Capturing Household Structure and Mobility within and between Remote Aboriginal Communities in Northern Australia Using Longitudinal Data: A Pilot Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area and Study Design
2.1.1. Study Participants
2.1.2. Study Procedures
- Dwelling contact—anyone who slept in the same dwelling as the participant the previous night.
- Social contact—anyone with whom the participant spent an hour or more during the previous 24 h, excluding dwelling contacts.
2.2. Data Analysis
2.2.1. Clustering Responses by Dwelling Location
2.2.2. Dwelling Contact Matrices
2.2.3. Intra-Community Mixing
2.2.4. Temporary Mobility
2.2.5. Individual Based Model Simulation
3. Results
3.1. Summary Statistics
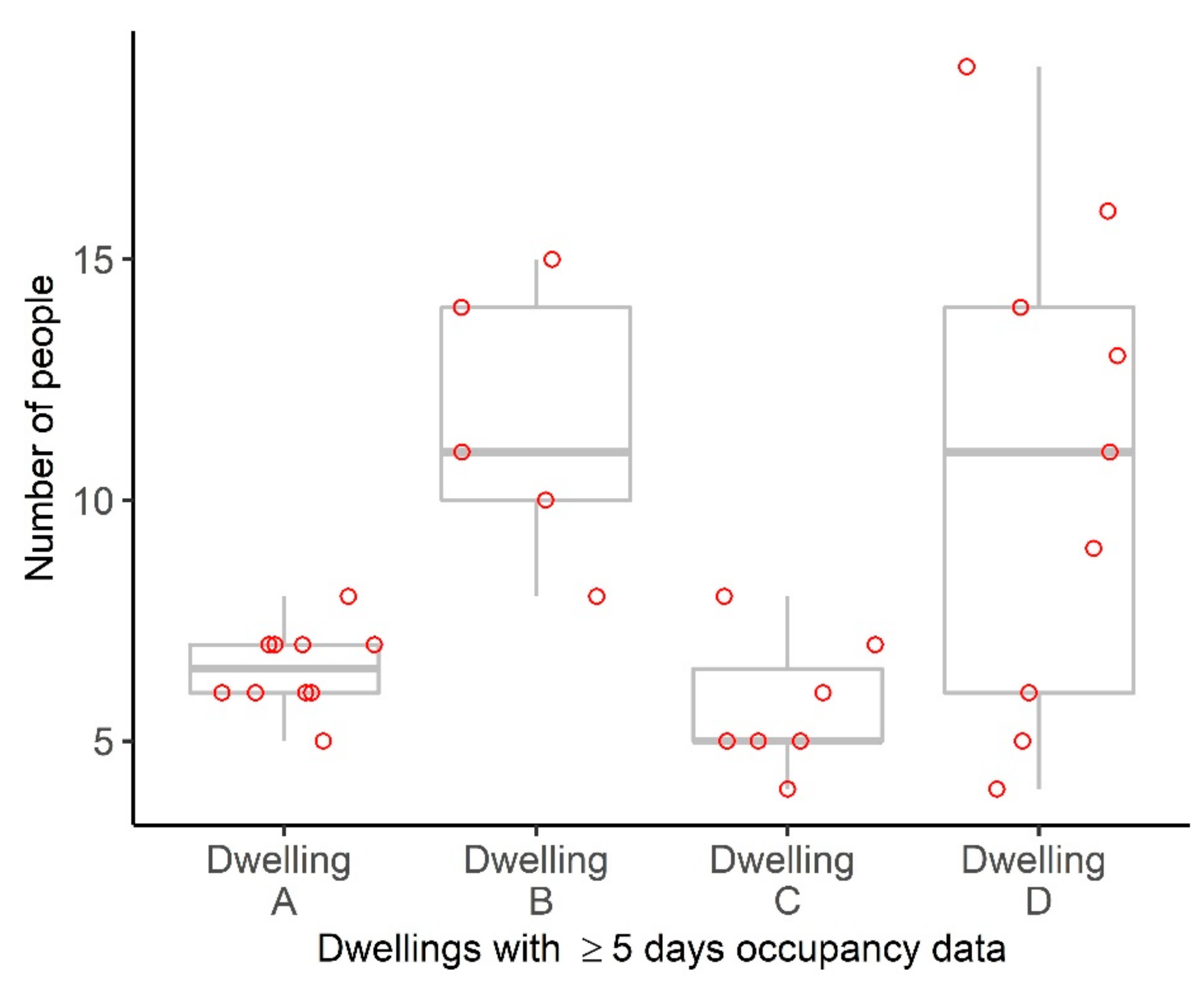
3.2. Household Structures and Size
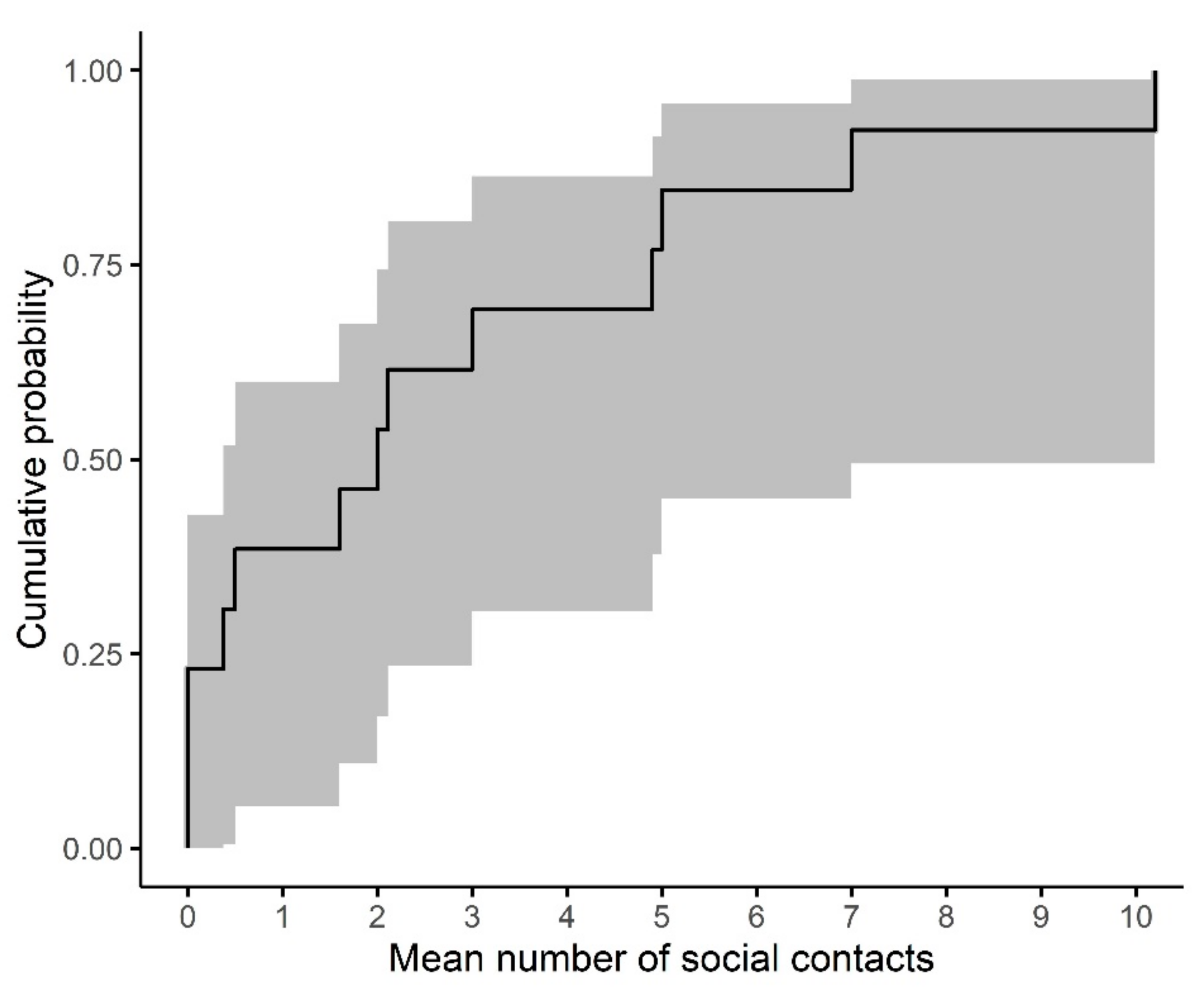
3.3. Dwelling Contacts
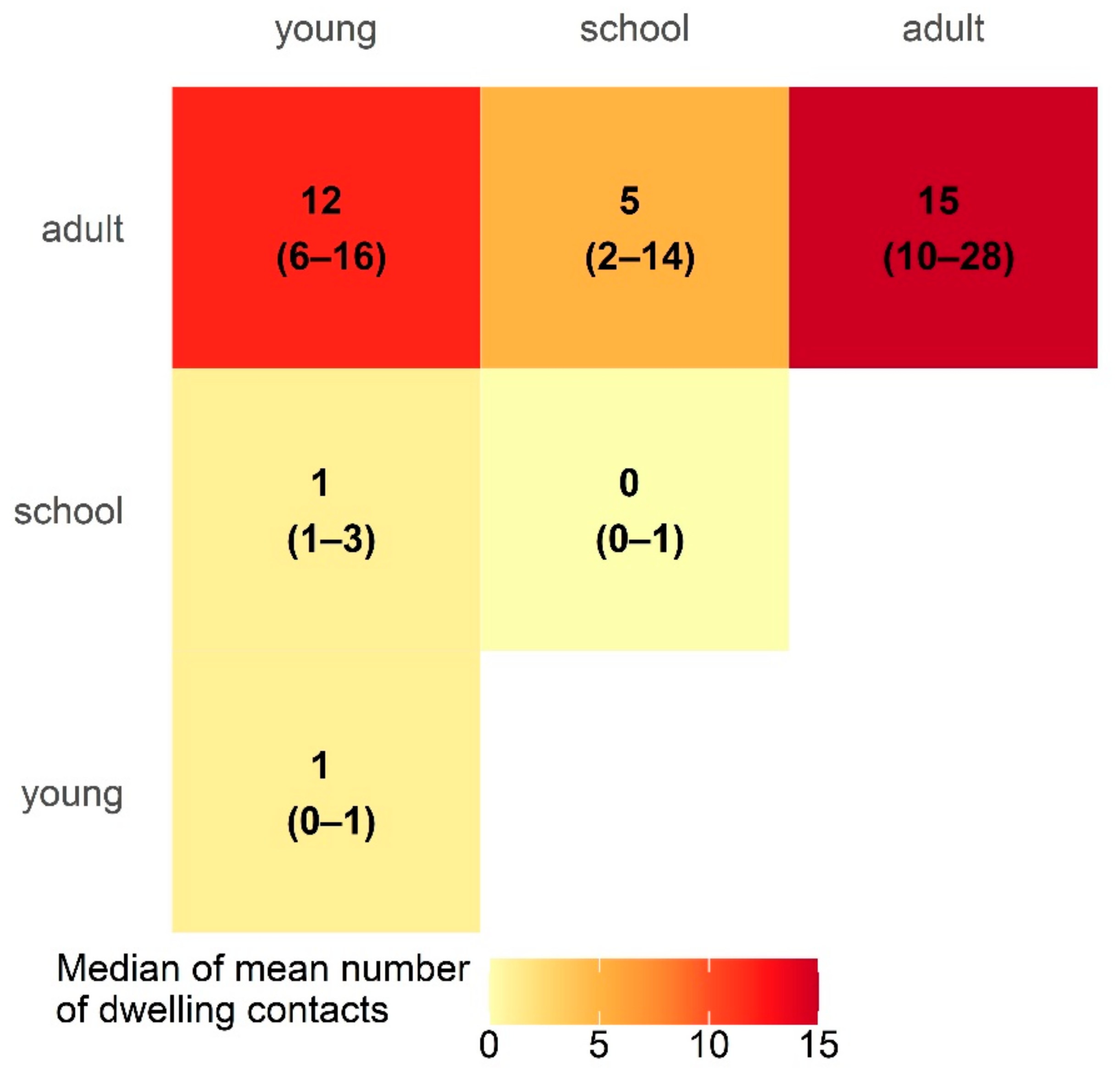
3.4. Intra-Community Mixing
3.5. Temporary Mobility
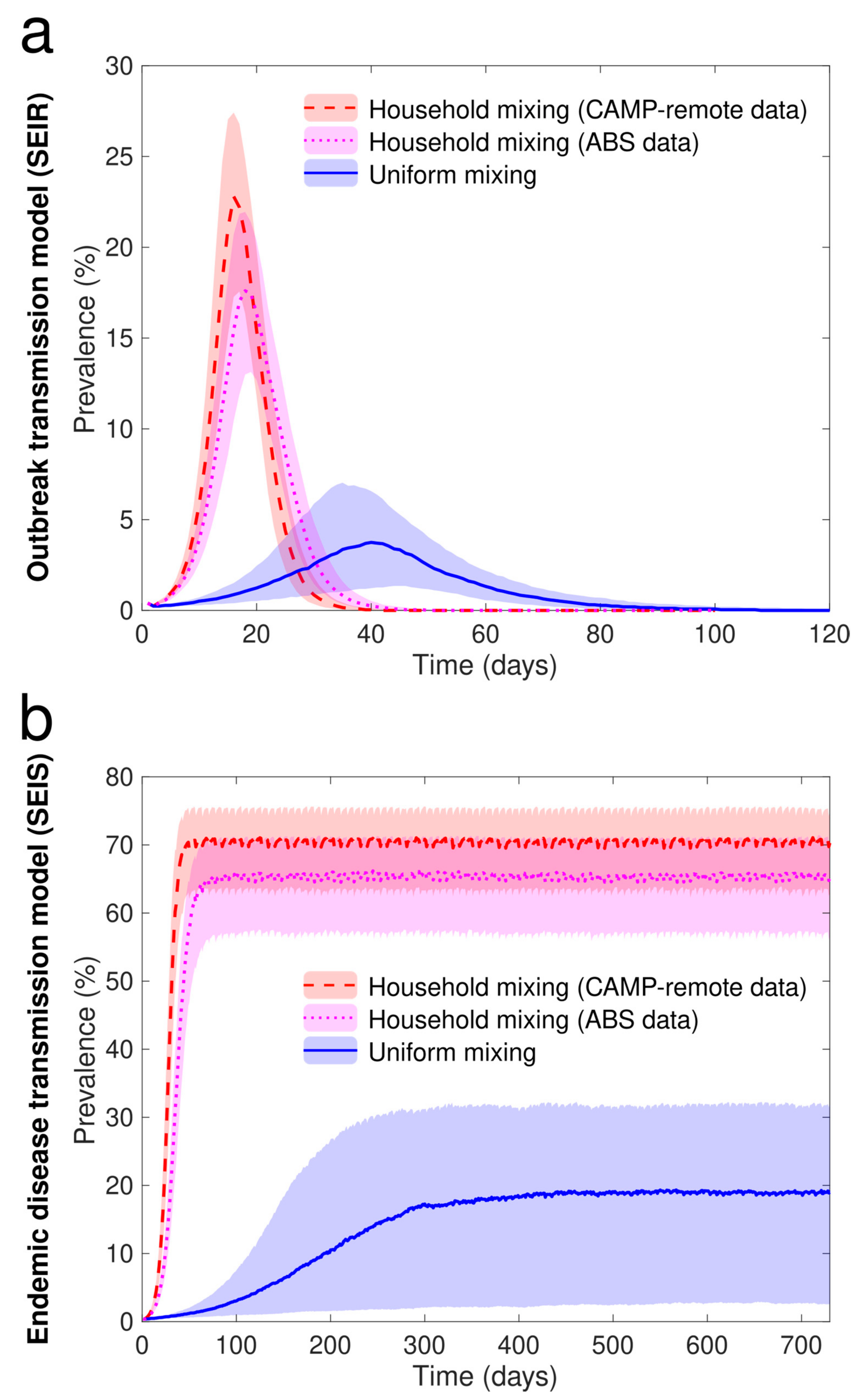
3.6. Individual Based Model Simulation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
Appendix A.1. Background Survey (First Interview)
- 1.
- What is your name?
- a.
- Balanda name [text]
- b.
- Yolngu first name [text]
- c.
- Yolngu last name [text]
- 2.
- What is your date of birth? [text]_______________ (enter age if DOB unknown)
- 3.
- How many days each week do you work in a paid job (on average)?
