A Proposed Method of Converting Gait Speed and TUG Test in Older Subjects
Abstract
:1. Introduction
2. Material and Methods
2.1. Subjects
2.2. Physical Performance
2.3. Statistical Methods
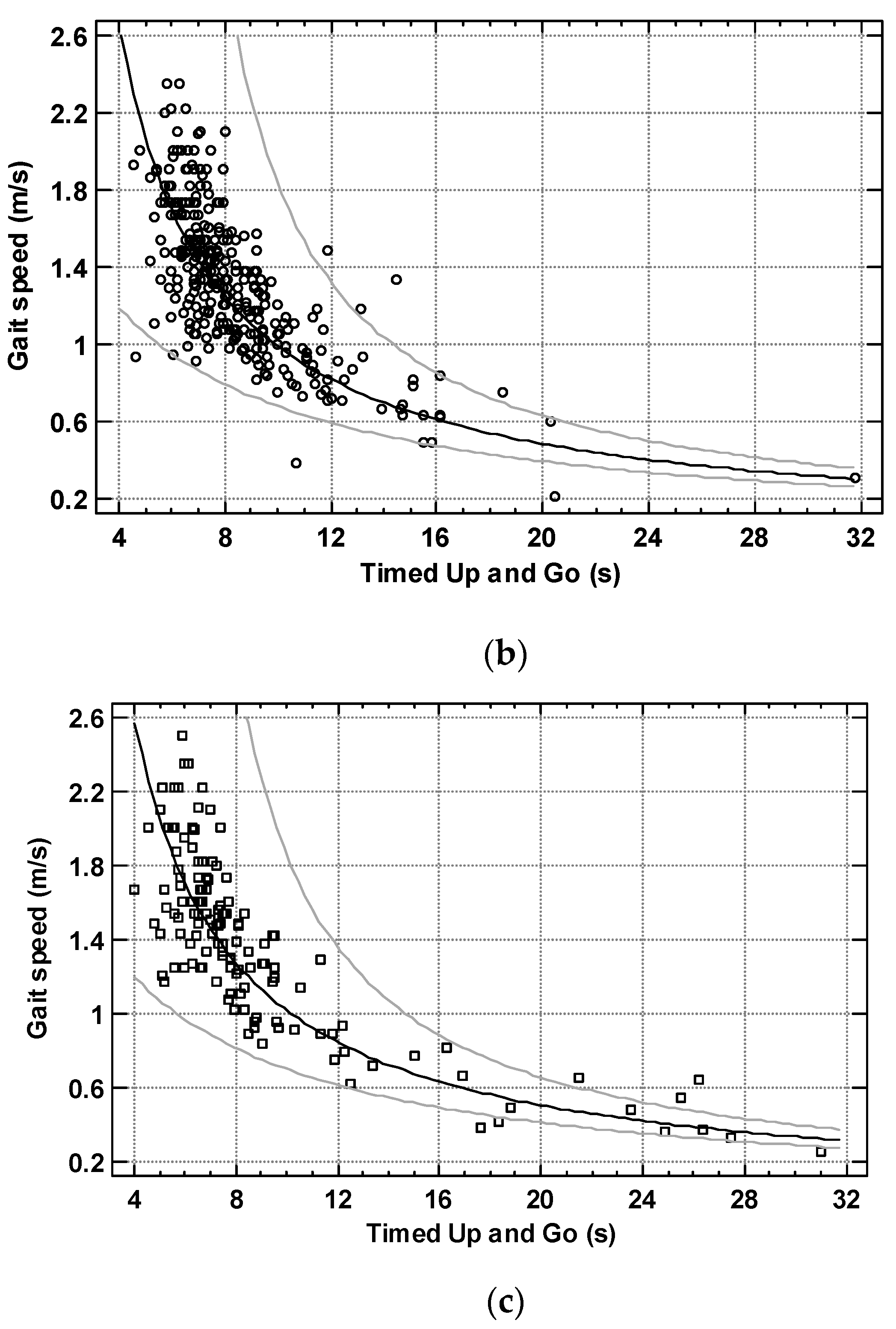
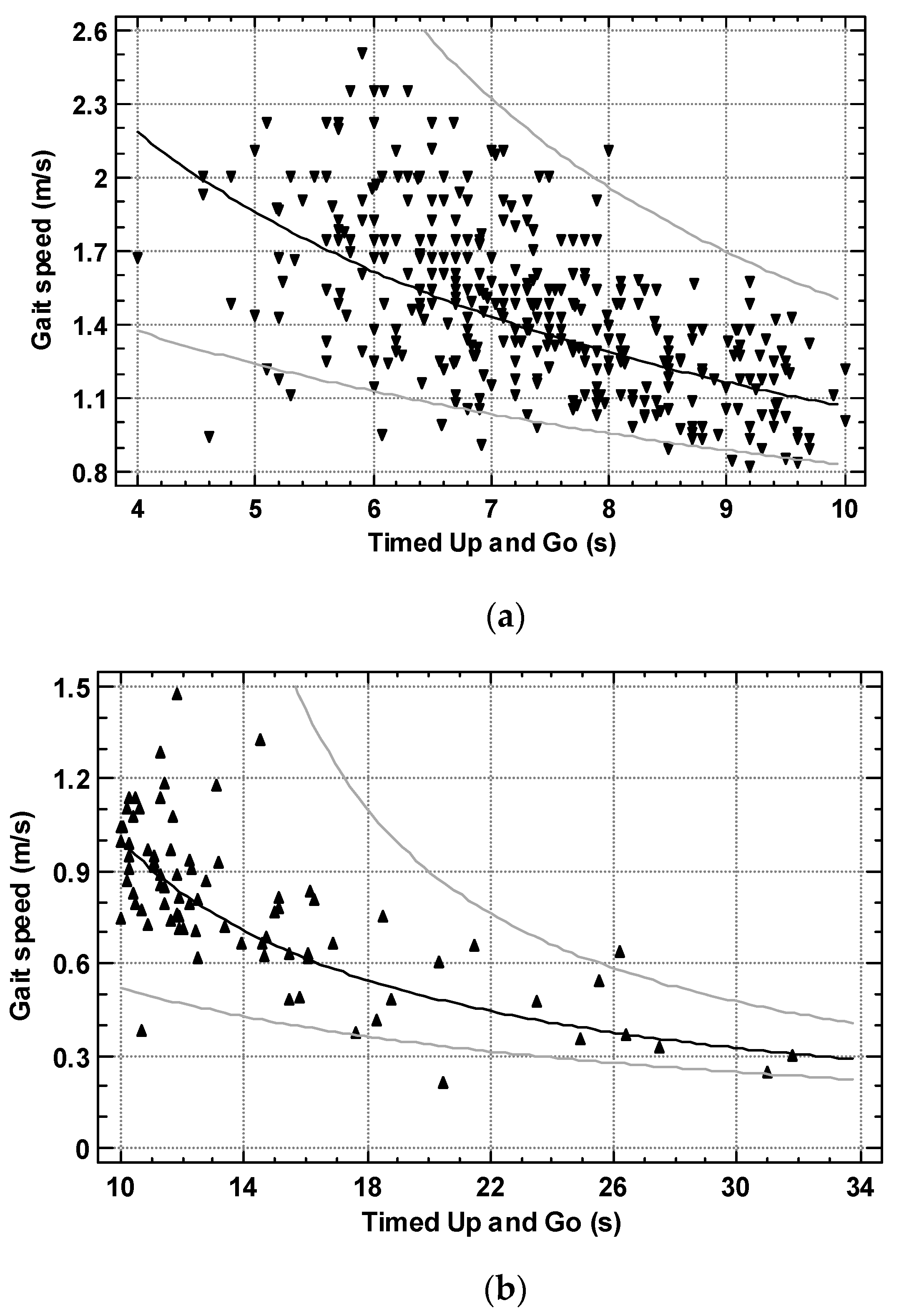
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lauretani, F.; Russo, C.R.; Bandinelli, S.; Bartali, B.; Cavazzini, C.; Di Iorio, A.; Corsi, A.M.; Rantanen, T.; Guralnik, J.M.; Ferrucci, L. Age-associated changes in skeletal muscles and their effect on mobility. J. Appl. Physiol. 2003, 95, 1851–1860. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, I.H. Sarcopenia: Origins and clinical relevance. J. Nutr. 1997, 127, 990s–991s. [Google Scholar] [CrossRef] [PubMed]
- Frontera, W.R.; Suh, D.; Krivickas, L.S.; Hughes, V.A.; Goldstein, R.; Roubenoff, R. Skeletal muscle fiber quality in older men and women. Am. J. Physiol. Cell Physiol. 2000, 279, C611–C618. [Google Scholar] [CrossRef] [PubMed]
- Sieber, C.C. Malnutrition and sarcopenia. Aging Clin. Exp. Res. 2019, 31, 793–798. [Google Scholar] [CrossRef]
- Russ, D.W.; Gregg-Cornell, K.; Conaway, M.J.; Clark, B.C. Evolving concepts on the age-related changes in “muscle quality”. J. Cachexia Sarcopenia Muscle 2012, 3, 95–109. [Google Scholar] [CrossRef]
- Jones, R.L.; Paul, L.; Steultjens, M.P.M.; Smith, S.L. Biomarkers associated with lower limb muscle function in individuals with sarcopenia: A systematic review. J. Cachexia Sarcopenia Muscle 2022. [Google Scholar] [CrossRef]
- Nishikawa, H.; Fukunishi, S.; Asai, A.; Yokohama, K.; Nishiguchi, S.; Higuchi, K. Pathophysiology and mechanisms of primary sarcopenia (Review). Int. J. Mol. Med. 2021, 48, 156. [Google Scholar] [CrossRef]
- Frontera, W.; Zayas, A.; Rodriguez, N. Aging of Human Muscle: Understanding Sarcopenia at the Single Muscle Cell Level. Phys. Med. Rehabil. Clin. N. Am. 2012, 23, 201–207. [Google Scholar] [CrossRef]
- Bilski, J.; Pierzchalski, P.; Szczepanik, M.; Bonior, J.; Zoladz, J.A. Multifactorial Mechanism of Sarcopenia and Sarcopenic Obesity. Role of Physical Exercise, Microbiota and Myokines. Cells 2022, 11, 160. [Google Scholar] [CrossRef]
