Efficacy of Mouth Rinses and Nasal Spray in the Inactivation of SARS-CoV-2: A Systematic Review and Meta-Analysis of In Vitro and In Vivo Studies
Abstract
:1. Introduction
2. Materials and Methods
2.1. Protocol and Registration
2.2. Focused Questions
2.3. Eligibility Criteria
2.4. Search Strategy and Data Extraction
2.5. Risk of Bias Assessment
2.6. Data Analysis
3. Results
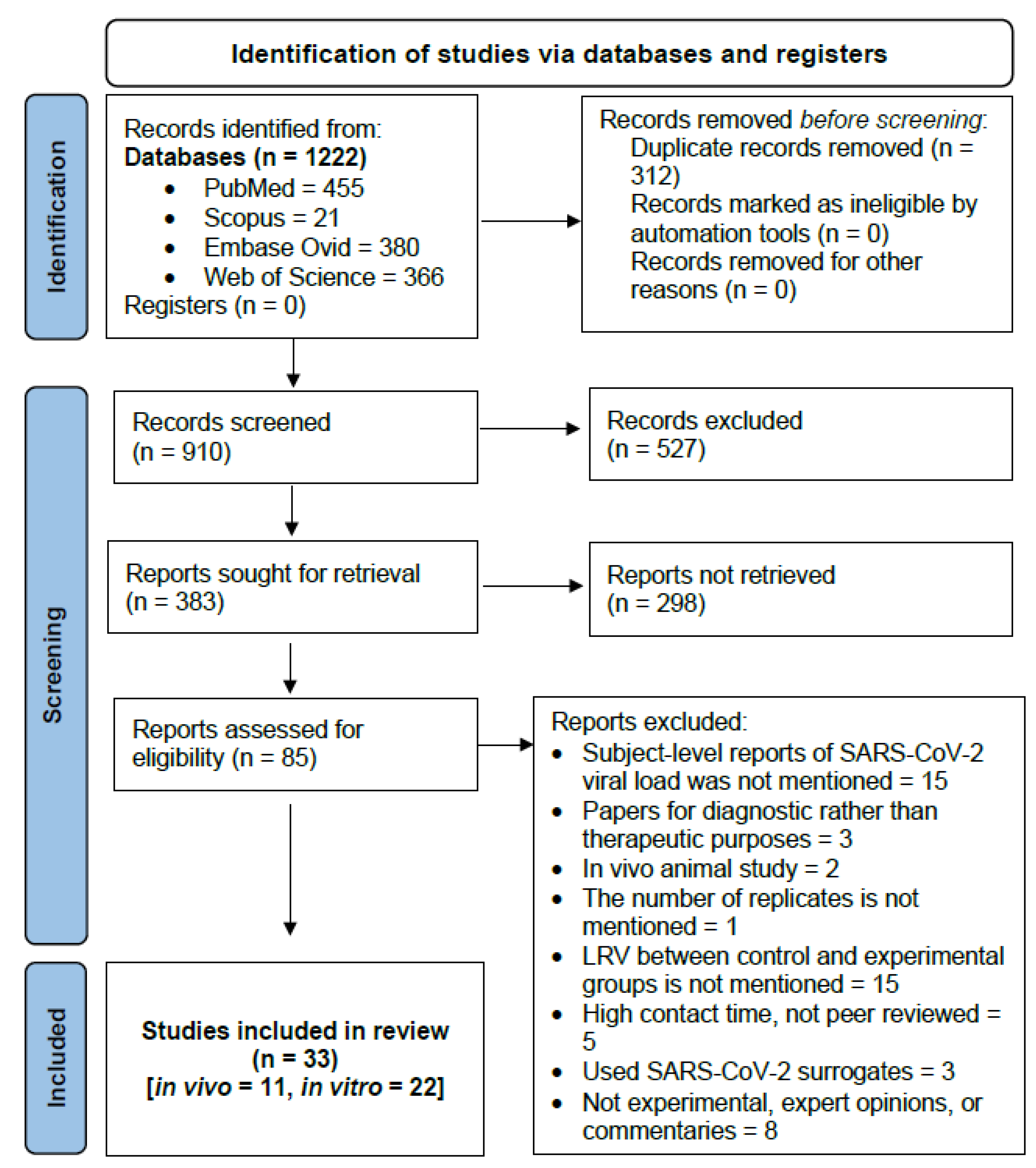
3.1. Results of Database Searches
3.2. General Characteristics of the Included Studies
3.2.1. In Vivo Studies
3.2.2. In Vitro Studies
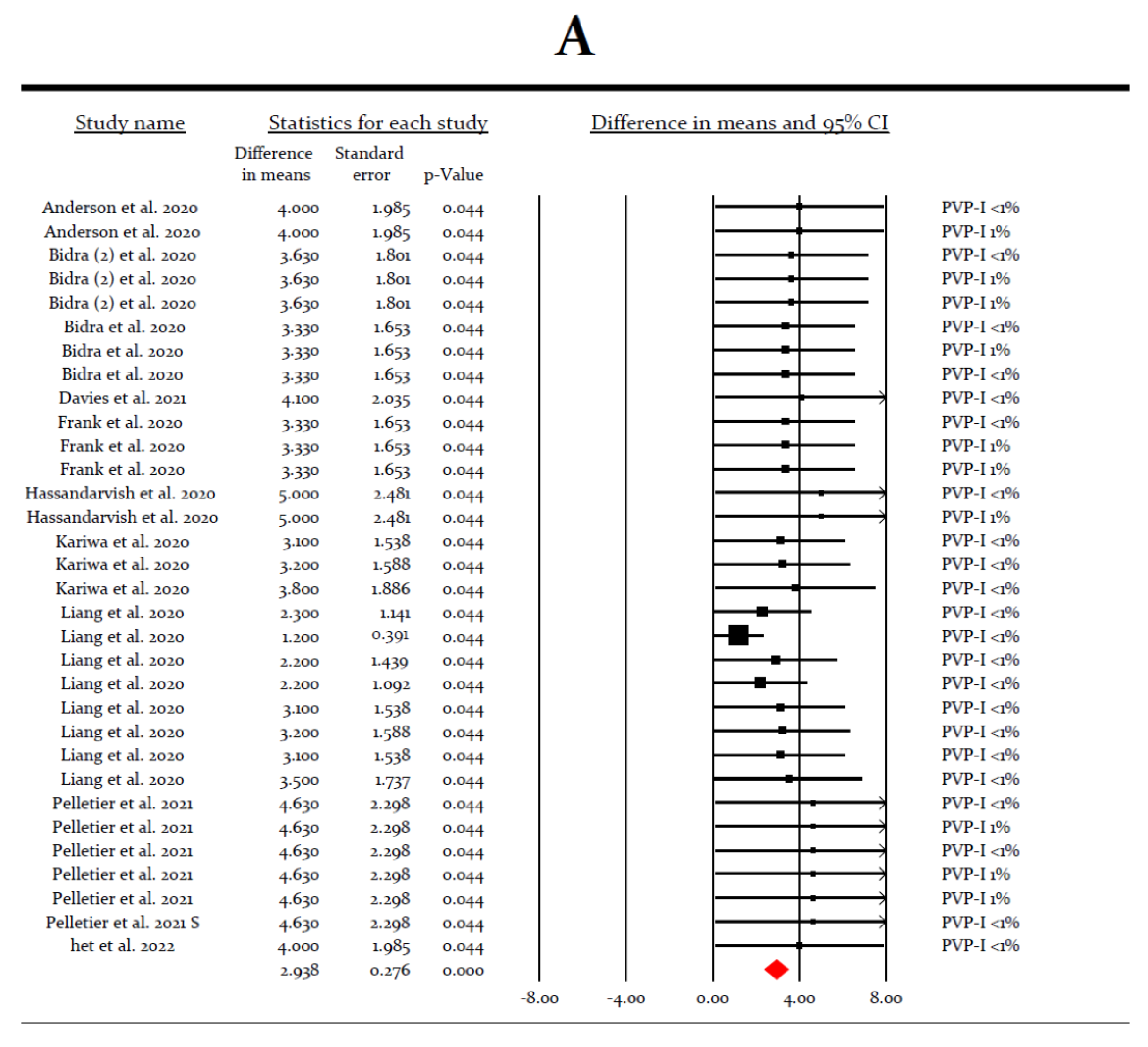
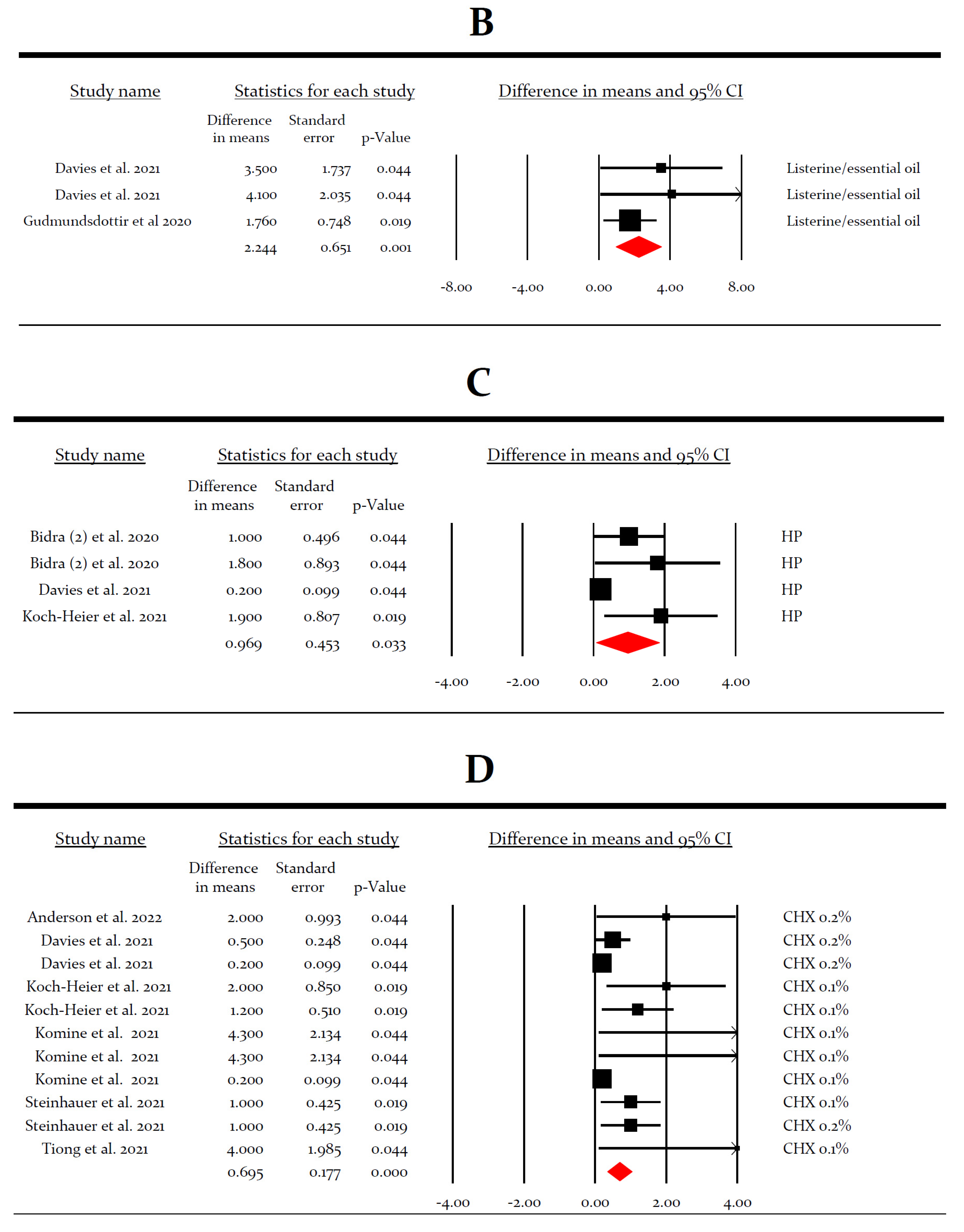
3.3. Meta-Analysis for the Virucidal Efficacy of Different Preparations against SARS-CoV-2 In Vivo
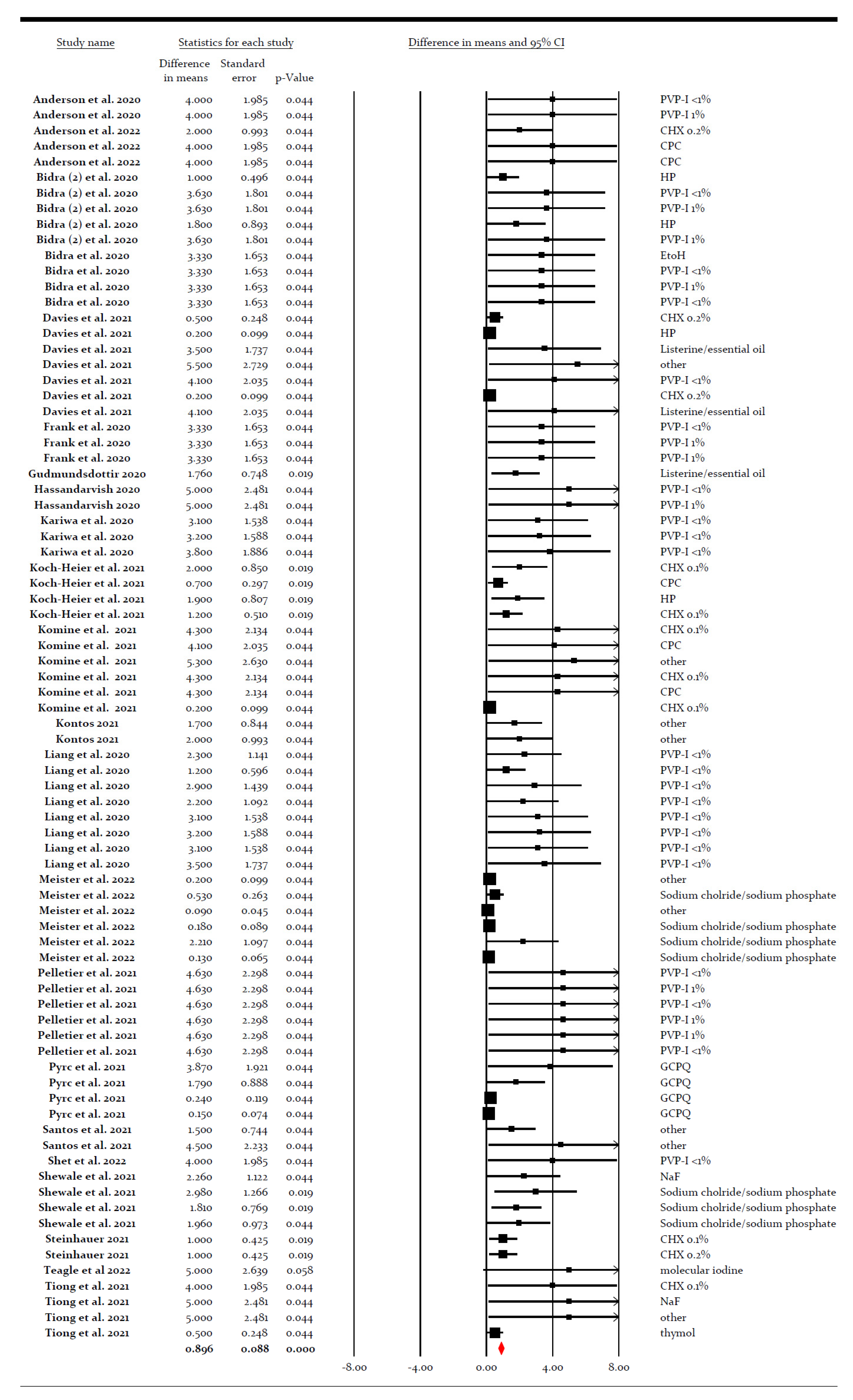
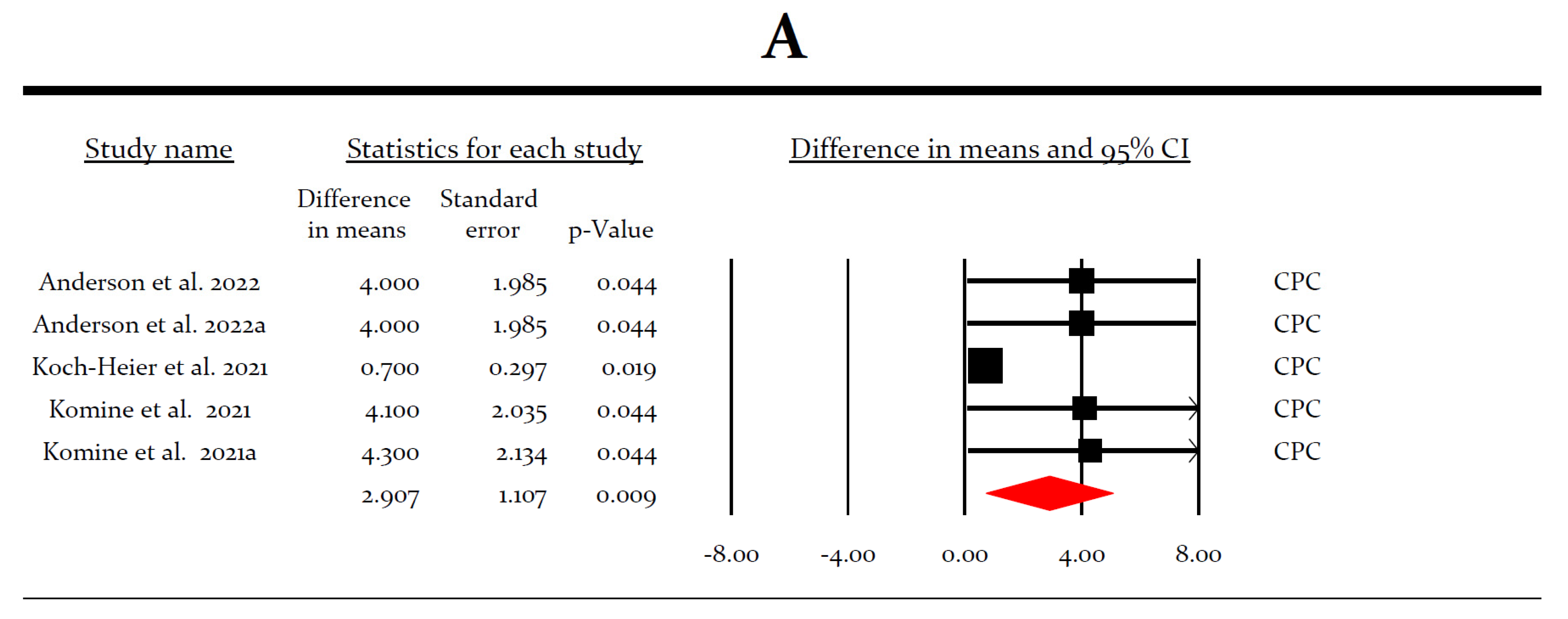
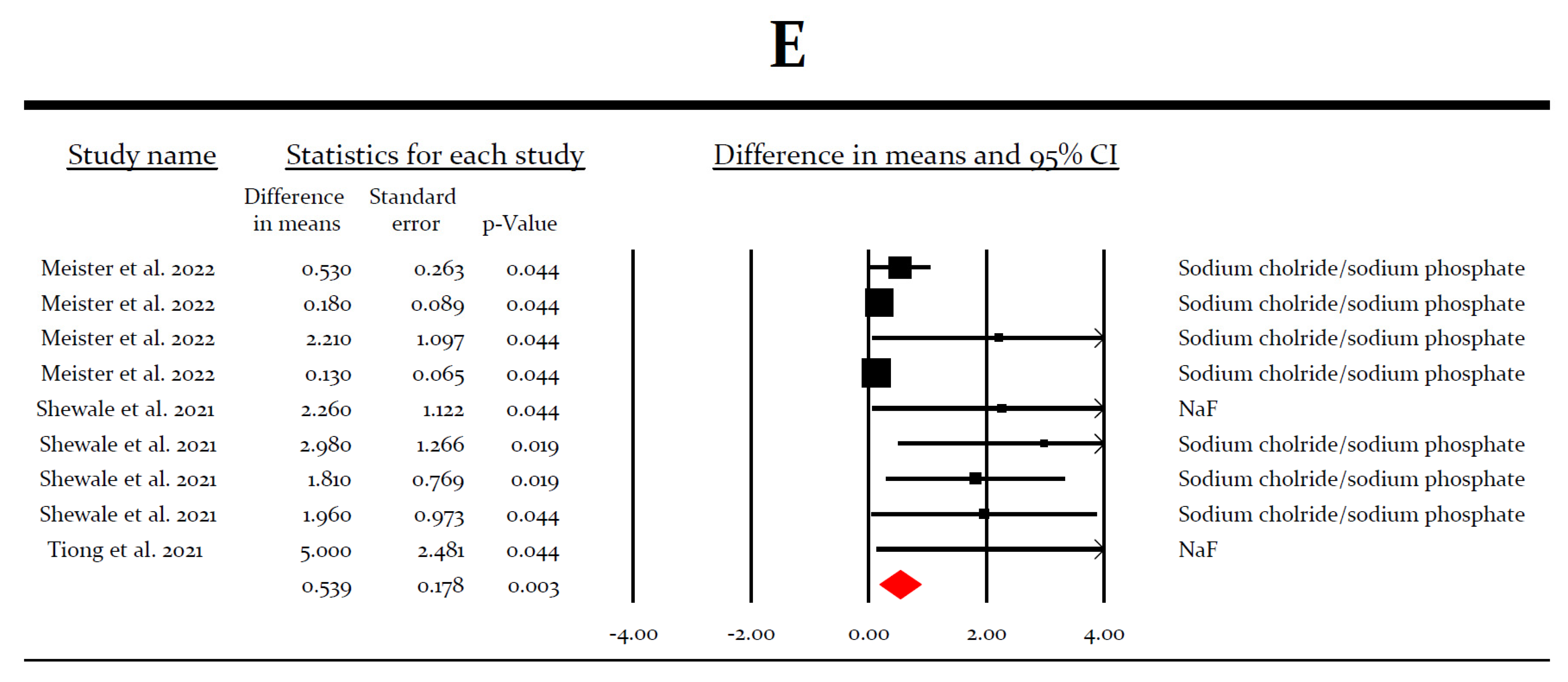
3.4. Meta-Analysis for the Virucidal Efficacy of Different Preparations against SARS-CoV-2 In Vitro
