Role of Atmospheric Temperature and Seismic Activity in Spring Water Hydrogeochemistry in Urumqi, China
Abstract
:1. Introduction
2. Geological and Hydrogeological Settings
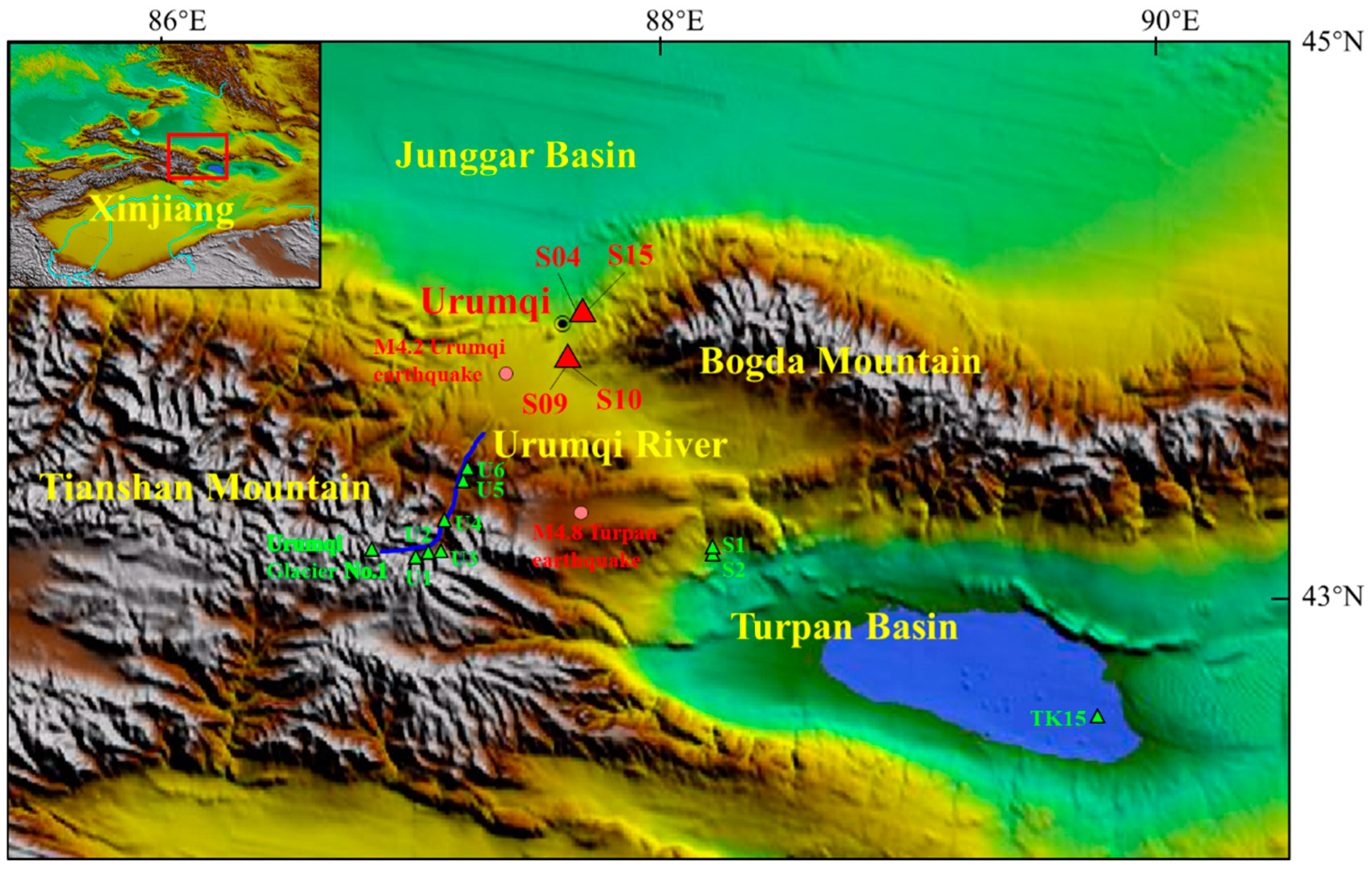
3. Samples and Methods
4. Results
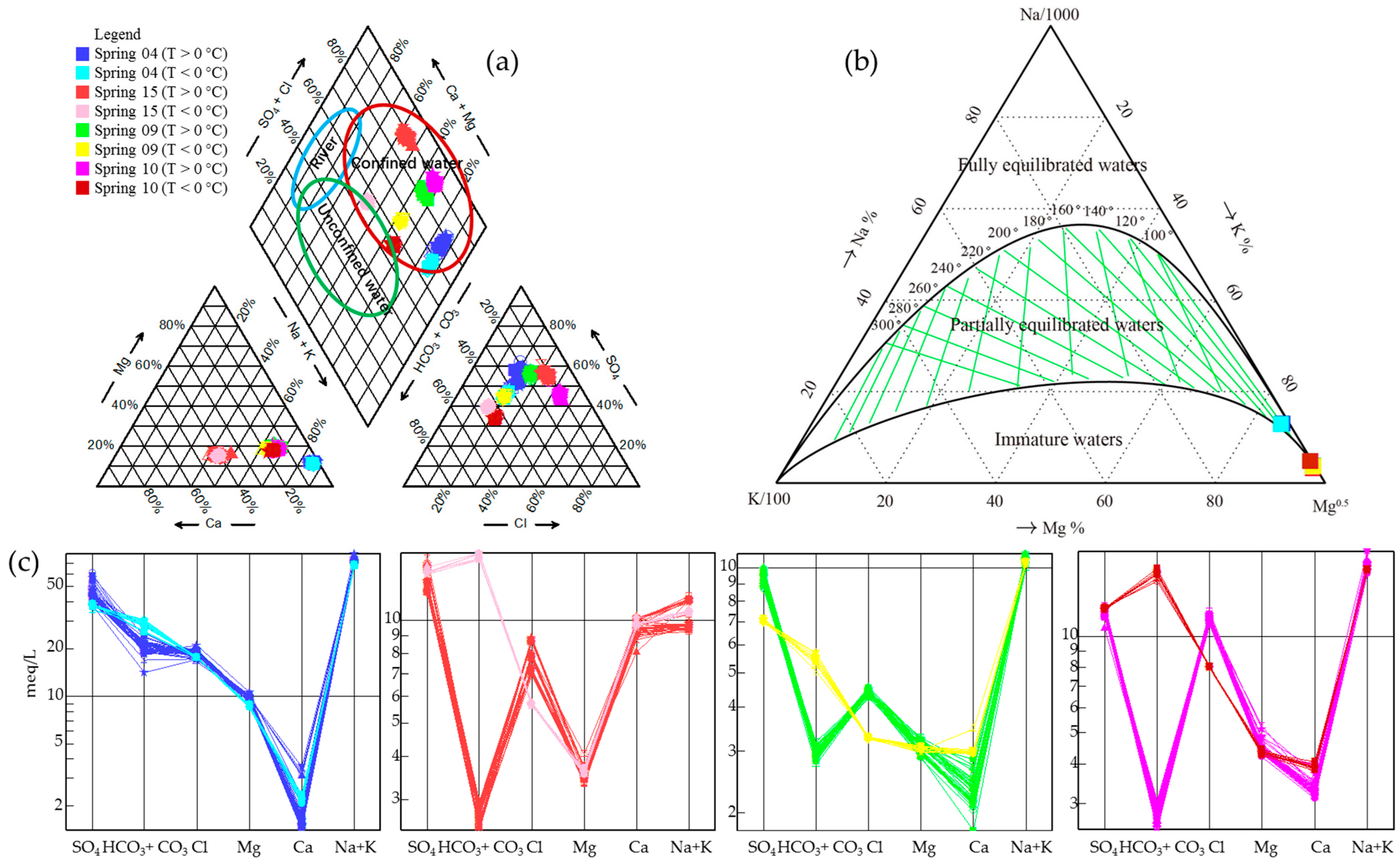
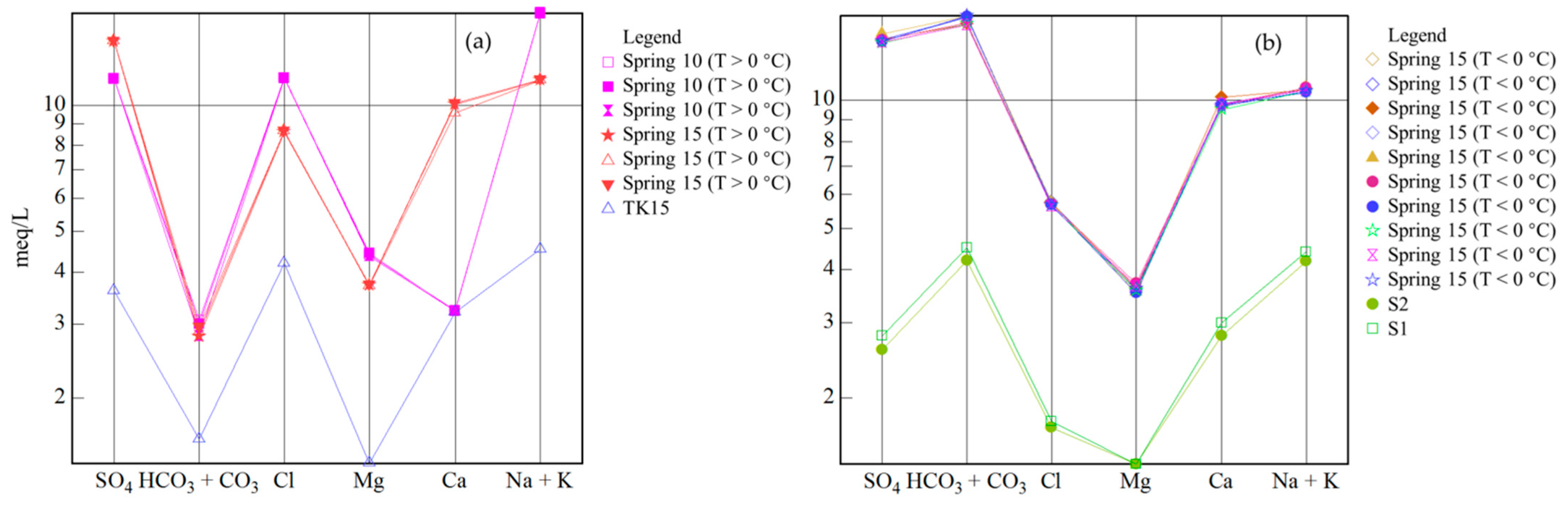
4.1. Hydrochemical Characteristics
4.2. Geothermometry and Circulation Depth
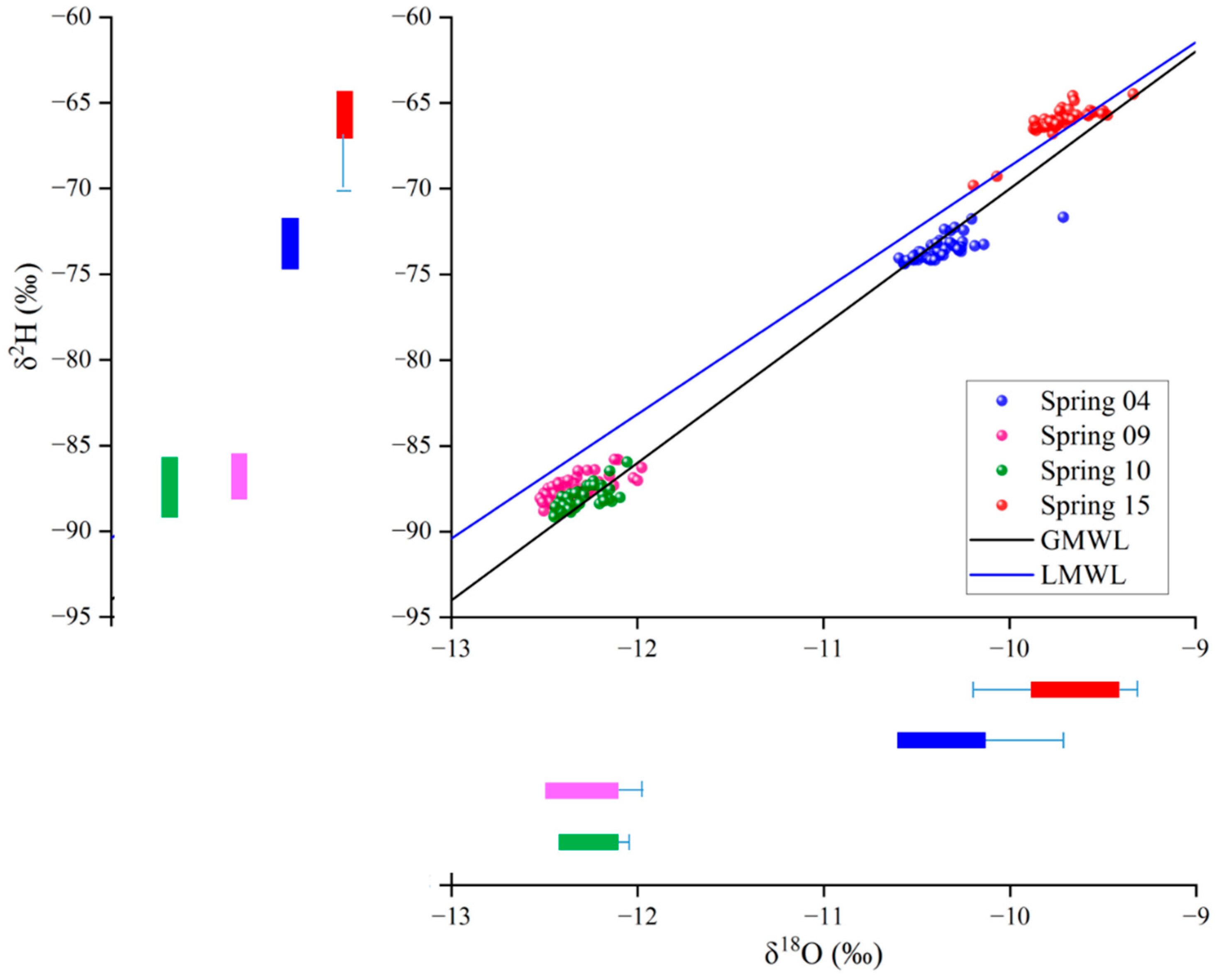
4.3. δ2H and δ18O Characteristics
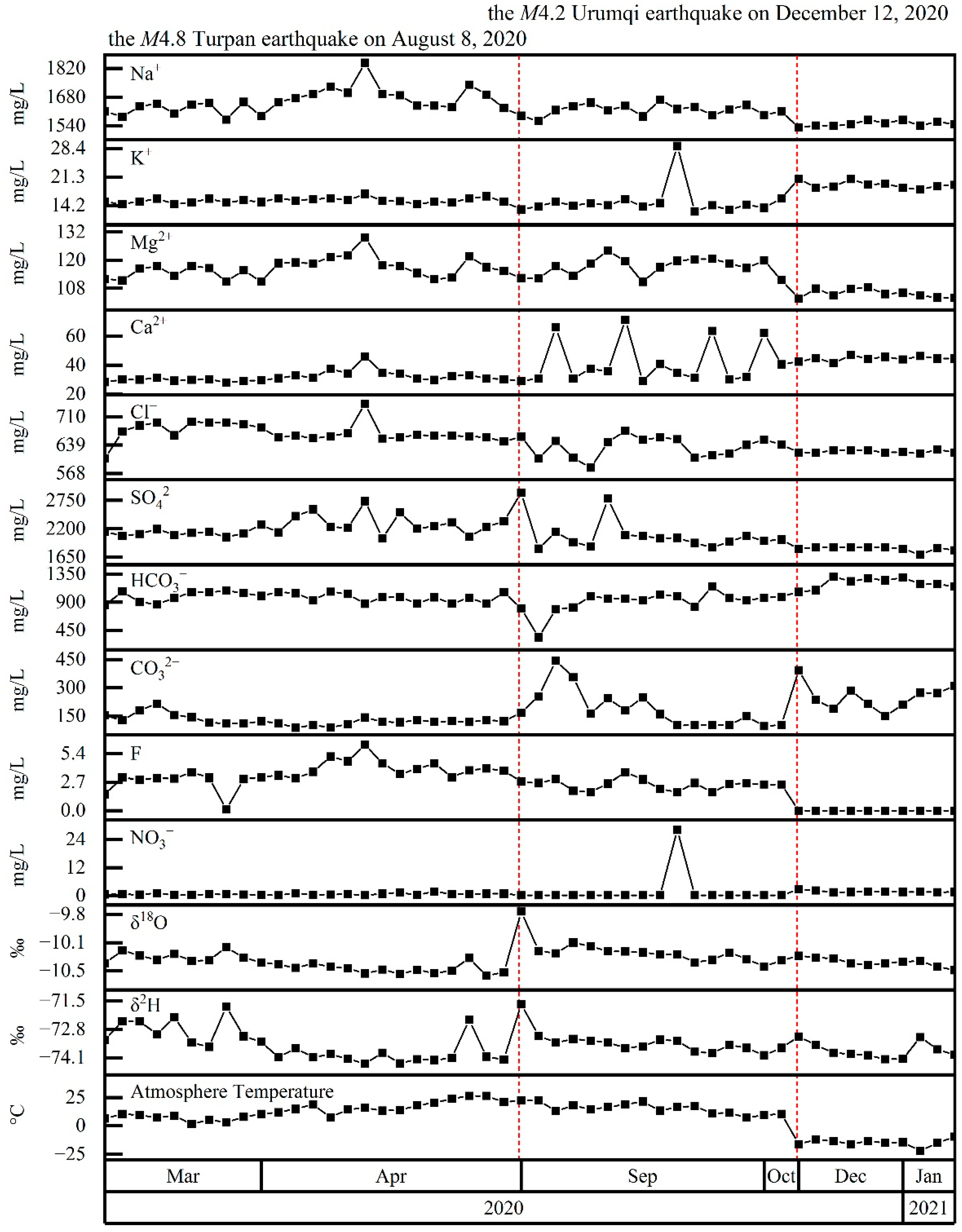
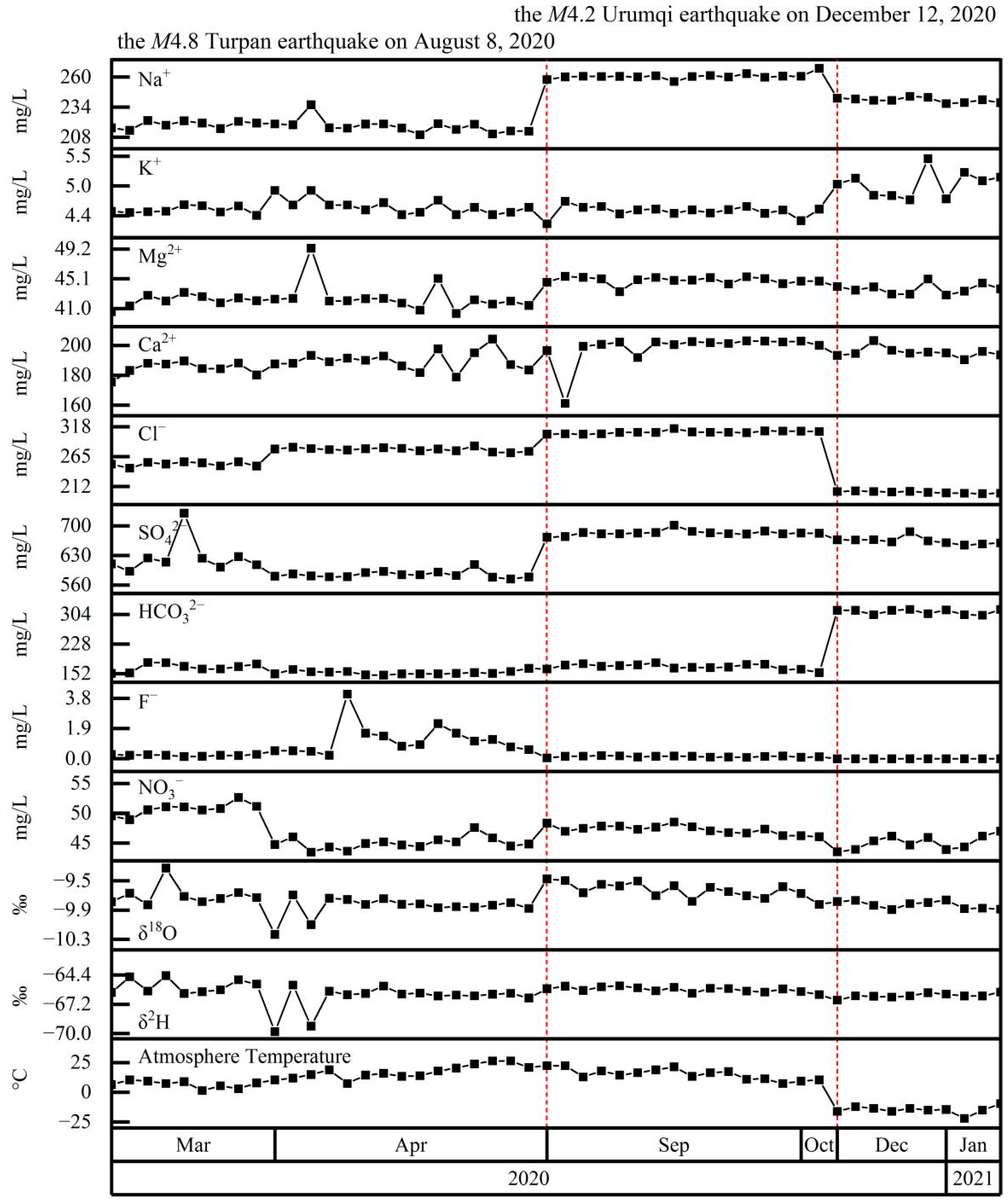
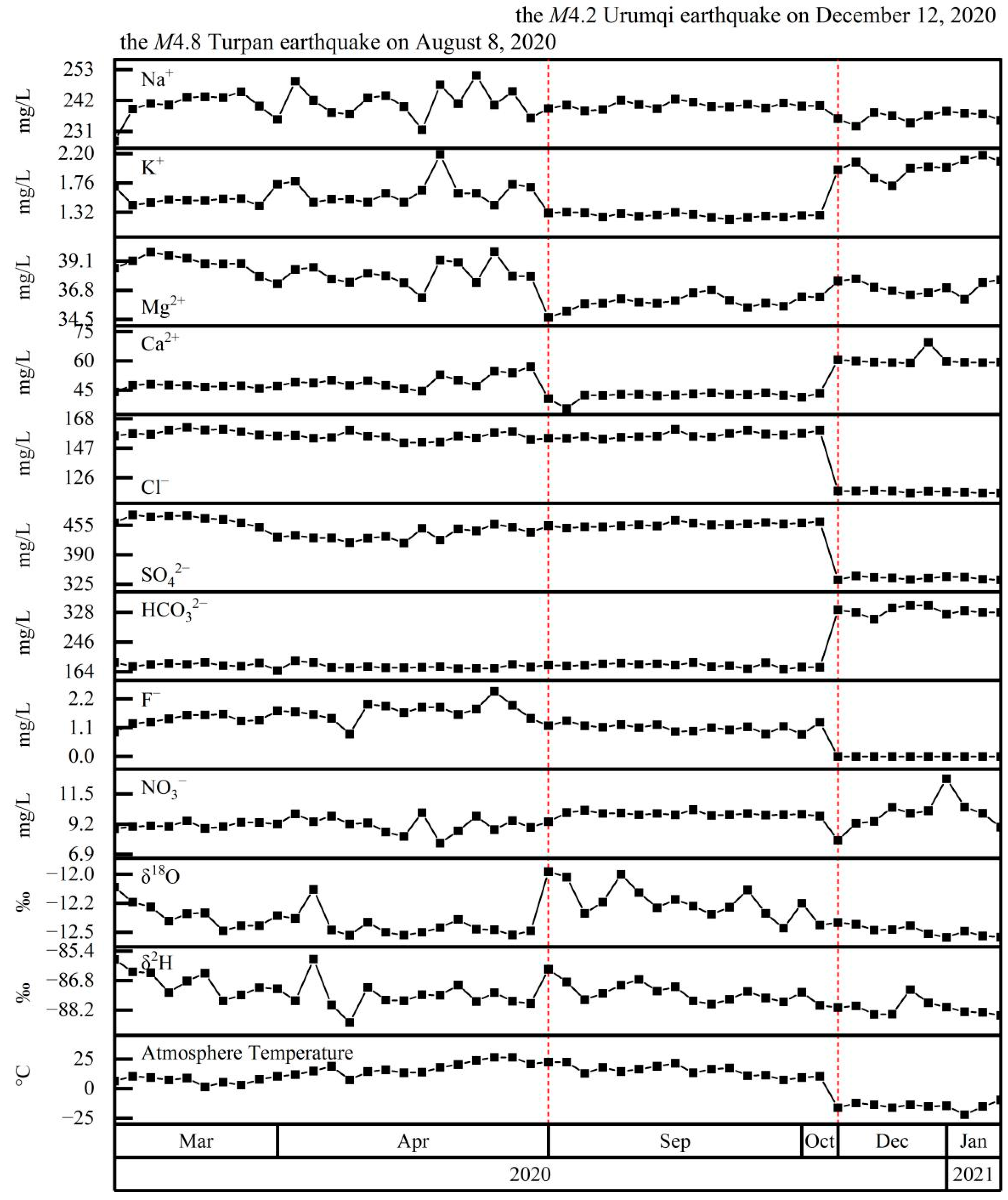
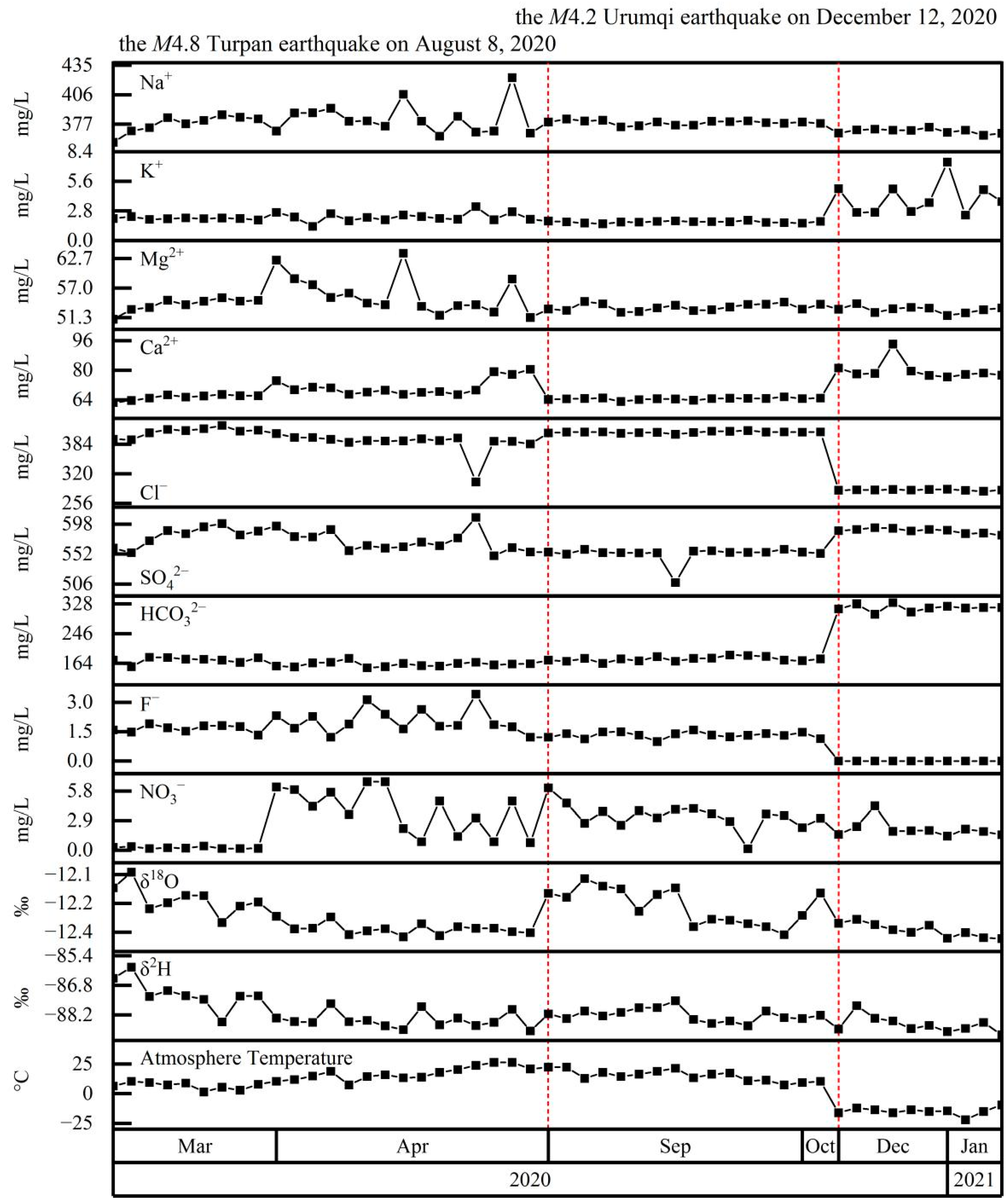
4.4. Coupling between Hydrochemistry and Earthquakes
5. Discussion
5.1. Groundwater Origin, Recharge Sources, and Circulation Characteristics
5.2. Hydrochemical Changes Coupled to Earthquakes
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, L.W.; Yin, X.X.; Liu, X. Tracing of recharge sources of deep aquifers in the concealed type colliery of North China by hydrochemistry and isotopes. Sci. Geogr. Sinica 2013, 33, 755–762. [Google Scholar]
- Clark, I.D.; Fritz, P. Environmental Isotopes in Hydrogeology; Lewis: Boca Raton, FL, USA, 1997. [Google Scholar]
- Huang, P.H.; Chen, J.S. Recharge sources and hydrogeochemical evolution of groundwater in the coal-mining district of Jiaozuo, China. Hydrogeol. J. 2012, 20, 739–754. [Google Scholar] [CrossRef]
- Huang, X.; Wang, G.; Liang, X.; Cui, K.; Ma, L.; Xu, Q. Hydrochemical and Stable Isotope (δD and δ18O) Characteristics of Groundwater and Hydrogeochemical Processes in the Ningtiaota Coalfield, Northwest China. Mine Water Environ. 2018, 37, 119–136. [Google Scholar] [CrossRef]
