieva, D.P.; et al. Glucose management for exercise using continuous glucose monitoring: Should sex and prandial state be additional considerations? Reply to Yardley JE and Sigal RJ. Diabetologia 2021, 63, 2501–2520. [Google Scholar] [CrossRef] [PubMed]
- Braune, K.; May, A.; Thurm, U. Safe and Successful Completion of a Half Marathon by an Adult with Type 1 Diabetes Using a Personalized Open Source Artificial Pancreas System. J. Diabetes Sci. Technol. 2020, 14, 1137–1138. [Google Scholar] [CrossRef]
- Tudor-Locke, C.; Bassett, D.R. How Many Steps/Day Are Enough? Sports Med. 2004, 34, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, M.D.; Cleland, V.J.; Shaw, K.; Dwyer, T.; Venn, A.J. Cardiometabolic Risk in Younger and Older Adults Across an Index of Ambulatory Activity. Am. J. Prev. Med. 2009, 37, 278–284. [Google Scholar] [CrossRef] [PubMed]
- McKercher, C.M.; Schmidt, M.D.; Sanderson, K.A.; Patton, G.C.; Dwyer, T.; Venn, A.J. Physical Activity and Depression in Young Adults. Am. J. Prev. Med. 2009, 36, 161–164. [Google Scholar] [CrossRef] [PubMed]
- Löllgen, H.; Wismach, J.; Bachl, N. Körperliche Aktivität als Medikament. In Arzneimittelverordnung in der Zukunft; Arzneimittelkommission der deutschen Ärzteschaft: Berlin, Germany, 2018; p. Band 45. [Google Scholar]
- Balducci, S.; Zanuso, S.; Nicolucci, A.; De Feo, P.; Cavallo, S.; Cardelli, P.; Fallucca, S.; Alessi, E.; Fallucca, F.; Pugliese, G.; et al. Effect of an Intensive Exercise Intervention Strategy on Modifiable Cardiovascular Risk Factors in Subjects with Type 2 Diabetes Mellitus: A Randomized Controlled Trial: The Italian Diabetes and Exercise Study (IDES). Arch. Intern. Med. 2010, 170, 1794–1803. [Google Scholar] [CrossRef]
- Riebe, D.; Franklin, B.A.; Thompson, P.D.; Garber, C.E.; Whitfield, G.P.; Magal, M.; Pescatello, L.S. Updating ACSM’s Recommendations for Exercise Preparticipation Health Screening. Med. Sci. Sports Exerc. 2015, 47, 2473–2479. [Google Scholar] [CrossRef]
- Nugroho, W.; Doewes, M.; Siswandari, S. Effect of Aerobic and Anaerobic Interval Training on Oxidative Stress. Int. J. Multicult. Multireligious Underst. 2018, 5, 58. [Google Scholar] [CrossRef]
- Borg, G.A.V.; Noble, B.J. Perceived Exertion. Exerc. Sport Sci. Rev. 1974, 2, 131–154. [Google Scholar] [CrossRef]
- Utter, A.C.; Robertson, R.J.; Green, J.M.; Suminski, R.R.; McAnulty, S.R.; Nieman, D.C. Validation of the Adult OMNI Scale of perceived exertion for walking/running exercise. Med. Sci. Sports Exerc. 2004, 36, 1776–1780. [Google Scholar] [CrossRef]
- Irving, B.A.; Rutkowski, J.; Brock, D.W.; Davis, C.K.; Barrett, E.J.; Gaesser, G.A.; Weltman, A. Comparison of Borg- and OMNI-RPE as markers of the blood lactate response to exercise. Med. Sci. Sports Exerc. 2006, 38, 1348–1352. [Google Scholar] [CrossRef] [PubMed]
- Fishman, E.; Cherry, C. E-bikes in the Mainstream: Reviewing a Decade of Research. Transp. Rev. 2016, 36, 72–91. [Google Scholar] [CrossRef]
- Cooper, A.R.; Tibbitts, B.; England, C.; Procter, D.; Searle, A.; Sebire, S.J.; Ranger, E.; Page, A.S. Potential of electric bicycles to improve the health of people with Type 2 diabetes: A feasibility study. Diabet. Med. A J. Br. Diabet. Assoc. 2018, 35, 1279–1282. [Google Scholar] [CrossRef] [PubMed]
- Nieß, A.M.; Thiel, A. Körperliche Aktivität und Sport bei Typ-2-Diabetes. Diabetol. Und Stoffwechs. 2017, 12, 112–126. [Google Scholar] [CrossRef]