0 1 2 3 4 5 6 7 - 4.
- How many children do you care for most of the time? {we will refer to these as your children and the youngest as your baby}
1 2 3 4 5 6 more than 6 [text] - 5.
- Please tell us about each of your children [start with baby and repeat for each child]
- a.
- Balanda name [text]
- b.
- Yolngu first name [text]
- c.
- Yolngu last name [text]
- d.
- Date of birth [text]___________ (enter age if unknown DOB)
- e.
- are you {child’s name} mother?
- ◦
- No
- ◦
- Yes
- f.
- How many days each week does {child’s name} attend (on average):
- School:
0 1 2 3 4 5 6 7 - Child care:
0 1 2 3 4 5 6 7 - Baby-hub:
0 1 2 3 4 5 6 7
Appendix A.2. Household, Contact and Travel Survey (Repeated Monthly)
- In the house you slept in last night, how many rooms do people sleep in (including the verandah)?
- How many people slept in each room of this house last night, including you and your children? [icons on house graphic]
- -
- young child, school aged, adult [usual residents of this house]
- -
- young child, school aged, adult [visitors to this house]
- How many of the ‘x’ visitors who slept in your house last night usually live outside the study community? 0, 1, 2……… ‘x’
- Last night, did you sleep on the same mattress or used the same bedding as:
- {baby}? Yes/No
- {child 2}? Yes/No
- {child 3}? Yes/No
- 5.
