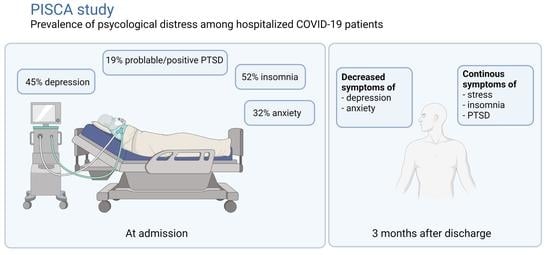
Psychological Distress among Hospitalized COVID-19 Patients in Denmark during the First 12 Months of the Pandemic
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Setting
2.2. Study Population
2.3. Data Collection
2.4. Sociodemographic and Clinical Variables
2.5. Statistical Analysis
2.6. Ethical Considerations
3. Results
3.1. Study Participants
3.2. Psychological Distress Outcomes
3.3. Potential Factors Associated with Symptoms of Anxiety and Depression and HRQoL
4. Discussion
4.1. Psychological Distress during and after Hospital Admission
4.2. Potential Factors Associated with Symptoms of Depression and Anxiety and HRQoL
4.3. Possible Practical Implications for Healthcare Professionals
4.4. Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Johns Hopkins University COVID-19 Dashboard by the Center for the Center for Systems Science and Engineering (CSSE). Available online: https://coronavirus.jhu.edu/map.html (accessed on 27 February 2022).
- Xiang, Y.-T.; Yang, Y.; Li, W.; Zhang, L.; Zhang, Q.; Cheung, T.; Ng, C.H. Timely Mental Health Care for the 2019 Novel Coronavirus Outbreak Is Urgently Needed. Lancet Psychiatry 2020, 7, 228–229. [Google Scholar] [CrossRef]
- Veazie, S.; Lafavor, B.; Vela, K.; Young, S.; Sayer, N.A.; Carlson, K.F.; O’Neil, M.E. Mental Health Outcomes of Adults Hospitalized for COVID-19: A Systematic Review. J. Affect Disord. Rep. 2022, 8, 100312. [Google Scholar] [CrossRef]
- Vindegaard, N.; Benros, M.E. COVID-19 Pandemic and Mental Health Consequences: Systematic Review of the Current Evidence. Brain Behav. Immun. 2020, 89, 531–542. [Google Scholar] [CrossRef]
- Kahl, K.G.; Correll, C.U. Management of Patients With Severe Mental Illness During the Coronavirus Disease 2019 Pandemic. JAMA Psychiatry 2020, 77, 977–978. [Google Scholar] [CrossRef]
- Vlake, J.H.; Wesselius, S.; van Genderen, M.E.; van Bommel, J.; Boxma-de Klerk, B.; Wils, E.-J. Psychological Distress and Health-Related Quality of Life in Patients after Hospitalization during the COVID-19 Pandemic: A Single-Center, Observational Study. PLoS ONE 2021, 16, e0255774. [Google Scholar] [CrossRef]
- Purssell, E.; Gould, D.; Chudleigh, J. Impact of Isolation on Hospitalised Patients Who Are Infectious: Systematic Review with Meta-Analysis. BMJ Open 2020, 10, e030371. [Google Scholar] [CrossRef]
- Dashiell-Earp, C.N.; Bell, D.S.; Ang, A.O.; Uslan, D.Z. Do Physicians Spend Less Time with Patients in Contact Isolation?: A Time-Motion Study of Internal Medicine Interns. JAMA Intern. Med. 2014, 174, 814–815. [Google Scholar] [CrossRef]
- Sharma, A.; Pillai, D.R.; Lu, M.; Doolan, C.; Leal, J.; Kim, J.; Hollis, A. Impact of Isolation Precautions on Quality of Life: A Meta-Analysis. J. Hosp. Infect. 2020, 105, 35–42. [Google Scholar] [CrossRef]
- Shigemura, J.; Ursano, R.J.; Morganstein, J.C.; Kurosawa, M.; Benedek, D.M. Public Responses to the Novel 2019 Coronavirus (2019-NCoV) in Japan: Mental Health Consequences and Target Populations. Psychiatry Clin. Neurosci. 2020, 74, 281–282. [Google Scholar] [CrossRef]