- Kendall, C. Tracing nitrogen source and cycling in catchments. In Isotope Tracers in Catchment Hydrology; Kendall, C., McDonnell, J.J., Eds.; Elsevier Science B.V.: Amsterdam, The Netherland, 1998; pp. 519–576. [Google Scholar]
- Cook, P.; Herczeg, A.L. Environmental Tracers in Subsurface Hydrology; Kluwer Academic Publishers: New York, NY, USA, 2000. [Google Scholar]
- Hosono, T.; Yamanaka, C. Origins and pathways of deeply derived carbon and fluids observed in hot spring waters from non-active volcanic fields, western Kumamoto, Japan. Earth Planets Space 2021, 73, 115. [Google Scholar] [CrossRef]
- Kendall, C.; McDonnell, J.J. Isotope Tracers in Catchment Hydrology; Elsevier: Amsterdam, The Netherland, 1998. [Google Scholar]
- Wang, P.; Yu, J.J.; Zhang, Y.C.; Liu, C.M. Groundwater recharge and hydrogeochemical evolution in the Ejina basin, northwest China. J. Hydrol. 2013, 476, 72–86. [Google Scholar] [CrossRef]
- Song, S.; Ku, W.Y.; Chen, Y.L.; Liu, C.M.; Chen, H.F.; Chan, P.S.; Chen, Y.G.; Yang, T.F.; Chen, C.H.; Liu, T.K.; et al. Hydrogeochemical anomalies in the springs of the Chiayi area in west-central Taiwan as possible precursors to earthquakes. Pure Appl. Geophys. 2006, 163, 675–691. [Google Scholar] [CrossRef]
- Ingebritsen, S.; Manga, M. Earthquakes: Hydrogeochemical precursors. Nat. Geosci. 2014, 7, 697–698. [Google Scholar] [CrossRef]
- André, L.; Franceschi, M.; Pouchan, P.; Atteia, O. Using geochemical data and modelling to enhance the understanding of groundwater flow in a regional deep aquifer, Aquitaine Basin, southwest of France. J. Hydrol. 2005, 305, 40–46. [Google Scholar] [CrossRef]
