Optical Motion Capture Systems for 3D Kinematic Analysis in Patients with Shoulder Disorders
Abstract
:1. Introduction
2. Material and Methods
2.1. Eligibility Criteria
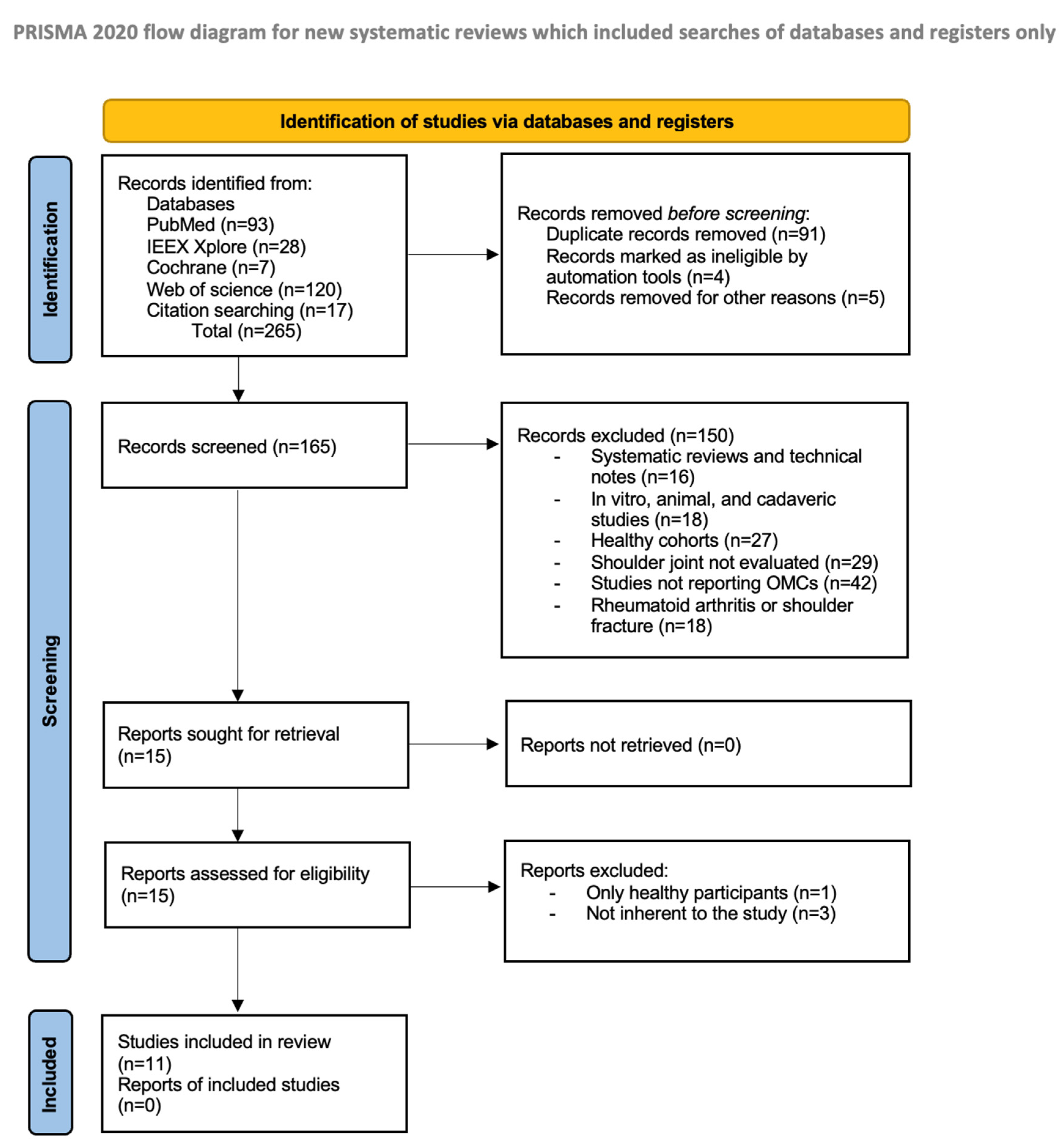
2.2. Search Strategy and Studies Selection Process
2.3. Data Synthesis
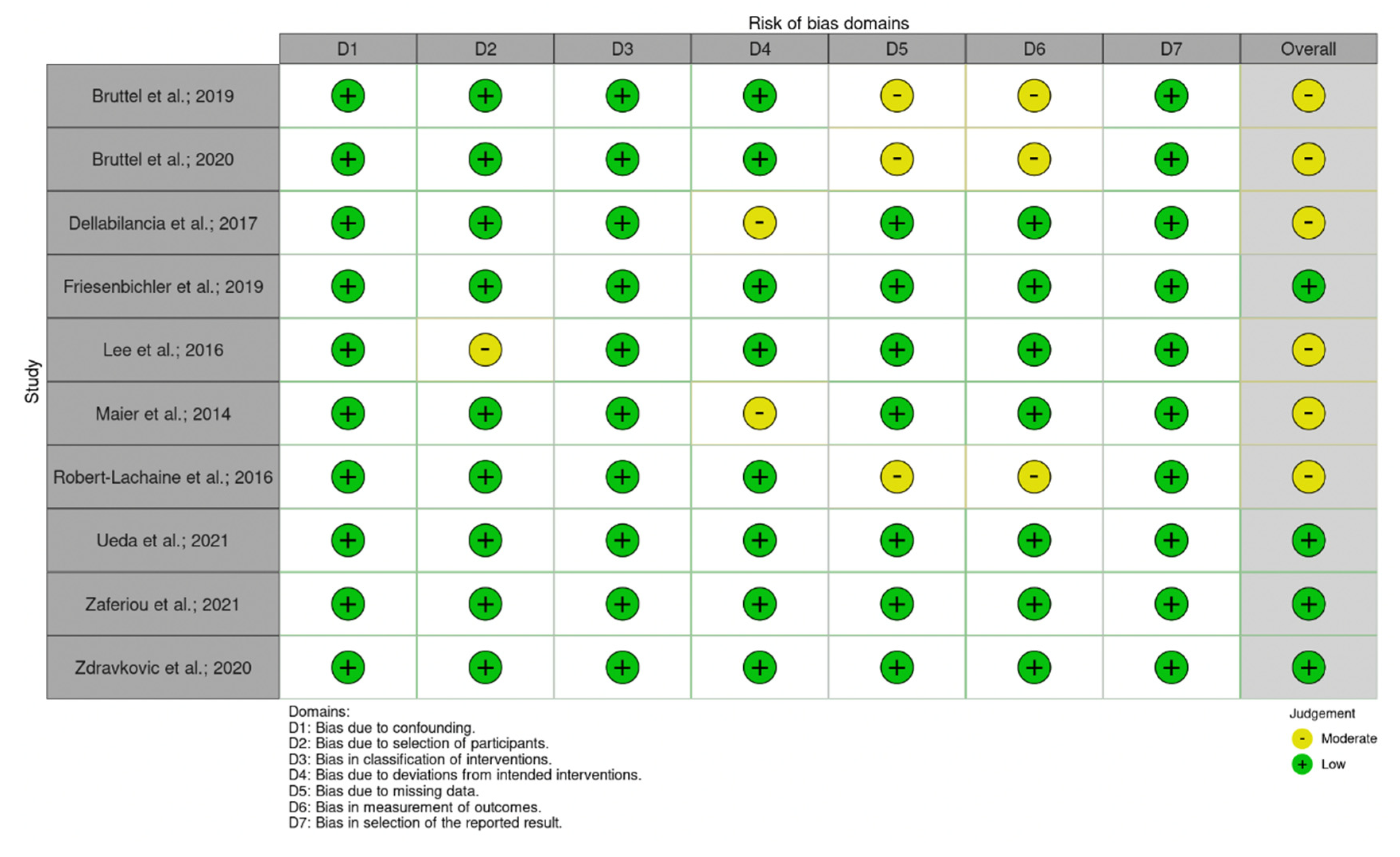
2.4. Quality Assessment
2.5. Statistical Analysis
2.6. Systematic Review Registration Statement
3. Results
3.1. Search Results
3.2. Study Characteristics and Population
3.3. Quality Assessment
3.4. Experimental Setup and Protocols
3.5. Degrees of Freedom and Kinematic Outcomes
4. Discussion
4.1. Markers Setup, Modelling, and Study Protocols
4.2. Degrees of Freedom and Kinematic Outcomes
4.3. Clinical Relevance and Limitations of 3D Kinematic Analysis of the Shoulder with OMCs
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ASES score | American Shoulder and Elbow Surgeons Standardized Shoulder Assessment Form |
| aTSA | Anatomic Total Shoulder Arthroplasty |
| CAS | Case-series |
| CS | Cohort study |
| CMS | Constant Murley score |
| CRS | Cross-sectional study |
| DASH questionnaire | Disabilities of the Arm, Shoulder and Hand questionnaire |
| FS | Frozen Shoulder |
| GOA | Glenohumeral Osteoarthritis |
| Mocap | Motion Capture |
| OMCs | Optoelectronic motion capture tracking systems |
| PLS | Prospective Laboratory Study |
| ROM | Range of motion |
| RCS | Retrospective case series |
| RCC | Retrospective case-control |
| RCS | Retrospective Cohort Study |
| RTSA | Reverse Total Shoulder Arthroplasty |
| RCTA | Rotator Cuff Tear Arthropathy |
| SAI | Shoulder Anterior Instability |
| SDY | Shoulder dysfunctions |
| SST | Simple Shoulder Test |
| VAS | Visual Analogue Scale |
References
- Luime, J.J.; Koes, B.W.; Hendriksen, I.J.; Burdorf, A.; Verhagen, A.P.; Miedema, H.S.; Verhaar, J.A. Prevalence and incidence of shoulder pain in the general population; a systematic review. Scand. J. Rheumatol. 2004, 33, 73–81. [Google Scholar] [CrossRef]
- De Baets, L.; Van der Straaten, R.; Matheve, T.; Timmermans, A. Shoulder assessment according to the international classification of functioning by means of inertial sensor technologies: A systematic review. Gait Posture 2017, 57, 278–294. [Google Scholar] [CrossRef]
- Meislin, R.J.; Sperling, J.W.; Stitik, T.P. Persistent shoulder pain: Epidemiology, pathophysiology, and diagnosis. Am. J. Orthop. 2005, 34, 5–9. [Google Scholar]
- Lietz, J.; Kozak, A.; Nienhaus, A. Prevalence and occupational risk factors of musculoskeletal diseases and pain among dental professionals in Western countries: A systematic literature review and meta-analysis. PLoS ONE 2018, 13, e0208628. [Google Scholar] [CrossRef]
- Longo, U.G.; Carnevale, A.; Piergentili, I.; Berton, A.; Candela, V.; Schena, E.; Denaro, V. Retear rates after rotator cuff surgery: A systematic review and meta-analysis. BMC Musculoskelet. Disord. 2021, 22, 749. [Google Scholar] [CrossRef] [PubMed]
- Longo, U.G.; Vasta, S.; Maffulli, N.; Denaro, V. Scoring systems for the functional assessment of patients with rotator cuff pathology. Sports Med. Arthrosc. Rev. 2011, 19, 310–320. [Google Scholar] [CrossRef]