- Nishikawa, H.; Asai, A.; Fukunishi, S.; Nishiguchi, S.; Higuchi, K. Metabolic Syndrome and Sarcopenia. Nutrients 2021, 13, 3519. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Baeyens, J.P.; Bauer, J.M.; Boirie, Y.; Cederholm, T.; Landi, F.; Martin, F.C.; Michel, J.P.; Rolland, Y.; Topinková, E.; et al. Sarcopenia: European consensus on definition and diagnosis. Report of the European Working Group on Sarcopenia in Older People. Age Ageing 2010, 39, 412–423. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Writing Group for the European Working Group on Sarcopenia in Older People 2 (EWGSOP2), and the Extended Group for EWGSOP2. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef] [PubMed]
- Abellan van Kan, G.; Rolland, Y.; Andrieu, S.; Bauer, J.; Beauchet, O.; Bonnefoy, M.; Cesari, M.; Donini, L.M.; Gillette Guyonnet, S.; Inzitari, M.; et al. Gait speed at usual pace as a predictor of adverse outcomes in community-dwelling older people an International Academy on Nutrition and Aging (IANA) Task Force. J. Nutr. Health Aging 2009, 13, 881–889. [Google Scholar] [CrossRef] [PubMed]
- Viccaro, L.J.; Perera, S.; Studenski, S.A. Is timed up and go better than gait speed in predicting health, function, and falls in older adults? J. Am. Geriatr. Soc. 2011, 59, 887–892. [Google Scholar] [CrossRef]
- Donoghue, O.A.; Savva, G.M.; Cronin, H.; Kenny, R.A.; Horgan, N.F. Using timed up and go and usual gait speed to predict incident disability in daily activities among community-dwelling adults aged 65 and older. Arch. Phys. Med. Rehabil. 2014, 95, 1954–1961. [Google Scholar] [CrossRef]
- Nguyen, A.T.; Nguyen, H.T.T.; Nguyen, H.T.T.; Nguyen, T.X.; Nguyen, T.N.; Nguyen, T.T.H.; Nguyen, A.L.; Pham, T.; Vu, H.T.T. Walking Speed Assessed by 4-Meter Walk Test in the Community-Dwelling Oldest Old Population in Vietnam. Int. J. Environ. Res. Public Health 2022, 19, 9788. [Google Scholar] [CrossRef]
- Cornish, S.M.; Cordingley, D.M.; Shaw, K.A.; Forbes, S.C.; Leonhardt, T.; Bristol, A.; Candow, D.G.; Chilibeck, P.D. Effects of Omega-3 Supplementation Alone and Combined with Resistance Exercise on Skeletal Muscle in Older Adults: A Systematic Review and Meta-Analysis. Nutrients 2022, 14, 2221. [Google Scholar] [CrossRef]
- Fielding, R.A.; Vellas, B.; Evans, W.J.; Bhasin, S.; Morley, J.E.; Newman, A.B.; Abellan van Kan, G.; Andrieu, S.; Bauer, J.; Breuille, D.; et al. Sarcopenia: An undiagnosed condition in older adults. Current consensus definition: Prevalence, etiology, and consequences. International working group on sarcopenia. J. Am. Med. Dir. Assoc. 2011, 12, 249–256. [Google Scholar] [CrossRef]