- Gastmans, D.; Chang, H.K.; Hutcheon, I. Groundwater geochemical evolution in the northern portion of the Guarani Aquifer System (Brazil) and its relationship to diagenetic features. Appl. Geochem. 2010, 25, 16–33. [Google Scholar] [CrossRef]
- Moral, F.; Cruz-Sanjulian, J.J.; Olias, M. Geochemical evolution of groundwater in the carbonate aquifers of Sierra de Segura (Betic Cordillera, southern Spain). J. Hydrol. 2008, 360, 281–296. [Google Scholar] [CrossRef]
- Sun, F.Q.; Hou, G.C.; Dou, Y.; Fang, C.S.; Jiang, J.; Zhang, L.Z. Hydrogeochemistry evidence of groundwater circulation features in Ordos Cretaceous basin—A case study in Chabu well field. J. Jilin U Earth Sci. 2009, 39, 269–275+293. (in Chinese). [Google Scholar]
- Wang, Y.X.; Guo, Q.H.; Su, C.L.; Ma, T. Strontium isotope characterization and major ion geochemistry of karst water flow, Shentou, northern China. J. Hydrol. 2006, 328, 592–603. [Google Scholar] [CrossRef]
- Chiodini, G.; Frondini, F.; Cardellini, C.; Parello, F.; Peruzzi, L. Rate of diffuse carbon dioxide Earth degassing estimated from carbon balance of regional aquifers: The case of central Apennine, Italy. J. Geophys. Res. Solid Earth 2000, 105, 8423–8434. [Google Scholar] [CrossRef]
- Yamada, M.; Ohsawa, S.; Kazahaya, K.; Yasuhara, M.; Takahashi, H.; Amita, K.; Mawatari, H.; Yoshikawa, S. Mixing of magmatic CO2 into volcano groundwater flow at Aso volcano assessed combining carbon and water stable isotopes. J. Geochem. Explor. 2011, 108, 81–87. [Google Scholar] [CrossRef]
- Rive, K.; Gaillardet, J.; Agrinier, P.; Rad, S. Carbon isotopes in the rivers from the Lesser Antilles: Origin of the carbonic acid consumed by weathering reactions in the Lesser Antilles. Earth Surf. Proc. Land 2013, 38, 1020–1035. [Google Scholar] [CrossRef]