- Roy, J.S.; MacDermid, J.C.; Woodhouse, L.J. Measuring shoulder function: A systematic review of four questionnaires. Arthritis Rheum. 2009, 61, 623–632. [Google Scholar] [CrossRef]
- Carnevale, A.; Longo, U.G.; Schena, E.; Massaroni, C.; Lo Presti, D.; Berton, A.; Candela, V.; Denaro, V. Wearable systems for shoulder kinematics assessment: A systematic review. BMC Musculoskelet. Disord. 2019, 20, 546. [Google Scholar] [CrossRef]
- Wagner, E.R.; Farley, K.X.; Higgins, I.; Wilson, J.M.; Daly, C.A.; Gottschalk, M.B. The incidence of shoulder arthroplasty: Rise and future projections compared with hip and knee arthroplasty. J. Shoulder Elb. Surg. 2020, 29, 2601–2609. [Google Scholar] [CrossRef]
- Ablove, R.H.; Aul, A.; Baer, G. The incidence and demographics of shoulder repair in Wisconsin, 2002–2010. WMJ 2014, 113, 223–226. [Google Scholar]
- Cutti, A.G.; Parel, I.; Pellegrini, A.; Paladini, P.; Sacchetti, R.; Porcellini, G.; Merolla, G. The Constant score and the assessment of scapula dyskinesis: Proposal and assessment of an integrated outcome measure. J. Electromyogr. Kinesiol. 2016, 29, 81–89. [Google Scholar] [CrossRef]
- Carnevale, A.; Schena, E.; Formica, D.; Massaroni, C.; Longo, U.G.; Denaro, V. Skin Strain Analysis of the Scapular Region and Wearables Design. Sensors 2021, 21, 5761. [Google Scholar] [CrossRef]
- McHugh, B.P.; Morton, A.M.; Akhbari, B.; Molino, J.; Crisco, J.J. Accuracy of an electrogoniometer relative to optical motion tracking for quantifying wrist range of motion. J. Med. Eng. Technol. 2020, 44, 49–54. [Google Scholar] [CrossRef]
- Valevicius, A.M.; Jun, P.Y.; Hebert, J.S.; Vette, A.H. Use of optical motion capture for the analysis of normative upper body kinematics during functional upper limb tasks: A systematic review. J. Electromyogr. Kinesiol. 2018, 40, 1–15. [Google Scholar] [CrossRef]
- Topley, M.; Richards, J.G. A comparison of currently available optoelectronic motion capture systems. J. Biomech. 2020, 106, 109820. [Google Scholar] [CrossRef]
- Robertson, D.G.E.; Caldwell, G.E.; Hamill, J.; Kamen, G.; Whittlesey, S. Research Methods in Biomechanics; Human Kinetics: Champaign, IL, USA, 2013. [Google Scholar]
- Bruttel, H.; Spranz, D.M.; Bülhoff, M.; Aljohani, N.; Wolf, S.I.; Maier, M.W. Comparison of glenohumeral and humerothoracical range of motion in healthy controls, osteoarthritic patients and patients after total shoulder arthroplasty performing different activities of daily living. Gait Posture 2019, 71, 20–25. [Google Scholar] [CrossRef]
- Spranz, D.M.; Bruttel, H.; Eckerle, J.M.; Wolf, S.I.; Berrsche, G.; Maier, M.W. Variation of the glenohumeral and scapulothoracic motion in progressive severity of glenohumeral osteoarthritis. Orthop. Traumatol. Surg. Res. 2019, 105, 1503–1507. [Google Scholar] [CrossRef]
- Lee, K.W.; Kim, Y.I.; Kim, H.Y.; Yang, D.S.; Lee, G.S.; Choy, W.S. Three-Dimensional Scapular Kinematics in Patients with Reverse Total Shoulder Arthroplasty during Arm Motion. Clin. Orthop. Surg. 2016, 8, 316–324. [Google Scholar] [CrossRef]