- Is this the house that you live in most of the time? (i.e., the house you spend most nights in)
- ◦
- No
- ◦
- Yes
- 6.
- How many nights in the last week did you sleep in this house?
1 2 3 4 5 6 7 - 7.
- How many nights in the last week did you sleep in the same house as:
- a.
- {baby}?:
0 1 2 3 4 5 6 7 - b.
- {child 2}?
0 1 2 3 4 5 6 7 - c.
- {child 3}?
0 1 2 3 4 5 6 7
- 8.
- On the map, please mark the house you slept in last night
- 9.
- On the map, please mark any other houses you slept in during the last week [skip if Q6 = ‘7’]
- 10.
- On the map, please mark any other houses your children slept in, when they were not with you [skip if Q7 all = ‘7’]
- a.
- {baby} [click on houses on the map] [skip if Qa = ‘7’]
- b.
- {baby} [click on houses on the map] [skip if Qb = ‘7’]
- c.
- {baby} [click on houses on the map] [skip if Qc = ‘7’]
- 11.
- Was {baby} with you all day yesterday? Yes/No [if yes, skip to Q13]
- 12.
- Who was {baby} with during the day yesterday: [check all that apply, must check at least one for each part of the day]
Morning: with me with friends/relatives at childcare other [text] Afternoon: with me with friends/relatives at childcare other [text] Evening: with me with friends/relatives at childcare other [text] - 13.
- During the day yesterday, did you spend at least one hour with anyone who was not in the same house as you last night? Yes/No [if no, skip Q15]
- 14.