- Han, G.; Yang, K.; Zeng, J.; Zhao, Y. Dissolved iron and isotopic geochemical characteristics in a typical tropical river across the floodplain: The potential environmental implication. Environ. Res. 2021, 200, 111452. [Google Scholar] [CrossRef]
- Xie, T.; Ye, Q.; Lu, J. Electrical resistivity of three phase cracked rock soil medium and its anisotropic changes caused by crack changes. Geomat. Nat. Hazards Risk 2020, 11, 1599–1618. [Google Scholar] [CrossRef]
- Zhang, W.; Kang, S.; Shen, Y.; He, J.; Chen, A. Response of snow hydrological processes to a changing climate during 1961 to 2016 in the headwater of Irtysh River Basin, Chinese Altai Mountains. J. Mt. Sci 2017, 14, 2295–2310. [Google Scholar] [CrossRef]
- Li, J.; Pang, Z. The elevation gradient of stable isotopes in precipitation in the eastern margin of Tibetan Plateau. Sci. China Earth Sci. 2022, 65, 1–13. [Google Scholar] [CrossRef]
- Tsunogai, U.; Wakita, H. Precursory chemical changes in ground water: Kobe earthquake, Japan. Science 1995, 269, 61–63. [Google Scholar] [CrossRef]
- Wang, C.-Y.; Manga, M. Hydrologic responses to earthquakes and a general metric. Geofluids 2010, 110, 206–216. [Google Scholar] [CrossRef]
- Skelton, A.; Liljedahl-Claesson, L.; Wästeby, N.; Andrén, M.; Stockmann, G.; Sturkell, E.; Mörth, C.-M.; Stefansson, A.; Tollefsen, E.; Siegmund, H.; et al. Hydrochemical Changes Before and After Earthquakes Based on Long- Term Measurements of Multiple Parameters at Two Sites in Northern Iceland—A Review. J. Geophys. Res. Solid Earth 2019, 124, 2702–2720. [Google Scholar] [CrossRef]
