Chlorpyrifos Occurrence and Toxicological Risk Assessment: A Review
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. The Behavior of Chlorpyrifos in the Environment
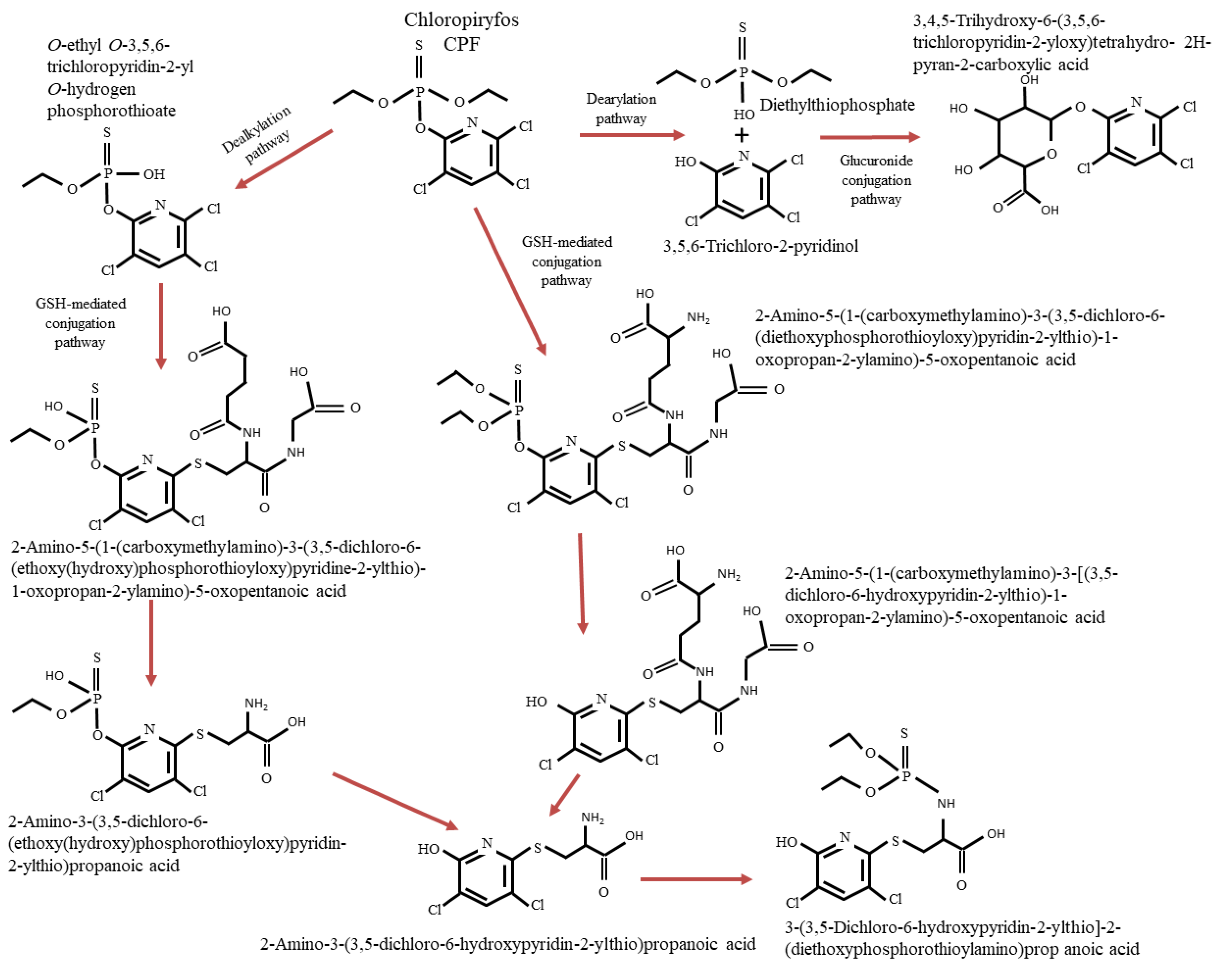
3.2. Degradation Pathways of CPF
3.3. Exposure Pathways of CPF in Humans
3.3.1. Exposure to CPF in Humans by Oral Administration
3.3.2. Exposure to CPF in Humans by Inhalation
3.3.3. Exposure to CPF in Humans by Dermal Absorption
3.3.4. Exposure to CPF in Children
3.4. Toxicity of CPF to Other Mammals
3.4.1. Acute Toxicity of CPF
3.4.2. In Vitro and In Vivo Analysis of CPF Toxicity
3.4.3. Genotoxicity of CPF
3.4.4. Endocrine-Disrupting Properties and Developmental/Reproductive Toxicity of CPF
3.4.5. CPF Developmental Neurotoxicity
3.5. Occurrence of CPF in Food and Risk Assessment
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Perry, J.; Cotton, J.; Rahman, M.A.; Brumby, S. Organophosphate Exposure and the Chronic Effects on Farmers: A Narrative Review. Rural. Remote Health 2020, 20, 4508. [Google Scholar] [CrossRef] [PubMed]
- George, N.; Singh, C.P.; Sondhi, S.; Saini, S.; Puri, N.; Gupta, N.; Gupta, N. Biodegradation and Analytical Methods for Detection of Organophosphorous Pesticide: Chlorpyrifos. Int. J. Pure Appl. Sci. Technol. 2014, 20, 79–94. [Google Scholar]
- Mackay, D.; Giesy, J.P.; Solomon, K.R. Fate in the Environment and Long-Range Atmospheric Transport of the Organophosphorus Insecticide, Chlorpyrifos and Its Oxon. Rev. Environ. Contam. Toxicol. 2014, 231, 35–76. [Google Scholar] [CrossRef]
- Lu, C.; Yang, Z.; Liu, J.; Liao, Q.; Ling, W.; Waigi, M.G.; Odinga, E.S. Chlorpyrifos Inhibits Nitrogen Fixation in Rice-Vegetated Soil Containing Pseudomonas Stutzeri A1501. Chemosphere 2020, 256, 127098. [Google Scholar] [CrossRef] [PubMed]
- Bernal-Rey, D.L.; Cantera, C.G.; Dos Santos Afonso, M.; Menéndez-Helman, R.J. Seasonal Variations in the Dose-Response Relationship of Acetylcholinesterase Activity in Freshwater Fish Exposed to Chlorpyrifos and Glyphosate. Ecotoxicol. Environ. Saf. 2020, 187, 109673. [Google Scholar] [CrossRef] [PubMed]
- Casida, J.E.; Quistad, G.B. Organophosphate Toxicology: Safety Aspects of Nonacetylcholinesterase Secondary Targets. Chem. Res. Toxicol. 2004, 17, 983–998. [Google Scholar] [CrossRef]
- Bootharaju, M.S.; Pradeep, T. Understanding the Degradation Pathway of the Pesticide, Chlorpyrifos by Noble Metal Nanoparticles. Langmuir 2012, 28, 2671–2679. [Google Scholar] [CrossRef]
- Rezg, R.; Mornagui, B.; El-Fazaa, S.; Gharbi, N. Organophosphorus Pesticides as Food Chain Contaminants and Type 2 Diabetes: A Review. Trends Food Sci. Technol. 2010, 21, 345–357. [Google Scholar] [CrossRef]
- Akoto, O.; Gavor, S.; Appah, M.K.; Apau, J. Estimation of Human Health Risk Associated with the Consumption of Pesticide-Contaminated Vegetables from Kumasi, Ghana. Environ. Monit. Assess. 2015, 187, 244. [Google Scholar] [CrossRef]
- Tudi, M.; Li, H.; Li, H.; Wang, L.; Lyu, J.; Yang, L.; Tong, S.; Yu, Q.J.; Ruan, H.D.; Atabila, A.; et al. Exposure Routes and Health Risks Associated with Pesticide Application. Toxics 2022, 10, 335. [Google Scholar] [CrossRef]
- Commission Implementing Regulation (EU) 2020/18 of 10 January 2020 Concerning the Non-Renewal of the Approval of the Active Substance Chlorpyrifos, in Accordance with Regulation (EC) No 1107/2009 of the European Parliament and of the Council Concerning the Placing of Plant Protection Products on the Market, and Amending the Annex to Commission Implementing Regulation (EU) No 540/2011 (Text with EEA Relevance). Available online: https://eur-lex.europa.eu/eli/reg_impl/2020/18/oj (accessed on 28 May 2021).