- Fried, L.P.; Tangen, C.M.; Walston, J.; Newman, A.B.; Hirsch, C.; Gottdiener, J.; Seeman, T.; Tracy, R.; Kop, W.J.; Burke, G.; et al. Frailty in older adults: Evidence for a phenotype. J. Gerontol. A Biol. Sci. Med. Sci. 2001, 56, M146–M156. [Google Scholar] [CrossRef]
- Middleton, A.; Fritz, S.L.; Lusardi, M. Walking speed: The functional vital sign. J. Aging Phys. Act. 2015, 23, 314–322. [Google Scholar] [CrossRef]
- Akbari Kamrani, A.A.; Zamani Sani, S.H.; Fathi Rezaie, Z.; Aghdasi, M.T. Concurrent Validity of Functional Gait Assessment, Timed Up and Go, and Gait Speed Tests in the Persian Community-Dwelling Elderly. Iran. Rehabil. J. 2010, 8, 15–20. [Google Scholar]
- Herman, T.; Giladi, N.; Hausdorff, J.M. Properties of the ‘timed up and go’ test: More than meets the eye. Gerontology 2011, 57, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Podsiadlo, D.; Richardson, S. The timed “up & go”: A test of basic functional mobility for frail elderly persons. J. Am. Geriatr. Soc. 1991, 39, 142–148. [Google Scholar] [PubMed]
- Botolfsen, P.; Helbostad, J.L.; Moe-Nilssen, R.; Wall, J.C. Reliability and concurrent validity of the Expanded Timed Up-and-Go test in older people with impaired mobility. Physiother. Res. Int. 2008, 13, 94–106. [Google Scholar] [CrossRef] [PubMed]
- Kon, S.S.; Patel, M.S.; Canavan, J.L.; Clark, A.L.; Jones, S.E.; Nolan, C.M.; Cullinan, P.; Polkey, M.I.; Man, W.D. Reliability and validity of 4-metre gait speed in COPD. Eur. Respir. J. 2013, 42, 333–340. [Google Scholar] [CrossRef]
- Morley, J.E.; Anker, S.D.; von Haehling, S. Prevalence, incidence, and clinical impact of sarcopenia: Facts, numbers, and epidemiology-update 2014. J. Cachexia Sarcopenia Muscle 2014, 5, 253–259. [Google Scholar] [CrossRef]
- Beaudart, C.; Rizzoli, R.; Bruyère, O.; Reginster, J.-Y.; Biver, E. Sarcopenia: Burden and challenges for public health. Arch. Public Health 2014, 72, 45. [Google Scholar] [CrossRef]
- Thomson, S.; Badiuk, B.; Parokaran Varghese, J.; Thai, V.; McIlroy, W.E.; Van Ooteghem, K. Standing, Transition, and Walking Ability in Older Adults: The Case for Independently Evaluating Different Domains of Mobility Function. Gerontology 2022, 1–12. [Google Scholar] [CrossRef]
- Batko-Szwaczka, A.; Wilczyński, K.; Hornik, B.; Janusz-Jenczeń, M.; Włodarczyk, I.; Wnuk, B.; Szołtysek, J.; Durmała, J.; Szuster-Kowolik, K.; Antoniak-Sobczak, K.; et al. Predicting Adverse Outcomes in Healthy Aging Community-Dwelling Early-Old Adults with the Timed Up and Go Test. Clin. Interv. Aging 2020, 15, 1263–1270. [Google Scholar] [CrossRef]