- Han, G.; Zeng, J. Iron isotope of suspended particulate matter in Zhujiang River, southwest China: Characteristics, sources, and environmental implications. Sci. Total Environ. 2021, 793, 148562. [Google Scholar] [CrossRef] [PubMed]
- Chiodini, G.; Caliro, S.; Cardellini, C.; Frondini, F.; Inguaggiato, S.; Matteucci, F. Geochemical evidence for and characterization of CO2 rich gas sources in the epicentral area of the Abruzzo 2009 earthquakes. Earth Planet. Sci. Lett. 2011, 304, 389–398. [Google Scholar] [CrossRef]
- Favara, R.; Grassa, F.; Inguaggiato, S.; Valenza, M. Hydrogeochemistry and stable isotopes of thermal springs: Earthquake-related chemical changes along Belice Fault (Western Sicily). Appl. Geochem. 2001, 16, 1–17. [Google Scholar] [CrossRef]
- Gou, X.; Yang, Y. Hydrogeochemical evaluation of urban groundwater in Urumqi. Xinjiang. Geology 2001, 19, 207–213. (In Chinese) [Google Scholar]
- Yang, Y.; Zhang, J.; Zhang, J.; Gao, Y.; Zhou, X.; Sun, X.; Wen, L.; Miao, M. Sedimentary characteristics and main controlling factors of the Middle-Upper Permian and Middle-Upper Triassic in the Bogda Mountain area of Xinjiang, NW China. Pet. Explor. Dev. 2022, 49, 770–784. [Google Scholar] [CrossRef]
- Qi, R.; Song, W.; Shen, R. Hydrogeological conditions and groundwater quality evaluation of Piedmont gravel plain in Urumqi. Ground Water 2017, 39, 143–145. (In Chinese) [Google Scholar]
- Xiang, Y.; Sun, X.; Gao, X. Different coseismic groundwater level changes in two adjacent wells in a fault-intersected aquifer system. J. Hydrol. 2019, 578, 124123. [Google Scholar] [CrossRef]
- Gao, X.; Li, X.; Xu, Q.; Li, Y.; Zhang, X.; Cui, Y. Characteristics of earthquake precursory for dissolved CH4 in ground water at the No.10 spring, Urumqi. South China J. Seismol. 2001, 21, 14–18, (In Chinese with English Abstract). [Google Scholar] [CrossRef]