- ECHA. Chlorpyrifos Draft Risk Profile. 2022. Available online: https://echa.europa.eu/documents/10162/8a51d7d9-e9a4-2513-e975-492fb70f825c (accessed on 28 May 2021).
- Database of Notifications of Final Regulatory Action. Available online: http://www.pic.int/Procedures/NotificationsofFinalRegulatoryActions/Database/tabid/1368/language/enUS/Default.aspx (accessed on 28 February 2022).
- Jaiswal, S.; Bara, J.K.; Soni, R.; Shrivastava, K. Bioremediation of Chlorpyrifos Contaminated Soil by Microorganism. Int. J. Environ. Agric. Biotechnol. 2017, 2, 1624–1630. [Google Scholar] [CrossRef]
- Huang, X.; Cui, H.; Duan, W. Ecotoxicity of Chlorpyrifos to Aquatic Organisms: A Review. Ecotoxicol. Environ. Saf. 2020, 200, 110731. [Google Scholar] [CrossRef]
- Singh, B.K.; Walker, A.; Morgan, J.A.W.; Wright, D.J. Effects of Soil PH on the Biodegradation of Chlorpyrifos and Isolation of a Chlorpyrifos-Degrading Bacterium. Appl. Environ. Microbiol. 2003, 69, 5198–5206. [Google Scholar] [CrossRef]
- Ajaz, M.; Jabeen, N.; Akhtar, S.; Rasool, S.A. Chlorpyrifos Resistant Bacteria from Pakistani Soils: Isolation, Identification, Resistance Profile and Growth Kinetics. Pak. J. Bot. 2005, 37, 381–388. [Google Scholar]
- Asamba, M.N.; Ezekiel, M.; Sifuna, O.P.; Essuman, S.; Chimbevo, L.M.; Norbert, A. Molecular Characterization of Chlorpyrifos Degrading Bacteria Isolated from Contaminated Dairy Farm Soils in Nakuru County, Kenya. SSRN Electron. J. 2021, 8, e09176. [Google Scholar] [CrossRef]
- Farhan, M.; Ahmad, M.; Kanwal, A.; Butt, Z.A.; Khan, Q.F.; Raza, S.A.; Qayyum, H.; Wahid, A. Biodegradation of Chlorpyrifos Using Isolates from Contaminated Agricultural Soil, Its Kinetic Studies. Sci. Rep. 2021, 11, 10320. [Google Scholar] [CrossRef]
- Sarnaik, S.S.; Kanekar, P.P.; Raut, V.M.; Taware, S.P.; Chavan, K.S.; Bhadbhade, B.J. Effect of Application of Different Pesticides to Soybean on the Soil Microflora. J. Environ. Biol. 2006, 27 (Suppl. 2), 423–426. [Google Scholar]
- John, E.M.; Shaike, J.M. Chlorpyrifos: Pollution and Remediation. Environ. Chem. Lett. 2015, 13, 269–291. [Google Scholar] [CrossRef]
- Riah, W.; Laval, K.; Laroche-Ajzenberg, E.; Mougin, C.; Latour, X.; Trinsoutrot-Gattin, I. Effects of Pesticides on Soil Enzymes: A Review. Environ. Chem. Lett. 2014, 12, 257–273. [Google Scholar] [CrossRef]
- Sanchez-Hernandez, J.C.; Notario del Pino, J.; Capowiez, Y.; Mazzia, C.; Rault, M. Soil Enzyme Dynamics in Chlorpyrifos-Treated Soils under the Influence of Earthworms. Sci. Total Environ. 2018, 612, 1407–1416. [Google Scholar] [CrossRef]
- Guo, A.; Pan, C.; Ma, J.; Bao, Y. Linkage of Antibiotic Resistance Genes, Associated Bacteria Communities and Metabolites in the Wheat Rhizosphere from Chlorpyrifos-Contaminated Soil. Sci. Total Environ. 2020, 741, 140457. [Google Scholar] [CrossRef] [PubMed]
- Zhong, J.; Shen, D.; Li, H.; He, Y.; Bao, Q.; Wang, W.; Ye, Q.; Gan, J. Fate of Chlorpyrifos Bound Residues in Paddy Soils: Release, Transformation, and Phytoavailability. Environ. Int. 2022, 166, 107338. [Google Scholar] [CrossRef] [PubMed]
- Rayu, S.; Nielsen, U.N.; Nazaries, L.; Singh, B.K. Isolation and Molecular Characterization of Novel Chlorpyrifos and 3,5,6-Trichloro-2-Pyridinol-Degrading Bacteria from Sugarcane Farm Soils. Front. Microbiol. 2017, 8, 518. [Google Scholar] [CrossRef] [PubMed]
- Feng, F.; Ge, J.; Li, Y.; He, S.; Zhong, J.; Liu, X.; Yu, X. Enhanced Degradation of Chlorpyrifos in Rice (Oryza sativa L.) by Five Strains of Endophytic Bacteria and Their Plant Growth Promotional Ability. Chemosphere 2017, 184, 505–513. [Google Scholar] [CrossRef]
- Ahmad, F.; Iqbal, S.; Anwar, S.; Afzal, M.; Islam, E.; Mustafa, T.; Khan, Q.M. Enhanced Remediation of Chlorpyrifos from Soil Using Ryegrass (Lollium multiflorum) and Chlorpyrifos-Degrading Bacterium Bacillus Pumilus C2A1. J. Hazard. Mater. 2012, 237–238, 110–115. [Google Scholar] [CrossRef] [PubMed]
- Akash, S.; Sivaprakash, B.; Rajamohan, N.; Pandiyan, C.M.; Vo, D.-V.N. Pesticide Pollutants in the Environment—A Critical Review on Remediation Techniques, Mechanism and Toxicological Impact. Chemosphere 2022, 301, 134754. [Google Scholar] [CrossRef] [PubMed]
- Schwantes, D.; Celso Gonçalves, A., Jr.; Conradi Junior, É.; Campagnolo, M.A.; Zimmermann, J. Determination of CHLORPYRIFOS by GC/ECD in Water and Its Sorption Mechanism Study in a RHODIC FERRALSOL. J. Environ. Health Sci. Eng. 2020, 18, 149–162. [Google Scholar] [CrossRef]
- Wang, D.; Singhasemanon, N.; Goh, K.S. A Statistical Assessment of Pesticide Pollution in Surface Waters Using Environmental Monitoring Data: Chlorpyrifos in Central Valley, California. Sci. Total Environ. 2016, 571, 332–341. [Google Scholar] [CrossRef]
- Marchesan, E.; Zanella, R.; de Avila, L.A.; Camargo, E.R.; de, O. Machado, S.L.; Macedo, V.R.M. Rice Herbicide Monitoring in Two Brazilian Rivers during the Rice Growing Season. Sci. Agric. 2007, 64, 131–137. [Google Scholar] [CrossRef]
- Dar, M.A.; Kaushik, G.; Villarreal-Chiu, J.F. Pollution Status and Bioremediation of Chlorpyrifos in Environmental Matrices by the Application of Bacterial Communities: A Review. J. Environ. Manag. 2019, 239, 124–136. [Google Scholar] [CrossRef]
- Hossain, M.S.; Chowdhury, M.A.Z.; Pramanik, M.K.; Rahman, M.A.; Fakhruddin, A.N.M.; Alam, M.K. Determination of Selected Pesticides in Water Samples Adjacent to Agricultural Fields and Removal of Organophosphorus Insecticide Chlorpyrifos Using Soil Bacterial Isolates. Appl. Water Sci. 2015, 5, 171–179. [Google Scholar] [CrossRef]
- Zhong, G.; Xie, Z.; Cai, M.; Möller, A.; Sturm, R.; Tang, J.; Zhang, G.; He, J.; Ebinghaus, R. Distribution and Air-Sea Exchange of Current-Use Pesticides (CUPs) from East Asia to the High Arctic Ocean. Environ. Sci. Technol. 2012, 46, 259–267. [Google Scholar] [CrossRef]
- Kumar, S.; Kaushik, G.; Villarreal-Chiu, J.F. Scenario of Organophosphate Pollution and Toxicity in India: A Review. Environ. Sci. Pollut. Res. Int. 2016, 23, 9480–9491. [Google Scholar] [CrossRef]
- Zhang, X.; Shen, Y.; Yu, X.-Y.; Liu, X.-J. Dissipation of Chlorpyrifos and Residue Analysis in Rice, Soil and Water under Paddy Field Conditions. Ecotoxicol. Environ. Saf. 2012, 78, 276–280. [Google Scholar] [CrossRef]