- Soubra, R.; Chkeir, A.; Novella, J.-L. A Systematic Review of Thirty-One Assessment Tests to Evaluate Mobility in Older Adults. Biomed. Res. Int. 2019, 2019, 1354362. [Google Scholar] [CrossRef]
- Bennell, K.; Dobson, F.; Hinman, R. Measures of physical performance assessments: Self-Paced Walk Test (SPWT), Stair Climb Test (SCT), Six-Minute Walk Test (6MWT), Chair Stand Test (CST), Timed Up & Go (TUG), Sock Test, Lift and Carry Test (LCT), and Car Task. Arthritis Care Res. 2011, 63 (Suppl. 11), S350–S370. [Google Scholar] [CrossRef]
- Ries, J.D.; Echternach, J.L.; Nof, L.; Gagnon Blodgett, M. Test-retest reliability and minimal detectable change scores for the timed “up & go” test, the six-minute walk test, and gait speed in people with Alzheimer disease. Phys. Ther. 2009, 89, 569–579. [Google Scholar] [CrossRef] [PubMed]
- Bridenbaugh, S.A.; Beauchet, O.; Annweiler, C.; Allali, G.; Herrmann, F.; Kressig, R.W. Association between dual task-related decrease in walking speed and real versus imagined Timed Up and Go test performance. Aging Clin. Exp. Res. 2013, 25, 283–289. [Google Scholar] [CrossRef]
- Ma, Y.; Zhang, W.; Han, P.; Kohzuki, M.; Guo, Q. Osteosarcopenic Obesity Associated with Poor Physical Performance in the Elderly Chinese Community. Clin. Interv. Aging 2020, 15, 1343–1352. [Google Scholar] [CrossRef] [PubMed]
- Kubicki, A. Functional assessment in older adults: Should we use timed up and go or gait speed test? Neurosci. Lett. 2014, 577, 89–94. [Google Scholar] [CrossRef]
- Montero-Odasso, M.; Muir, S.W.; Gopaul, K.; Annweiler, C.; Beauchet, O. Gait velocity versus the timed up and go test: Which one to use for the prediction of falls and other adverse health outcomes in primary care? J. Am. Geriatr. Soc. 2011, 59, 2191–2192. [Google Scholar] [CrossRef]
- Barry, E.; Galvin, R.; Keogh, C.; Horgan, F.; Fahey, T. Is the Timed Up and Go test a useful predictor of risk of falls in community dwelling older adults: A systematic review and meta-analysis. BMC Geriatr. 2014, 14, 14. [Google Scholar] [CrossRef]
- Lee, S.-P.; Dufek, J.S.; Hickman, R.; Schuerman, S. Influence of Procedural Factors on the Reliability and Performance of the Timed Up-and-go Test in Older Adults. Int. J. Gerontol. 2016, 10, 37–42. [Google Scholar] [CrossRef]