- Boser, Q.A.; Valevicius, A.M.; Lavoie, E.B.; Chapman, C.S.; Pilarski, P.M.; Hebert, J.S.; Vette, A.H. Cluster-based upper body marker models for three-dimensional kinematic analysis: Comparison with an anatomical model and reliability analysis. J. Biomech. 2018, 72, 228–234. [Google Scholar] [CrossRef]
- Cappozzo, A.; Cappello, A.; Della Croce, U.; Pensalfini, F. Surface-marker cluster design criteria for 3-D bone movement reconstruction. IEEE Trans. Biomed. Eng. 1997, 44, 1165–1174. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Bruttel, H.; Spranz, D.M.; Wolf, S.I.; Maier, M.W. Scapulohumeral rhythm in patients after total shoulder arthroplasty compared to age-matched healthy individuals. Gait Posture 2020, 82, 38–44. [Google Scholar] [CrossRef]
- Dellabiancia, F.; Parel, I.; Filippi, M.V.; Porcellini, G.; Merolla, G. Glenohumeral and scapulohumeral kinematic analysis of patients with traumatic anterior instability wearing a shoulder brace: A prospective laboratory study. Musculoskelet. Surg. 2017, 101, 159–167. [Google Scholar] [CrossRef]
- Friesenbichler, B.; Grassi, A.; Grobet, C.; Audigé, L.; Wirth, B. Is limited shoulder abduction associated with poor scapulothoracic mobility after reverse shoulder arthroplasty? Arch. Orthop. Trauma Surg. 2021, 141, 587–591. [Google Scholar] [CrossRef]
- Maier, M.W.; Niklasch, M.; Dreher, T.; Zeifang, F.; Rettig, O.; Klotz, M.C.; Wolf, S.I.; Kasten, P. Motion patterns in activities of daily living: 3- year longitudinal follow-up after total shoulder arthroplasty using an optical 3D motion analysis system. BMC Musculoskelet. Disord. 2014, 15, 244. [Google Scholar] [CrossRef]
- Robert-Lachaine, X.; Allard, P.; Godbout, V.; Tétreault, P.; Begon, M. Scapulohumeral rhythm relative to active range of motion in patients with symptomatic rotator cuff tears. J. Shoulder Elb. Surg. 2016, 25, 1616–1622. [Google Scholar] [CrossRef]
- Ueda, A.; Matsumura, A.; Shinkuma, T.; Oki, T.; Nakamura, Y. Scapular dyskinesis type is associated with glenohumeral joint and scapular kinematic alteration during pitching motion in baseball players. J. Bodyw. Mov. Ther. 2021, 28, 332–340. [Google Scholar] [CrossRef]
- Zaferiou, A.M.; Knowlton, C.B.; Jang, S.H.; Saltzman, B.M.; Verma, N.N.; Forsythe, B.; Nicholson, G.P.; Romeo, A.A. Scapular and humeral elevation coordination patterns used before vs. after Reverse Total Shoulder Arthroplasty. J. Biomech. 2021, 125, 110550. [Google Scholar] [CrossRef]
- Zdravkovic, V.; Alexander, N.; Wegener, R.; Spross, C.; Jost, B. How Do Scapulothoracic Kinematics During Shoulder Elevation Differ Between Adults with and without Rotator Cuff Arthropathy? Clin. Orthop. Relat. Res. 2020, 478, 2640–2649. [Google Scholar] [CrossRef]
- Robert-Lachaine, X.; Marion, P.; Godbout, V.; Bleau, J.; Begon, M. Elucidating the scapulo-humeral rhythm calculation: 3D joint contribution method. Comput. Methods Biomech. Biomed. Eng. 2015, 18, 249–258. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef]