- Lam, M.H.-B.; Wing, Y.-K.; Yu, M.W.-M.; Leung, C.-M.; Ma, R.C.W.; Kong, A.P.S.; So, W.Y.; Fong, S.Y.-Y.; Lam, S.-P. Mental Morbidities and Chronic Fatigue in Severe Acute Respiratory Syndrome Survivors: Long-Term Follow-Up. Arch. Intern. Med. 2009, 169, 2142–2147. [Google Scholar] [CrossRef]
- Lee, A.M.; Wong, J.G.W.S.; McAlonan, G.M.; Cheung, V.; Cheung, C.; Sham, P.C.; Chu, C.-M.; Wong, P.-C.; Tsang, K.W.T.; Chua, S.E. Stress and Psychological Distress among SARS Survivors 1 Year after the Outbreak. Can J. Psychiatry 2007, 52, 233–240. [Google Scholar] [CrossRef]
- Rogers, J.P.; Chesney, E.; Oliver, D.; Pollak, T.A.; McGuire, P.; Fusar-Poli, P.; Zandi, M.S.; Lewis, G.; David, A.S. Psychiatric and Neuropsychiatric Presentations Associated with Severe Coronavirus Infections: A Systematic Review and Meta-Analysis with Comparison to the COVID-19 Pandemic. Lancet Psychiatry 2020, 7, 611–627. [Google Scholar] [CrossRef]
- Liu, C.; Pan, W.; Li, L.; Li, B.; Ren, Y.; Ma, X. Prevalence of Depression, Anxiety, and Insomnia Symptoms among Patients with COVID-19: A Meta-Analysis of Quality Effects Model. J. Psychosom. Res. 2021, 147, 110516. [Google Scholar] [CrossRef]
- Statens Serum Institut COVID-19 in Denmark. Epidemiological Trend and Focus: Hospitalizations June 2020. Available online: https://files.ssi.dk/COVID-19-epi-trendogfokus-04062020-zp84 (accessed on 1 March 2022).
- The Danish Health Data Authority The National Cause of Death Register (Dødsårsagsregisteret)/COVID-19. Available online: https://www.esundhed.dk/Registre/Doedsaarsagsregisteret#tabpanel97BF922C33EB4D969EA49D0D9D4ABC1A (accessed on 10 May 2022).
- HADS—Danish Translation, Mapi Research Trust. Available online: https://eprovide.mapi-trust.org/instruments/hospital-anxiety-and-depression-scale (accessed on 5 July 2022).
- Zigmond, A.S.; Snaith, R.P. The Hospital Anxiety and Depression Scale. Acta Psychiatr. Scand. 1983, 67, 361–370. [Google Scholar] [CrossRef]
- Bjelland, I.; Dahl, A.A.; Haug, T.T.; Neckelmann, D. The Validity of the Hospital Anxiety and Depression Scale. An Updated Literature Review. J. Psychosom. Res. 2002, 52, 69–77. [Google Scholar] [CrossRef]
- Herrmann, C. International Experiences with the Hospital Anxiety and Depression Scale—A Review of Validation Data and Clinical Results. J. Psychosom. Res. 1997, 42, 17–41. [Google Scholar] [CrossRef]
- Snaith, R.P. The Hospital Anxiety and Depression Scale. Health Qual. Life Outcomes 2003, 1, 29. [Google Scholar] [CrossRef]
- Christensen, A.V.; Dixon, J.K.; Juel, K.; Ekholm, O.; Rasmussen, T.B.; Borregaard, B.; Mols, R.E.; Thrysøe, L.; Thorup, C.B.; Berg, S.K. Psychometric Properties of the Danish Hospital Anxiety and Depression Scale in Patients with Cardiac Disease: Results from the DenHeart Survey. Health Qual. Life Outcomes 2020, 18, 9. [Google Scholar] [CrossRef]
- Cohen, S.; Kamarck, T.; Mermelstein, R. A Global Measure of Perceived Stress. J. Health Soc. Behav. 1983, 24, 385–396. [Google Scholar] [CrossRef]