- Celis-Morales, C.A.; Lyall, D.M.; Welsh, P.; Anderson, J.; Steell, L.; Guo, Y.; Maldonado, R.; Mackay, D.F.; Pell, J.P.; Sattar, N.; et al. Association between active commuting and incident cardiovascular disease, cancer, and mortality: Prospective cohort study. BMJ 2017, 357, j1456. [Google Scholar] [CrossRef]
- Hoevenaar-Blom, M.P.; Wendel-Vos, G.C.W.; Spijkerman, A.M.W.; Kromhout, D.; Verschuren, W.M.M. Cycling and sports, but not walking, are associated with 10-year cardiovascular disease incidence: The MORGEN Study. Eur. J. Cardiovasc. Prev. Rehabil. 2010, 18, 41–47. [Google Scholar] [CrossRef]
- Lusk, A.C.; Mekary, R.A.; Feskanich, D.; Willett, W.C. Bicycle riding, walking, and weight gain in premenopausal women. Arch. Intern. Med. 2010, 170, 1050–1056. [Google Scholar] [CrossRef]
- Huy, C.; Becker, S.; Gomolinsky, U.; Klein, T.; Thiel, A. Health, Medical Risk Factors, and Bicycle Use in Everyday Life in the Over-50 Population. 2008, 16, 454. J. Aging Phys. Act. 2018, 16, 454. [Google Scholar] [CrossRef]
- Lièvre, M.M.; Moulin, P.; Thivolet, C.; Rodier, M.; Rigalleau, V.; Penfornis, A.; Pradignac, A.; Ovize, M. Detection of silent myocardial ischemia in asymptomatic patients with diabetes: Results of a randomized trial and meta-analysis assessing the effectiveness of systematic screening. Trials 2011, 12, 23. [Google Scholar] [CrossRef]
- Young, L.H.; Wackers, F.J.T.; Chyun, D.A.; Davey, J.A.; Barrett, E.J.; Taillefer, R.; Heller, G.V.; Iskandrian, A.E.; Wittlin, S.D.; Filipchuk, N.; et al. Cardiac outcomes after screening for asymptomatic coronary artery disease in patients with type 2 diabetes: The DIAD study: A randomized controlled trial. JAMA 2009, 301, 1547–1555. [Google Scholar] [CrossRef]
| Blood Glucose before Physical Activity | Specifications for Additional Carbohydrate Intake |
|---|---|
| <90 mg/dL (<5.0 mmol/L) |
|
| 90–150 mg/dL (5.0–8.3 mmol/L) |
|
| 150–250 mg/dL (8.3–13.9 mmol/L) |
|
| 250–350 mg/dL (13.9–19.4 mmol/L) |
|
| ≥350 mg/dL (≥19.4 mmol/L) |
|
| Training Duration | ||
|---|---|---|
| 30 min | 60 min | |
| Intensity of physical activity | ||
| Low (ca. 25% VO2max *) | Minus 25% | Minus 50% |
| Moderate (ca. 50% VO2max) | Minus 50% | Minus 75% |
| High (ca. 70–75% VO2max) | Minus 75% | Not specified |
| Intensive (>80% VO2max) | Reduction is not recommended | Not specified |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schubert-Olesen, O.; Kröger, J.; Siegmund, T.; Thurm, U.; Halle, M. Continuous Glucose Monitoring and Physical Activity. Int. J. Environ. Res. Public Health 2022, 19, 12296. https://doi.org/10.3390/ijerph191912296
Schubert-Olesen O, Kröger J, Siegmund T, Thurm U, Halle M. Continuous Glucose Monitoring and Physical Activity. International Journal of Environmental Research and Public Health. 2022; 19(19):12296. https://doi.org/10.3390/ijerph191912296
Chicago/Turabian StyleSchubert-Olesen, Oliver, Jens Kröger, Thorsten Siegmund, Ulrike Thurm, and Martin Halle. 2022. "Continuous Glucose Monitoring and Physical Activity" International Journal of Environmental Research and Public Health 19, no. 19: 12296. https://doi.org/10.3390/ijerph191912296
APA StyleSchubert-Olesen, O., Kröger, J., Siegmund, T., Thurm, U., & Halle, M. (2022). Continuous Glucose Monitoring and Physical Activity. International Journal of Environmental Research and Public Health, 19(19), 12296. https://doi.org/10.3390/ijerph191912296