- Sterne, J.A.; Hernán, M.A.; Reeves, B.C.; Savović, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I.; et al. ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016, 355, i4919. [Google Scholar] [CrossRef] [Green Version]
- Wu, G.; Van der Helm, F.C.; Veeger, H.E.; Makhsous, M.; Van Roy, P.; Anglin, C.; Nagels, J.; Karduna, A.R.; McQuade, K.; Wang, X.; et al. ISB recommendation on definitions of joint coordinate systems of various joints for the reporting of human joint motion--Part II: Shoulder, elbow, wrist and hand. J. Biomech. 2005, 38, 981–992. [Google Scholar] [CrossRef]
- McHugh, B.; Akhbari, B.; Morton, A.M.; Moore, D.C.; Crisco, J.J. Optical motion capture accuracy is task-dependent in assessing wrist motion. J. Biomech. 2021, 120, 110362. [Google Scholar] [CrossRef]
- Medina-Mirapeix, F.; Martín-San Agustín, R.; Cánovas-Ambit, G.; García-Vidal, J.A.; Gacto-Sánchez, M.; Escolar-Reina, P. An Optoelectronic System for Measuring the Range of Motion in Healthy Volunteers: A Cross-Sectional Study. Medicina 2019, 55, 516. [Google Scholar] [CrossRef]
- Meskers, C.G.M.; Van der Helm, F.C.T.; Rozendaal, L.A.; Rozing, P.M. In vivo estimation of the glenohumeral joint rotation center from scapular bony landmarks by linear regression. J. Biomech. 1997, 31, 93–96. [Google Scholar] [CrossRef]
- Stokdijk, M.; Nagels, J.; Rozing, P.M. The glenohumeral joint rotation centre in vivo. J. Biomech. 2000, 33, 1629–1636. [Google Scholar] [CrossRef]
- Cappozzo, A.; Catani, F.; Della Croce, U.; Leardini, A. Position and orientation in space of bones during movement: Anatomical frame definition and determination. Clin. Biomech. 1995, 10, 171–178. [Google Scholar] [CrossRef]
- Karduna, A.R.; McClure, P.W.; Michener, L.A.; Sennett, B. Dynamic measurements of three-dimensional scapular kinematics: A validation study. J. Biomech. Eng. 2001, 123, 184–190. [Google Scholar] [CrossRef]
- McClure, P.W.; Michener, L.A.; Sennett, B.J.; Karduna, A.R. Direct 3-dimensional measurement of scapular kinematics during dynamic movements in vivo. J. Shoulder Elb. Surg. 2001, 10, 269–277. [Google Scholar] [CrossRef]
- Lempereur, M.; Brochard, S.; Leboeuf, F.; Rémy-Néris, O. Validity and reliability of 3D marker based scapular motion analysis: A systematic review. J. Biomech. 2014, 47, 2219–2230. [Google Scholar] [CrossRef]
- Warner, M.B.; Chappell, P.H.; Stokes, M.J. Measurement of dynamic scapular kinematics using an acromion marker cluster to minimize skin movement artifact. J. Vis. Exp. 2015, 96, e51717. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Andel, C.J.; Wolterbeek, N.; Doorenbosch, C.A.M.; Veeger, D.H.E.J.; Harlaar, J. Complete 3D kinematics of upper extremity functional tasks. Gait Posture 2008, 27, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Šenk, M.; Cheze, L. Rotation sequence as an important factor in shoulder kinematics. Clin. Biomech. 2006, 21, S3–S8. [Google Scholar] [CrossRef]
- Bonnefoy-Mazure, A.; Slawinski, J.; Riquet, A.; Lévèque, J.M.; Miller, C.; Cheze, L. Rotation sequence is an important factor in shoulder kinematics. Application to the elite players’ flat serves. J. Biomech. 2010, 43, 2022–2025. [Google Scholar] [CrossRef]