- Please tell us about each person that you spent at least one hour with yesterday, who was not in the same house as you last night
- Record each contact person only once, even if you saw them more than once
- Don’t include people who were in your household last night
| Contact No. | Age group Young Child School Aged Adult | Did They Touch You? | Did They Touch {Baby}? | ||
|---|---|---|---|---|---|
| Y | N | Y | N | ||
| 1 | Young child | ⊠ | □ | ⊠ | □ |
| 2 | School | □ | ⊠ | ⊠ | □ |
| 3 | Adult | □ | ⊠ | ⊠ | □ |
| 4 | Adult | □ | ⊠ | □ | ⊠ |
- 15.
- Did you travel outside the study community in the last month?
- ◦
- No [skip to Q20]
- ◦
- Yes
- 16.
- Where did you spend at least one night? [check all that apply]
- □
- East Arnhem (outside the study community)
- □
- Darwin
- □
- Elsewhere in the NT
- □
- Outside the NT
- 17.
- How many nights did you spend away from the study community?
1 2 3 4 5 6 7 8 9 10 more [text] - 18.
- Was {baby} with you on the nights you travelled out of the study community?
- ◦
- Yes, all nights I was away
- ◦
- Some nights I was away
- ◦
- No {baby} did not travel with me
- 19.
- What was the main reason for this travel?
- ◦
- Access services (e.g., shopping, health services)
- ◦
- Work
- ◦
- Visit family
- ◦
- Attend a funeral
- ◦
- Other cultural business
- ◦
- Holiday
- 20.
- Have you had to go to clinic or hospital because you were unwell in the last month?
- ◦
- No [skip to Q21]
- ◦
- Yes
- a.
- What type of illness did you have?
- □
- Skin infection or scabies
- □
- Cold or ‘flu-like illness
- □
- Chest infection
- □
- Diarrhoea
- □
- Other illness
- 21.
- Have you taken antibiotics in the last month?
- ◦
- No
- □
- Antibiotic injection
- □
- Antibiotic syrup
- 22.
- Has your baby had to go to the clinic because they were unwell in the last month?
- ◦
- No [skip to Q24]
- ◦
- Yes
- 23.
- What type of illness did your baby have? [check all that apply]
- □
- Skin infection or scabies
- □
- Cold or ‘flu-like illness
- □
- Chest infection
- □
- Diarrhoea
- □
- Other illness
- 24.
- Has your baby taken antibiotics in the last month? [check all that apply]
- ◦
- No
- □
- Antibiotic injection
- □
- Antibiotic syrup
Appendix B
| Pre-School Aged | School Aged | Adult | |
|---|---|---|---|
| Adult | ab | ac | a (a − 1)/2 |
| School aged | cb | c (c − 1)/2 | |
| Pre-school aged | b (b − 1)/2 |
Appendix C
Appendix C.1. Transmission Scenarios (Defined by Mixing Model and Disease Model)
| Model | Infection Parameters | Model Inputs * |
|---|---|---|
| SEIR (outbreak) | Duration of latency | U (1, 3) days |
| Duration of infectiousness | U (1, 3) days | |
| Probability of transmission in the household/community mixing models (chosen so that the median attack rate in uniform model is ~66%) | U (0.01, 0.015) | |
| SEIS (endemic): | Duration of latency | U (1, 3) days |
| Duration of infectiousness | U (7, 21) days | |
| Probability of transmission in the household/community mixing models (chosen so that the median population prevalence at endemic equilibrium in uniform model is ~20%) | U (0.0014, 0.0021) |
Appendix C.1.1. Household/Community Mixing
- The ABS model: which is parameterized by ABS (census) data only,
- The CAMP remote model: which is supplemented by CAMP remote data.
Appendix C.1.2. Uniform Mixing between All Agents in the Population
- store the distribution of the current occupancy of houses, O.
