Microbial Fuel Cell-Based Biosensor for Simultaneous Test of Sodium Acetate and Glucose in a Mixed Solution
Abstract
:1. Introduction
2. Materials and Methods
2.1. Construction and Operation of MFC Sensor
2.2. Analytical Methods
2.2.1. Electrochemical Measurement
2.2.2. Anode Biofilm Analysis
3. Results and Discussion
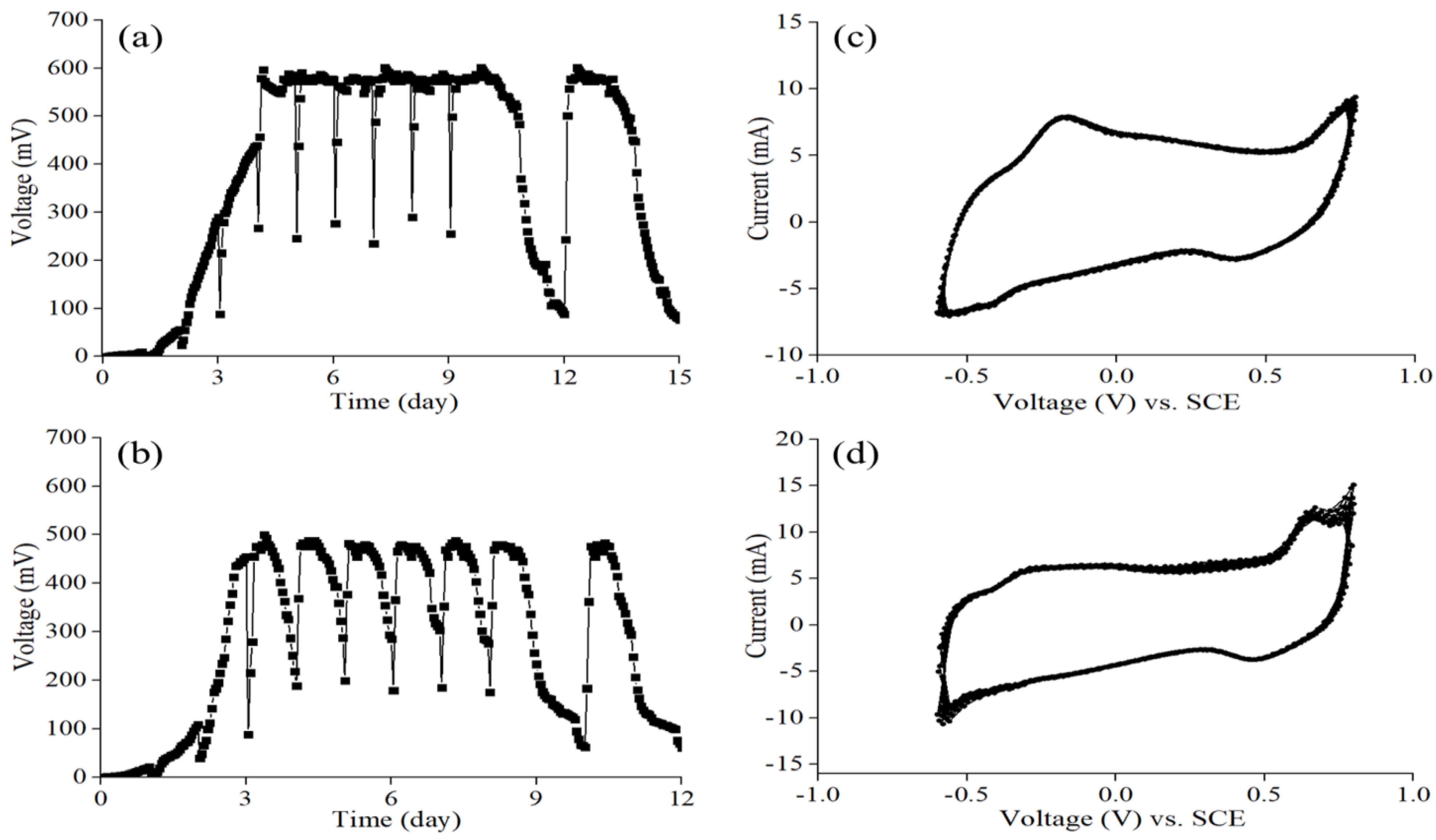
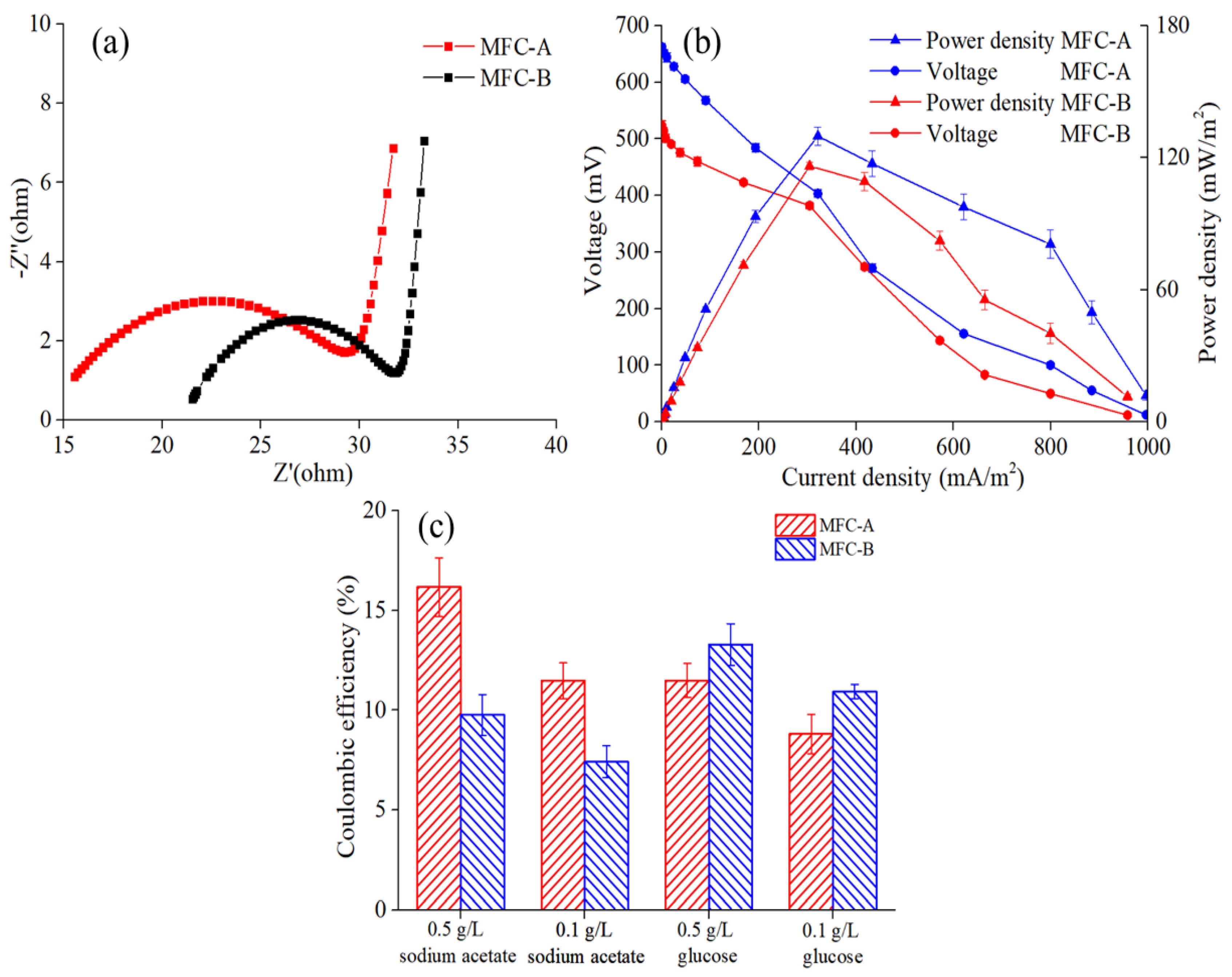
3.1. Startup of MFC Sensors and Performance Characterization
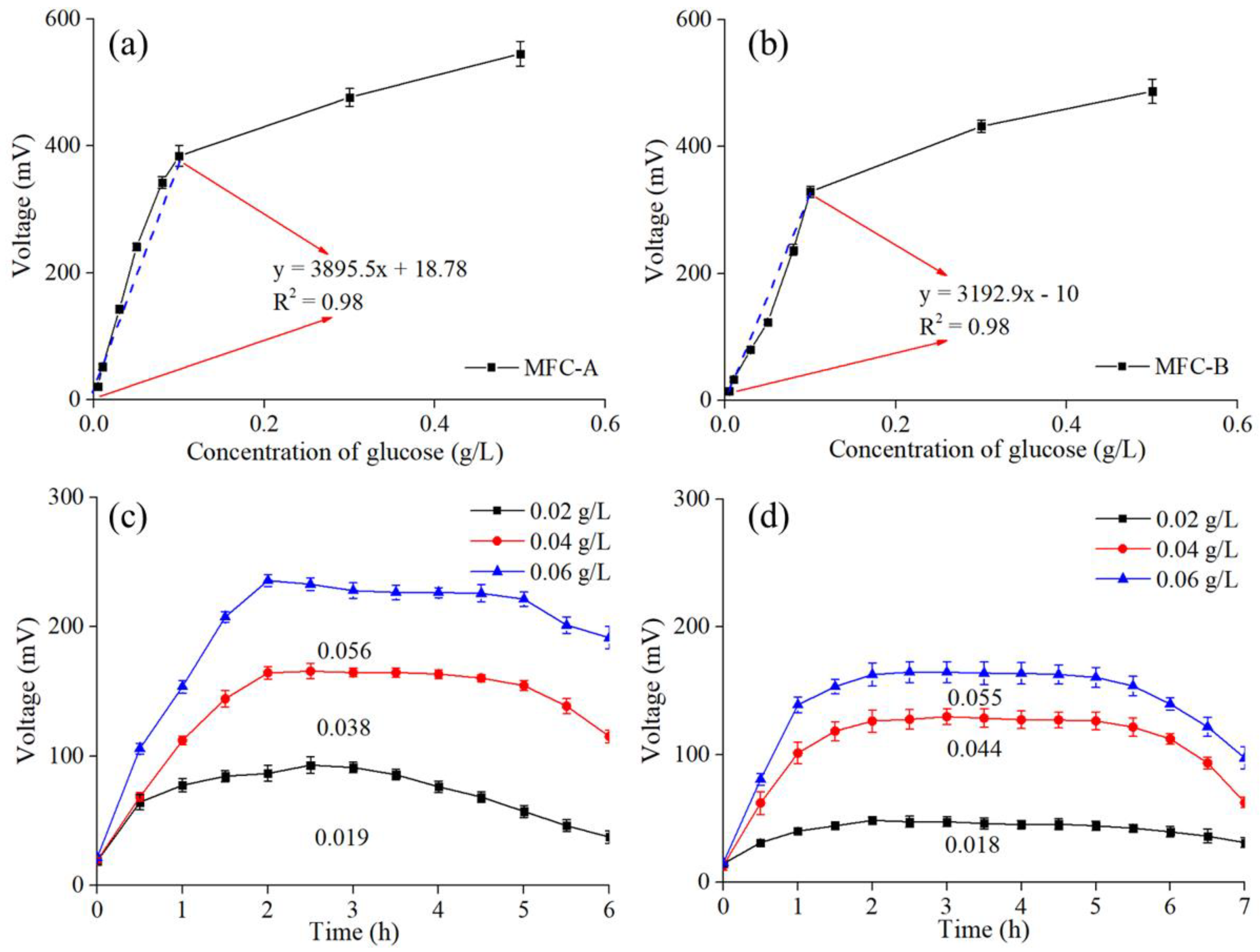
3.2. Individual Test of Sodium Acetate and Glucose
3.3. Simultaneous Test of Sodium Acetate and Glucose in a Mixed Solution
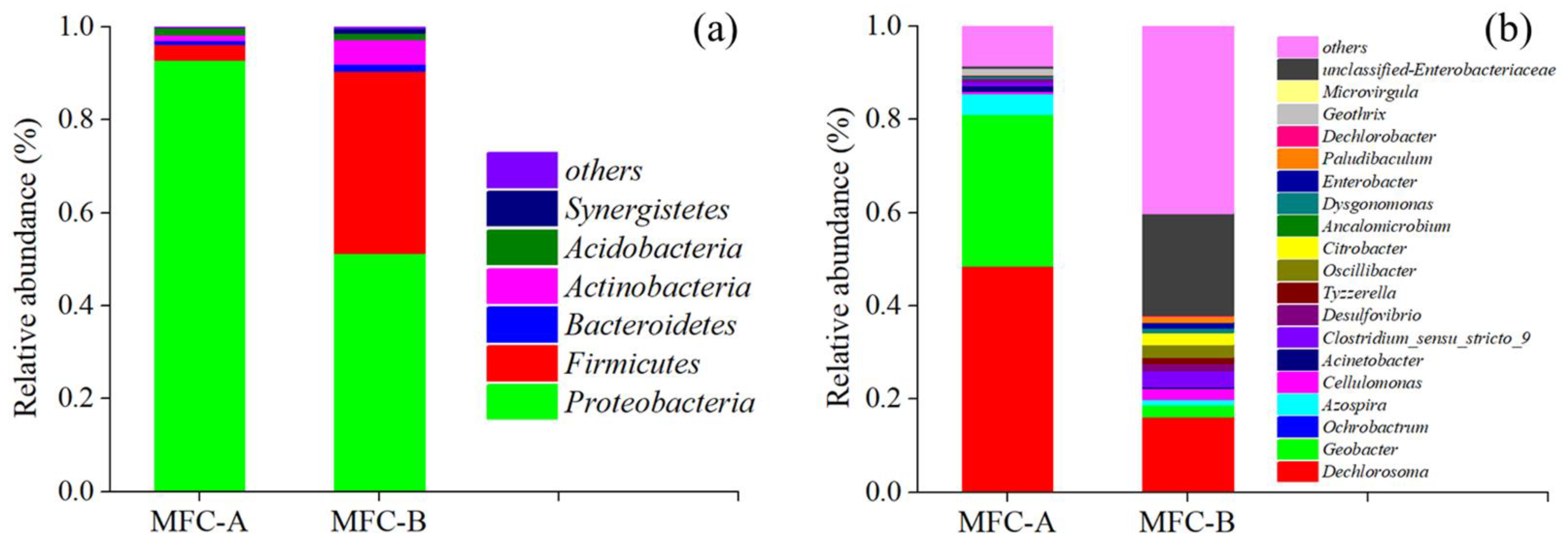
3.4. Analysis of Anode Biofilm
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xin, S.; Shen, J.; Liu, G.; Chen, Q.; Xiao, Z.; Zhang, G.; Xin, Y. Electricity generation and microbial community of single-chamber microbial fuel cells in response to Cu2O nanoparticles/reduced graphene oxide as cathode catalyst. Chem. Eng. J. 2019, 380, 122446. [Google Scholar] [CrossRef]
- Logan, B.E.; Hamelers, B.; Rozendal, R.; Schröder, U.; Keller, J.; Freguia, S.; Aelterman, P.; Verstraete, W.; Rabaey, K. Microbial fuel cell: Methodology and technology. Environ. Sci. Technol. 2006, 40, 5181–5192. [Google Scholar] [CrossRef]
- Qiu, S.; Guo, Z.; Naz, F.; Yang, Z.; Yu, C. An overview in the development of cathode materials for the improvement in power generation of microbial fuel cells. Bioelectrochemistry 2021, 141, 107834. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.; Zhao, Y.; Kang, C.; Yang, Y.; Morgan, D.; Xu, L. Towards concurrent pollutants removal and high energy harvesting in a pilot-scale CW-MFC: Insight into the cathode conditions and electrodes connection. Chem. Eng. J. 2019, 373, 150–160. [Google Scholar] [CrossRef]