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yuki, K.; Fujiogi, M.; Koutsogiannaki, S. COVID-19 pathophysiology: A review. Clin. Immunol. 2020, 215, 108427. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.Y.; Zhao, R.; Gao, L.J.; Gao, X.F.; Wang, D.P.; Cao, J.M. SARS-CoV-2: Structure, Biology, and Structure-Based Therapeutics Development. Front. Cell. Infect. Microbiol. 2020, 10, 587269. [Google Scholar] [CrossRef] [PubMed]
- Harrel, S.K.; Molinari, J. Aerosols and splatter in dentistry: A brief review of the literature and infection control implications. J. Am. Dent. Assoc. 2004, 135, 429–437. [Google Scholar] [CrossRef] [PubMed]
- Hasanoglu, I.; Korukluoglu, G.; Asilturk, D.; Cosgun, Y.; Kalem, A.K.; Altas, A.B.; Kayaaslan, B.; Eser, F.; Kuzucu, E.A.; Guner, R. Higher viral loads in asymptomatic COVID-19 patients might be the invisible part of the iceberg. Infection 2021, 49, 117–126. [Google Scholar] [CrossRef]
- Fallahi, H.R.; Keyhan, S.O.; Zandian, D.; Kim, S.G.; Cheshmi, B. Being a front-line dentist during the COVID-19 pandemic: A literature review. Maxillofac. Plast. Reconstr. Surg. 2020, 42, 12. [Google Scholar] [CrossRef]
- van Doremalen, N.; Bushmaker, T.; Morris, D.H.; Holbrook, M.G.; Gamble, A.; Williamson, B.N.; Tamin, A.; Harcourt, J.L.; Thornburg, N.J.; Gerber, S.I.; et al. Aerosol and Surface Stability of SARS-CoV-2 as Compared with SARS-CoV-1. N. Engl. J. Med. 2020, 382, 1564–1567. [Google Scholar] [CrossRef]
- Fears, A.C.; Klimstra, W.B.; Duprex, P.; Hartman, A.; Weaver, S.C.; Plante, K.S.; Mirchandani, D.; Plante, J.A.; Aguilar, P.V.; Fernandez, D.; et al. Persistence of Severe Acute Respiratory Syndrome Coronavirus 2 in Aerosol Suspensions. Emerg. Infect. Dis. 2020, 26, 2168–2171. [Google Scholar] [CrossRef]
- Australian Dental Association: Risk Management Principles for Dentistry during the COVID-19 Pandemic. Available online: https://www.ada.org.au/getdoc/d3eecaba-d0aa-4803-a7ea-89facae6f274/Risk-Management-Principles-for-Dentistry-(1).aspx (accessed on 10 April 2022).