- Eskildsen, A.; Dalgaard, V.L.; Nielsen, K.J.; Andersen, J.H.; Zachariae, R.; Olsen, L.R.; Jørgensen, A.; Christiansen, D.H. Cross-Cultural Adaptation and Validation of the Danish Consensus Version of the 10-Item Perceived Stress Scale. Scand J. Work Environ. Health 2015, 41, 486–490. [Google Scholar] [CrossRef]
- Morin, C.M.; Belleville, G.; Bélanger, L.; Ivers, H. The Insomnia Severity Index: Psychometric Indicators to Detect Insomnia Cases and Evaluate Treatment Response. Sleep 2011, 34, 601–608. [Google Scholar] [CrossRef]
- Bastien, C.H.; Vallières, A.; Morin, C.M. Validation of the Insomnia Severity Index as an Outcome Measure for Insomnia Research. Sleep Med. 2001, 2, 297–307. [Google Scholar] [CrossRef]
- Dieperink, K.B.; Elnegaard, C.M.; Winther, B.; Lohman, A.; Zerlang, I.; Möller, S.; Zangger, G. Preliminary Validation of the Insomnia Severity Index in Danish Outpatients with a Medical Condition. J. Patient Rep. Outcomes 2020, 4, 18. [Google Scholar] [CrossRef]
- Mollica, R.F.; Caspi-Yavin, Y.; Bollini, P.; Truong, T.; Tor, S.; Lavelle, J. The Harvard Trauma Questionnaire. Validating a Cross-Cultural Instrument for Measuring Torture, Trauma, and Posttraumatic Stress Disorder in Indochinese Refugees. J. Nerv. Ment. Dis. 1992, 180, 111–116. [Google Scholar] [CrossRef]
- Bach, M. En empirisk Belysning og Analyse af “Emotional Numbing” som Eventuel Selvstændig factor i PTSD. [An Empirical Description and Analysis of “Emotional Numbing”, as a Potential Independent Factor in PTSD]; Psykologisk Studieskriftserie; Psykologisk Institut, Aarhus Universitet: Risskov, Denmark, 2003; pp. 1–132. [Google Scholar]
- Ware, J.; Kosinski, M.; Keller, S.D. A 12-Item Short-Form Health Survey: Construction of Scales and Preliminary Tests of Reliability and Validity. Med. Care 1996, 34, 220–233. [Google Scholar] [CrossRef]
- Ware, J.; Kosinski, M.; Keller, S. How to Score the SF-2 Physical and Mental Summary Scales, 2nd ed.; The Health Insitute, New England Medical Center: Boston, MA, USA, 1995. [Google Scholar]
- Bjorner, J.B.; Thunedborg, K.; Kristensen, T.S.; Modvig, J.; Bech, P. The Danish SF-36 Health Survey: Translation and Preliminary Validity Studies. J. Clin. Epidemiol. 1998, 51, 991–999. [Google Scholar] [CrossRef]
- Şahan, E.; Ünal, S.M.; Kırpınar, İ. Can We Predict Who Will Be More Anxious and Depressed in the COVID-19 Ward? J. Psychosom. Res. 2021, 140, 110302. [Google Scholar] [CrossRef]
- Kong, X.; Kong, F.; Zheng, K.; Tang, M.; Chen, Y.; Zhou, J.; Li, Y.; Diao, L.; Wu, S.; Jiao, P.; et al. Effect of Psychological-Behavioral Intervention on the Depression and Anxiety of COVID-19 Patients. Front. Psychiatry 2020, 11, 586355. [Google Scholar] [CrossRef]
- Li, X.; Tian, J.; Xu, Q. The Associated Factors of Anxiety and Depressive Symptoms in COVID-19 Patients Hospitalized in Wuhan, China. Psychiatr. Q 2021, 92, 879–887. [Google Scholar] [CrossRef]