- Lockridge, O.; Verdier, L.; Schopfer, L.M. Half-Life of Chlorpyrifos Oxon and Other Organophosphorus Esters in Aqueous Solution. Chem. Biol. Interact. 2019, 311, 108788. [Google Scholar] [CrossRef]
- Khan, M.S.I.; Lee, N.R.; Ahn, J.; Kim, J.Y.; Kim, J.H.; Kwon, K.H.; Kim, Y.-J. Degradation of Different Pesticides in Water by Microplasma: The Roles of Individual Radicals and Degradation Pathways. Environ. Sci. Pollut. Res. Int. 2021, 28, 8296–8309. [Google Scholar] [CrossRef]
- Romeh, A.A. Synergistic Effect of Ficus-Zero Valent Iron Supported on Adsorbents and Plantago Major for Chlorpyrifos Phytoremediation from Water. Int. J. Phytoremediation 2021, 23, 151–161. [Google Scholar] [CrossRef]
- Rajmohan, K.S.; Chandrasekaran, R.; Varjani, S. A Review on Occurrence of Pesticides in Environment and Current Technologies for Their Remediation and Management. Ind. J. Microbiol. 2020, 60, 125–138. [Google Scholar] [CrossRef]
- Kashyap, V.; Kumar, M. Studies on the Effects of Chlorpyrifos on Growth and Yield in Green Gram (Vigna radiata L.) at Different Phenological Stages. J. Biol. Chem. Res. 2013, 30, 734–740. [Google Scholar]
- Parween, T.; Jan, S.; Mahmooduzzafar; Fatma, T. Variation in Elemental Composition as Influenced by Chlorpyrifos Application in Mung Bean (Vigna radiata L.). Saudi J. Biol. Sci. 2018, 25, 1439–1445. [Google Scholar] [CrossRef]
- Nandhini, A.R.; Harshiny, M.; Gummadi, S.N. Chlorpyrifos in Environment and Food: A Critical Review of Detection Methods and Degradation Pathways. Environ. Sci. Process. Impacts 2021, 23, 1255–1277. [Google Scholar] [CrossRef] [PubMed]
- Romeh, A.A.; Hendawi, M.Y. Chlorpyrifos Insecticide Uptake by Plantain from Polluted Water and Soil. Environ. Chem. Lett. 2013, 11, 163–170. [Google Scholar] [CrossRef]
- Eaton, D.L.; Daroff, R.B.; Autrup, H.; Bridges, J.; Buffler, P.; Costa, L.G.; Coyle, J.; McKhann, G.; Mobley, W.C.; Nadel, L.; et al. Review of the Toxicology of Chlorpyrifos with an Emphasis on Human Exposure and Neurodevelopment. Crit. Rev. Toxicol. 2008, 38 (Suppl. 2), 1–125. [Google Scholar] [CrossRef] [PubMed]
- US EPA (United States Environmental Protection Agency). Chlorpyrifos: Preliminary Human Health Risk Assessment for Registration. DP No. D388070. Office of Chemical Safety and Polution Prevention. 2011. Available online: https://archive.epa.gov/pesticides/news/web/html/chlorpyrifos.html (accessed on 30 April 2021).
- Timchalk, C.; Nolan, R.J.; Mendrala, A.L.; Dittenber, D.A.; Brzak, K.A. Mattsson A Physiologically Based Pharmacokinetic and Pharmacodynamic (PBPK/PD) Model for the Organophosphate Insecticide Chlorpyrifos in Rats and Humans. Toxicol. Sci. 2002, 66, 34–53. [Google Scholar] [CrossRef]
- Choi, K.; Joo, H.; Rose, R.L.; Hodgson, E. Metabolism of Chlorpyrifos and Chlorpyrifos Oxon by Human Hepatocytes. J. Biochem. Mol. Toxicol. 2006, 20, 279–291. [Google Scholar] [CrossRef]
- Chebab, S.; Mekircha, F.; Leghouchi, E. Potential Protective Effect of Pistacia Lentiscus Oil against Chlorpyrifos-Induced Hormonal Changes and Oxidative Damage in Ovaries and Thyroid of Female Rats. Biomed. Pharmacother. 2017, 96, 1310–1316. [Google Scholar] [CrossRef]
- Shenouda, J.; Green, P.; Sultatos, L. An Evaluation of the Inhibition of Human Butyrylcholinesterase and Acetylcholinesterase by the Organophosphate Chlorpyrifos Oxon. Toxicol. Appl. Pharmacol. 2009, 241, 135–142. [Google Scholar] [CrossRef]
- Mehta, A.; Verma, R.S.; Srivastava, N. Chlorpyrifos-Induced DNA Damage in Rat Liver and Brain. Environ. Mol. Mutagen. 2008, 49, 426–433. [Google Scholar] [CrossRef]
- Smith, J.N.; Timchalk, C.; Bartels, M.J.; Poet, T.S. In Vitro Age-Dependent Enzymatic Metabolism of Chlorpyrifos and Chlorpyrifos-Oxon in Human Hepatic Microsomes and Chlorpyrifos-Oxon in Plasma. Drug Metab. Dispos. 2011, 39, 1353–1362. [Google Scholar] [CrossRef]
- Elersek, T.; Filipic, M. Organophosphorous Pesticides—Mechanisms of Their Toxicity. In Pesticides—The Impacts of Pesticides Exposure; InTech: London, UK, 2011. [Google Scholar]
- Chambers, J.E.; Chambers, H.W. Oxidative Desulfuration of Chlorpyrifos, Chlorpyrifos-Methyl, and Leptophos by Rat Brain and Liver. J. Biochem. Toxicol. 1989, 4, 201–203. [Google Scholar] [CrossRef]
- Bradman, A.; Whitaker, D.; Quirós, L.; Castorina, R.; Claus Henn, B.; Nishioka, M.; Morgan, J.; Barr, D.B.; Harnly, M.; Brisbin, J.A.; et al. Pesticides and Their Metabolites in the Homes and Urine of Farmworker Children Living in the Salinas Valley, CA. J. Expo. Sci. Environ. Epidemiol. 2007, 17, 331–349. [Google Scholar] [CrossRef]
- EL-Nahhal, Y.; Lubbad, R. Acute and Single Repeated Dose Effects of Low Concentrations of Chlorpyrifos, Diuron, and Their Combination on Chicken. Environ. Sci. Pollut. Res. Int. 2018, 25, 10837–10847. [Google Scholar] [CrossRef]
- Eskenazi, B.; Marks, A.R.; Bradman, A.; Harley, K.; Barr, D.B.; Johnson, C.; Morga, N.; Jewell, N.P. Organophosphate Pesticide Exposure and Neurodevelopment in Young Mexican-American Children. Environ. Health Perspect. 2007, 115, 792–798. [Google Scholar] [CrossRef]
- Sapbamrer, R.; Hongsibsong, S. Effects of Prenatal and Postnatal Exposure to Organophosphate Pesticides on Child Neurodevelopment in Different Age Groups: A Systematic Review. Environ. Sci. Pollut. Res. Int. 2019, 26, 18267–18290. [Google Scholar] [CrossRef]
- Lehman-Mckeeman, L.D. Casaret and Doull’s Toxicology: The Basic Science of Poisons; Klaasen, C.D., Ed.; McGraw-Hill: New York, NY, USA, 2008; Volume 5. [Google Scholar]
- Mattsson, J.L. Lack of Differential Sensitivity to Cholinesterase Inhibition in Fetuses and Neonates Compared to Dams Treated Perinatally with Chlorpyrifos. Toxicol. Sci. 2000, 53, 438–446. [Google Scholar] [CrossRef]
- EFSA (European Food Safety Authority). Statement on the available outcomes of the human health assessment in the context of the pesticides peer review of the active substance chlorpyrifos. EFSA J. 2019, 17, e05809. [Google Scholar] [CrossRef]
- US EPA (Environmental Protection Agency). Chlorpyrifos: Revised Human Health Risk Assessment for Registration Review. EPA-HQ-OPP-2015-0653-0454. 2016. Available online: https://www.regulations.gov/document/EPA-HQ-OPP-2015-0653-0454 (accessed on 9 March 2021).
