Different Exercise Types Produce the Same Acute Inhibitory Control Improvements When the Subjective Intensity Is Equal
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Participants
2.3. Genetic Analysis
2.4. Inhibitory Control Test
2.5. Exercise Sessions
2.6. Statistical Analysis
3. Results
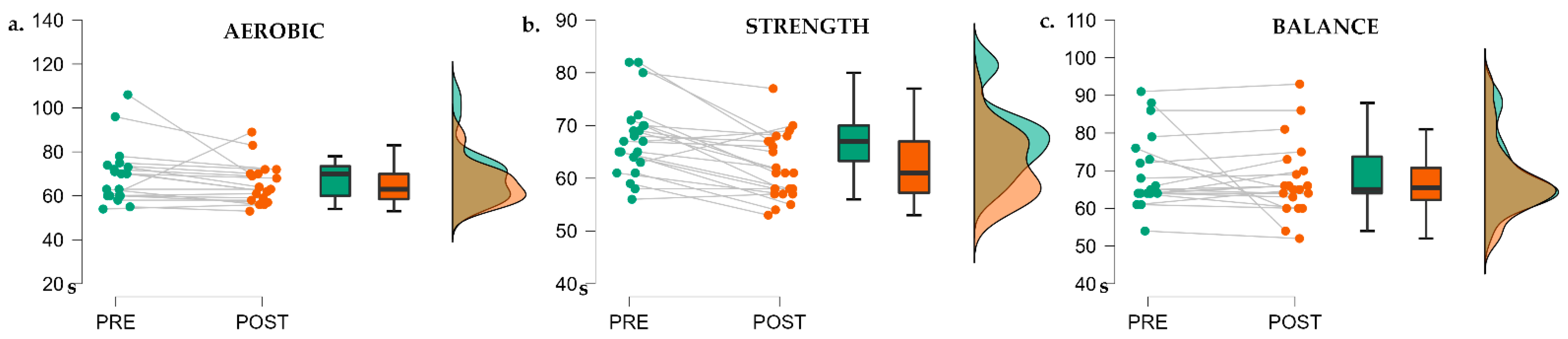
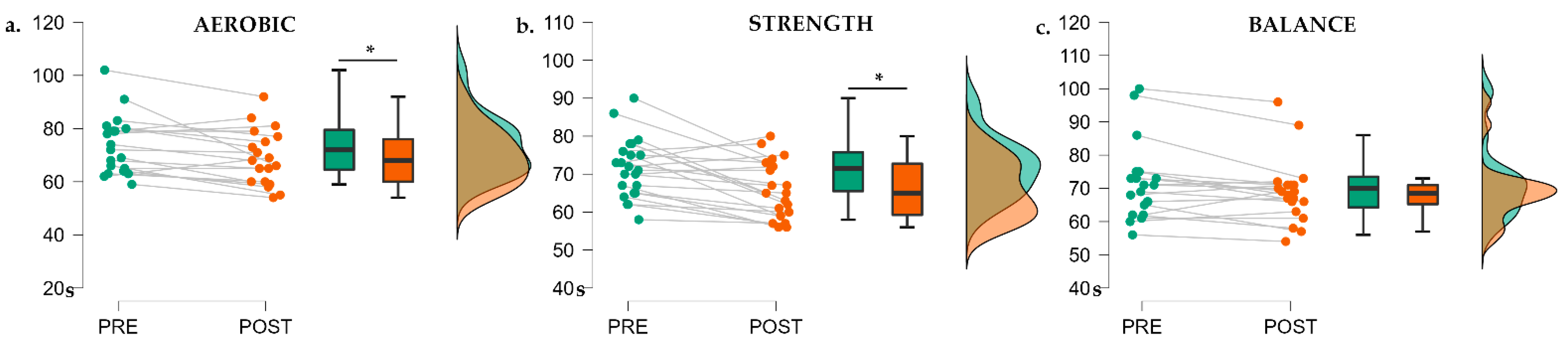
3.1. Exercise Session Intensity
3.2. Stroop Test: Successes, Failures, and Time
3.3. Stroop Test: Word Condition
3.4. Stroop Test: Color Condition
3.5. Stroop Test: Word + Color Condition
3.6. BDNF SNP Influence
4. Discussion
5. Conclusions
6. Limitations of the Study
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Park, D.C.; Yeo, S.G. Aging. Korean J. Audiol. 2013, 17, 39–44. [Google Scholar] [CrossRef][Green Version]
- Raz, N.; Rodrigue, K.M. Differential aging of the brain: Patterns, cognitive correlates and modifiers. Neurosci. Biobehav. Rev. 2006, 30, 730–748. [Google Scholar] [CrossRef]
- Bherer, L.; Erickson, K.I.; Liu-Ambrose, T. A Review of the Effects of Physical Activity and Exercise on Cognitive and Brain Functions in Older Adults. J. Aging Res. 2013, 2013, 657508. [Google Scholar] [CrossRef]
- Izquierdo, M.; Merchant, R.A.; Morley, J.E.; Anker, S.D.; Aprahamian, I.; Arai, H.; Aubertin-Leheudre, M.; Bernabei, R.; Cadore, E.L.; Cesari, M.; et al. International Exercise Recommendations in Older Adults (ICFSR): Expert Consensus Guidelines. J. Nutr. Health Aging 2021, 25, 824–853. [Google Scholar] [CrossRef]
- De Asteasu, M.L.S.; Martínez-Velilla, N.; Zambom-Ferraresi, F.; Casas-Herrero, Á.; Izquierdo, M. Role of physical exercise on cognitive function in healthy older adults: A systematic review of randomized clinical trials. Ageing Res. Rev. 2017, 37, 117–134. [Google Scholar] [CrossRef]
- Diamond, A. Executive Functions. Annu. Rev. Psychol. 2013, 64, 135–168. [Google Scholar] [CrossRef]
- Titz, C.; Karbach, J. Working memory and executive functions: Effects of training on academic achievement. Psychol. Res. 2014, 78, 852–868. [Google Scholar] [CrossRef]
- Stevens, C.; Bavelier, D. The role of selective attention on academic foundations: A cognitive neuroscience perspective. Dev. Cogn. Neurosci. 2012, 2, S30–S48. [Google Scholar] [CrossRef]
- Wilke, J.; Giesche, F.; Klier, K.; Vogt, L.; Herrmann, E.; Banzer, W. Acute Effects of Resistance Exercise on Cognitive Function in Healthy Adults: A Systematic Review with Multilevel Meta-Analysis. Sports Med. 2019, 49, 905–916. [Google Scholar] [CrossRef]