- Beck, K.; Vincent, A.; Becker, C.; Keller, A.; Cam, H.; Schaefert, R.; Reinhardt, T.; Sutter, R.; Tisljar, K.; Bassetti, S.; et al. Prevalence and Factors Associated with Psychological Burden in COVID-19 Patients and Their Relatives: A Prospective Observational Cohort Study. PLoS ONE 2021, 16, e0250590. [Google Scholar] [CrossRef]
- Bao, Y.; Sun, Y.; Meng, S.; Shi, J.; Lu, L. 2019-NCoV Epidemic: Address Mental Health Care to Empower Society. Lancet 2020, 395, e37–e38. [Google Scholar] [CrossRef]
- Guo, M.; Kong, M.; Shi, W.; Wang, M.; Yang, H. Listening to COVID-19 Survivors: What They Need after Early Discharge from Hospital—A Qualitative Study. Int. J. Qual. Stud. Health Well-Being 2022, 17, 2030001. [Google Scholar] [CrossRef] [PubMed]
- Charles, S.T.; Piazza, J.R.; Mogle, J.; Sliwinski, M.J.; Almeida, D.M. The Wear and Tear of Daily Stressors on Mental Health. Psychol. Sci. 2013, 24, 733–741. [Google Scholar] [CrossRef]
- Piazza, J.R.; Charles, S.T.; Sliwinski, M.J.; Mogle, J.; Almeida, D.M. Affective Reactivity to Daily Stressors and Long-Term Risk of Reporting a Chronic Physical Health Condition. Ann. Behav. Med. 2013, 45, 110–120. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhu, L.-Y.; Ma, Y.-F.; Bo, H.-X.; Deng, H.-B.; Cao, J.; Wang, Y.; Wang, X.-J.; Xu, Y.; Lu, Q.-D.; et al. Association of Insomnia Disorder with Sociodemographic Factors and Poor Mental Health in COVID-19 Inpatients in China. Sleep Med. 2020, 75, 282–286. [Google Scholar] [CrossRef]
- Jahrami, H.A.; Alhaj, O.A.; Humood, A.M.; Alenezi, A.F.; Fekih-Romdhane, F.; AlRasheed, M.M.; Saif, Z.Q.; Bragazzi, N.L.; Pandi-Perumal, S.R.; BaHammam, A.S.; et al. Sleep Disturbances during the COVID-19 Pandemic: A Systematic Review, Meta-Analysis, and Meta-Regression. Sleep Med. Rev. 2022, 62, 101591. [Google Scholar] [CrossRef]
- De Lorenzo, R.; Conte, C.; Lanzani, C.; Benedetti, F.; Roveri, L.; Mazza, M.G.; Brioni, E.; Giacalone, G.; Canti, V.; Sofia, V.; et al. Residual Clinical Damage after COVID-19: A Retrospective and Prospective Observational Cohort Study. PLoS ONE 2020, 15, e0239570. [Google Scholar] [CrossRef]
- Einvik, G.; Dammen, T.; Ghanima, W.; Heir, T.; Stavem, K. Prevalence and Risk Factors for Post-Traumatic Stress in Hospitalized and Non-Hospitalized COVID-19 Patients. Int. J. Environ. Res. Public Health 2021, 18, 2079. [Google Scholar] [CrossRef]
- Bryant, R.A. Post-Traumatic Stress Disorder: A State-of-the-Art Review of Evidence and Challenges. World Psychiatry 2019, 18, 259–269. [Google Scholar] [CrossRef]
- Nandasena, H.M.R.K.G.; Pathirathna, M.L.; Atapattu, A.M.M.P.; Prasanga, P.T.S. Quality of Life of COVID-19 Patients after Discharge: Systematic Review. PLoS ONE 2022, 17, e0263941. [Google Scholar] [CrossRef]
- Alkodaymi, M.S.; Omrani, O.A.; Fawzy, N.A.; Shaar, B.A.; Almamlouk, R.; Riaz, M.; Obeidat, M.; Obeidat, Y.; Gerberi, D.; Taha, R.M.; et al. Prevalence of Post-Acute COVID-19 Syndrome Symptoms at Different Follow-up Periods: A Systematic Review and Meta-Analysis. Clin. Microbiol. Infect. 2022, 28, 657–666. [Google Scholar] [CrossRef] [PubMed]