- Tanikkul, P.; Pisutpaisal, N. Membrane-less MFC based biosensor for monitoring wastewater quality. Int. J. Hydrog. Energy 2018, 43, 483–489. [Google Scholar] [CrossRef]
- Peera, S.G.; Maiyalagan, T.; Liu, C.; Ashmath, S.; Lee, T.G.; Jiang, Z.; Mao, S. A review on carbon and non-precious metal based cathode catalysts in microbial fuel cells. Int. J. Hydrog. Energy 2021, 46, 3056–3089. [Google Scholar] [CrossRef]
- Zhang, Q.; Kaisti, M.; Prabhu, A.; Yu, Y.; Song, Y.A.; Rafailovich, M.H.; Rahman, A.; Levon, K. Polyaniline-functionalized ion-sensitive floating-gate FETs for the on-chip monitoring of peroxidase-catalyzed redox reactions. Electrochim. Acta 2018, 261, 256–264. [Google Scholar] [CrossRef]
- Yu, Y.; Zhang, Q.; Buscaglia, J.; Chang, C.-C.; Liu, Y.; Yang, Z.; Guo, Y.; Wang, Y.; Levon, K.; Rafailovich, M. Quantitative real-time detection of carcinoembryonic antigen (CEA) from pancreatic cyst fluid using 3-D surface molecular imprinting. Analyst 2016, 141, 4424–4431. [Google Scholar] [CrossRef]
- Yu, Y.; Wu, T.; Johnson-Buck, A.; Li, L.; Su, X. A two-layer assay for single-nucleotide variants utilizing strand displacement and selective digestion. Biosens. Bioelectron. 2016, 82, 248–254. [Google Scholar] [CrossRef]
- Lu, H.; Yu, Y.; Xi, H.; Wang, C.; Zhou, Y. Bacterial response to formaldehyde in an MFC toxicity sensor. Enzym. Microb. Technol. 2020, 140, 109565. [Google Scholar] [CrossRef]