- Chen, L.; Wang, G.; Hu, F.; Wang, Y.; Liu, L. Groundwater hydrochemistry and isotope geochemistry in the Turpan Basin, northwestern China. J. Arid Land 2014, 6, 378–388. [Google Scholar] [CrossRef]
- Zhong, J.; Chen, S.; Wang, W.; Yan, Z.; Ellam, R.M.; Li, S. Unravelling the hydrological effects on patiotemporal variability of water chemistry in mountainous rivers from Southwest China. Hydrol. Process. 2020, 34, 5595–5605. [Google Scholar] [CrossRef]
- Chen, S.; Zhong, J.; Li, C.; Wang, W.-F.; Xu, S.; Yan, Z.-L.; Li, S.L. The chemical weathering characteristics of different lithologic mixed small watersheds in Southwest China. Chin. J. Ecol. 2020, 39, 1288–1299, (In Chinese with English Abstract). [Google Scholar]
- Fournier, R.O.; Truesdell, A.H. Empirical Na-K-Ca geothermometer for natural waters. Geochim. Cosmochim. Acta 1973, 37, 1255–1275. [Google Scholar] [CrossRef]
- Jin, J.; Liu, M.; Liu, Y.; Wang, J.; Gao, C.; Luo, Z.; Lu, F.; Ren, Y. Present-day temperature-pressure field and its controlling factors of the lower composite reservoir in the southern margin of Junggar Basin. Chin. J. Geol. 2021, 56, 28–43. [Google Scholar] [CrossRef]
- Ndikubwimana, I.; Mao, X.; Zhu, D.; He, Y.; Shi, Z. Geothermal evolution of deep parent fluid in Western Guangdong, China: Evidence from water chemistry, stable isotopes and geothermometry. Hydrogeol. J. 2020, 28, 2947–2961. [Google Scholar] [CrossRef]
- Li, W.; Jiao, Y.; Zuo, Y.; Song, X.; Qiu, N. Effect of deposition rate on geothermal field in Bozhong Depression, Bohai Bay Basin. Chin. J. Geophys. 2014, 57, 1568–1577. [Google Scholar]