- Ministry of Health. Denmark New Clinics for Patients with Severe Long-Term Effects Following COVID-19 [Nye Klinikker Til Patienter Med Svære Senfølger efter COVID-19]. November 2020. Available online: https://www.sst.dk/da/nyheder/2020/nye-klinikker-til-patienter-med-svaere-senfoelger-efter-covid-19 (accessed on 6 June 2022).
- Smith, K.; Bhui, K.; Cipriani, A. COVID-19, Mental Health and Ethnic Minorities. Evid. Based Ment. Health 2020, 23, 89–90. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.; Huo, Y.; Tassiopoulos, K.; Rutstein, R.; Kapetanovic, S.; Mellins, C.; Kacanek, D.; Malee, K. Pediatric HIV/AIDS Cohort Study (PHACS) Mental Health Diagnoses, Symptoms, and Service Utilization in US Youth with Perinatal HIV Infection or HIV Exposure. AIDS Patient Care STDS 2019, 33, 1–13. [Google Scholar] [CrossRef]
- Tasleem, A.; Wang, Y.; Li, K.; Jiang, X.; Krishnan, A.; He, C.; Sun, Y.; Wu, Y.; Fan, S.; Boruff, J.T.; et al. Effects of Mental Health Interventions among People Hospitalized with COVID-19 Infection: A Systematic Review of Randomized Controlled Trials. Gen. Hosp. Psychiatry 2022, 77, 40–68. [Google Scholar] [CrossRef] [PubMed]
- Priyamvada, R.; Ranjan, R.; Chaudhury, S. Efficacy of Psychological Intervention in Patients with Post-COVID-19 Anxiety. Ind Psychiatry J. 2021, 30, S41–S44. [Google Scholar] [CrossRef] [PubMed]
- Stewart, D.E.; Appelbaum, P.S. COVID-19 and Psychiatrists’ Responsibilities: A WPA Position Paper. World Psychiatry 2020, 19, 406–407. [Google Scholar] [CrossRef]
- Kuzman, M.R.; Curkovic, M.; Wasserman, D. Principles of Mental Health Care during the COVID-19 Pandemic. Eur. Psychiatry 2020, 63, e45. [Google Scholar] [CrossRef]
- Schaefert, R.; Stein, B.; Meinlschmidt, G.; Roemmel, N.; Huber, C.G.; Hepp, U.; Saillant, S.; Fazekas, C.; Vitinius, F. COVID-19-Related Psychosocial Care in General Hospitals: Results of an Online Survey of Psychosomatic, Psychiatric, and Psychological Consultation and Liaison Services in Germany, Austria, and Switzerland. Front. Psychiatry 2022, 13, 870984. [Google Scholar] [CrossRef]
| Age | |
| Median (IQR) | 61 (49–72) |
| Range | 24–90 |
| Sex, n (%) | |
| Male | 68 (72) |
| Female | 27 (28) |
| Born in Denmark | |
| Yes | 70 (75) |
| No | 24 (25) |
| Relationship status | |
| Married/living with partner | 72 (76) |
| Has a partner, but not living together | 7 (7) |
| No partner | 16 (17) |
| Nursing home resident, n (%) | <3 (1) |
| Education | |
| Compulsory (middle/high school) | 40 (43) |
| Higher education (college/university) | 51 (55) |
| Missing | <3 (2) |
| Currently employed | |
| Yes | 53 (56) |
| No | 40 (42) |
| Missing | <3 (2) |
| BMI | |
| Median (IQR) | 29.4 (25.8–32.0) |
| Range | 20.4–46.1 |
| Missing | 4 |
| Smoking | |
| Never smoked | 37 (39) |
| Former smoker | 54 (57) |
| Current smoker | 4 (4) |
| Alcohol use | |
| Greater than recommendations | 12 (13) |
| Less than recommendations | 83 (87) |
| Charlson’s Comorbidity Index | |
| 0 | 41 (43) |
| 1 | 32 (34) |
| ≥2 | 22 (23) |
| Ever treated for a psychological problem | |
| Yes | 26 (28) |
| No | 65 (69) |
| Missing | 3 (3) |
| Temperature | |