- American Dental Association: ADA Interim Guidance for Minimizing Risk of COVID-19 Transmission. Available online: https://snlg.iss.it/wp-content/uploads/2020/04/ADA_COVID_Int_Guidance_Treat_Pts.pdf (accessed on 10 April 2022).
- Burton, M.J.; Clarkson, J.E.; Goulao, B.; Glenny, A.M.; McBain, A.J.; Schilder, A.G.; Webster, K.E.; Worthington, H.V. Antimicrobial mouthwashes (gargling) and nasal sprays to protect healthcare workers when undertaking aerosol-generating procedures (AGPs) on patients without suspected or confirmed COVID-19 infection. Cochrane Database Syst. Rev. 2020, 9, CD013628. [Google Scholar]
- Amber, A.; Abhishek, P.; Nikita, R. Efficacy of Mouth Rinses against SARS-CoV-2: A Scoping Review. Front. Dent. Med. 2021, 2, 648547. [Google Scholar]
- Cavalcante-Leao, B.L.; de Araujo, C.M.; Basso, I.B.; Schroder, A.G.; Guariza-Filho, O.; Ravazzi, G.C.; Goncalves, F.M.; Zeigelboim, B.S.; Santos, R.S.; Stechman-Neto, J. Is there scientific evidence of the mouthwashes effectiveness in reducing viral load in COVID-19? A systematic review. J. Clin. Exp. Dent. 2021, 13, e179–e189. [Google Scholar] [CrossRef]
- Garcia-Sanchez, A.; Pena-Cardelles, J.F.; Ruiz, S.; Robles, F.; Ordonez-Fernandez, E.; Salgado-Peralvo, A.O.; Balloch, J.; Simon, J.C. Efficacy of Pre-Procedural Mouthwashes against SARS-CoV-2: A Systematic Review of Randomized Controlled Trials. J. Clin. Med. 2022, 11, 1692. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- European Standard: EN14476:2013+A1:2015, Chemical Disinfectants and Antiseptics—Quantitative Suspension Test for the Evaluation of Virucidal Activity in the Medical Area—Test Method and Requirements (Phase 2/Step 1). Available online: https://standards.iteh.ai/catalog/standards/cen/5e78911a-aedf-4456-90b7-39e1649f8acf/en-14476-2013a1-2015 (accessed on 12 April 2022).
- Gentilini, F.; Turba, M.E.; Taddei, F.; Gritti, T.; Fantini, M.; Dirani, G.; Sambri, V. Modelling RT-qPCR cycle-threshold using digital PCR data for implementing SARS-CoV-2 viral load studies. PLoS ONE 2021, 16, e0260884. [Google Scholar] [CrossRef]
- Schneider, K.; Schwarz, M.; Burkholder, I.; Kopp-Schneider, A.; Edler, L.; Kinsner-Ovaskainen, A.; Hartung, T.; Hoffmann, S. “ToxRTool”, a new tool to assess the reliability of toxicological data. Toxicol. Lett. 2009, 189, 138–144. [Google Scholar] [CrossRef] [PubMed]
- Klimisch, H.J.; Andreae, M.; Tillmann, U. A systematic approach for evaluating the quality of experimental toxicological and ecotoxicological data. Regul. Toxicol. Pharmacol. 1997, 25, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Carrouel, F.; Valette, M.; Gadea, E.; Esparcieux, A.; Illes, G.; Langlois, M.E.; Perrier, H.; Dussart, C.; Tramini, P.; Ribaud, M.; et al. Use of an antiviral mouthwash as a barrier measure in the SARS-CoV-2 transmission in adults with asymptomatic to mild COVID-19: A multicentre, randomized, double-blind controlled trial. Clin. Microbiol. Infect. 2021, 27, 1494–1501. [Google Scholar] [CrossRef]
- Costa, D.D.; Brites, C.; Vaz, S.N.; de Santana, D.S.; Dos Santos, J.N.; Cury, P.R. Chlorhexidine mouthwash reduces the salivary viral load of SARS-CoV-2: A randomized clinical trial. Oral Dis. 2021. [Google Scholar] [CrossRef]
- Eduardo, F.P.; Correa, L.; Heller, D.; Daep, C.A.; Benitez, C.; Malheiros, Z.; Stewart, B.; Ryan, M.; Machado, C.M.; Hamerschlak, N.; et al. Salivary SARS-CoV-2 load reduction with mouthwash use: A randomized pilot clinical trial. Heliyon 2021, 7, e07346. [Google Scholar] [CrossRef]
- Elzein, R.; Abdel-Sater, F.; Fakhreddine, S.; Hanna, P.A.; Feghali, R.; Hamad, H.; Ayoub, F. In vivo evaluation of the virucidal efficacy of chlorhexidine and povidone-iodine mouthwashes against salivary SARS-CoV-2. A randomized-controlled clinical trial. J. Evid. Based Dent. Pract. 2021, 21, 101584. [Google Scholar] [CrossRef]
- Gottsauner, M.J.; Michaelides, I.; Schmidt, B.; Scholz, K.J.; Buchalla, W.; Widbiller, M.; Hitzenbichler, F.; Ettl, T.; Reichert, T.E.; Bohr, C.; et al. A prospective clinical pilot study on the effects of a hydrogen peroxide mouthrinse on the intraoral viral load of SARS-CoV-2. Clin. Oral Investig. 2020, 24, 3707–3713. [Google Scholar] [CrossRef]
- Guimaraes, T.C.; Marques, B.B.F.; Castro, M.V.; Secco, D.A.; Porto, L.; Tinoco, J.M.M.; Tinoco, E.M.B.; Fletcher, P.; Fischer, R.G. Reducing the viral load of SARS-CoV-2 in the saliva of patients with COVID-19. Oral Dis. 2021. [Google Scholar] [CrossRef] [PubMed]
- Martinez Lamas, L.; Diz Dios, P.; Perez Rodriguez, M.T.; Del Campo Perez, V.; Cabrera Alvargonzalez, J.J.; Lopez Dominguez, A.M.; Fernandez Feijoo, J.; Diniz Freitas, M.; Limeres Posse, J. Is povidone iodine mouthwash effective against SARS-CoV-2? First in vivo tests. Oral Dis. 2020, 28 (Suppl. 1), 908–911. [Google Scholar] [CrossRef] [PubMed]
- Seneviratne, C.J.; Balan, P.; Ko, K.K.K.; Udawatte, N.S.; Lai, D.; Ng, D.H.L.; Venkatachalam, I.; Lim, K.S.; Ling, M.L.; Oon, L.; et al. Efficacy of commercial mouth-rinses on SARS-CoV-2 viral load in saliva: Randomized control trial in Singapore. Infection 2021, 49, 305–311. [Google Scholar] [CrossRef] [PubMed]
- Schurmann, M.; Aljubeh, M.; Tiemann, C.; Sudhoff, H. Mouthrinses against SARS-CoV-2: Anti-inflammatory effectivity and a clinical pilot study. Eur. Arch. Otorhinolaryngol. 2021, 278, 5059–5067. [Google Scholar] [CrossRef]
- Yoon, J.G.; Yoon, J.; Song, J.Y.; Yoon, S.Y.; Lim, C.S.; Seong, H.; Noh, J.Y.; Cheong, H.J.; Kim, W.J. Clinical Significance of a High SARS-CoV-2 Viral Load in the Saliva. J. Korean Med. Sci. 2020, 35, e195. [Google Scholar] [CrossRef]