- Atabila, A.; Phung, D.T.; Hogarh, J.N.; Osei-Fosu, P.; Sadler, R.; Connell, D.; Chu, C. Dermal Exposure of Applicators to Chlorpyrifos on Rice Farms in Ghana. Chemosphere 2017, 178, 350–358. [Google Scholar] [CrossRef]
- Garabrant, D.H.; Aylward, L.L.; Berent, S.; Chen, Q.; Timchalk, C.; Burns, C.J.; Hays, S.M.; Albers, J.W. Cholinesterase Inhibition in Chlorpyrifos Workers: Characterization of Biomarkers of Exposure and Response in Relation to Urinary TCPy. J. Expo. Sci. Environ. Epidemiol. 2009, 19, 634–642. [Google Scholar] [CrossRef]
- Oostingh, G.J.; Wichmann, G.; Schmittner, M.; Lehmann, I.; Duschl, A. The Cytotoxic Effects of the Organophosphates Chlorpyrifos and Diazinon Differ from Their Immunomodulating Effects. J. Immunotoxicol. 2009, 6, 136–145. [Google Scholar] [CrossRef]
- Albers, J.W.; Berent, S.; Garabrant, D.H.; Giordani, B.; Schweitzer, S.J.; Garrison, R.P.; Richardson, R.J. The Effects of Occupational Exposure to Chlorpyrifos on the Neurologic Examination of Central Nervous System Function: A Prospective Cohort Study. J. Occup. Environ. Med. 2004, 46, 367–378. [Google Scholar] [CrossRef]
- Kumar, N. Dermal Exposure to Sub-Toxic Amount of Chlorpyrifos—Is It Neurotoxic. In Pesticides in the Modern World—Effects of Pesticides Exposure; InTech: Rijeka, Croatia, 2011. [Google Scholar]
- Meuling, W.J.; Ravensbergl, C.; Van Hemmenj, J. Absorption of Chlorpyrifos in Human Volunteers. Int. Arch. Occup. Environ. Health 2005, 78, 44–50. [Google Scholar] [CrossRef]
- Abu-Qare, A.W.; Abdel-Rahman, A.; Brownie, C.; Kishk, A.M.; Abou-Donia, M.B. Inhibition of Cholinesterase Enzymes Following a Single Dermal Dose of Chlorpyrifos and Methyl Parathion, Alone and in Combination, in Pregnant Rats. J. Toxicol. Environ. Health Part A. 2001, 63, 173–189. [Google Scholar] [CrossRef]
- Bernardes, M.F.F.; Pazin, M.; Pereira, L.C.; Dorta, D.J. Impact of Pesticides on Environmental and Human Health. In Toxicology Studies—Cells, Drugs and Environment; InTech: London, UK, 2015. [Google Scholar]
- Lim, K.L.; Tay, A.; Nadarajah, V.D.; Mitra, N.K. The Effect of Consequent Exposure of Stress and Dermal Application of Low Doses of Chlorpyrifos on the Expression of Glial Fibrillary Acidic Protein in the Hippocampus of Adult Mice. J. Occup. Med. Toxicol. 2011, 6, 4. [Google Scholar] [CrossRef]
- Hines, C.J.; Deddens, J.A. Determinants of Chlorpyrifos Exposures and Urinary 3,5,6-Trichloro-2-Pyridinol Levels among Termiticide Applicators. Ann. Occup. Hyg. 2001, 45, 309–321. [Google Scholar] [CrossRef]
- Sexton, K.; Needham, L.; Pirkle, J. Human Biomonitoring of Environmental Chemicals. Am. Sci. 2004, 92, 38. [Google Scholar] [CrossRef]
- Burke, R.D.; Todd, S.W.; Lumsden, E.; Mullins, R.J.; Mamczarz, J.; Fawcett, W.P.; Gullapalli, R.P.; Randall, W.R.; Pereira, E.F.R.; Albuquerque, E.X. Developmental Neurotoxicity of the Organophosphorus Insecticide Chlorpyrifos: From Clinical Findings to Preclinical Models and Potential Mechanisms. J. Neurochem. 2017, 142 (Suppl. 2), 162–177. [Google Scholar] [CrossRef]
- Das, K.; Sarkar, K.; Tarafder, P.; Nath, P.P.; Paul, G. Chlorpyrifos Suppresses Female Reproductive Function in Rat. Int. J. Pharma Bio Sci. 2014, 5, 810–818. [Google Scholar]
- Rauh, V.A.; Perera, F.P.; Horton, M.K.; Whyatt, R.M.; Bansal, R.; Hao, X.; Liu, J.; Barr, D.B.; Slotkin, T.A.; Peterson, B.S. Brain Anomalies in Children Exposed Prenatally to a Common Organophosphate Pesticide. Proc. Natl. Acad. Sci. USA 2012, 109, 7871–7876. [Google Scholar] [CrossRef]
- Bölte, S.; Girdler, S.; Marschik, P.B. The Contribution of Environmental Exposure to the Etiology of Autism Spectrum Disorder. Cell. Mol. Life Sci. 2019, 76, 1275–1297. [Google Scholar] [CrossRef]
- Hertz-Picciotto, I.; Croen, L.A.; Hansen, R.; Jones, C.R.; van de Water, J.; Pessah, I.N. The CHARGE Study: An Epidemiologic Investigation of Genetic and Environmental Factors Contributing to Autism. Environ. Health Perspect. 2006, 114, 1119–1125. [Google Scholar] [CrossRef]
- Maheshwari, D.G.; Shaikh, N.K. An Overview on Toxicity Testing Method. Int. J. Pharm Technol. 2016, 8, 3834–3849. [Google Scholar]
- Erhirhie, E.O.; Ihekwereme, C.P.; Ilodigwe, E.E. Advances in Acute Toxicity Testing: Strengths, Weaknesses and Regulatory Acceptance. Interdiscip. Toxicol. 2018, 11, 5–12. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, A.K.; Kesavachandran, C. Health Effects of Pesticides; CRC Press: Boca Raton, FL, USA, 2019. [Google Scholar] [CrossRef]
- EFSA (European Food Safety Authority). Conclusion on the peer review of the pesticide human health risk assessment of the active substance chlorpyrifos. EFSA J. 2014, 12, 3640. [Google Scholar] [CrossRef]
- Mie, A.; Rudén, C.; Grandjean, P. Safety of Safety Evaluation of Pesticides: Developmental Neurotoxicity of Chlorpyrifos and Chlorpyrifos-Methyl. Environ. Health 2018, 17, 77. [Google Scholar] [CrossRef]
- Tweedale, A.C. The Inadequacies of Pre-Market Chemical Risk Assessment’s Toxicity Studies-the Implications. J. Appl. Toxicol. 2017, 37, 92–104. [Google Scholar] [CrossRef]
- Grandjean, P.; Landrigan, P.J. Neurobehavioural Effects of Developmental Toxicity. Lancet Neurol. 2014, 13, 330–338. [Google Scholar] [CrossRef]
- Goh, J.-Y.; Weaver, R.J.; Dixon, L.; Platt, N.J.; Roberts, R.A. Development and Use of in Vitro Alternatives to Animal Testing by the Pharmaceutical Industry 1980–2013. Toxicol. Res. (Camb.) 2015, 4, 1297–1307. [Google Scholar] [CrossRef]
- Parasuraman, S. Toxicological Screening. J. Pharmacol. Pharmacother. 2011, 2, 74–79. [Google Scholar] [CrossRef]
- Begum, S.A.; Upadhyaya, T.N.; Baruah, G.K.; Rahman, T.; Pathak, D.C.; Sarma, K.; Bora, R.S. Hematobiochemical Alterations of Acute Chlorpyriphos Intoxication in Indigenous Chicken. Vet. World 2015, 8, 750–754. [Google Scholar] [CrossRef]
- Samsam, T.E.; Hunter, D.L.; Bushnell, P.J. Effects of Chronic Dietary and Repeated Acute Exposure to Chlorpyrifos on Learning and Sustained Attention in Rats. Toxicol. Sci. 2005, 87, 460–468. [Google Scholar] [CrossRef]
- Moore, D.R.J.; Teed, R.S.; Greer, C.D.; Solomon, K.R.; Giesy, J.P. Refined Avian Risk Assessment for Chlorpyrifos in the United States. Rev. Environ. Contam. Toxicol. 2014, 231, 163–217. [Google Scholar] [CrossRef]
- Betancourt, A.M.; Burgess, S.C.; Carr, R.L. Effect of Developmental Exposure to Chlorpyrifos on the Expression of Neurotrophin Growth Factors and Cell-Specific Markers in Neonatal Rat Brain. Toxicol. Sci. 2006, 92, 500–506. [Google Scholar] [CrossRef][Green Version]