- Pedersen, B.K. The Physiology of Optimizing Health with a Focus on Exercise as Medicine. Annu. Rev. Physiol. 2019, 81, 607–627. [Google Scholar] [CrossRef]
- Chang, Y.-K.; Etnier, J.L. Exploring the Dose-Response Relationship between Resistance Exercise Intensity and Cognitive Function. J. Sport Exerc. Psychol. 2009, 31, 640–656. [Google Scholar] [CrossRef]
- Costigan, S.A.; Eather, N.; Plotnikoff, R.; Hillman, C.; Lubans, D. High-Intensity Interval Training for Cognitive and Mental Health in Adolescents. Med. Sci. Sports Exerc. 2016, 48, 1985–1993. [Google Scholar] [CrossRef]
- Davranche, K.; Brisswalter, J.; Radel, R. Where are the limits of the effects of exercise intensity on cognitive control? J. Sport Health Sci. 2015, 4, 56–63. [Google Scholar] [CrossRef]
- Jamnick, N.A.; Pettitt, R.W.; Granata, C.; Pyne, D.B.; Bishop, D.J. An Examination and Critique of Current Methods to Determine Exercise Intensity. Sports Med. 2020, 50, 1729–1756. [Google Scholar] [CrossRef]
- Jeon, Y.K.; Ha, C.H. The effect of exercise intensity on brain derived neurotrophic factor and memory in adolescents. Environ. Health Prev. Med. 2017, 22, 27. [Google Scholar] [CrossRef]
- Pastor, D.; Cervelló, E.; Peruyero, F.; Biddle, S.; Montero, C. Acute physical exercise intensity, cognitive inhibition and psychological well-being in adolescent physical education students. Curr. Psychol. 2021, 40, 5030–5039. [Google Scholar] [CrossRef]
- Peruyero, F.; Zapata, J.; Pastor, D.; Cervelló, E. The Acute Effects of Exercise Intensity on Inhibitory Cognitive Control in Adolescents. Front. Psychol. 2017, 8, 921. [Google Scholar] [CrossRef]
- I Erickson, K.; Kramer, A.F. Aerobic exercise effects on cognitive and neural plasticity in older adults. Br. J. Sports Med. 2008, 43, 22–24. [Google Scholar] [CrossRef]
- Wayne, P.M.; Bs, J.N.W.; Taylor-Piliae, R.E.; Wells, R.E.; Papp, K.V.; Donovan, N.J.; Yeh, G.Y. Effect of Tai Chi on Cognitive Performance in Older Adults: Systematic Review and Meta-Analysis. J. Am. Geriatr. Soc. 2014, 62, 25–39. [Google Scholar] [CrossRef]
- Martínez-Velilla, N.; Casas-Herrero, A.; Zambom-Ferraresi, F.; De Asteasu, M.L.S.; Lucia, A.; Galbete, A.; García-Baztán, A.; Alonso-Renedo, J.; González-Glaría, B.; Gonzalo-Lázaro, M.; et al. Effect of Exercise Intervention on Functional Decline in Very Elderly Patients During Acute Hospitalization. JAMA Intern. Med. 2019, 179, 28–36. [Google Scholar] [CrossRef]