- Chew, J.; Yeo, A.; Yew, S.; Lim, J.P.; Tay, L.; Ding, Y.Y.; Lim, W.S. Muscle Strength Definitions Matter: Prevalence of Sarcopenia and Predictive Validity for Adverse Outcomes Using the European Working Group on Sarcopenia in Older People 2 (EWGSOP2) Criteria. J. Nutr. Health Aging 2020, 24, 614–618. [Google Scholar] [CrossRef]
- Rockwood, K.; Awalt, E.; Carver, D.; MacKnight, C. Feasibility and measurement properties of the functional reach and the timed up and go tests in the Canadian study of health and aging. J. Gerontol. A Biol. Sci. Med. Sci. 2000, 55, M70–M73. [Google Scholar] [CrossRef]
- Beauchamp, M.K.; Hao, Q.; Kuspinar, A.; D’Amore, C.; Scime, G.; Ma, J.; Mayhew, A.; Bassim, C.; Wolfson, C.; Kirkland, S.; et al. Reliability and Minimal Detectable Change Values for Performance-Based Measures of Physical Functioning in the Canadian Longitudinal Study on Aging. J. Gerontol. A Biol. Sci. Med. Sci. 2021, 76, 2030–2038. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.-H.; Chang, Q.-X.; Liang, C.-C.; Chen, J.-C. The Effects of Using a Low-Cost and Easily Accessible Exercise Toolkit Incorporated to the Governmental Health Program on Community-Dwelling Older Adults: A Quasi-Experimental Study. Int. J. Environ. Res. Public Health 2022, 19, 9614. [Google Scholar] [CrossRef] [PubMed]
- Freter, S.H.; Fruchter, N. Relationship between timed ‘up and go’ and gait time in an elderly orthopaedic rehabilitation population. Clin. Rehabil. 2000, 14, 96–101. [Google Scholar] [CrossRef] [PubMed]
- Verdecchia, D.H.; Monzón, A.M.; Urbina Jaimes, V.; da Silva Paiva, L.; Oliveira, F.R.; de Carvalho, T.D. Correlation between timed up and go, usual gait speed and dizziness handicap inventory in elderly with vestibular disorders: A retrospective and analytical study. Arch. Physiother. 2020, 10, 12. [Google Scholar] [CrossRef]
- Wrisley, D.M.; Kumar, N.A. Functional Gait Assessment: Concurrent, Discriminative, and Predictive Validity in Community-Dwelling Older Adults. Phys. Ther. 2010, 90, 761–773. [Google Scholar] [CrossRef] [PubMed]
- Quach, L.; Galica, A.M.; Jones, R.N.; Procter-Gray, E.; Manor, B.; Hannan, M.T.; Lipsitz, L.A. The Nonlinear Relationship Between Gait Speed and Falls: The Maintenance of Balance, Independent Living, Intellect, and Zest in the Elderly of Boston Study. J. Am. Geriatr. Soc. 2011, 59, 1069–1073. [Google Scholar] [CrossRef]
- Kramer, D.B.; Tsai, T.; Natarajan, P.; Tewksbury, E.; Mitchell, S.L.; Travison, T.G. Frailty, Physical Activity, and Mobility in Patients With Cardiac Implantable Electrical Devices. J. Am. Heart Assoc. 2017, 6, e004659. [Google Scholar] [CrossRef]