- Carr, R.L.; Alugubelly, N.; de Leon, K.; Loyant, L.; Mohammed, A.N.; Patterson, M.E.; Ross, M.K.; Rowbotham, N.E. Inhibition of Fatty Acid Amide Hydrolase by Chlorpyrifos in Juvenile Rats Results in Altered Exploratory and Social Behavior as Adolescents. Neurotoxicology 2020, 77, 127–136. [Google Scholar] [CrossRef]
- Li, J.-W.; Fang, B.; Pang, G.-F.; Zhang, M.; Ren, F.-Z. Age- and Diet-Specific Effects of Chronic Exposure to Chlorpyrifos on Hormones, Inflammation and Gut Microbiota in Rats. Pestic. Biochem. Physiol. 2019, 159, 68–79. [Google Scholar] [CrossRef]
- Silva, M.H. Effects of Low-Dose Chlorpyrifos on Neurobehavior and Potential Mechanisms: A Review of Studies in Rodents, Zebrafish, and Caenorhabditis Elegans. Birth Defects Res. 2020, 112, 445–479. [Google Scholar] [CrossRef]
- Silva, J.G.; Boareto, A.C.; Schreiber, A.K.; Redivo, D.D.B.; Gambeta, E.; Vergara, F.; Morais, H.; Zanoveli, J.M.; Dalsenter, P.R. Chlorpyrifos Induces Anxiety-like Behavior in Offspring Rats Exposed during Pregnancy. Neurosci. Lett. 2017, 641, 94–100. [Google Scholar] [CrossRef]
- Baba, N.A.; Raina, R.; Verma, P.K.; Sultana, M.; Prawez, S.; Nisara, N.A. Toxc Effects of Fluoride and Chlorpyrifos on Antioxidant Parameters in Rats: Protective Effects of Vitamins C and E. Fluoride 2013, 46, 73–79. [Google Scholar]
- Muller, M.; Hess, L.; Tardivo, A.; Lajmanovich, R.; Attademo, A.; Poletta, G.; Simoniello, M.F.; Yodice, A.; Lavarello, S.; Chialvo, D.; et al. Neurologic Dysfunction and Genotoxicity Induced by Low Levels of Chlorpyrifos. Neurotoxicology 2014, 45, 22–30. [Google Scholar] [CrossRef]
- Aldridge, J.E.; Levin, E.D.; Seidler, F.J.; Slotkin, T.A. Developmental Exposure of Rats to Chlorpyrifos Leads to Behavioral Alterations in Adulthood, Involving Serotonergic Mechanisms and Resembling Animal Models of Depression. Environ. Health Perspect. 2005, 113, 527–531. [Google Scholar] [CrossRef]
- Venerosi, A.; Ricceri, L.; Rungi, A.; Sanghez, V.; Calamandrei, G. Gestational Exposure to the Organophosphate Chlorpyrifos Alters Social-Emotional Behaviour and Impairs Responsiveness to the Serotonin Transporter Inhibitor Fluvoxamine in Mice. Psychopharmacology 2010, 208, 99–107. [Google Scholar] [CrossRef]
- Liang, Y.; Zhan, J.; Liu, D.; Luo, M.; Han, J.; Liu, X.; Liu, C.; Cheng, Z.; Zhou, Z.; Wang, P. Organophosphorus Pesticide Chlorpyrifos Intake Promotes Obesity and Insulin Resistance through Impacting Gut and Gut Microbiota. Microbiome 2019, 7, 19. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.; Eriksson, P.; Fredriksson, A.; Buratovic, S.; Viberg, H. Developmental Neurotoxic Effects of Two Pesticides: Behavior and Biomolecular Studies on Chlorpyrifos and Carbaryl. Toxicol. Appl. Pharmacol. 2015, 288, 429–438. [Google Scholar] [CrossRef] [PubMed]
- Sai, L.; Li, X.; Liu, Y.; Guo, Q.; Xie, L.; Yu, G.; Bo, C.; Zhang, Z.; Li, L. Effects of Chlorpyrifos on Reproductive Toxicology of Male Rats: Effects of Chlorpyrifos on Reproductive Toxicology. Environ. Toxicol. 2014, 29, 1083–1088. [Google Scholar] [CrossRef] [PubMed]
- EFSA (PPR Panel EFSA Panel on Plant Protection Products and their Residues); Ockleford, C.; Adriaanse, P.; Berny, P.; Brock, T.; Duquesne, S.; Grilli, S.; Hernandez-Jerez, A.F.; Bennekou, S.H.; Klein, M.; et al. Scientific Opinion on the investigation into experimental toxicological properties of plant protection products having a potential link to Parkinson’s disease and childhood leukaemia. EFSA J. 2017, 15, e04691. [Google Scholar] [CrossRef]
- Grabovska, S.; Salyha, Y. ADHD-like Behaviour in the Offspring of Female Rats Exposed to Low Chlorpyrifos Doses before Pregnancy / Ponašanje Nalik ADHD-u u Potomaka Ženki Štakora Izloženih Niskim Dozama Klorpirifosa Prije Trudnoće. Arh. Hig. Rada Toksikol. 2015, 66, 121–127. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Abdelaziz, K.B.; Makawy, E.; Elsalam, A.I.; Darwish, A.-A. Genotoxicity of Chlorpyrifos and the Antimutagenic Role of Lettuce Leaves in Male Mice. Com. Sci. 2010, 1, 137–145. [Google Scholar]
- Kopjar, N.; Žunec, S.; Mendaš, G.; Micek, V.; Kašuba, V.; Mikolić, A.; Lovaković, B.T.; Milić, M.; Pavičić, I.; Čermak, A.M.M.; et al. Evaluation of Chlorpyrifos Toxicity through a 28-Day Study: Cholinesterase Activity, Oxidative Stress Responses, Parent Compound/Metabolite Levels, and Primary DNA Damage in Blood and Brain Tissue of Adult Male Wistar Rats. Chem. Biol. Interact. 2018, 279, 51–63. [Google Scholar] [CrossRef]
- Sandhu, M.A.; Saeed, A.A.; Khilji, M.S.; Ahmed, A.; Latif, M.S.Z.; Khalid, N. Genotoxicity Evaluation of Chlorpyrifos: A Gender Related Approach in Regular Toxicity Testing. J. Toxicol. Sci. 2013, 38, 237–244. [Google Scholar] [CrossRef]
- Cui, Y.; Guo, J.; Xu, B.; Chen, Z. Genotoxicity of Chlorpyrifos and Cypermethrin to ICR Mouse Hepatocytes. Toxicol. Mech. Methods 2011, 21, 70–74. [Google Scholar] [CrossRef]
- OECD (Organisation for Economic Co-operation and Development). Test No. 489: In Vivo Mammalian Alkaline Comet Assay; OECD Publishing: Paris, France, 2014. [Google Scholar] [CrossRef]
- EFSA (European Food Safety Authority). Statement on the available outcomes of the human health assessment in the context of the pesticides peer review of the active substance chlorpyrifos-methyl. EFSA J. 2019, 17, e5810. [Google Scholar] [CrossRef]
- EFSA (European Food Safety Authority). Review of the existing maximum residue levels for chlorpyrifos according to Article 12 of Regulation (EC) No 396/2005. EFSA J. 2017, 15, e04733. [Google Scholar] [CrossRef][Green Version]
- Ojha, A.; Yaduvanshi, S.K.; Pant, S.C.; Lomash, V.; Srivastava, N. Evaluation of DNA Damage and Cytotoxicity Induced by Three Commonly Used Organophosphate Pesticides Individually and in Mixture, in Rat Tissues: Evaluation of DNA Damage And Cytotoxicity. Environ. Toxicol. 2013, 28, 543–552. [Google Scholar] [CrossRef]
- Lu, C.; Liu, X.; Liu, C.; Wang, J.; Li, C.; Liu, Q.; Li, Y.; Li, S.; Sun, S.; Yan, J.; et al. Chlorpyrifos Induces MLL Translocations through Caspase 3-Dependent Genomic Instability and Topoisomerase II Inhibition in Human Fetal Liver Hematopoietic Stem Cells. Toxicol. Sci. 2015, 147, 588–606. [Google Scholar] [CrossRef]