- store the distribution of the number of current residents (temporary and present, permanent) in each age group i, Ai.
- For each age group i, calculate the mean number of contacts per day per individual in age group i, Ci.
- Calculate C, the mean number of community contacts per individual for this population, C = sumi(Ci·Ai)/sumi(Ai).
Appendix D
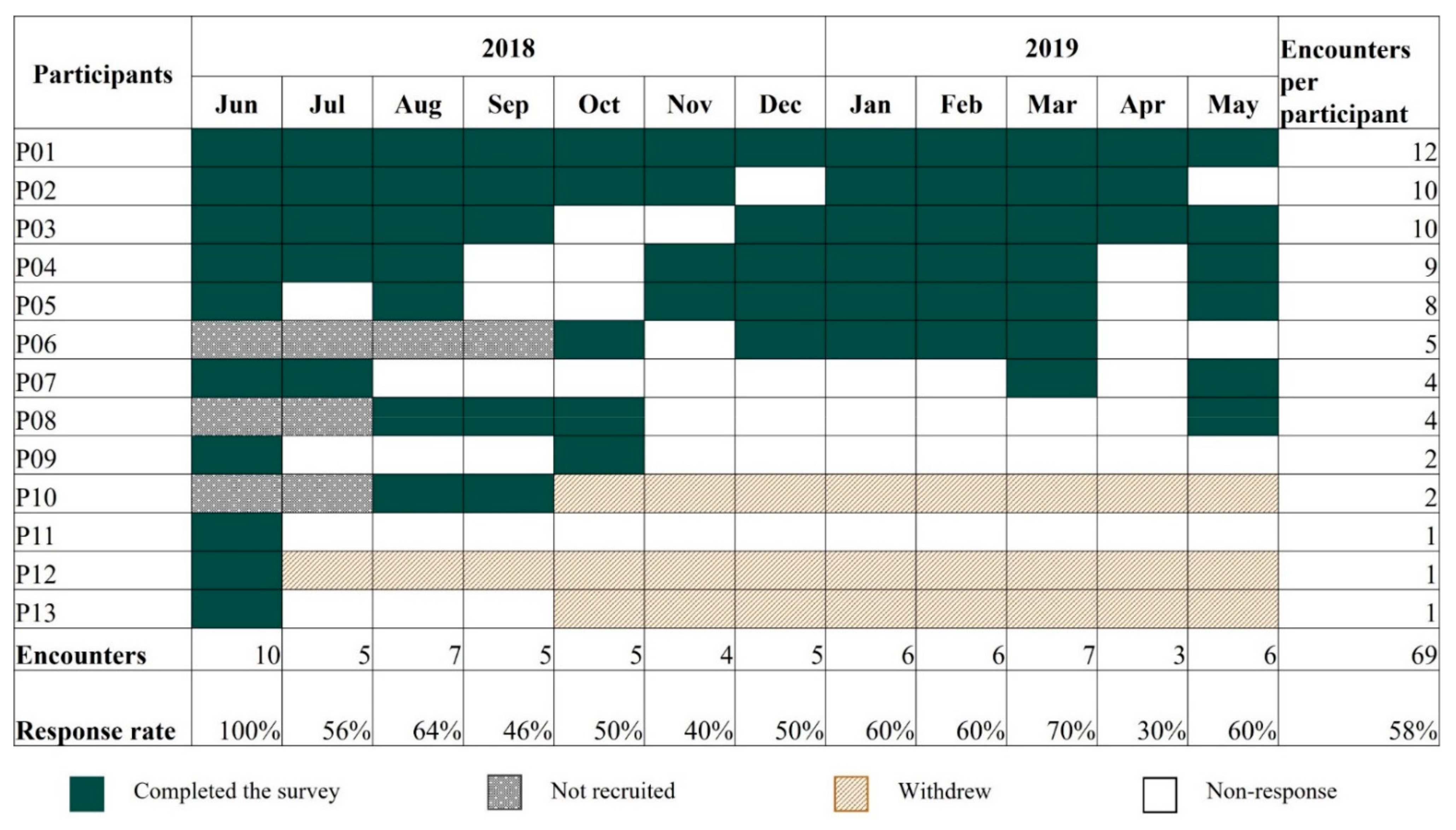
Appendix E
| Season | Month | Responded | Did Not Respond | No. of Enrolled Participants | Total who Travelled out of the Community | ||||
|---|---|---|---|---|---|---|---|---|---|
| Travelled | Did Not Travel | % | Travelling | Not Travelling | No | % | |||
| Dry | June | 2 | 8 | 20% | - | - | 10 | 2 | 20% |
| July | 1 | 4 | 20% | 1 | 2 | 8 | 2 | 25% | |
| August | 1 | 6 | 14% | 2 | 0 | 10 | 3 | 30% | |
| September | 0 | 5 | 0% | 0 | 3 | 10 | 0 | 0% | |
| October | 3 | 2 | 60% | 1 | 1 | 8 | 4 | 50% | |
| Wet | November | 3 | 1 | 75% | 2 | 1 | 8 | 5 | 63% |
| December | 3 | 2 | 60% | 1 | 0 | 8 | 4 | 50% | |
| January | 2 | 4 | 33% | 0 | 0 | 8 | 2 | 25% | |
| February | 0 | 6 | 0% | 0 | 0 | 8 | 0 | 0% | |
| March | 3 | 4 | 43% | 0 | 0 | 8 | 3 | 38% | |
| April | 1 | 2 | 33% | 0 | 5 | 8 | 1 | 13% | |
| Dry | May | 3 | 3 | 50% | 1 | 0 | 8 | 4 | 50% |
Appendix F
| Disease Model | Mixing Model | Outbreak Duration (Days) | Final Size (Number Infected) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 2.5p | 25p | 50p | 75p | 97.5p | 2.5p | 25p | 50p | 75p | 97.5p * | ||
| SEIR | HH | 30 | 36 | 41 | 45.5 | 56 | 2421 | 2472 | 2483 | 2487 | 2492 |
| SEIR | HH (ABS) | 36 | 43.5 | 49 | 55 | 69 | 2246 | 2386 | 2432 | 2457 | 2478 |
| SEIR | U | 34.5 | 89 | 107 | 134 | 220.5 | 45 | 1151 | 1638 | 1938 | 2211 |
| Endemic Prevalence | |||||||||||
| 2.5p | 25p | 50p | 75p | 97.5p | |||||||
| SEIS | HH | 52.0% | 63.3% | 70.3% | 75.3% | 80.7% | |||||
| SEIS | HH (ABS) | 43.3% | 57.1% | 65.5% | 70.9% | 76.6% | |||||
| SEIS | U | 0.2% | 2.8% | 19.0% | 31.7% | 44.9% | |||||
Appendix G
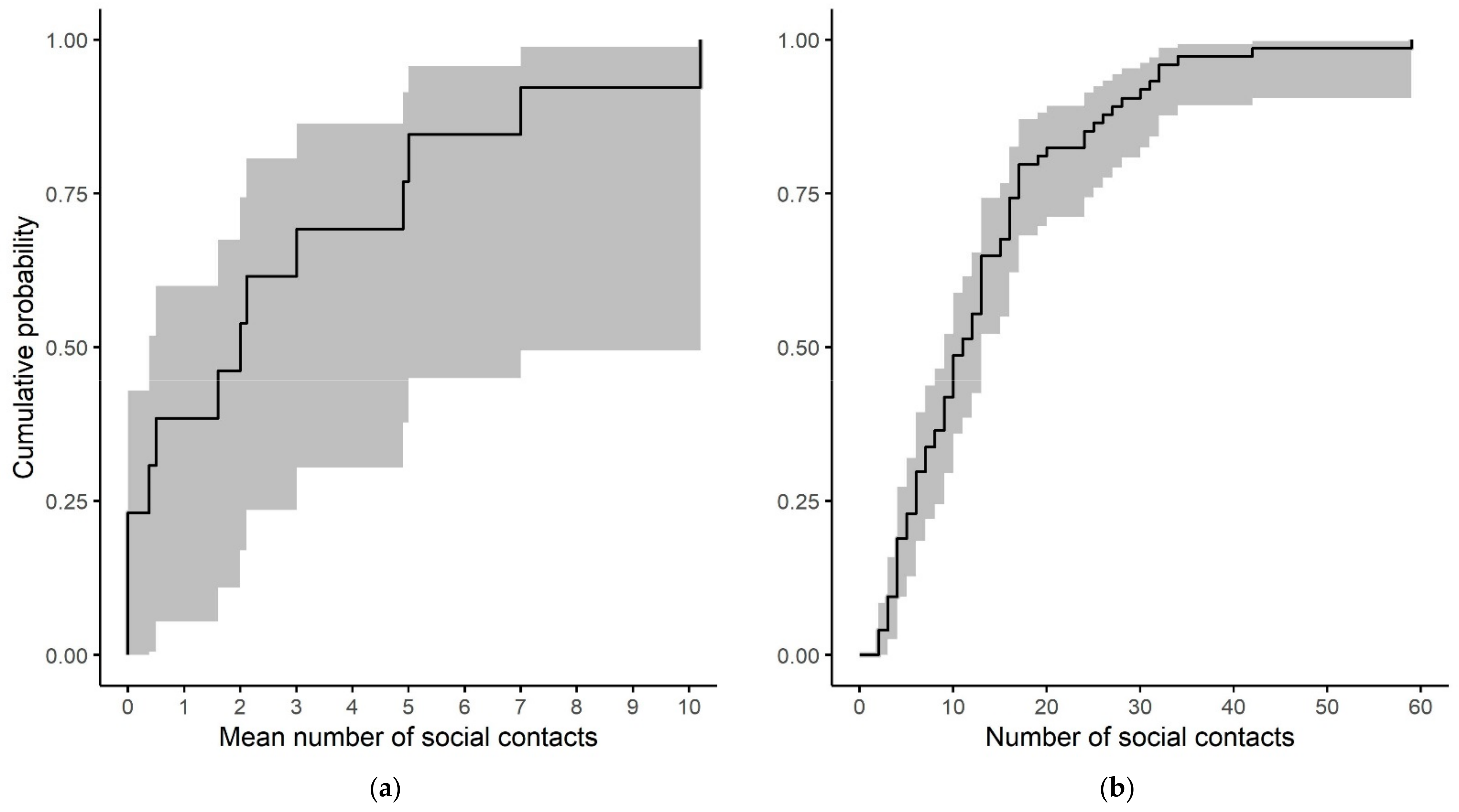
References
- Read, J.M.; Edmunds, W.J.; Riley, S.; Lessler, J.; Cummings, D.A.T. Close encounters of the infectious kind: Methods to measure social mixing behaviour. Epidemiol. Infect. 2012, 140, 2117–2130. [Google Scholar] [CrossRef] [PubMed]
- Musharbash, Y. Yuendumu Everyday: Contemporary Life in Remote Aboriginal Australia; Aboriginal Studies Press: Canberra, Australia, 2008. [Google Scholar]
- Biddle, N. Measures of Indigenous social capital and their relationship with well-being. Aust. J. Rural. Health 2012, 20, 298–304. [Google Scholar] [CrossRef] [PubMed]
- Biddle, N.; Prout, S. The geography and demography of Indigenous temporary mobility: An analysis of the 2006 census snapshot. J. Popul. Res. 2009, 26, 305–326. [Google Scholar] [CrossRef]
- Hosegood, V.; Benzler, J.; Solarsh, G.C. Population mobility and household dynamics in rural South Africa: Implications for demographic and health research. S. Afr. J. Demogr. 2005, 10, 43–68. [Google Scholar] [CrossRef]
- Jenkins, A.L.; Gyorkos, T.W.; Culman, K.N.; Ward, B.J.; Pekeles, G.S.; Mills, E.L. An overview of factors influencing the health of Canadian Inuit infants. Int. J. Circumpolar Health 2003, 62, 17–39. [Google Scholar] [CrossRef]
- Randall, S.; Coast, E. Poverty in African households: The limits of survey and census representations. J. Dev. Stud. 2015, 51, 162–177. [Google Scholar] [CrossRef]