- Cadore, E.L.; Casas-Herrero, A.; Zambom-Ferraresi, F.; Idoate, F.; Millor, N.; Gómez, M.; Rodríguez-Mañas, L.; Izquierdo, M. Multicomponent exercises including muscle power training enhance muscle mass, power output, and functional outcomes in institutionalized frail nonagenarians. AGE 2014, 36, 773–785. [Google Scholar] [CrossRef]
- Huang, E.J.; Reichardt, L.F. Neurotrophins: Roles in Neuronal Development and Function. Annu. Rev. Neurosci. 2001, 24, 677–736. [Google Scholar] [CrossRef] [PubMed]
- Kowiański, P.; Lietzau, G.; Czuba, E.; Waśkow, M.; Steliga, A.; Moryś, J. BDNF: A Key Factor with Multipotent Impact on Brain Signaling and Synaptic Plasticity. Cell. Mol. Neurobiol. 2018, 38, 579–593. [Google Scholar] [CrossRef] [PubMed]
- Bramham, C.R.; Messaoudi, E. BDNF function in adult synaptic plasticity: The synaptic consolidation hypothesis. Prog. Neurobiol. 2005, 76, 99–125. [Google Scholar] [CrossRef] [PubMed]
- Egan, M.F.; Kojima, M.; Callicott, J.H.; Goldberg, T.E.; Kolachana, B.S.; Bertolino, A.; Zaitsev, E.; Gold, B.; Goldman, D.; Dean, M.; et al. The BDNF val66met Polymorphism Affects Activity-Dependent Secretion of BDNF and Human Memory and Hippocampal Function. Cell 2003, 112, 257–269. [Google Scholar] [CrossRef]
- Canivet, A.; Albinet, C.; André, N.; Pylouster, J.; Rodríguez-Ballesteros, M.; Kitzis, A.; Audiffren, M. Effects of BDNF polymorphism and physical activity on episodic memory in the elderly: A cross sectional study. Eur. Rev. Aging Phys. Act. 2015, 12, 15. [Google Scholar] [CrossRef]
- Sánchez-Romero, M.A.; Dorado, P.; Guarino, E.; Llerena, A. Development of a new genotyping assay for detection of the BDNF Val66Met polymorphism using melting-curve analysis. Pharmacogenomics 2009, 10, 989–995. [Google Scholar] [CrossRef]
- Pastor, D.; Cervello, E.; Jimenez, M. Test Stroop UMH-MEMTRAIN v0.1; AppAndAbout: Comunidad Valenciana, Spain, 2018. [Google Scholar]
- Golden, C.J. STROOP: Test de Colores y Palabras: Manual; TEA Ediciones: Madrid, Spain, 1994. [Google Scholar]
- Martin, R.; Hernández, S.; Rodríguez, C.; García, E.; Díaz, A.; Jiménez, J.E. Datos normativos para el Test de Stroop: Patrón de desarrollo de la inhibición y formas alternativas para su evaluación. Eur. J. Psychol. Educ. 2012, 5, 39–51. [Google Scholar] [CrossRef]
- Tanaka, H.; Monahan, K.D.; Seals, D.R. Age-predicted maximal heart rate revisited. J. Am. Coll. Cardiol. 2001, 37, 153–156. [Google Scholar] [CrossRef]
- Cohen, J. Statistical power analysis. Curr. Dir. Psychol. Sci. 1992, 1, 98–101. [Google Scholar] [CrossRef]