- Hernández, A.F.; Menéndez, P. Linking Pesticide Exposure with Pediatric Leukemia: Potential Underlying Mechanisms. Int. J. Mol. Sci. 2016, 17, 461. [Google Scholar] [CrossRef]
- Serpa, E.A.; Schmitt, E.G.; Zuravski, L.; Machado, M.M.; de Oliveira, L.F.S. Chlorpyrifos Induces Genotoxic Effects in Human Leukocytes in Vitro at Low Concentrations. Acta Sci. Health Sci. 2019, 41, 44291. [Google Scholar] [CrossRef]
- Encarnação, T.; Pais, A.A.; Campos, M.G.; Burrows, H.D. Endocrine Disrupting Chemicals: Impact on Human Health, Wildlife and the Environment. Sci. Prog. 2019, 102, 3–42. [Google Scholar] [CrossRef]
- Kalliora, C.; Mamoulakis, C.; Vasilopoulos, E.; Stamatiades, G.A.; Kalafati, L.; Barouni, R.; Karakousi, T.; Abdollahi, M.; Tsatsakis, A. Association of Pesticide Exposure with Human Congenital Abnormalities. Toxicol. Appl. Pharmacol. 2018, 346, 58–75. [Google Scholar] [CrossRef]
- Gapp, K.; Jawaid, A.; Sarkies, P.; Bohacek, J.; Pelczar, P.; Prados, J.; Farinelli, L.; Miska, E.; Mansuy, I.M. Implication of Sperm RNAs in Transgenerational Inheritance of the Effects of Early Trauma in Mice. Nat. Neurosci. 2014, 17, 667–669. [Google Scholar] [CrossRef]
- Fontelles, C.C.; Carney, E.; Clarke, J.; Nguyen, N.M.; Yin, C.; Jin, L.; Cruz, M.I.; Ong, T.P.; Hilakivi-Clarke, L.; de Assis, S. Paternal Overweight Is Associated with Increased Breast Cancer Risk in Daughters in a Mouse Model. Sci. Rep. 2016, 6, 28602. [Google Scholar] [CrossRef]
- Ng, S.-F.; Lin, R.C.Y.; Laybutt, D.R.; Barres, R.; Owens, J.A.; Morris, M.J. Chronic High-Fat Diet in Fathers Programs β-Cell Dysfunction in Female Rat Offspring. Nature 2010, 467, 963–966. [Google Scholar] [CrossRef]
- Suwannakul, B.; Sapbamrer, R.; Wiwattanadittakul, N.; Hongsibsong, S. Organophosphate Pesticide Exposures in Early and Late Pregnancy Influence Different Aspects of Infant Developmental Performance. Toxics 2021, 9, 99. [Google Scholar] [CrossRef]
- Soldin, O.P.; Nsouli-Maktabi, H.; Genkinger, J.M.; Loffredo, C.A.; Ortega-Garcia, J.A.; Colantino, D.; Barr, D.B.; Luban, N.L.; Shad, A.T.; Nelson, D. Pediatric Acute Lymphoblastic Leukemia and Exposure to Pesticides. Ther. Drug Monit. 2009, 31, 495–501. [Google Scholar] [CrossRef]
- Yang, K.J.; Lee, J.; Park, H.L. Organophosphate Pesticide Exposure and Breast Cancer Risk: A Rapid Review of Human, Animal, and Cell-Based Studies. Int. J. Environ. Res. Public Health 2020, 17, 5030. [Google Scholar] [CrossRef]
- Jeong, S.-H.; Kim, B.-Y.; Kang, H.-G.; Ku, H.-O.; Cho, J.-H. Effect of Chlorpyrifos-Methyl on Steroid and Thyroid Hormones in Rat F0- and F1-Generations. Toxicology 2006, 220, 189–202. [Google Scholar] [CrossRef]
- Slotkin, T.A.; Brown, K.K.; Seidler, F.J. Developmental Exposure of Rats to Chlorpyrifos Elicits Sex-Selective Hyperlipidemia and Hyperinsulinemia in Adulthood. Environ. Health Perspect. 2005, 113, 1291–1294. [Google Scholar] [CrossRef][Green Version]
- Salazar-Arredondo, E.; de Jesús Solís-Heredia, M.; Rojas-García, E.; Hernández-Ochoa, I.; Quintanilla-Vega, B. Sperm Chromatin Alteration and DNA Damage by Methyl-Parathion, Chlorpyrifos and Diazinon and Their Oxon Metabolites in Human Spermatozoa. Reprod. Toxicol. 2008, 25, 455–460. [Google Scholar] [CrossRef]
- Ventura, C.; Nieto, M.R.R.; Bourguignon, N.; Lux-Lantos, V.; Rodriguez, H.; Cao, G.; Randi, A.; Cocca, C.; Núñez, M. Pesticide Chlorpyrifos Acts as an Endocrine Disruptor in Adult Rats Causing Changes in Mammary Gland and Hormonal Balance. J. Steroid Biochem. Mol. Biol. 2016, 156, 1–9. [Google Scholar] [CrossRef]
- EFSA (European Food Safety Authority). The 2016 European Union report on pesticide residues in food. EFSA J. 2018, 16, 5348. [Google Scholar] [CrossRef]
- Tian, Y.; Ishikawa, H.; Yamaguchi, T.; Yamauchi, T.; Yokoyama, K. Teratogenicity and Developmental Toxicity of Chlorpyrifos. Reprod. Toxicol. 2005, 20, 267–270. [Google Scholar] [CrossRef] [PubMed]
- Marasinghe, J.; Yu, Q.; Connell, D. Assessment of Health Risk in Human Populations Due to Chlorpyrifos. Toxics 2014, 2, 92–114. [Google Scholar] [CrossRef]
- Peiris, D.C.; Dhanushka, T. Low Doses of Chlorpyrifos Interfere with Spermatogenesis of Rats through Reduction of Sex Hormones. Environ. Sci. Pollut. Res. Int. 2017, 24, 20859–20867. [Google Scholar] [CrossRef]
- Mandal, T.K.; Das, N.S. Testicular Gametogenic and Steroidogenic Activities in Chlorpyrifos Insecticide-Treated Rats: A Correlation Study with Testicular Oxidative Stress and Role of Antioxidant Enzyme Defence Systems in Sprague-Dawley Rats: Chlorpyrifos and Testicular Oxidative Stress. Andrologia 2012, 44, 102–115. [Google Scholar] [CrossRef]
- Pallotta, M.M.; Barbato, V.; Pinton, A.; Acloque, H.; Gualtieri, R.; Talevi, R.; Jammes, H.; Capriglione, T. In Vitro Exposure to CPF Affects Bovine Sperm Epigenetic Gene Methylation Pattern and the Ability of Sperm to Support Fertilization and Embryo Development: In Vitro CPF Exposure Affects Spermatozoa Methylation. Environ. Mol. Mutagen. 2019, 60, 85–95. [Google Scholar] [CrossRef]
- Heikal, T.M.; H. Mossa, A.-T.; Ibrahim, A.W.; Abdel-Hami, H.F. Oxidative Damage and Reproductive Toxicity Associated with Cyromazine and Chlorpyrifos in Male Rats: The Protective Effects of Green Tea Extract. Res. J. Environ. Toxicol. 2014, 8, 53–67. [Google Scholar] [CrossRef]
- Mittal, R.; Debs, L.H.; Patel, A.P.; Nguyen, D.; Patel, K.; O’Connor, G.; Grati, M.; Mittal, J.; Yan, D.; Eshraghi, A.A.; et al. Neurotransmitters: The Critical Modulators Regulating Gut–Brain Axis. J. Cell. Physiol. 2017, 232, 2359–2372. [Google Scholar] [CrossRef]
- Kalupahana, N.S.; Moustaid-Moussa, N.; Claycombe, K.J. Immunity as a Link between Obesity and Insulin Resistance. Mol. Aspects Med. 2012, 33, 26–34. [Google Scholar] [CrossRef]
- Peris-Sampedro, F.; Cabré, M.; Basaure, P.; Reverte, I.; Domingo, J.L.; Teresa Colomina, M. Adulthood Dietary Exposure to a Common Pesticide Leads to an Obese-like Phenotype and a Diabetic Profile in ApoE3 Mice. Environ. Res. 2015, 142, 169–176. [Google Scholar] [CrossRef]
- Joly Condette, C.; Khorsi-Cauet, H.; Morlière, P.; Zabijak, L.; Reygner, J.; Bach, V.; Gay-Quéheillard, J. Increased Gut Permeability and Bacterial Translocation after Chronic Chlorpyrifos Exposure in Rats. PLoS ONE 2014, 9, e102217. [Google Scholar] [CrossRef]
- Tirelli, V.; Catone, T.; Turco, L.; Di Consiglio, E.; Testai, E.; De Angelis, I. Effects of the Pesticide Clorpyrifos on an in Vitro Model of Intestinal Barrier. Toxicol. In Vitro 2007, 21, 308–313. [Google Scholar] [CrossRef]