- Zarabanda, D.; Vukkadala, N.; Phillips, K.M.; Qian, Z.J.; Mfuh, K.O.; Hatter, M.J.; Lee, I.T.; Rao, V.K.; Hwang, P.H.; Domb, G.; et al. The Effect of Povidone-Iodine Nasal Spray on Nasopharyngeal SARS-CoV-2 Viral Load: A Randomized Control Trial. Laryngoscope 2021. [Google Scholar] [CrossRef]
- Anderson, E.R.; Patterson, E.I.; Richards, S.; Pitol, A.K.; Edwards, T.; Wooding, D.; Buist, K.; Green, A.; Mukherjee, S.; Hoptroff, M.; et al. CPC-containing oral rinses inactivate SARS-CoV-2 variants and are active in the presence of human saliva. J. Med. Microbiol. 2022, 71, 001508. [Google Scholar] [CrossRef]
- Bidra, A.S.; Pelletier, J.S.; Westover, J.B.; Frank, S.; Brown, S.M.; Tessema, B. Rapid In-Vitro Inactivation of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Using Povidone-Iodine Oral Antiseptic Rinse. J. Prosthodont. 2020, 29, 529–533. [Google Scholar] [CrossRef]
- Bidra, A.S.; Pelletier, J.S.; Westover, J.B.; Frank, S.; Brown, S.M.; Tessema, B. Comparison of In Vitro Inactivation of SARS CoV-2 with Hydrogen Peroxide and Povidone-Iodine Oral Antiseptic Rinses. J. Prosthodont. 2020, 29, 599–603. [Google Scholar] [CrossRef]
- Frank, S.; Brown, S.M.; Capriotti, J.A.; Westover, J.B.; Pelletier, J.S.; Tessema, B. In Vitro Efficacy of a Povidone-Iodine Nasal Antiseptic for Rapid Inactivation of SARS-CoV-2. JAMA Otolaryngol. Head Neck Surg. 2020, 146, 1054–1058. [Google Scholar] [CrossRef]
- Gudmundsdottir, A.; Scheving, R.; Lindberg, F.; Stefansson, B. Inactivation of SARS-CoV-2 and HCoV-229E in vitro by ColdZyme(R) a medical device mouth spray against the common cold. J. Med. Virol. 2021, 93, 1792–1795. [Google Scholar] [CrossRef]
- Kontos, Z. Efficacy of “Essential Iodine Drops” against Severe Acute Respiratory Syndrome-Coronavirus 2 (SARS-CoV-2). PLoS ONE 2021, 16, e0254341. [Google Scholar] [CrossRef] [PubMed]
- Liang, B.; Yuan, X.; Wei, G.; Wang, W.; Zhang, M.; Peng, H.; Javer, A.; Mendenhall, M.; Julander, J.; Huang, S.; et al. In-Vivo Toxicity Studies and In-Vitro Inactivation of SARS-CoV-2 by Povidone-iodine In-situ Gel Forming Formulations. bioRxiv 2020. [Google Scholar] [CrossRef]
- Pelletier, J.S.; Tessema, B.; Frank, S.; Westover, J.B.; Brown, S.M.; Capriotti, J.A. Efficacy of Povidone-Iodine Nasal and Oral Antiseptic Preparations Against Severe Acute Respiratory Syndrome-Coronavirus 2 (SARS-CoV-2). Ear Nose Throat J. 2021, 100 (Suppl. 2), 192S–196S. [Google Scholar] [CrossRef]
- Shet, M.; Westover, J.; Hong, R.; Igo, D.; Cataldo, M.; Bhaskar, S. In vitro inactivation of SARS-CoV-2 using a povidone-iodine oral rinse. BMC Oral Health 2022, 22, 47. [Google Scholar] [CrossRef]
- Shewale, J.G.; Gelhaus, H.C.; Ratcliff, J.L.; Hernandez-Kapila, Y.L. In vitro antiviral activity of stabilized chlorine dioxide containing oral care products. Oral Dis. 2021. [Google Scholar] [CrossRef] [PubMed]
- Teagle, V.; Clem, D.S.; Yoon, T. Virucidal Properties of Molecular Iodine Oral Rinse Against SARS-CoV-2. Compend. Contin. Educ. Dent. 2022, 43, e13–e16. [Google Scholar] [PubMed]
- Anderson, D.E.; Sivalingam, V.; Kang, A.E.Z.; Ananthanarayanan, A.; Arumugam, H.; Jenkins, T.M.; Hadjiat, Y.; Eggers, M. Povidone-Iodine Demonstrates Rapid In Vitro Virucidal Activity against SARS-CoV-2, the Virus Causing COVID-19 Disease. Infect. Dis. Ther. 2020, 9, 669–675. [Google Scholar] [CrossRef]
- Davies, K.; Buczkowski, H.; Welch, S.R.; Green, N.; Mawer, D.; Woodford, N.; Roberts, A.D.G.; Nixon, P.J.; Seymour, D.W.; Killip, M.J. Effective in vitro inactivation of SARS-CoV-2 by commercially available mouthwashes. J. Gen. Virol. 2021, 102, 001578. [Google Scholar] [CrossRef]
- Hassandarvish, P.; Tiong, V.; Mohamed, N.A.; Arumugam, H.; Ananthanarayanan, A.; Qasuri, M.; Hadjiat, Y.; Abubakar, S. In vitro virucidal activity of povidone iodine gargle and mouthwash against SARS-CoV-2: Implications for dental practice. Br. Dent. J. 2020. [Google Scholar] [CrossRef]
- Komine, A.; Yamaguchi, E.; Okamoto, N.; Yamamoto, K. Virucidal activity of oral care products against SARS-CoV-2 in vitro. J. Oral Maxillofac. Surg. Med. Pathol. 2021, 33, 475–477. [Google Scholar] [CrossRef] [PubMed]
- Kariwa, H.; Sawa, H.; Kobayashi, S. Inactivation of SARS-CoV-2 by povidone-iodine products: Implications for effective mouth rinsing and gargling. Jpn. J. Vet. Res. 2021, 69, 183–187. [Google Scholar]
- Koch-Heier, J.; Hoffmann, H.; Schindler, M.; Lussi, A.; Planz, O. Inactivation of SARS-CoV-2 through Treatment with the Mouth Rinsing Solutions ViruProX((R)) and BacterX((R)) Pro. Microorganisms 2021, 9, 521. [Google Scholar] [CrossRef] [PubMed]
- Meister, T.L.; Todt, D.; Bruggemann, Y.; Steinmann, J.; Banava, S.; Brill, F.H.H.; Steinmann, J.; Pfaender, S.; Steinmann, E. Virucidal activity of nasal sprays against severe acute respiratory syndrome coronavirus-2. J. Hosp. Infect. 2022, 120, 9–13. [Google Scholar] [CrossRef] [PubMed]
- Pyrc, K.; Milewska, A.; Duran, E.B.; Botwina, P.; Dabrowska, A.; Jedrysik, M.; Benedyk, M.; Lopes, R.; Arenas-Pinto, A.; Badr, M.; et al. SARS-CoV-2 inhibition using a mucoadhesive, amphiphilic chitosan that may serve as an anti-viral nasal spray. Sci. Rep. 2021, 11, 20012. [Google Scholar] [CrossRef]
- Santos, C.; da Fonseca Orcina, B.; Brito Reia, V.C.; Ribeiro, L.G.; Grotto, R.M.T.; Prudenciatti, A.; de Moraes, L.N.; Ragghianti Zangrando, M.; Vilhena, F.V.; da Silva Santos, P.S. Virucidal Activity of the Antiseptic Mouthwash and Dental Gel Containing Anionic Phthalocyanine Derivative: In vitro Study. Clin. Cosmet. Investig. Dent. 2021, 13, 269–274. [Google Scholar] [CrossRef]
- Tiong, V.; Hassandarvish, P.; Bakar, S.A.; Mohamed, N.A.; Wan Sulaiman, W.S.; Baharom, N.; Abdul Samad, F.N.; Isahak, I. The effectiveness of various gargle formulations and salt water against SARS-CoV-2. Sci. Rep. 2021, 11, 20502. [Google Scholar] [CrossRef]