- Corbella, C.; Hartl, M.; Fernandez-Gatell, M.; Puigagut, J. MFC-based biosensor for domestic wastewater COD assessment in constructed wetlands. Sci. Total Environ. 2018, 660, 218–226. [Google Scholar] [CrossRef] [PubMed]
- Abrevaya, X.C.; Sacco, N.J.; Bonetto, M.C.; Hilding-Ohlsson, A.; Cortón, E. Analytical applications of microbial fuel cells. Part I: Biochemical oxygen demand. Biosens. Bioelectron. 2015, 63, 580–590. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Wang, S.; Yin, F.; Hu, P.; Wang, X.; Liu, Y.; Liu, H. Enhancing sensitivity of microbial fuel cell sensors for low concentration biodegradable organic matter detection: Regulation of substrate concentration, anode area and external resistance. J. Environ. Sci. 2020, 101, 227–235. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Yang, X.; Liang, P.; Liu, P.; Huang, X. Microbial fuel cell sensors for water quality early warning systems: Fundamentals, signal resolution, optimization and future challenges. Renew. Sustain. Energy Rev. 2018, 81, 292–305. [Google Scholar] [CrossRef]
- Schneider, G.; Czeller, M.; Rostás, V.; Kovács, T. Microbial fuel cell-based diagnostic platform to reveal antibacterial effect of beta-lactam antibiotics. Enzym. Microb. Technol. 2015, 73–74, 59–64. [Google Scholar] [CrossRef]
- Stein, N.E.; Hamelers, H.; Buisman, C.N. Influence of membrane type, current and potential on the response to chemical toxicants of a microbial fuel cell based biosensor. Sens. Actuators B Chem. 2012, 163, 1–7. [Google Scholar] [CrossRef]