| Median (IQR) | 37.4 (36.6–38.5) |
| Range | 35.7–40.9 |
| Oxygen need | |
| <5 L O2/min | 62 (65) |
| 5–10 L O2/min | 19 (20) |
| 11–29 L O2/min | 13 (14) |
| >30 L O2/min | <3 (1) |
| Hospital length of stay, days, median (IQR) | 6 (2–24) |
| Admitted to ICU for mechanical intervention, n (%) | 4 (4) |
| Died during hospital admission | 9 (9) |
| T1 | T2 | T3 | Difference between T1 and T3 | Difference between T1 and T3 | ||||
|---|---|---|---|---|---|---|---|---|
| Range | Mean (95% CI) | p-Value | Mean (95% CI) | p-Value | ||||
| Symptoms of depression (HADS-D) | 0–21 | n = 95 | n = 51 | n = 45 | ||||
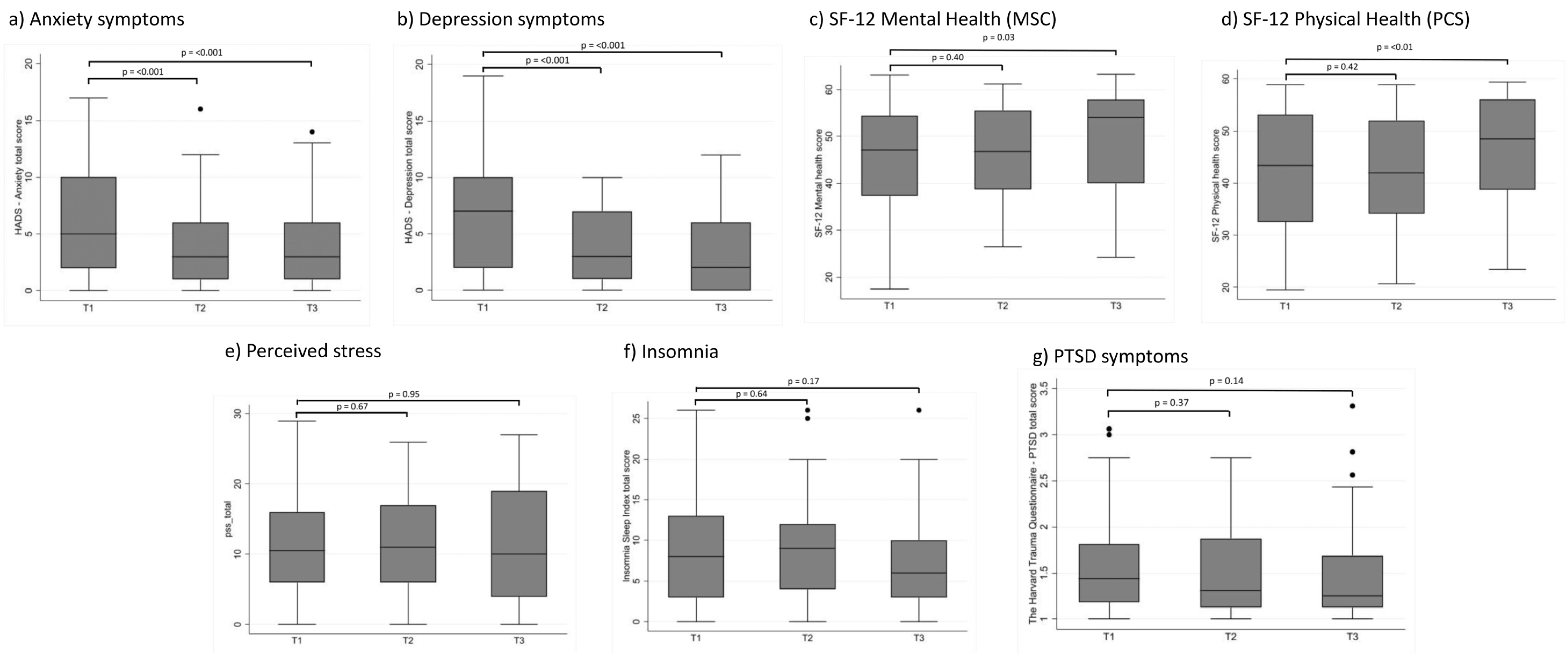
| Mean score (95% CI) | 6.54 (5.60–7.47) | 3.71 (2.78–4.63) | 3.29 (2.17–4.41) | −2.77 (−3.87 to −1.67) | <0.001 | −3.12 (−4.25 to −1.98) | <0.001 | |
| No depression, (score < 8), n (%) | 54 (57) | 41 (84) | 37 (82) | |||||
| Possible depression, (score ≥ 8), n (%) | 41 (43) | 8 (16) | 8 (18) | |||||
| Symptoms of anxiety (HADS-A) | 0–21 | n = 95 | n = 51 | n = 45 | ||||
| Mean score (95% CI) | 5.87 (4.97–6.78) | 3.90 (2.82–4.98) | 3.93 (2.77–5.09) | −2.27 (−3.35 to −1.20) | <0.001 | −2.26 (−3.33 to −1.19) | <0.001 | |
| No anxiety (score < 8), n (%) | 65 (68) | 43 (84) | 37 (82) | |||||
| Possible anxiety, (score ≥ 8), n (%) | 30 (32) | 8 (16) | 8 (18) | |||||
| Perceived Stress (PSS) | 0–40 | n = 94 | n = 49 | n = 45 | ||||
| Total score, mean (95% CI) | 11.11 (9.70–12.51) | 11.47 (9.42–13.52) | 10.93 (8.56–13.29) | 0.41 (−1.56 to 2.38) | 0.67 | −0.09 (−2.60 to 2.43) | 0.95 | |
| Low stress (score 0–13), n (%) | 57 (61) | 33 (67) | 28 (62) | |||||
| Moderate stress (score 14–26), n (%) | 35 (37) | 16 (33) | 16 (36) | |||||
| High stress (score 27–40), n (%) | 2 (2) | 0 | 1 (2) | |||||
| Insomnia (ISI) | 0–28 | n = 95 | n = 49 | n = 45 | ||||
| Mean score (95% CI) | 8.58 (7.25–9.90) | 9.41 (7.59–11.22) | 7.33 (5.51–9.16) | 0.34 (−1.11 to 1.79) | 0.64 | −1.24 (−3.02 to 0.53) | 0.17 | |
| No insomnia (score 0–7), n (%) | 45 (48) | 19 (39) | 28 (62) | |||||
| Subthreshold insomnia (score 8–14), n (%) | 36 (38) | 20 (41) | 12 (27) | |||||
| Clinical insomnia—moderate (score 15–21), n (%) | 11 (11) | 8 (16) | 4 (9) | |||||
| Clinical insomnia—severe (score 22–28), n (%) | 3 (3) | 2 (4) | 1 (2) | |||||
| PTSD Symptoms (HTQ) | 1–4 | n = 94 | n = 49 | n = 45 | ||||
| Mean score (95% CI) | 1.55 (1.45–1.65) | 1.53 (1.38–1.68) | 1.47 (1.31–1.63) | −0.05 (−0.15 to 0.06) | 0.37 | −0.10 (−0.24 to 0.03) | 0.14 | |
| No PTSD symptoms, (score < 2), n (%) | 76 (81) | 40 (82) | 38 (84) | |||||
| Probable PTSD Symptoms, (score 2–2.4), n (%) | 12 (13) | 4 (8) | 3 (7) | |||||
| Positive PTSD Symptoms, (score > 2.4), n (%) | 6 (6) | 5 (10) | 4 (9) | |||||
| SF-12: Mental health score (MSC) | 0–100 | n = 95 | n = 51 | n = 45 | ||||
| Mean score (95% CI) | 46.35 (44.15–48.55) | 46.42 (43.60–49.23) | 49.33 (46.05–52.62) | 1.06 (−1.39 to 3.51) | 0.40 | 3.47 (0.42 to6.52) | 0.03 | |
| Affected mental health (score ≤ 42), n (%) | 66 (69) | 34 (67) | 26 (58) | |||||