- Steinhauer, K.; Meister, T.L.; Todt, D.; Krawczyk, A.; Passvogel, L.; Becker, B.; Paulmann, D.; Bischoff, B.; Pfaender, S.; Brill, F.H.H.; et al. Comparison of the in-vitro efficacy of different mouthwash solutions targeting SARS-CoV-2 based on the European Standard EN 14476. J. Hosp. Infect. 2021, 111, 180–183. [Google Scholar] [CrossRef]
- Hoffmann, M.; Kleine-Weber, H.; Schroeder, S.; Kruger, N.; Herrler, T.; Erichsen, S.; Schiergens, T.S.; Herrler, G.; Wu, N.H.; Nitsche, A.; et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell 2020, 181, 271–280 e278. [Google Scholar] [CrossRef]
- Funnell, S.G.P.; Afrough, B.; Baczenas, J.J.; Berry, N.; Bewley, K.R.; Bradford, R.; Florence, C.; Duff, Y.L.; Lewis, M.; Moriarty, R.V.; et al. A cautionary perspective regarding the isolation and serial propagation of SARS-CoV-2 in Vero cells. NPJ Vaccines 2021, 6, 83. [Google Scholar] [CrossRef]
- Abdelalim, A.A.; Mohamady, A.A.; Elsayed, R.A.; Elawady, M.A.; Ghallab, A.F. Corticosteroid nasal spray for recovery of smell sensation in COVID-19 patients: A randomized controlled trial. Am. J. Otolaryngol. 2021, 42, 102884. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, P.; Melkonyan, A.; Meethil, A.; Saraswat, S.; Hall, D.L.; Cottle, J.; Wenzel, M.; Ayouty, N.; Bense, S.; Casanova, F.; et al. Estimating salivary carriage of severe acute respiratory syndrome coronavirus 2 in nonsymptomatic people and efficacy of mouthrinse in reducing viral load: A randomized controlled trial. J. Am. Dent. Assoc. 2021, 152, 903–908. [Google Scholar] [CrossRef] [PubMed]
- Burgos-Ramos, E.; Urbieta, I.R.; Rodriguez, D. Is hydrogen peroxide an effective mouthwash for reducing the viral load of SARS-CoV-2 in dental clinics? Saudi Dent. J. 2022, 34, 237–242. [Google Scholar] [CrossRef] [PubMed]
- da Fonseca Orcina, B.; Vilhena, F.V.; Cardoso de Oliveira, R.; Marques da Costa Alves, L.; Araki, K.; Toma, S.H.; Ragghianti Zangrando, M.S.; da Silva Santos, P.S. A Phthalocyanine Derivate Mouthwash to Gargling/Rinsing as an Option to Reduce Clinical Symptoms of COVID-19: Case Series. Clin. Cosmet. Investig. Dent. 2021, 13, 47–50. [Google Scholar] [CrossRef]
- Domenico, M.B.D.; Collares, K.; Santos, R.B.D.; Lenz, U.; Antunes, V.P.; Godinho, V.W.; Cesca, H.; Ponciano, T.H.J.; Corazza, P.H. Hydrogen peroxide as an auxiliary treatment for COVID-19 in Brazil: A randomized double-blind clinical trial. Epidemiol. Health 2021, 43, e2021051. [Google Scholar] [CrossRef] [PubMed]
- Figueroa, J.M.; Lombardo, M.E.; Dogliotti, A.; Flynn, L.P.; Giugliano, R.; Simonelli, G.; Valentini, R.; Ramos, A.; Romano, P.; Marcote, M.; et al. Efficacy of a Nasal Spray Containing Iota-Carrageenan in the Postexposure Prophylaxis of COVID-19 in Hospital Personnel Dedicated to Patients Care with COVID-19 Disease. Int. J. Gen. Med. 2021, 14, 6277–6286. [Google Scholar] [CrossRef]
- Guenezan, J.; Garcia, M.; Strasters, D.; Jousselin, C.; Leveque, N.; Frasca, D.; Mimoz, O. Povidone Iodine Mouthwash, Gargle, and Nasal Spray to Reduce Nasopharyngeal Viral Load in Patients With COVID-19: A Randomized Clinical Trial. JAMA Otolaryngol. Head Neck Surg. 2021, 147, 400–401. [Google Scholar] [CrossRef]
- Huang, Y.H.; Huang, J.T. Use of chlorhexidine to eradicate oropharyngeal SARS-CoV-2 in COVID-19 patients. J. Med. Virol. 2021, 93, 4370–4373. [Google Scholar] [CrossRef]
- Kasiri, H.; Rouhani, N.; Salehifar, E.; Ghazaeian, M.; Fallah, S. Mometasone furoate nasal spray in the treatment of patients with COVID-19 olfactory dysfunction: A randomized, double blind clinical trial. Int. Immunopharmacol. 2021, 98, 107871. [Google Scholar] [CrossRef]
- Arefin, M.K.; Rumi, S.; Uddin, A.; Banu, S.S.; Khan, M.; Kaiser, A.; Chowdhury, J.A.; Khan, M.A.S.; Hasan, M.J. Virucidal effect of povidone iodine on COVID-19 in the nasopharynx: An open-label randomized clinical trial. Indian J. Otolaryngol. Head Neck Surg. 2021, 1–5. [Google Scholar] [CrossRef]
- Khan, M.M.; Parab, S.R.; Paranjape, M. Repurposing 0.5% povidone iodine solution in otorhinolaryngology practice in COVID-19 pandemic. Am. J. Otolaryngol. 2020, 41, 102618. [Google Scholar] [CrossRef]
- Saud, Z.; Tyrrell, V.J.; Zaragkoulias, A.; Protty, M.B.; Statkute, E.; Rubina, A.; Bentley, K.; White, D.A.; Rodrigues, P.D.S.; Murphy, R.C.; et al. The SARS-CoV2 envelope differs from host cells, exposes procoagulant lipids, and is disrupted in vivo by oral rinses. J. Lipid Res. 2022, 63, 100208. [Google Scholar] [CrossRef] [PubMed]
- Avhad, S.K.; Bhanushali, M.; Sachdev, S.S.; Save, S.S.; Kalra, D.; Kamala, D.N. Comparison of effectiveness of chlorine dioxide mouthwash and chlorhexidine gluconate mouthwash in reduction of oral viral load in patients with COVID-19. Indian J. Public Health Res. Dev. 2020, 11, 27–32. [Google Scholar]
- Almanza-Reyes, H.; Moreno, S.; Plascencia-Lopez, I.; Alvarado-Vera, M.; Patron-Romero, L.; Borrego, B.; Reyes-Escamilla, A.; Valencia-Manzo, D.; Brun, A.; Pestryakov, A.; et al. Evaluation of silver nanoparticles for the prevention of SARS-CoV-2 infection in health workers: In vitro and in vivo. PLoS ONE 2021, 16, e0256401. [Google Scholar] [CrossRef] [PubMed]
- Aref, Z.F.; Bazeed, S.; Hassan, M.H.; Hassan, A.S.; Rashad, A.; Hassan, R.G.; Abdelmaksoud, A.A. Clinical, Biochemical and Molecular Evaluations of Ivermectin Mucoadhesive Nanosuspension Nasal Spray in Reducing Upper Respiratory Symptoms of Mild COVID-19. Int. J. Nanomed. 2021, 16, 4063–4072. [Google Scholar] [CrossRef]