- Morphy, F. Population, People and Place: The Fitzroy Valley Population Project; Centre for Aboriginal Economic Policy Research, The Australian National University: Canberra, Australia, 2018. [Google Scholar]
- Chisholm, R.H.; Crammond, B.; Wu, Y.; Bowen, A.C.; Campbell, P.T.; Tong, S.Y.C.; McVernon, J.; Geard, N. A model of population dynamics with complex household structure and mobility: Implications for transmission and control of communicable diseases. PeerJ 2020, 8, e10203. [Google Scholar] [CrossRef]
- House, T.; Keeling, M.J. Household structure and infectious disease transmission. Epidemiol. Infect. 2009, 137, 654–661. [Google Scholar] [CrossRef]
- Jing, Q.-L.; Liu, M.-J.; Zhang, Z.-B.; Fang, L.-Q.; Yuan, J.; Zhang, A.-R.; Dean, N.E.; Luo, L.; Ma, M.-M.; Longini, I.; et al. Household secondary attack rate of COVID-19 and associated determinants in Guangzhou, China: A retrospective cohort study. Lancet Infect. Dis. 2020, 20, 1141–1150. [Google Scholar] [CrossRef]
- Vino, T.; Singh, G.R.; Davison, B.; Campbell, P.T.; Lydeamore, M.J.; Robinson, A.; McVernon, J.; Tong, S.Y.C.; Geard, N. Indigenous Australian household structure: A simple data collection tool and implications for close contact transmission of communicable diseases. PeerJ 2017, 5, e3958. [Google Scholar] [CrossRef] [Green Version]
- Australian Bureau of Statistics. Census of Population and Housing: Characteristics of Aboriginal and Torres Strait Islander Australians, 2016, ‘East Arnhem 2016 Census Story’—Technical Report Cat no. 2076.0; Australian Bureau of Statistics: Canberra, Australia, 2018.
- Jackson, M.L.; Hart, G.R.; McCulloch, D.J.; Adler, A.; Brandstetter, E.; Fay, K.; Han, P.; Lacombe, K.; Lee, J.; Sibley, T.R.; et al. Effects of weather-related social distancing on city-scale transmission of respiratory viruses: A retrospective cohort study. BMC Infect. Dis. 2021, 21, 335. [Google Scholar] [CrossRef]
- Mossong, J.; Hens, N.; Jit, M.; Beutels, P.; Auranen, K.; Mikolajczyk, R.; Massari, M.; Salmaso, S.; Tomba, G.S.; Wallinga, J.; et al. Social contacts and mixing patterns relevant to the spread of infectious diseases. PLoS Med. 2008, 5, e74. [Google Scholar] [CrossRef]
- Kiti, M.C.; Kinyanjui, T.M.; Koech, D.C.; Munywoki, P.K.; Medley, G.F.; Nokes, D.J. Quantifying age-related rates of social contact using diaries in a rural coastal population of Kenya. PLoS ONE 2014, 9, e104786. [Google Scholar] [CrossRef]
- Read, J.M.; Lessler, J.; Riley, S.; Wang, S.; Tan, L.J.; Kwok, K.O.; Guan, Y.; Jiang, C.Q.; Cummings, D.A. Social mixing patterns in rural and urban areas of southern China. Proc. R. Soc. B 2014, 281, 20140268. [Google Scholar] [CrossRef]
- Le Polain de Waroux, O.; Cohuet, S.; Ndazima, D.; Kucharski, A.J.; Juan-Giner, A.; Flasche, S.; Tumwesigye, E.; Arinaitwe, R.; Mwanga-Amumpaire, J.; Boum, Y.; et al. Characteristics of human encounters and social mixing patterns relevant to infectious diseases spread by close contact: A survey in Southwest Uganda. BMC Infect. Dis. 2018, 18, 172. [Google Scholar] [CrossRef]
- Wesolowski, A.; Buckee, C.O.; Engø-Monsen, K.; Metcalf, C.J.E. Connecting mobility to infectious diseases: The promise and limits of mobile phone data. J. Infect. Dis. 2016, 214, S414–S420. [Google Scholar] [CrossRef]
- Australian Bureau of Statistics. Census of Population and Housing: Understanding the Census and Census Data, Australia, 2016; Australian Bureau of Statistics: Canberra, Australia, 2017.