- Gifford, R.; Siribaddana, S.; Forbes, S.; Eddleston, M. Endocrine-Disrupting Chemicals and the Diabetes Epidemic in Countries in the WHO South-East Asia Region. Lancet Diabetes Endocrinol. 2015, 3, 925–927. [Google Scholar] [CrossRef]
- Venerosi, A.; Tait, S.; Stecca, L.; Chiarotti, F.; De Felice, A.; Cometa, M.F.; Volpe, M.T.; Calamandrei, G.; Ricceri, L. Effects of Maternal Chlorpyrifos Diet on Social Investigation and Brain Neuroendocrine Markers in the Offspring-a Mouse Study. Environ. Health 2015, 14, 32. [Google Scholar] [CrossRef] [PubMed]
- Viswanath, G.; Chatterjee, S.; Dabral, S.; Nanguneri, S.R.; Divya, G.; Roy, P. Anti-Androgenic Endocrine Disrupting Activities of Chlorpyrifos and Piperophos. J. Steroid Biochem. Mol. Biol. 2010, 120, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Moyano, P.; García, J.; García, J.M.; Pelayo, A.; Muñoz-Calero, P.; Frejo, M.T.; Anadon, M.J.; Lobo, M.; Del Pino, J. Chlorpyrifos-Induced Cell Proliferation in Human Breast Cancer Cell Lines Differentially Mediated by Estrogen and Aryl Hydrocarbon Receptors and KIAA1363 Enzyme after 24 h and 14 Days Exposure. Chemosphere 2020, 251, 126426. [Google Scholar] [CrossRef] [PubMed]
- Zárate, L.V.; Pontillo, C.A.; Español, A.; Miret, N.V.; Chiappini, F.; Cocca, C.; Álvarez, L.; de Pisarev, D.K.; Sales, M.E.; Randi, A.S. Angiogenesis Signaling in Breast Cancer Models Is Induced by Hexachlorobenzene and Chlorpyrifos, Pesticide Ligands of the Aryl Hydrocarbon Receptor. Toxicol. Appl. Pharmacol. 2020, 401, 115093. [Google Scholar] [CrossRef] [PubMed]
- Pistollato, F.; de Gyves, E.M.; Carpi, D.; Bopp, S.K.; Nunes, C.; Worth, A.; Bal-Price, A. Assessment of Developmental Neurotoxicity Induced by Chemical Mixtures Using an Adverse Outcome Pathway Concept. Environ. Health 2020, 19, 23. [Google Scholar] [CrossRef] [PubMed]
- Guodong, D.; Pei, W.; Ying, T.; Jun, Z.; Yu, G.; Xiaojin, W.; Rong, S.; Guoquan, W.; Xiaoming, S. Organophosphate Pesticide Exposure and Neurodevelopment in Young Shanghai Children. Environ. Sci. Technol. 2012, 46, 2911–2917. [Google Scholar] [CrossRef]
- Kousba, A.A.; Sultatos, L.G.; Poet, T.S.; Timchalk, C. Comparison of Chlorpyrifos-Oxon and Paraoxon Acetylcholinesterase Inhibition Dynamics: Potential Role of a Peripheral Binding Site. Toxicol. Sci. 2004, 80, 239–248. [Google Scholar] [CrossRef][Green Version]
- Topal, A.; Şişecioğlu, M.; Atamanalp, M.; Işık, A.; Yılmaz, B. Thein Vitroandin Vivoeffects of Chlorpyrifos on Acetylcholinesterase Activity of Rainbow Trout Brain. J. Appl. Anim. Res. 2016, 44, 243–247. [Google Scholar] [CrossRef]
- Shaker, E.M.; Elsharkawy, E.E. Organochlorine and Organophosphorus Pesticide Residues in Raw Buffalo Milk from Agroindustrial Areas in Assiut, Egypt. Environ. Toxicol. Pharmacol. 2015, 39, 433–440. [Google Scholar] [CrossRef]
- Crépet, A.; Héraud, F.; Béchaux, C.; Gouze, M.E.; Pierlot, S.; Fastier, A.; Leblanc, J.C.; Le Hégarat, L.; Takakura, N.; Fessard, V.; et al. The PERICLES Research Program: An Integrated Approach to Characterize the Combined Effects of Mixtures of Pesticide Residues to Which the French Population Is Exposed. Toxicology 2013, 313, 83–93. [Google Scholar] [CrossRef]
- Jankowska, M.; Łozowicka, B.; Kaczyński, P. Comprehensive Toxicological Study over 160 Processing Factors of Pesticides in Selected Fruit and Vegetables after Water, Mechanical and Thermal Processing Treatments and Their Application to Human Health Risk Assessment. Sci. Total Environ. 2019, 652, 1156–1167. [Google Scholar] [CrossRef]
- Solomon, K.R.; Williams, W.M.; Mackay, D.; Purdy, J.; Giddings, J.M.; Giesy, J.P. Properties and Uses of Chlorpyrifos in the United States. In Ecological Risk Assessment for Chlorpyrifos in Terrestrial and Aquatic Systems in the United States; Springer International Publishing: Cham, Switzerland, 2014; pp. 13–34. [Google Scholar]
- Yuan, Y.; Chen, C.; Zheng, C.; Wang, X.; Yang, G.; Wang, Q.; Zhang, Z. Residue of Chlorpyrifos and Cypermethrin in Vegetables and Probabilistic Exposure Assessment for Consumers in Zhejiang Province, China. Food Control 2014, 36, 63–68. [Google Scholar] [CrossRef]
- Slotkin, T.A. Does Early-Life Exposure to Organophosphate Insecticides Lead to Prediabetes and Obesity? Reprod. Toxicol. 2011, 31, 297–301. [Google Scholar] [CrossRef]
- Nasreddine, L.; Rehaime, M.; Kassaify, Z.; Rechmany, R.; Jaber, F. Dietary exposure to pesticide residues from foods of plant origin and drinks in Lebanon. Environ. Monit. Assess. 2016, 188, 485. [Google Scholar] [CrossRef]
- Riederer, A.M.; Hunter, R.E., Jr.; Hayden, S.W.; Ryan, P.B. Pyrethroid and Organophosphorus Pesticides in Composite Diet Samples from Atlanta, USA Adults. Environ. Sci. Technol. 2010, 44, 483–490. [Google Scholar] [CrossRef]
- Łozowicka, B.; Kaczyński, P.; Mojsak, P.; Rusiłowska, J.; Beknazarova, Z.; Ilyasova, G.; Absatarova, D. Systemic and Non-Systemic Pesticides in Apples from Kazakhstan and Their Impact on Human Health. J. Food Compost. Anal. 2020, 90, 103494. [Google Scholar] [CrossRef]
- Mojsak, P.; Łozowicka, B.; Kaczyński, P. Estimating Acute and Chronic Exposure of Children and Adults to Chlorpyrifos in Fruit and Vegetables Based on the New, Lower Toxicology Data. Ecotoxicol. Environ. Saf. 2018, 159, 182–189. [Google Scholar] [CrossRef]
- EFSA (European Food Safety Authority). The 2020 European Union report on pesticide residues in food. EFSA J. 2022, 20, 7215. [Google Scholar] [CrossRef]
- Quijano, L.; Yusà, V.; Font, G.; Pardo, O. Chronic Cumulative Risk Assessment of the Exposure to Organophosphorus, Carbamate and Pyrethroid and Pyrethrin Pesticides through Fruit and Vegetables Consumption in the Region of Valencia (Spain). Food Chem. Toxicol. 2016, 89, 39–46. [Google Scholar] [CrossRef]
- Swarnam, T.P.; Velmurugan, A. Pesticide Residues in Vegetable Samples from the Andaman Islands, India. Environ. Monit. Assess. 2013, 185, 6119–6127. [Google Scholar] [CrossRef]
- EC European Commission. Regulation (EC) No 396/2005 of the European Parliament and of the Council of 23 February 2005 on maximum residue levels of pesticides in or on food and feed of plant and animal origin and amending council directive 91/414/EEC, Annex II i III (SANTE/10367/2015). OJ L 70. Available online: https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=CONSLEG:2005R0396:20121026:EN:PDF (accessed on 30 April 2021).