- Phadke, V.; Braman, J.P.; LaPrade, R.F.; Ludewig, P.M. Comparison of glenohumeral motion using different rotation sequences. J. Biomech. 2011, 44, 700–705. [Google Scholar] [CrossRef]
- Kontaxis, A.; Cutti, A.G.; Johnson, G.R.; Veeger, H.E.J. A framework for the definition of standardized protocols for measuring upper-extremity kinematics. Clin. Biomech. 2009, 24, 246–253. [Google Scholar] [CrossRef]
- Cutti, A.G.; Giovanardi, A.; Rocchi, L.; Davalli, A.; Sacchetti, R. Ambulatory measurement of shoulder and elbow kinematics through inertial and magnetic sensors. Med. Biol. Eng. Comput. 2008, 46, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Jildeh, T.R.; Ference, D.A.; Abbas, M.J.; Jiang, E.X.; Okoroha, K.R. Scapulothoracic Dyskinesis: A Concept Review. Curr. Rev. Musculoskelet. Med. 2021, 14, 246–254. [Google Scholar] [CrossRef] [PubMed]
- Lefèvre-Colau, M.M.; Nguyen, C.; Palazzo, C.; Srour, F.; Paris, G.; Vuillemin, V.; Poiraudeau, S.; Roby-Brami, A.; Roren, A. Kinematic patterns in normal and degenerative shoulders. Part II: Review of 3-D scapular kinematic patterns in patients with shoulder pain, and clinical implications. Ann. Phys. Rehabil. Med. 2018, 61, 46–53. [Google Scholar] [CrossRef]
- Rockwood, C.A. The Shoulder; Elsevier Health Sciences: Philadelphia, PA, USA, 2009; Volume 1. [Google Scholar]
- Longo, U.G.; De Salvatore, S.; Sassi, M.; Carnevale, A.; De Luca, G.; Denaro, V. Motion Tracking Algorithms Based on Wearable Inertial Sensor: A Focus on Shoulder. Electronics 2022, 11, 1741. [Google Scholar] [CrossRef]

| Author and Year | Type of Study | LOE | Population | |||
|---|---|---|---|---|---|---|
| Patients | Gender | Age (Mean ± SD or Mean (Range)) | ||||
| M | F | |||||
| Bruttel et al., 2019 [17] | CS | III | HC: 11 | 4 | 7 | 69.6 ± 5.3 |
| aTSA: 16 * | 7 | 9 | 71.2 ± 5.2 | |||
| GOA: 12 * | 8 | 4 | 66.3 ± 7.5 | |||
| Bruttel et al., 2020 [23] | CS | III | HC: 11 ** | 4 | 7 | 69.6 ± 5.3 |
| aTSA: 16 ** | 7 | 9 | 71.2 ± 5.2 | |||
| Dellabiancia et al., 2017 [24] | PLS involving Human subjects | III | SAI: 12 | 8 | 4 | 26 (18–32) |
| Friesenbichler et al., 2019 [25] | CS | III | RTSA: 23 | 11 | 12 | 74 ± 7 |
| Lee et al., 2016 [19] | RCS | III | RTSA: 13 | 1 | 12 | 72 (69–79) |
| Maier et al., 2014 [26] | RCS | III | HC: 10 | 5 | 5 | 64.0 ± 7.3 |
| aTSA (GOA): 10 | 3 | 7 | 65.0 ± 4.7 | |||
| Robert-Lachaine et al., 2016 [27] | CS | III | HC: 14 | 12 | 2 | 25.2 ± 4.1 |
| RCTA: 14 | 12 | 2 | 56.4 ± 6.3 | |||
| Spranz et al., 2019 [18] | CAS | III | GOA: 21 | 13 | 8 | 64.3 ± 9.2 (46–72) |
| Ueda et al., 2021 [28] | CRS | IV | TI-SD: 24 | 24 | - | 20 ± 1 |
| TIV-SD: 27 | 27 | - | 20 ± 1 | |||
| Zaferiou et al., 2021 [29] | CS | III | RTSA: 11 | 6 | 5 | 71 ± 7 |
| Zdravkovic et al., 2020 [30] | CS | III | HC: 20 | 13 | 7 | 27 ± 3.5 |
| RCTA: 20 | 10 | 10 | 74 ± 6.2 | |||
| Author and Year | OMCs (Brand, Number of Cameras, Sampling Frequency | Marker Set (Number of Markers) | Markers Position | Tasks |