- Jiang, Y.; Liang, P.; Huang, X.; Ren, Z.J. A novel microbial fuel cell sensor with a gas diffusion biocathode sensing element for water and air quality monitoring. Chemosphere 2018, 203, 21–25. [Google Scholar] [CrossRef]
- Abrevaya, X.C.; Sacco, N.J.; Bonetto, M.C.; Hilding-Ohlsson, A.; Cortón, E. Analytical applications of microbial fuel cells. Part II: Toxicity, microbial activity and quantification, single analyte detection and other uses. Biosens. Bioelectron. 2015, 63, 591–601. [Google Scholar] [CrossRef]
- Pan, J.; Hu, J.; Liu, B.; Li, J.; Wang, D.; Bu, C.; Wang, X.; Xiao, K.; Liang, S.; Yang, J.; et al. Enhanced quorum sensing of anode biofilm for better sensing linearity and recovery capability of microbial fuel cell toxicity sensor. Environ. Res. 2019, 181, 108906. [Google Scholar] [CrossRef]
- Shen, Y.; Wang, M.; Chang, I.S.; Ng, H.Y. Effect of shear rate on the response of microbial fuel cell toxicity sensor to Cu(II). Bioresour. Technol. 2013, 136, 707–710. [Google Scholar] [CrossRef]
- Gao, Y.; Yin, F.; Ma, W.; Wang, S.; Liu, Y.; Liu, H. Rapid detection of biodegradable organic matter in polluted water with microbial fuel cell sensor: Method of partial coulombic yield. Bioelectrochemistry 2020, 133, 107488. [Google Scholar] [CrossRef] [PubMed]
- Kaur, A.; Ibrahim, S.; Pickett, C.J.; Michie, I.S.; Dinsdale, R.M.; Guwy, A.J.; Premier, G.C. Anode modification to improve the performance of a microbial fuel cell volatile fatty acid biosensor. Sens. Actuators B Chem. 2014, 201, 266–273. [Google Scholar] [CrossRef]