| SF-12: Physical health score (PSC) | n = 95 | n = 51 | n = 45 | |||||
| Mean score (95% CI) | 42.39 (40.15–44.63) | 42.48 (39.42–45.54) | 46.26 (43.04–49.47) | 1.21 (−1.71 to 4.13) | 0.42 | 4.24 (1.28 to7.19) | <0.01 | |
| Affected physical health (score ≤ 50), n (%) | 32 (34) | 16 (31) | 14 (31) | |||||
| Severity of Anxiety Symptoms | Severity of Depressive Symptoms | |||||||
|---|---|---|---|---|---|---|---|---|
| Univariate | Multivariate | Univariate | Multivariate | |||||
| Beta (95% CI) | p-Value | Beta (95% CI) | p-Value | Beta (95% CI) | p-Value | Beta (95% CI) | p-Value | |
| Time since discharge | ||||||||
| 1 month | −2.27 (−3.34 to −1.20) | <0.001 | −2.11 (−3.18 to −1.04) | <0.001 | −2.77 (−3.87 to −1.67) | <0.001 | −2.62 (−3.74 to −1.49) | <0.001 |
| 3 months | −2.26 (−3.33 to −1.19) | <0.001 | −2.15 (−3.23 to −1.08) | <0.001 | −3.12 (−4.25 to −1.98) | <0.001 | −3.02 (−4.17 to −1.87) | <0.001 |
| Age, years | −0.06 (−0.09 to −0.03) | <0.001 | −0.03 (−0.08 to 0.01) | 0.17 | −0.02 (−0.06 to 0.02) | 0.38 | ||
| Sex (female) | 1.17 (−0.88 to 2.46) | 0.07 | 1.03 (−0.35 to 2.41) | 0.15 | 1.21 (−0.18 to 2.60) | 0.09 | 0.64 (−0.69 to 1.98) | 0.35 |
| County of birth outside DK | 3.51 (2.10–4.92) | <0.001 | 2.18 (0.36 to3.40) | 0.02 | 2.76 (1.27 to4.24) | <0.001 | 2.27 (0.48 to4.04) | 0.01 |
| Education | ||||||||
| Compulsory (middle/high school) | ref | ref | ref | Ref | ||||
| Higher education (College/University) | −1.43 (−2.61 to −0.24) | 0.02 | −0.60 (−1.88 to 0.72) | 0.38 | −1.77 (−2.95 to −0.59) | <0.01 | 1.05 (−0.98 to 3.07) | 0.31 |
| Charlson’s Comorbidity | ||||||||
| 0 | ref | ref | ||||||
| 1 | 0.34 (−1.11 to 1.80) | 0.64 | 1.67 (0.24 to3.09) | 0.02 | 1.74 (0.37 to3.11) | 0.01 | ||
| 2 | −0.62 (−1.95 to 0.71) | 0.35 | 0.94 (−0.41 to 2.28) | 0.17 | 1.24 (−0.36 to 2.83) | 0.13 | ||
| Hospital stay, days | 0.01 (−0.02 to 0.03) | 0.62 | −0.01 (−0.08 to 0.07) | 0.95 | ||||
| ICU admission/mechanical intervention | −0.98 (−2.40 to 0.45) | 0.18 | 3.17 (−0.57 to 6.93) | 0.10 | ||||
| Prior psychiatric treatment (yes) | −1.33 (−2.60 to −0.07) | 0.04 | −0.79 (−2.23 to 0.66) | 0.28 | −0.48 (−1.98 to 1.01) | 0.53 | ||
| SF-12 Mental Health | SF-12 Physical Health | |||||||
|---|---|---|---|---|---|---|---|---|
| Univariate | Multivariate | Univariate | Multivariate | |||||
| Beta (95% CI) | p-Value | Beta (95% CI) | p-Value | Beta (95% CI) | p-Value | Beta (95% CI) | p-Value | |
| Time since discharge | ||||||||
| 1 month | 1.06 (−1.43 to 3.55) | 0.41 | −1.50 (−3.76 to 0.77) | 0.20 | 1.21 (−1.66 to 4.08) | 0.41 | 0.17 (−2.38 to 2.73) | 0.89 |
| 3 months | 3.47 (0.30 to6.64) | 0.03 | −0.06 (−2.32 to 2.20) | 0.96 | 4.24 (1.31 to7.16) | <0.01 | 2.19 (−0.14 to 4.52) | 0.07 |
| Age, years | 0.01 (−0.07 to 0.10) | 0.75 | 0.03 (−0.06 to 0.13) | 0.53 | ||||
| Sex (female) | −1.97 (−5.14 to 1.20) | 0.22 | −4.46 (−8.04 to −0.88) | 0.02 | −3.50 (−6.28 to −0.72) | 0.01 | ||
| County of birth outside DK | −2.88 (−6.41 to 0.65) | 0.11 | −3.19 (−6.95 to 0.60) | 0.10 | ||||
| Education | ||||||||