|---|---|---|---|---|
| Bruttel et al., 2019 [17] | Vicon 12 NR | Anatomical Cluster | Clavicle, forearm, humerus, scapula, thorax B, ** | Perineal care Washing axilla Combing hair Taking a book from a shelf |
| Bruttel et al., 2020 [23] | Vicon 12 120 Hz | Anatomical Cluster (4 markers for humerus and scapular clusters) | C7, T10, IJ, PX, acromion cluster, humerus cluster, digitized anatomical landmarks B, ** | E, sagittal plane E, frontal plane |
| Dellabiancia et al., 2017 [24] | Vicon 8 100 Hz | Anatomical (20) Cluster (4 markers for humerus and scapular clusters; 3 markers for thorax and scapular clusters) | C7, T8, IJ, PX, TS, AI, AA, PC, LE, ME, RS, US 4 markers cluster on humerus and forearm 3 markers cluster on scapula and thorax B, ** | AB-AD, frontal plane IR-ER, transverse plane (arm adducted, elbow 90° of flexion) |
| Friesenbichler et al.; 2019 [25] | Vicon 12 200 Hz | NR | C7, T10, IJ, PX, AI, US, RS, U, TD B | E, scapular plane |
| Lee et al., 2016 [19] | Motion Analysis Co. 6 120 Hz | Anatomical (16) | TS, AI, AA, midpoint between the most anterosuperior aspect of the acromioclavicular joint and the angle of the acromion, C7, T8, IJ, PX, LE, ME B, ** | E, sagittal plane E, scapular plane |
| Maier et al., 2014 [26] | Vicon 312 12 120 Hz | Anatomical (14) | IJ, PX, C7, T10, AC, ulna distally to the olecranon, RS, US, tuberositas deltoidea | Combing the hair Washing the opposite armpit Tying an apron Taking a book from a shelf |
| Robert-Lachaine et al., 2016 [31] | Vicon 8 60 Hz | Anatomical (35) | Pelvis (4), trunk (6), clavicle (5), scapula (9), upper arm (7), lower arm (4) B, ** | E, scapular plane |
| Spranz et al., 2019 [18] | Vicon 12 120 Hz | Anatomical (18) Cluster (4 markers for humerus and scapular clusters) | C7, T8, IJ, PX, TS, AI, AA, AC, LE, ME, SC, Acromion clusters, humerus clusters B, ** | E, sagittal plane E, frontal plane |
| Ueda et al., 2021 [28] | MAC3D system NR 240 Hz | Anatomical Cluster (4 markers for scapular clusters) | C7, T10, IJ, PX, LE, ME, acromion clusters, hand B, ** | Pitching motion |
| Zaferiou et al., 2021 [29] | Optitrack system NR 100 Hz | Anatomical Cluster (3 markers for scapular clusters) | C7, T8, IJ, PX, LE, ME, AC, acromion clusters B, ** | E, scapular plane |
| Zdravkovic et al., 2020 [30] | Vicon 8 200 Hz | Anatomical Cluster (25) | Trunk, arms, shoulders, B, ** | E, scapular plane |
| Author and Year | Aim | Degrees of Freedom | Kinematic Outcomes | Conclusions |
|---|---|---|---|---|
| Bruttel et al., 2019 [17] | To examine how aTSA improves the performance in daily activities compared with patients with GOA and healthy controls | Humerothoracic elevation Glenohumeral elevation | ROM Peak angles Kinematic time series | Total shoulder arthroplasty improves the performance of activities of daily living in patients with primary GOA but cannot restore the full ROM compared with healthy controls. |