- Feng, Y.; Harper, W.F. Biosensing with microbial fuel cells and artificial neural networks: Laboratory and field investigations. J. Environ. Manag. 2013, 130, 369–374. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.C.; Kharkwal, S.; Chew, K.K.W.; Alwi, R.; Mak, S.F.W.; Ng, H.Y. Enhancing the robustness of microbial fuel cell sensor for continuous copper(II) detection against organic strength fluctuations by acetate and glucose addition. Bioresour. Technol. 2018, 259, 357–364. [Google Scholar] [CrossRef]
- Khan, A.; Salama, E.-S.; Chen, Z.; Ni, H.; Zhao, S.; Zhou, T.; Pei, Y.; Sani, R.K.; Ling, Z.; Liu, P.; et al. A novel biosensor for zinc detection based on microbial fuel cell system. Biosens. Bioelectron. 2019, 147, 111763. [Google Scholar] [CrossRef]
- Oveisi, F.; Fallah, N.; Nasernejad, B. Biodegradation of synthetic wastewater containing styrene in microbial fuel cell: Effect of adaptation of microbial community. Fuel 2021, 305, 121382. [Google Scholar] [CrossRef]
- Li, M.; Bi, Y.-G.; Xiang, L.; Chen, X.-T.; Qin, Y.-J.; Mo, C.-H.; Zhou, S.-Q. Improved cathodic oxygen reduction and bioelectricity generation of electrochemical reactor based on reduced graphene oxide decorated with titanium-based composites. Bioresour. Technol. 2019, 296, 122319. [Google Scholar] [CrossRef]
- Narayanasamy, S.; Jayaprakash, J. Improved performance of Pseudomonas aeruginosa catalyzed MFCs with graphite/polyester composite electrodes doped with metal ions for azo dye degradation. Chem. Eng. J. 2018, 343, 258–269. [Google Scholar] [CrossRef]