| Compulsory (middle/high school) | ref | ref | ||||||
| Higher education (College/University) | 1.23 (−1.74 to 4.20) | 0.42 | 0.96 (−2.26 to 4.19) | 0.56 | ||||
| Charlson’s Comorbidity | ||||||||
| 0 | ref | ref | ref | ref | ||||
| 1 | −3.32 (−6.78 to 0.14) | 0.06 | −1.20 (−3.47 to 1.07) | 0.30 | −5.24 (−8.74 to −1.73) | <0.01 | −2.98 (−5.68 to −0.29) | 0.03 |
| 2 | −5.54 (−9.11 to −1.96) | <0.01 | −4.77 (−7.14 to −2.39) | <0.001 | −6.03 (−9.83 to −2.22) | <0.01 | −4.69 (−7.94 to −1.45) | <0.01 |
| Hospital stay, days | 0.02 (−0.18 to 0.22) | 0.84 | 0.02 (−0.18 to 0.22) | 0.82 | ||||
| ICU admission/mechanical intervention | −2.88 (−20.96 to 15.21) | 0.76 | −6.42 (−22.38 to 9.55) | 0.43 | ||||
| Prior psychiatric treatment (yes) | 2.05 (−1.11 to 5.21) | 0.20 | 3.12 (−0.50 to 6.75) | 0.09 | ||||
| Severity of anxiety symptoms | −1.33 (−1.73 to −0.93) | <0.001 | −0.14 (−0.60 to 0.33) | 0.56 | −1.23 (−1.66 to −0.79) | <0.001 | 0.03 (−0.40 to 0.47) | 0.87 |
| Severity of depressive symptoms | −1.34 (−1.74 to −0.94) | <0.001 | −0.77 (−1.16 to −0.37) | <0.001 | −1.25 (−1.72 to −0.78) | <0.001 | −0.58 (−0.97 to −0.18) | <0.01 |
| Severity of perceived Stress | −0.92 (−1.12 to −0.67) | <0.001 | −0.43 (−0.68 to −0.19) | <0.01 | −0.85 (−1.07 to −0.63) | <0.001 | −0.50 (−0.79 to −0.22) | <0.01 |
| Severity of insomnia | −0.82 (−1.09 to −0.54) | <0.001 | −0.17 (−0.43 to 0.09) | 0.20 | −0.86 (−1.08 to −0.64) | <0.001 | −0.32 (−0.57 to −0.07) | 0.01 |
| Severity of PTSD symptoms | −12.71 (−16.40 to −9.01) | <0.001 | −3.10 (−8.03 to 1.83) | 0.22 | −12.25 (−15.23 to −9.26) | <0.001 | −2.03 (−6.04 to 1.97) | 0.32 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moseholm, E.; Midtgaard, J.; Bollerup, S.; Apol, Á.D.; Olesen, O.B.; Jespersen, S.; Weis, N. Psychological Distress among Hospitalized COVID-19 Patients in Denmark during the First 12 Months of the Pandemic. Int. J. Environ. Res. Public Health 2022, 19, 10097. https://doi.org/10.3390/ijerph191610097
Moseholm E, Midtgaard J, Bollerup S, Apol ÁD, Olesen OB, Jespersen S, Weis N. Psychological Distress among Hospitalized COVID-19 Patients in Denmark during the First 12 Months of the Pandemic. International Journal of Environmental Research and Public Health. 2022; 19(16):10097. https://doi.org/10.3390/ijerph191610097
Chicago/Turabian StyleMoseholm, Ellen, Julie Midtgaard, Signe Bollerup, Ása D. Apol, Oskar B. Olesen, Sofie Jespersen, and Nina Weis. 2022. "Psychological Distress among Hospitalized COVID-19 Patients in Denmark during the First 12 Months of the Pandemic" International Journal of Environmental Research and Public Health 19, no. 16: 10097. https://doi.org/10.3390/ijerph191610097
APA StyleMoseholm, E., Midtgaard, J., Bollerup, S., Apol, Á. D., Olesen, O. B., Jespersen, S., & Weis, N. (2022). Psychological Distress among Hospitalized COVID-19 Patients in Denmark during the First 12 Months of the Pandemic. International Journal of Environmental Research and Public Health, 19(16), 10097. https://doi.org/10.3390/ijerph191610097