| Bruttel et al., 2020 [23] | To confirm a higher amount of scapula lateral rotation to compensate for reduced glenohumeral elevation after aTSA and examine additional effects on the sternoclavicular and acromioclavicular joints’ kinematics | Sternoclavicular pro-/retraction Sternoclavicular elevation/depression Glenohumeral elevation/depression Acromioclavicular pro-/retraction Acromioclavicular lateral/medial rotation Acromioclavicular posterior/anterior tilting Humerothoracic elevation | cSG = AUC (SG elevation)/AUC (HT elevation) cGH = 1 − cSG SHR = (1 − cSG)/cSG | The SG relative contribution to the elevation movements in patients after aTSA is higher than in healthy controls. Kinematics of sternoclavicular and acromioclavicular joints showed significantly different patterns. |
| Dellabiancia et al., 2017 [24] | To assess the effectiveness of a novel glenohumeral joint immobilizer | Scapular pro-/retraction Scapular lateral/medial rotation Scapular posterior/anterior tilting Humeral abduction–adduction Humeral internal–external rotation | ROM Glenohumeral translation in superior–inferior direction Euclidean distance between glenohumeral centre of rotation and the geometrical centre (i.e., the trunk). | The immobilizer significantly limited joint excursion in all planes of movement except internal rotation. |
| Friesenbichler et al., 2019 [25] | To demonstrate the differences in scapulothoracic joint contribution to shoulder abduction in RTSA patients with poor-to-excellent function | Scapular lateral/medial rotation Glenohumeral abduction | ROM Peak angles SHR | Limited shoulder abduction is not associated with insufficient scapulothoracic mobility after RTSA. |
| Lee et al., 2016 [19] | To evaluate the dynamic 3D scapular motion in addition to the SHR in the RTSA and contralateral shoulders during dynamic arm motion. | Scapular lateral rotation Scapular internal rotation Scapular posterior tilting Humeral elevation | Peak angles SHR | Increased scapular lateral rotation and decreased SHR after RTSA indicate that RTSA shoulders use more scapulothoracic motion and less glenohumeral motion to elevate the arm. |
| Maier et al., 2014 [26] | To examine whether total shoulder arthroplasty is able to restore normal ROM in ADLs in patients with degenerative GOA over the course of 3 years. | Humerothoracic abduction/adduction Humerothoracic external rotation Humerothoracic flexion/extension | Maximum angles Minimum angles ROM | aTSA improves the ability to perform ADLs in patients with degenerative GOA. However, these patients do not use their maximum available abduction ROM in performing ADLs. |
| Robert-Lachaine et al., 2016 [27] | To identify the SHR patterns of compensation to reach the maximal arm elevation without pain in patients with symptomatic rotator cuff tears compared with a control healthy group. | Scapular lateral rotation Scapular internal rotation Scapular posterior tilting Humeral elevation | SHR ROM | Patients who reached at least 85° showed reduced SHR as they compensated for the loss of glenohumeral motion by increased scapulothoracic contribution. Patients who reached at least 40° showed increased SHR since they underused the scapulothoracic joint. |