- Pontifex, M.B.; McGowan, A.L.; Chandler, M.C.; Gwizdala, K.L.; Parks, A.C.; Fenn, K.; Kamijo, K. A primer on investigating the after effects of acute bouts of physical activity on cognition. Psychol. Sport Exerc. 2019, 40, 1–22. [Google Scholar] [CrossRef]
- Biazus-Sehn, L.F.; Schuch, F.B.; Firth, J.; Stigger, F.D.S. Effects of physical exercise on cognitive function of older adults with mild cognitive impairment: A systematic review and meta-analysis. Arch. Gerontol. Geriatr. 2020, 89, 104048. [Google Scholar] [CrossRef] [PubMed]
- Herold, F.; Müller, P.; Gronwald, T.; Müller, N.G. Dose–Response Matters!—A Perspective on the Exercise Prescription in Exercise–Cognition Research. Front. Psychol. 2019, 10, 2338. [Google Scholar] [CrossRef] [PubMed]
- Lim, K.H.-L.; Pysklywec, A.; Plante, M.; Demers, L. The effectiveness of Tai Chi for short-term cognitive function improvement in the early stages of dementia in the elderly: A systematic literature review. Clin. Interv. Aging 2019, 14, 827–839. [Google Scholar] [CrossRef]
- ACSM. ACSM´s Guidelines for Exercise Testing and Prescription, 8th ed.; Wilkins, L.W., Ed.; Wolters Kluwer: New York, NY, USA, 2010. [Google Scholar]
- Chang, Y.K.; Labban, J.D.; Gapin, J.I.; Etnier, J.L. The effects of acute exercise on cognitive performance: A meta-analysis. Brain Res. 2012, 1453, 87–101. [Google Scholar] [CrossRef] [PubMed]
- Day, M.L.; McGuigan, M.R.; Brice, G.; Foster, C. Monitoring Exercise Intensity During Resistance Training Using the Session RPE Scale. J. Strength Cond. Res. 2004, 18, 353–358. [Google Scholar] [CrossRef]
- Foster, J.P.; Carl, H.; Kara, M.; Esten, P.L.; Brice, G. Differences in perceptions of training by coaches and athletes. S. Afr. J. Sports Med. 2001, 8, 3–7. [Google Scholar]
- Laforgia, J.; Withers, R.T.; Gore, C.J. Effects of exercise intensity and duration on the excess post-exercise oxygen consumption. J. Sports Sci. 2006, 24, 1247–1264. [Google Scholar] [CrossRef]
- Robertson, R.J.; Noble, B.J. Perception of physical exertion: Methods, mediators, and applications. Exerc. Sport Sci. Rev. 1997, 25, 407–452. [Google Scholar] [CrossRef]
- Gallardo-Gómez, D.; del Pozo-Cruz, J.; Noetel, M.; Álvarez-Barbosa, F.; Alfonso-Rosa, R.M.; Cruz, B.D.P. Optimal Dose and Type of Exercise to Improve Cognitive Function in Older Adults: A Systematic Review and Bayesian Model-Based Network Meta-Analysis of RCTs. Ageing Res. Rev. 2022, 76, 101591. [Google Scholar] [CrossRef]