- Xiao, N.; Wu, R.; Huang, J.J.; Selvaganapathy, P.R. Anode surface modification regulates biofilm community population and the performance of micro-MFC based biochemical oxygen demand sensor. Chem. Eng. Sci. 2020, 221, 115691. [Google Scholar] [CrossRef]
- Ayyaru, S.; Dharmalingam, S. Enhanced response of microbial fuel cell using sulfonated poly ether ether ketone membrane as a biochemical oxygen demand sensor. Anal. Chim. Acta 2014, 818, 15–22. [Google Scholar] [CrossRef]
- Yang, S.; Du, F.; Liu, H. Characterization of mixed-culture biofilms established in microbial fuel cells. Biomass-Bioenergy 2012, 46, 531–537. [Google Scholar] [CrossRef]
- Do, M.H.; Ngo, H.H.; Guo, W.; Chang, S.W.; Nguyen, D.D.; Liu, Y.; Varjani, S.; Kumar, M. Microbial fuel cell-based biosensor for online monitoring wastewater quality: A critical review. Sci. Total Environ. 2019, 712, 135612. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Tian, S.; Zhang, P.; Ye, J.; Tao, X.; Li, F.; Zhou, Z.; Nabi, M. Enhancement of biological oxygen demand detection with a microbial fuel cell using potassium permanganate as cathodic electron acceptor. J. Environ. Manag. 2019, 252, 109682. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.; Liu, P.; Liang, P.; Hao, W.; Li, M.; Li, Q.; Zhou, Y.; Huang, X. Biofilm’s morphology design for high sensitivity of bioelectrochemical sensor: An experimental and modeling study. Sci. Total Environ. 2020, 729, 138908. [Google Scholar] [CrossRef]