- Zachreson, C.; Mitchell, L.; Lydeamore, M.J.; Rebuli, N.; Tomko, M.; Geard, N. Risk mapping for COVID-19 outbreaks in Australia using mobility data. J. R. Soc. Interface 2021, 18, 20200657. [Google Scholar] [CrossRef]
- Morphy, F. Uncontained subjects: Population and household in remote aboriginal Australia. J. Popul. Res. 2007, 24, 163–184. [Google Scholar] [CrossRef]
- Morphy, F. Mobility and its consequences: The 2006 enumeration in the north-east Arnhem Land region. In Agency, Contingency and Census Process: Observations of the 2006 Indigenous Enumeration Strategy in Remote Aboriginal Australia; Morphy, F., Ed.; Centre for Aboriginal Economic Policy Research: Canberra, Australia, 2007. [Google Scholar] [CrossRef]
- Dockery, A.; Hampton, K. The Dynamics of Services, Housing, Jobs and Mobility in Remote Aboriginal and Torres Strait Islander Communities in Central Australia; CRC-REP Working Paper; Ninti One Limited: Alice Springs, Australia, 2015. [Google Scholar]
- Bolton, K.J.; McCaw, J.M.; Forbes, K.; Nathan, P.; Robins, G.; Pattison, P.; Nolan, T.; McVernon, J. Influence of contact definitions in assessment of the relative importance of social settings in disease transmission risk. PLoS ONE 2012, 7, e30893. [Google Scholar] [CrossRef] [Green Version]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Wickham, H. ggplot2. Wiley Interdiscip. Rev. Comput. Stat. 2011, 3, 180–185. [Google Scholar] [CrossRef]
- Helwig, N.E. Nptest: Nonparametric Bootstrap and Permutation Tests, 1.0-3 ed. 2021. Available online: https://cran.r-project.org/web/packages/nptest/index.html (accessed on 1 November 2020).
- Australian Bureau of Statistics. Life Tables for Aboriginal and Torres Strait Islander Australians, 2015–2017; Australian Bureau of Statistics: Canberra, Australia, 2018.
- Hoang, T.; Coletti, P.; Melegaro, A.; Wallinga, J.; Grijalva, C.G.; Edmunds, J.W.; Beutels, P.; Hens, N. A systematic review of social contact surveys to inform transmission models of close-contact infections. Epidemiology 2019, 30, 723–736. [Google Scholar] [CrossRef] [PubMed]