| Spranz et al., 2019 [18] | To investigate the variation of the glenohumeral and scapulothoracic motion in progressive severity GOA. | Scapulothoracic elevation Humeral elevation | cST = AUC (ST elevation)/AUC (HT elevation) SHR | In the progressive severity of GOA, the contribution of the scapulothoracic joint to the total humeral elevation between 30° and 90° increased to compensate the loss of glenohumeral joint movement. |
| Ueda et al., 2021 [28] | To clarify the incidence of scapular dyskinesis types in baseball players and investigate kinematic alterations in glenohumeral joint and scapular motion during pitching in baseball players with type I scapular dyskinesis. | Scapular internal rotation Scapular lateral rotation Scapular posterior tilting Glenohumeral horizontal abduction Glenohumeral external rotation Glenohumeral abduction Humerothoracic horizontal abduction Humerothoracic external rotation Humerothoracic abduction | ROM Joint angles Peak values | Baseball players in the abnormal group showed increased glenohumeral motion and decreased scapular motion during pitching compared with the normal group. |
| Zaferiou et al., 2021 [29] | To compare SHR used before and after RTSA during the ascent phase of scapular plane arm elevation tasks performed with varied shoulder rotation. | Scapular pro-/retraction Scapular lateral/medial rotation Scapular posterior/anterior tilting Humeral elevation | SHR Joint angular displacements | This study showed significant differences in scapulohumeral coordination before vs. after RTSA aligned with the hypothesis of increased SHR post-RTSA. |
| Zdravkovic et al., 2020 [30] | To evaluate the SHR variations in adults with and without RCA during arm elevation | Scapular pro-/retraction Scapular lateral/medial rotation Scapular posterior/anterior tilting Humeral elevation Humeral flexion | SHR Peak values | Patients with RCA exhibited more scapulothoracic motion during arm elevation than the control group. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Longo, U.G.; De Salvatore, S.; Carnevale, A.; Tecce, S.M.; Bandini, B.; Lalli, A.; Schena, E.; Denaro, V. Optical Motion Capture Systems for 3D Kinematic Analysis in Patients with Shoulder Disorders. Int. J. Environ. Res. Public Health 2022, 19, 12033. https://doi.org/10.3390/ijerph191912033
Longo UG, De Salvatore S, Carnevale A, Tecce SM, Bandini B, Lalli A, Schena E, Denaro V. Optical Motion Capture Systems for 3D Kinematic Analysis in Patients with Shoulder Disorders. International Journal of Environmental Research and Public Health. 2022; 19(19):12033. https://doi.org/10.3390/ijerph191912033
Chicago/Turabian StyleLongo, Umile Giuseppe, Sergio De Salvatore, Arianna Carnevale, Salvatore Maria Tecce, Benedetta Bandini, Alberto Lalli, Emiliano Schena, and Vincenzo Denaro. 2022. "Optical Motion Capture Systems for 3D Kinematic Analysis in Patients with Shoulder Disorders" International Journal of Environmental Research and Public Health 19, no. 19: 12033. https://doi.org/10.3390/ijerph191912033