- Craig, H. Isotopic variations in meteoric waters. Science 1961, 133, 1702–1703. [Google Scholar] [CrossRef] [PubMed]
- Feng, F.; Li, Z.; Zhang, M.; Jin, S.; Dong, Z. Deuterium and oxygen 18 in precipitation and atmospheric moisture in the upper Urumqi River Basin, eastern Tianshan Mountains. Environ. Earth Sci. 2013, 68, 1199–1209. [Google Scholar] [CrossRef]
- Dobrovolsky, I.P.; Zubkov, S.I.; Miachkin, V.I. Estimation of the size of earthquake preparation zones. Pure Appl. Geophys. 1979, 117, 1025–1044. [Google Scholar] [CrossRef]
- Li, X.; Liu, X.; Du, J.; Cui, Y.; Sun, F. Hydrogeochemical Characteristics of the Haiyuan-Liupanshan Seismic Belt at the Northeastern Edge of the Tibet Plateau. Geofluids 2022, 2022, 6894229. [Google Scholar] [CrossRef]
- Taylor, H.P., Jr. Water/rock interactions and the origin of H2O in granitic batholiths: Thirtieth William Smith lecture. J. Geol. Soc. 1977, 133, 509–558. [Google Scholar] [CrossRef]
- Sun, C.J.; Li, W.H.; Chen, Y.N.; Li, X.G.; Yang, Y.H. Isotopic and hydrochemical composition of runoff in the Urumqi River, Tianshan Mountains, China. Environ. Earth Sci. 2015, 74, 1521–1537. [Google Scholar] [CrossRef]
- Cai, C.F.; Mei, B.; Li, W.; Zeng, F.G. Water-Rock Interaction in Tarim Basin: Constraints from Oilfieid Water Geochemistry. Chin. J. Geochem. 1997, 16, 289–303. [Google Scholar] [CrossRef]
- Garrels, R.M.; Mackenzie, F.T. Origin of the chemical compositions of some springs and lakes. In Equilibrium Concepts in Natural Water Systems; Gould, R.F., Ed.; American Chemical Society: Washington, DC, USA, 1967; Volume 67, pp. 222–242. [Google Scholar]