- Gareta, D.; Baisley, K.; Mngomezulu, T.; Smit, T.; Khoza, T.; Nxumalo, S.; Dreyer, J.; Dube, S.; Majozi, N.; Ording-Jesperson, G.; et al. Cohort profile update: Africa Centre Demographic Information System (ACDIS) and population-based HIV survey. Int. J. Epidemiol. 2021, 50, 33–34. [Google Scholar] [CrossRef] [PubMed]
- Tanser, F.; Hosegood, V.; Bärnighausen, T.; Herbst, K.; Nyirenda, M.; Muhwava, W.; Newell, C.; Viljoen, J.; Mutevedzi, T.; Newell, M.-L. Cohort Profile: Africa Centre Demographic Information System (ACDIS) and population-based HIV survey. Int. J. Epidemiol. 2008, 37, 956–962. [Google Scholar] [CrossRef] [PubMed]
- Blower, S.M.; Dowlatabadi, H. Sensitivity and uncertainty analysis of complex models of disease transmission: An HIV model, as an example. Int. Stat. Rev. 1994, 62, 229–243. [Google Scholar] [CrossRef]
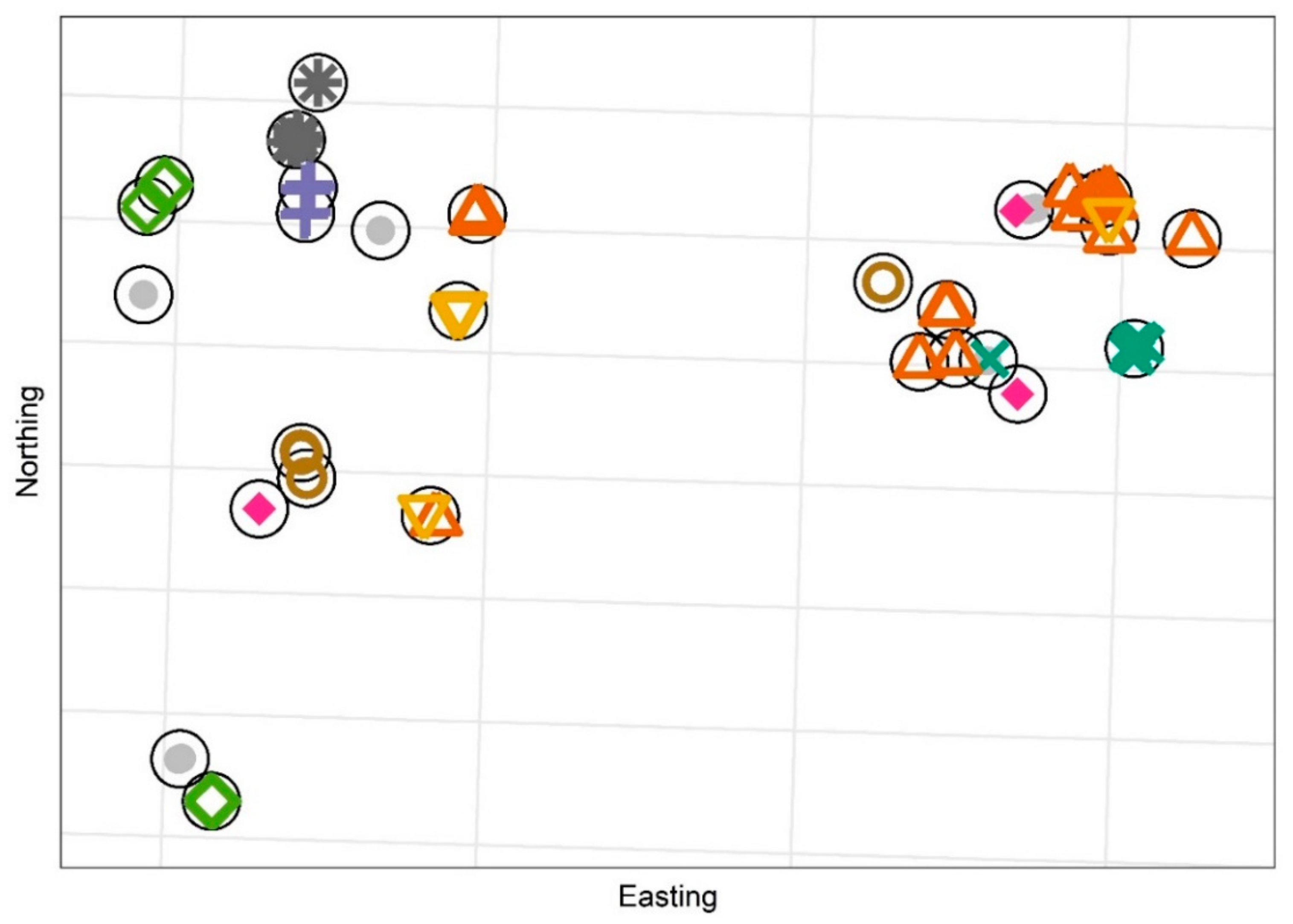
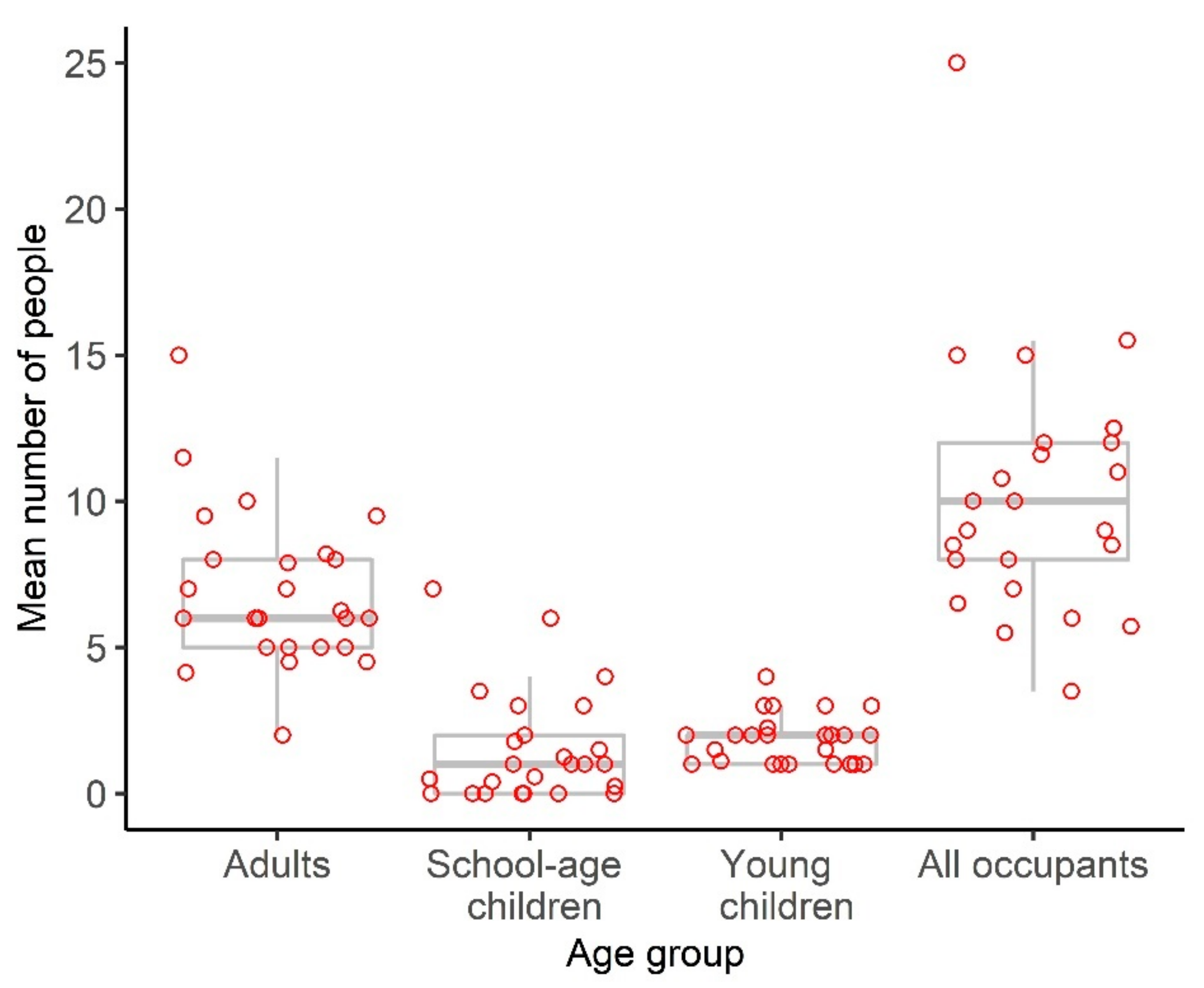
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goldsmith, J.J.; Campbell, P.T.; Villanueva-Cabezas, J.P.; Chisholm, R.H.; McKinnon, M.; Gurruwiwi, G.G.; Dhurrkay, R.G.; Dockery, A.M.; Geard, N.; Tong, S.Y.C.; et al. Capturing Household Structure and Mobility within and between Remote Aboriginal Communities in Northern Australia Using Longitudinal Data: A Pilot Study. Int. J. Environ. Res. Public Health 2022, 19, 12002. https://doi.org/10.3390/ijerph191912002
Goldsmith JJ, Campbell PT, Villanueva-Cabezas JP, Chisholm RH, McKinnon M, Gurruwiwi GG, Dhurrkay RG, Dockery AM, Geard N, Tong SYC, et al. Capturing Household Structure and Mobility within and between Remote Aboriginal Communities in Northern Australia Using Longitudinal Data: A Pilot Study. International Journal of Environmental Research and Public Health. 2022; 19(19):12002. https://doi.org/10.3390/ijerph191912002
Chicago/Turabian StyleGoldsmith, Jessie J., Patricia T. Campbell, Juan Pablo Villanueva-Cabezas, Rebecca H. Chisholm, Melita McKinnon, George G. Gurruwiwi, Roslyn G. Dhurrkay, Alfred M. Dockery, Nicholas Geard, Steven Y. C. Tong, and et al. 2022. "Capturing Household Structure and Mobility within and between Remote Aboriginal Communities in Northern Australia Using Longitudinal Data: A Pilot Study" International Journal of Environmental Research and Public Health 19, no. 19: 12002. https://doi.org/10.3390/ijerph191912002