- Laferl, H.; Seitz, T.; Baier-Grabner, S.; Kelani, H.; Scholz, E.; Heger, F.; Gotzinger, F.; Frischer, P.T.; Wenisch, C.; Allerberger, P.F. Evaluation of RT-qPCR of mouthwash and buccal swabs for detection of SARS-CoV-2 in children and adults. Am. J. Infect. Control 2022, 50, 176–181. [Google Scholar] [CrossRef]
- Michel, W.; Farber, J.; Dilas, M.; Heuft, H.G.; Tammer, I.; Baar, J.; Kaasch, A.J. A combined oro-nasopharyngeal swab is more sensitive than mouthwash in detecting SARS-CoV-2 by a high-throughput PCR assay. Infection 2021, 49, 527–531. [Google Scholar] [CrossRef]
- Mora-Aguilera, G.; Martinez-Bustamante, V.; Acevedo-Sanchez, G.; Coria-Contreras, J.J.; Guzman-Hernandez, E.; Flores-Colorado, O.E.; Mendoza-Ramos, C.; Hernandez-Nava, G.; Alvarez-Maya, I.; Gutierrez-Espinosa, M.A.; et al. Surveillance Web System and Mouthwash-Saliva qPCR for Labor Ambulatory SARS-CoV-2 Detection and Prevention. Int. J. Environ. Res. Public Health 2022, 19, 1271. [Google Scholar] [CrossRef]
- Paull, J.R.A.; Luscombe, C.A.; Castellarnau, A.; Heery, G.P.; Bobardt, M.D.; Gallay, P.A. Protective Effects of Astodrimer Sodium 1% Nasal Spray Formulation against SARS-CoV-2 Nasal Challenge in K18-hACE2 Mice. Viruses 2021, 13, 1656. [Google Scholar] [CrossRef]
- Errecalde, J.; Lifschitz, A.; Vecchioli, G.; Ceballos, L.; Errecalde, F.; Ballent, M.; Marin, G.; Daniele, M.; Turic, E.; Spitzer, E.; et al. Safety and Pharmacokinetic Assessments of a Novel Ivermectin Nasal Spray Formulation in a Pig Model. J. Pharm. Sci. 2021, 110, 2501–2507. [Google Scholar] [CrossRef]
- Jain, A.; Grover, V.; Singh, C.; Sharma, A.; Das, D.K.; Singh, P.; Thakur, K.G.; Ringe, R.P. Chlorhexidine: An effective anticovid mouth rinse. J. Indian Soc. Periodontol. 2021, 25, 86–88. [Google Scholar] [CrossRef] [PubMed]
- Bano-Polo, M.; Martinez-Gil, L.; Sanchez Del Pino, M.M.; Massoli, A.; Mingarro, I.; Leon, R.; Garcia-Murria, M.J. Cetylpyridinium chloride promotes disaggregation of SARS-CoV-2 virus-like particles. J. Oral Microbiol. 2022, 14, 2030094. [Google Scholar] [CrossRef] [PubMed]
- Bansal, S.; Jonsson, C.B.; Taylor, S.L.; Figueroa, J.M.; Dugour, A.V.; Palacios, C.; Vega, J.C. Iota-carrageenan and xylitol inhibit SARS-CoV-2 in Vero cell culture. PLoS ONE 2021, 16, e0259943. [Google Scholar] [CrossRef] [PubMed]
- Bentley, K.; Stanton, R.J. Hydroxypropyl Methylcellulose-Based Nasal Sprays Effectively Inhibit In Vitro SARS-CoV-2 Infection and Spread. Viruses 2021, 13, 2345. [Google Scholar] [CrossRef] [PubMed]
- Bovard, D.; van der Toorn, M.; Schlage, W.K.; Constant, S.; Renggli, K.; Peitsch, M.C.; Hoeng, J. Iota-carrageenan extracted from red algae is a potent inhibitor of SARS-CoV-2 infection in reconstituted human airway epithelia. Biochem. Biophys. Rep. 2022, 29, 101187. [Google Scholar] [CrossRef] [PubMed]
- Haridas, M.; Sasidhar, V.; Nath, P.; Abhithaj, J.; Sabu, A.; Rammanohar, P. Compounds of Citrus medica and Zingiber officinale for COVID-19 inhibition: In silico evidence for cues from Ayurveda. Future J. Pharm. Sci. 2021, 7, 13. [Google Scholar] [CrossRef]
- Moakes, R.J.A.; Davies, S.P.; Stamataki, Z.; Grover, L.M. Formulation of a Composite Nasal Spray Enabling Enhanced Surface Coverage and Prophylaxis of SARS-COV-2. Adv. Mater. 2021, 33, e2008304. [Google Scholar] [CrossRef]
- Morokutti-Kurz, M.; Froba, M.; Graf, P.; Grosse, M.; Grassauer, A.; Auth, J.; Schubert, U.; Prieschl-Grassauer, E. Iota-carrageenan neutralizes SARS-CoV-2 and inhibits viral replication in vitro. PLoS ONE 2021, 16, e0237480. [Google Scholar] [CrossRef]
- Munoz-Basagoiti, J.; Perez-Zsolt, D.; Leon, R.; Blanc, V.; Raich-Regue, D.; Cano-Sarabia, M.; Trinite, B.; Pradenas, E.; Blanco, J.; Gispert, J.; et al. Mouthwashes with CPC Reduce the Infectivity of SARS-CoV-2 Variants In Vitro. J. Dent. Res. 2021, 100, 1265–1272. [Google Scholar] [CrossRef]
- Paolacci, S.; Ergoren, M.C.; De Forni, D.; Manara, E.; Poddesu, B.; Cugia, G.; Dhuli, K.; Camilleri, G.; Tuncel, G.; Kaya Suer, H.; et al. In vitro and clinical studies on the efficacy of alpha-cyclodextrin and hydroxytyrosol against SARS-CoV-2 infection. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 81–89. [Google Scholar] [CrossRef]
- Rodriguez, K.; Saunier, F.; Rigaill, J.; Audoux, E.; Botelho-Nevers, E.; Prier, A.; Dickerscheit, Y.; Pillet, S.; Pozzetto, B.; Bourlet, T.; et al. Evaluation of in vitro activity of copper gluconate against SARS-CoV-2 using confocal microscopy-based high content screening. J. Trace Elem. Med. Biol. 2021, 68, 126818. [Google Scholar] [CrossRef] [PubMed]
- Sharad, S.; Kapur, S. Indian Herb-Derived Phytoconstituent-Based Antiviral, Antimicrobial and Antifungal Formulation: An Oral Rinse Candidate for Oral Hygiene and the Potential Prevention of COVID-19 Outbreaks. Pathogens 2021, 10, 1130. [Google Scholar] [CrossRef] [PubMed]
- Tateyama-Makino, R.; Abe-Yutori, M.; Iwamoto, T.; Tsutsumi, K.; Tsuji, M.; Morishita, S.; Kurita, K.; Yamamoto, Y.; Nishinaga, E.; Tsukinoki, K. The inhibitory effects of toothpaste and mouthwash ingredients on the interaction between the SARS-CoV-2 spike protein and ACE2, and the protease activity of TMPRSS2 in vitro. PLoS ONE 2021, 16, e0257705. [Google Scholar] [CrossRef] [PubMed]
- Yadalam, P.K.; Varatharajan, K.; Rajapandian, K.; Chopra, P.; Arumuganainar, D.; Nagarathnam, T.; Sohn, H.; Madhavan, T. Antiviral Essential Oil Components against SARS-CoV-2 in Pre-procedural Mouth Rinses for Dental Settings during COVID-19: A Computational Study. Front. Chem. 2021, 9, 642026. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Casanovas, H.J.; la Rosa, M.; Bello-Lemus, Y.; Rasperini, G.; Acosta-Hoyos, A.J. Virucidal Activity of Different Mouthwashes Using a Novel Biochemical Assay. Healthcare 2021, 10, 63. [Google Scholar] [CrossRef] [PubMed]