- Lei, X.; Wang, G. Is clustered seismicity an indicator of regional stress? Insights from earthquake sequences in Yongning-Luguhu faulted basin, Southwest China. Earthq. Res. Adv. 2022, 2022, 100138. [Google Scholar] [CrossRef]
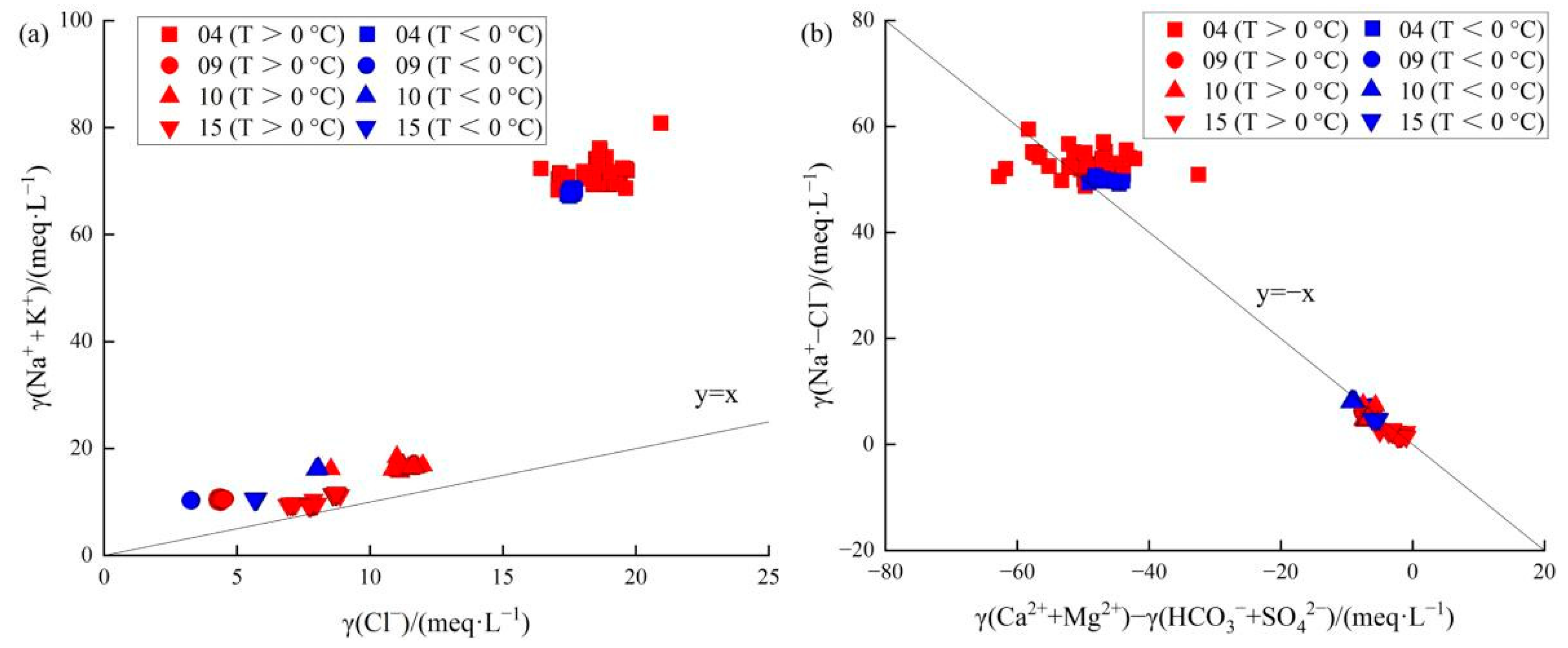
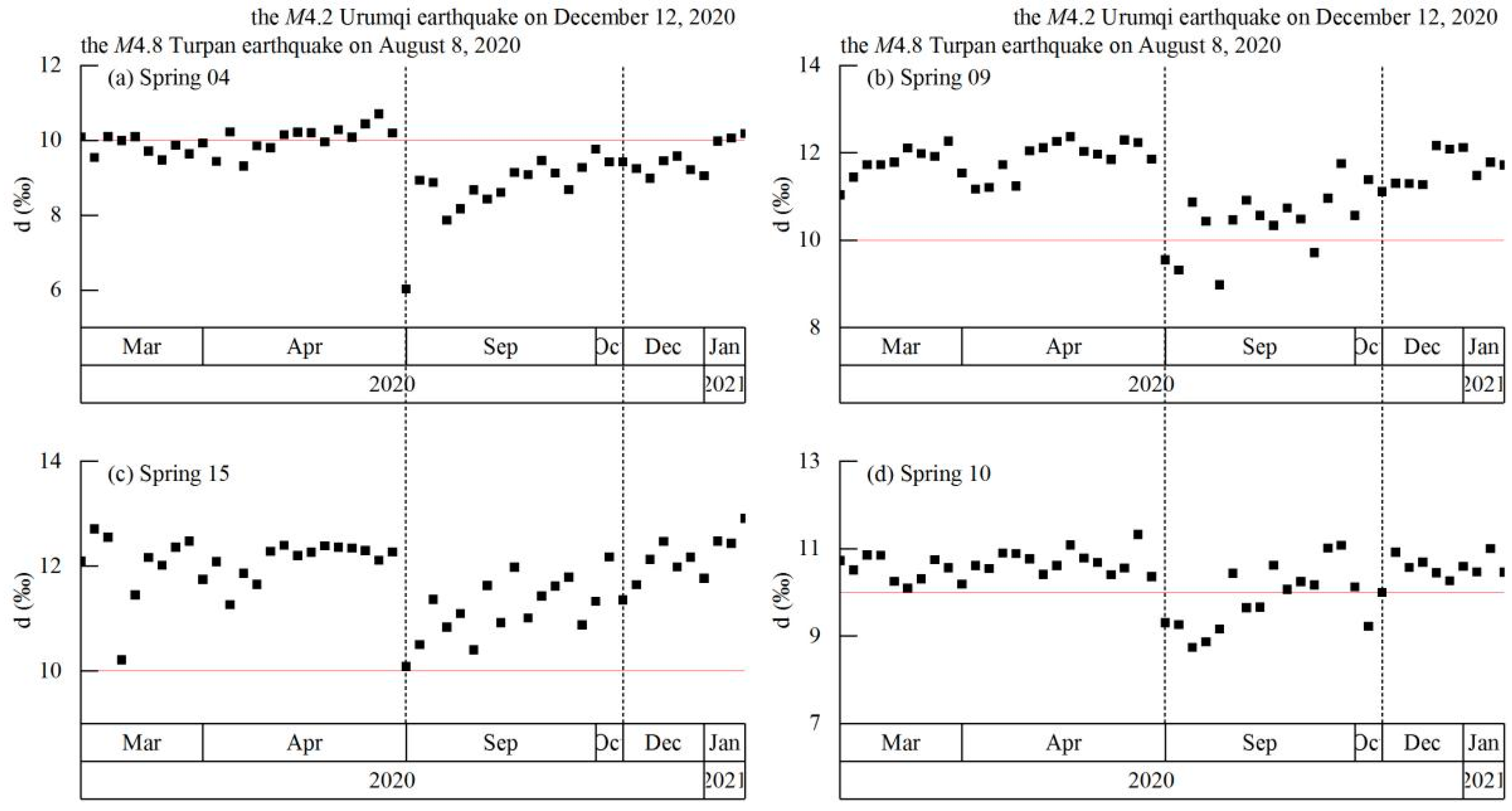
| Hydrochemical Type | Average Altitude of Recharge Water (km) | Temperature (Na-K-Ca Geothermometer; °C) | Average Circulation Depth (km) | |
|---|---|---|---|---|
| Spring 04 (T > 0 °C) | SO4–Na | 3.99 | 103.4 | 4.71 |
| Spring 04 (T < 0 °C) | SO4–Na SO4·HCO3–Na | 4.00 | 112.4 | 5.21 |
| Spring 15 (T > 0 °C) | SO4·Cl–Na·Ca SO4·Cl–Ca·Na | 3.70 | 38.04 | 1.12 |
| Spring 15 (T < 0 °C) | SO4–Na·Ca | 3.71 | 40.43 | 1.25 |
| Spring 09 (T > 0 °C) | SO4·Cl–Na | 4.52 | 33.67 | 0.88 |
| Spring 09 (T < 0 °C) | SO4·HCO3–Na | 4.55 | 36.83 | 1.05 |
| Spring 10 (T > 0 °C) | SO4·Cl–Na Cl·SO4–Na | 4.55 | 39.06 | 1.18 |
| Spring 10 (T < 0 °C) | SO4·Cl–Na | 4.57 | 52.93 | 1.94 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, Z.; Zhong, J. Role of Atmospheric Temperature and Seismic Activity in Spring Water Hydrogeochemistry in Urumqi, China. Int. J. Environ. Res. Public Health 2022, 19, 12004. https://doi.org/10.3390/ijerph191912004
Zhou Z, Zhong J. Role of Atmospheric Temperature and Seismic Activity in Spring Water Hydrogeochemistry in Urumqi, China. International Journal of Environmental Research and Public Health. 2022; 19(19):12004. https://doi.org/10.3390/ijerph191912004
Chicago/Turabian StyleZhou, Zhihua, and Jun Zhong. 2022. "Role of Atmospheric Temperature and Seismic Activity in Spring Water Hydrogeochemistry in Urumqi, China" International Journal of Environmental Research and Public Health 19, no. 19: 12004. https://doi.org/10.3390/ijerph191912004