- Słowik-Borowiec, M.; Szpyrka, E.; Podbielska, M.; Kurdziel, A.; Matyaszek, A. Pesticide Residues in Root Vegetables and Potatoes in South-Eastern Poland (2009–2011). J. Agron 2009, 11, 47–51. [Google Scholar]
- Essumang, D.K.; Asare, E.A.; Dodoo, D.K. Pesticides Residues in Okra (Non-Target Crop) Grown Close to a Watermelon Farm in Ghana. Environ. Monit. Assess. 2013, 185, 7617–7625. [Google Scholar] [CrossRef] [PubMed]
- Hongsibsong, S.; Prapamontol, T.; Xu, T.; Hammock, B.D.; Wang, H.; Chen, Z.-J.; Xu, Z.-L. Monitoring of the Organophosphate Pesticide Chlorpyrifos in Vegetable Samples from Local Markets in Northern Thailand by Developed Immunoassay. Int. J. Environ. Res. Public Health 2020, 17, 4723. [Google Scholar] [CrossRef] [PubMed]
- EFSA (European Food Safety Authority). National summary reports on pesticide residue analysis performed in 2019. EFSA Support. Publ. 2021, N-6487, 1–198. [Google Scholar] [CrossRef]
- Kumar, S.; Kaushik, G.; Dar, M.A.; Nimesh, S.; López-Chuken, U.J.; Villarreal-Chiu, J.F. Microbial Degradation of Organophosphate Pesticides: A Review. Pedosphere 2018, 28, 190–208. [Google Scholar] [CrossRef]
- Am, S. Monitoring of Some Organophosphorus and Organochlorine Pesticides Residue in Beef Meat from Khartoum State Slaughterhouses. Am. J. Biomed. Sci. Res. 2019, 6, 405–409. [Google Scholar] [CrossRef]
- Dallegrave, A.; Pizzolato, T.M.; Barreto, F.; Bica, V.C.; Eljarrat, E.; Barceló, D. Residue of Insecticides in Foodstuff and Dietary Exposure Assessment of Brazilian Citizens. Food Chem. Toxicol. 2018, 115, 329–335. [Google Scholar] [CrossRef]
- Gazzotti, T.; Sticca, P.; Zironi, E.; Lugoboni, B.; Serraino, A.; Pagliuca, G. Determination of 15 Organophosphorus Pesticides in Italian Raw Milk. Bull. Environ. Contam. Toxicol. 2009, 82, 251–254. [Google Scholar] [CrossRef]
- Hartle, J.C.; Cohen, R.S.; Sakamoto, P.; Barr, D.B.; Carmichael, S.L. Chemical Contaminants in Raw and Pasteurized Human Milk. J. Hum. Lact. 2018, 34, 340–349. [Google Scholar] [CrossRef]
- Oliveira, F.A.; Reis, L.P.G.; Soto-Blanco, B.; Melo, M.M. Pesticides Residues in TheProchilodus Costatus(Valenciennes, 1850) Fish Caught in the São Francisco River, Brazil. J. Environ. Sci. Health B 2015, 50, 398–405. [Google Scholar] [CrossRef]
- Sun, F.; Chen, H.-S. Monitoring of Pesticide Chlorpyrifos Residue in Farmed Fish: Investigation of Possible Sources. Chemosphere 2008, 71, 1866–1869. [Google Scholar] [CrossRef]
- Gao, Y.; Chen, S.; Hu, M.; Hu, Q.; Luo, J.; Li, Y. Purification and Characterization of a Novel Chlorpyrifos Hydrolase from Cladosporium Cladosporioides Hu-01. PLoS ONE 2012, 7, e38137. [Google Scholar] [CrossRef]


| Species | Dosage/Route/Type | Effect | Ref. |
|---|---|---|---|
| Rats | Initial dose of 60 mg/kg, followed every 2 months with a single dose of 45 mg/kg | Deficits in learning | [90] |
| Rat pups on PND 1–6 | Diet at a daily dose1234567of 1.5 or 3.0 mg/kg (by gavage in corn oil) | Decreased levels of mRNA for nerve growth factor, muscarinic M1, and reelin receptors, and an increase in glial fibrillary acidic protein mRNA and inhibited brain AChE activity | [92] |
| Rat pups PND 11–16 | Daily dose 0.5, 0.75, and 1.0 mg/kg b.w. | For brain AChE inhibition being 1.0 mg/kg b.w./day | [93] |
| 3-month-old Long-Evans rats | Diet at a daily dose of 0, 1, or 5 mg/kg for 1 year | No effects on learning or memory | [90] |
| 3-week-old male Wistar rats | Daily dose 0.30 mg/kg b. w. normal fat | Significant increase in various hormones such as pancreatic polypeptide, gastric inhibitory polypeptide and monocyte chemoattractant protein 1 and tumor necrosis factor α | [94] |
| Daily dose 0.30 mg/kg b.w. high fat | Significant influence on the gut microbiome and increased glucagon-like peptide-1 | ||
| Pregnant rats from GD 6 to PND 10 | Daily dose at 0.3, 1, and 5 mg/kg b.w. | 5 mg/kg: led to a decrease in pup weights and viability index (%) and presented cholinergic signs (fasciculatins, ataxia, tremors, etc.)12345670.3 and 1 mg/kg: 8–11% decrease in the cerebellum height to brain weight ratio | [95] |
| Pregnant rats from GD 14–20 | 10 mg/kg CPF (oral) | Reduced body mass gain in mothers during treatment and increased body weight gain in male offspring from PND42 | [96] |
| Adult Wistar rats weighing 150–200 g | 10 mg/kg b.w. 28-day oral exposure | decreased GSH-Px activity in blood | [97] |
| Male Sprague-Dawley adult rats | Doses of 0.1, 1, and 10 mg/kg b.w. once daily for 7 days (sunflower oil) | Inhibition of AChE activity by approximately 20% | [98] |
| CD-1 mice from GD 15–18 | 3 or 6 mg/kg/day (peanut oil) | 3 mg/kg: approximately 10% brain AChE inhibition12345676 mg/kg: 40% brain AChE inhibition 24 h after last dose | [99] |
| Pregnant CD-1 mice from GD 14–17 | 6 mg/kg CPF (oral) | Concentration of 3,5,6-TCP found in the brains of fetuses was 250 ng/g and revealed decreased cognition in males and females | [100] |
| Pregnant guinea pigs starting approximately GD 53–55 | 25 mg/kg/day formulated in peanut oil, 10th day | Decrease in AChE activity in red blood cells by approximately 75% | [75] |
| 3-week-old male C57Bl/6 and CD-1 (ICR) mice | diet at daily doses of 5 mg/kg (dissolved in corn oil) for 12 weeks | Can disturb glucose homeostasis and induce insulin resistance and effects on intestinal inflammation | [101] |
| Neonatal mice PND 10 | a single oral dose (0.1, 1.0 or 5 mg/kg b.w.) | Induced effects are not related to the classical mechanism of acute cholinergic hyperstimulation, as the AChE inhibition level (8–12%) remained below the threshold required to cause systemic toxicity | [102] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wołejko, E.; Łozowicka, B.; Jabłońska-Trypuć, A.; Pietruszyńska, M.; Wydro, U. Chlorpyrifos Occurrence and Toxicological Risk Assessment: A Review. Int. J. Environ. Res. Public Health 2022, 19, 12209. https://doi.org/10.3390/ijerph191912209
Wołejko E, Łozowicka B, Jabłońska-Trypuć A, Pietruszyńska M, Wydro U. Chlorpyrifos Occurrence and Toxicological Risk Assessment: A Review. International Journal of Environmental Research and Public Health. 2022; 19(19):12209. https://doi.org/10.3390/ijerph191912209
Chicago/Turabian StyleWołejko, Elżbieta, Bożena Łozowicka, Agata Jabłońska-Trypuć, Marta Pietruszyńska, and Urszula Wydro. 2022. "Chlorpyrifos Occurrence and Toxicological Risk Assessment: A Review" International Journal of Environmental Research and Public Health 19, no. 19: 12209. https://doi.org/10.3390/ijerph191912209
APA StyleWołejko, E., Łozowicka, B., Jabłońska-Trypuć, A., Pietruszyńska, M., & Wydro, U. (2022). Chlorpyrifos Occurrence and Toxicological Risk Assessment: A Review. International Journal of Environmental Research and Public Health, 19(19), 12209. https://doi.org/10.3390/ijerph191912209