- Robinson, T.E.; Moakes, R.J.A.; Grover, L.M. Low Acyl Gellan as an Excipient to Improve the Sprayability and Mucoadhesion of Iota Carrageenan in a Nasal Spray to Prevent Infection With SARS-CoV-2. Front. Med. Technol. 2021, 3, 687681. [Google Scholar] [CrossRef]
- Westover, J.B.; Ferrer, G.; Vazquez, H.; Bethencourt-Mirabal, A.; Go, C.C. In Vitro Virucidal Effect of Intranasally Delivered Chlorpheniramine Maleate Compound against Severe Acute Respiratory Syndrome Coronavirus 2. Cureus 2020, 12, e10501. [Google Scholar] [CrossRef]
- Cannon, M.L.; Westover, J.B.; Bleher, R.; Sanchez-Gonzalez, M.A.; Ferrer, G. In Vitro Analysis of the Anti-viral Potential of nasal spray constituents against SARS-CoV-2. bioRxiv 2020. [Google Scholar] [CrossRef]
- Mohamed, N.A.; Baharom, N.; Sulaiman, W.S.W.; Rashid, Z.Z.; Ken, W.K.; Ali, U.K.; Othman, S.N.; Samat, M.N.; Kori, N.; Periyasamy, P.; et al. Early viral clearance among COVID-19 patients when gargling with povidone-iodine and essential oils—A clinical trial. medRvix 2020. [Google Scholar] [CrossRef]
- Statkute, E.; Rubina, A.; O’Donnell, V.B.; Thomas, D.W.; Stanton, R.J. Brief Report: The Virucidal Efficacy of Oral Rinse Components against SARS-CoV-2 In Vitro. bioRxiv 2020. [Google Scholar] [CrossRef]
- Xu, C.; Wang, A.; Hoskin, E.R.; Cugini, C.; Markowitz, K.; Chang, T.L.; Fine, D.H. Differential effects of antiseptic mouth rinses on SARS-CoV-2 infectivity in vitro. bioRxiv 2020, 10, 272. [Google Scholar] [CrossRef] [PubMed]
- Buonavoglia, A.; Camero, M.; Lanave, G.; Catella, C.; Trombetta, C.M.; Gandolfi, M.G.; Palazzo, G.; Martella, V.; Prati, C. Virucidal activity in vitro of mouthwashes against a feline coronavirus type II. Oral Dis. 2021. [Google Scholar] [CrossRef] [PubMed]
- Green, A.; Roberts, G.; Tobery, T.; Vincent, C.; Barili, M.; Jones, C. In vitro assessment of the virucidal activity of four mouthwashes containing Cetylpyridinium Chloride, ethanol, zinc and a mix of enzyme and proteins against a human coronavirus. bioRxiv 2020. [Google Scholar] [CrossRef]
- Shet, M.; Hong, R.; Igo, D.; Cataldo, M.; Bhaskar, S. In Vitro Evaluation of the Virucidal Activity of Different Povidone-Iodine Formulations against Murine and Human Coronaviruses. Infect. Dis. Ther. 2021, 10, 2777–2790. [Google Scholar] [CrossRef] [PubMed]
- Balouch, B.; Vontela, S.; Yeakel, H.; Alnouri, G.; Sataloff, R.T. Role of Famotidine and Other Acid Reflux Medications for SARS-CoV-2: A Pilot Study. J. Voice 2021. [Google Scholar] [CrossRef]
- Frank, S.; Capriotti, J.; Brown, S.M.; Tessema, B. Povidone-Iodine Use in Sinonasal and Oral Cavities: A Review of Safety in the COVID-19 Era. Ear Nose Throat J. 2020, 99, 586–593. [Google Scholar] [CrossRef]
- Vergara-Buenaventura, A.; Castro-Ruiz, C. Use of mouthwashes against COVID-19 in dentistry. Br. J. Oral Maxillofac. Surg. 2020, 58, 924–927. [Google Scholar] [CrossRef]
- de Toledo Telles-Araujo, G.; Caminha, R.D.G.; Kallas, M.S.; Sipahi, A.M.; da Silva Santos, P.S. Potential mouth rinses and nasal sprays that reduce SARS-CoV-2 viral load: What we know so far? Clinics 2020, 75, e2328. [Google Scholar] [CrossRef]
- Gerlach, M.; Wolff, S.; Ludwig, S.; Schafer, W.; Keiner, B.; Roth, N.J.; Widmer, E. Rapid SARS-CoV-2 inactivation by commonly available chemicals on inanimate surfaces. J. Hosp. Infect. 2020, 106, 633–634. [Google Scholar] [CrossRef]
- Carrouel, F.; Conte, M.P.; Fisher, J.; Goncalves, L.S.; Dussart, C.; Llodra, J.C.; Bourgeois, D. COVID-19: A Recommendation to Examine the Effect of Mouthrinses with beta-Cyclodextrin Combined with Citrox in Preventing Infection and Progression. J. Clin. Med. 2020, 9, 1126. [Google Scholar] [CrossRef]
- Peng, X.; Xu, X.; Li, Y.; Cheng, L.; Zhou, X.; Ren, B. Transmission routes of 2019-nCoV and controls in dental practice. Int. J. Oral Sci. 2020, 12, 9. [Google Scholar] [CrossRef] [PubMed]
- Pattanshetty, S.; Narayana, A.; Radhakrishnan, R. Povidone-iodine gargle as a prophylactic intervention to interrupt the transmission of SARS-CoV-2. Oral Dis. 2021, 27 (Suppl. 3), 752–753. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Idrees, M.; McGowan, B.; Fawzy, A.; Abuderman, A.A.; Balasubramaniam, R.; Kujan, O. Efficacy of Mouth Rinses and Nasal Spray in the Inactivation of SARS-CoV-2: A Systematic Review and Meta-Analysis of In Vitro and In Vivo Studies. Int. J. Environ. Res. Public Health 2022, 19, 12148. https://doi.org/10.3390/ijerph191912148
Idrees M, McGowan B, Fawzy A, Abuderman AA, Balasubramaniam R, Kujan O. Efficacy of Mouth Rinses and Nasal Spray in the Inactivation of SARS-CoV-2: A Systematic Review and Meta-Analysis of In Vitro and In Vivo Studies. International Journal of Environmental Research and Public Health. 2022; 19(19):12148. https://doi.org/10.3390/ijerph191912148
Chicago/Turabian StyleIdrees, Majdy, Bridget McGowan, Amr Fawzy, Abdulwahab Ali Abuderman, Ramesh Balasubramaniam, and Omar Kujan. 2022. "Efficacy of Mouth Rinses and Nasal Spray in the Inactivation of SARS-CoV-2: A Systematic Review and Meta-Analysis of In Vitro and In Vivo Studies" International Journal of Environmental Research and Public Health 19, no. 19: 12148. https://doi.org/10.3390/ijerph191912148
APA StyleIdrees, M., McGowan, B., Fawzy, A., Abuderman, A. A., Balasubramaniam, R., & Kujan, O. (2022). Efficacy of Mouth Rinses and Nasal Spray in the Inactivation of SARS-CoV-2: A Systematic Review and Meta-Analysis of In Vitro and In Vivo Studies. International Journal of Environmental Research and Public Health, 19(19), 12148. https://doi.org/10.3390/ijerph191912148