- Saba, B.; Christy, A.D.; Yu, Z.; Co, A.C.; Islam, R.; Tuovinen, O.H. Characterization and performance of anodic mixed culture biofilms in submersed microbial fuel cells. Bioelectrochemistry 2017, 113, 79–84. [Google Scholar] [CrossRef]
- Wang, J.; Song, X.; Wang, Y.; Abayneh, B.; Li, Y.; Yan, D.; Bai, J. Nitrate removal and bioenergy production in constructed wetland coupled with microbial fuel cell: Establishment of electrochemically active bacteria community on anode. Bioresour. Technol. 2016, 221, 358–365. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qiu, S.; Wang, L.; Zhang, Y.; Yu, Y. Microbial Fuel Cell-Based Biosensor for Simultaneous Test of Sodium Acetate and Glucose in a Mixed Solution. Int. J. Environ. Res. Public Health 2022, 19, 12297. https://doi.org/10.3390/ijerph191912297
Qiu S, Wang L, Zhang Y, Yu Y. Microbial Fuel Cell-Based Biosensor for Simultaneous Test of Sodium Acetate and Glucose in a Mixed Solution. International Journal of Environmental Research and Public Health. 2022; 19(19):12297. https://doi.org/10.3390/ijerph191912297
Chicago/Turabian StyleQiu, Song, Luyang Wang, Yimei Zhang, and Yingjie Yu. 2022. "Microbial Fuel Cell-Based Biosensor for Simultaneous Test of Sodium Acetate and Glucose in a Mixed Solution" International Journal of Environmental Research and Public Health 19, no. 19: 12297. https://doi.org/10.3390/ijerph191912297
APA StyleQiu, S., Wang, L., Zhang, Y., & Yu, Y. (2022). Microbial Fuel Cell-Based Biosensor for Simultaneous Test of Sodium Acetate and Glucose in a Mixed Solution. International Journal of Environmental Research and Public Health, 19(19), 12297. https://doi.org/10.3390/ijerph191912297