- Sosowska, N.; Piglowska, M.; Guligowska, A.; Soltysik, B.; Kostka, T. Comparison of Agreement between Several Diagnostic Criteria of Sarcopenia in Community-Dwelling Older Adults. J. Frailty Aging 2021, 11, 33–39. [Google Scholar] [CrossRef]
- Li, Z.; Wang, T.; Shen, M.; Song, T.; He, J.; Guo, W.; Wang, Z.; Zhuang, J. Comparison of Wuqinxi Qigong with Stretching on Single- and Dual-Task Gait, Motor Symptoms and Quality of Life in Parkinson’s Disease: A Preliminary Randomized Control Study. Int. J. Environ. Res. Public Health 2022, 19, 8042. [Google Scholar] [CrossRef]
- Kimura, M.; Moriyasu, A.; Makizako, H. Positive Association between High Protein Food Intake Frequency and Physical Performance and Higher-Level Functional Capacity in Daily Life. Nutrients 2022, 14, 72. [Google Scholar] [CrossRef]

| All (n = 450) | Women (n = 315) | Men (n = 135) | Significance | |
|---|---|---|---|---|
| Age (years) | 79.3 ± 5.4 80 (75–83) | 79.2 ± 5.3 79 (75–83) | 79.6 ± 5.5 80 (75–84) | p = 0.56 |
| TUG (seconds) | 8.60 ± 3.64 7.6 (6.7–9.2) | 8.46 ± 2.83 7.8 (6.8–9.3) | 8.94 ± 5.05 7.4 (6.3–9.1) | p = 0.20 |
| Gait speed (m/s) | 1.34 ± 0.41 1.33 (1.07–1.6) | 1.32 ± 0.38 1.3 (1.05–1.54) | 1.38 ± 0.47 1.42 (1.11–1.67) | p = 0.13 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kostka, J.; Sosowska, N.; Guligowska, A.; Kostka, T. A Proposed Method of Converting Gait Speed and TUG Test in Older Subjects. Int. J. Environ. Res. Public Health 2022, 19, 12145. https://doi.org/10.3390/ijerph191912145
Kostka J, Sosowska N, Guligowska A, Kostka T. A Proposed Method of Converting Gait Speed and TUG Test in Older Subjects. International Journal of Environmental Research and Public Health. 2022; 19(19):12145. https://doi.org/10.3390/ijerph191912145
Chicago/Turabian StyleKostka, Joanna, Natalia Sosowska, Agnieszka Guligowska, and Tomasz Kostka. 2022. "A Proposed Method of Converting Gait Speed and TUG Test in Older Subjects" International Journal of Environmental Research and Public Health 19, no. 19: 12145. https://doi.org/10.3390/ijerph191912145
APA StyleKostka, J., Sosowska, N., Guligowska, A., & Kostka, T. (2022). A Proposed Method of Converting Gait Speed and TUG Test in Older Subjects. International Journal of Environmental Research and Public Health, 19(19), 12145. https://doi.org/10.3390/ijerph191912145