- Heras, B.D.L.; Rodrigues, L.; Cristini, J.; Weiss, M.; Prats-Puig, A.; Roig, M. Does the Brain-Derived Neurotrophic Factor Val66Met Polymorphism Modulate the Effects of Physical Activity and Exercise on Cognition? Neuroscientist 2020, 28, 69–86. [Google Scholar] [CrossRef] [PubMed]
- Norman, J.E.; Rutkowsky, J.; Bodine, S.; Rutledge, J.C. The Potential Mechanisms of Exercise-induced Cognitive Protection: A Literature Review. Curr. Pharm. Des. 2018, 24, 1827–1831. [Google Scholar] [CrossRef] [PubMed]
- Van Praag, H. Neurogenesis and Exercise: Past and Future Directions. NeuroMolecular Med. 2008, 10, 128–140. [Google Scholar] [CrossRef]
- McMorris, T. Developing the catecholamines hypothesis for the acute exercise-cognition interaction in humans: Lessons from animal studies. Physiol. Behav. 2016, 165, 291–299. [Google Scholar] [CrossRef]
- Hashimoto, T.; Tsukamoto, H.; Ando, S.; Ogoh, S. Effect of Exercise on Brain Health: The Potential Role of Lactate as a Myokine. Metabolites 2021, 11, 813. [Google Scholar] [CrossRef]
- Cervelló, E.; Peruyero, F.; Montero, C.; González-Cutre, D.; Beltrán-Carrillo, V.J.; Moreno-Murcia, J.A. Exercise, psy-chological well-being, sleep quality and situational motivation in physical education students. Cuad. Psicol. 2014, 14, 31–38. [Google Scholar]
- Garcia, D.; Jimmefors, A.; Mousavi, F.; Adrianson, L.; Rosenberg, P.; Archer, T. Self-regulatory mode (locomotion and assessment), well-being (subjective and psychological), and exercise behavior (frequency and intensity) in relation to high school pupils’ academic achievement. PeerJ 2015, 3, e847. [Google Scholar] [CrossRef]
- Yu, L.; Shek, D.T.L.; Zhu, X. The Influence of Personal Well-Being on Learning Achievement in University Students Over Time: Mediating or Moderating Effects of Internal and External University Engagement. Front. Psychol. 2018, 8, 2287. [Google Scholar] [CrossRef]
- Stillman, C.M.; Cohen, J.; Lehman, M.E.; Erickson, K.I. Mediators of Physical Activity on Neurocognitive Function: A Review at Multiple Levels of Analysis. Front. Hum. Neurosci. 2016, 10, 626. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carbonell-Hernandez, L.; Ballester-Ferrer, J.A.; Sitges-Macia, E.; Bonete-Lopez, B.; Roldan, A.; Cervello, E.; Pastor, D. Different Exercise Types Produce the Same Acute Inhibitory Control Improvements When the Subjective Intensity Is Equal. Int. J. Environ. Res. Public Health 2022, 19, 9748. https://doi.org/10.3390/ijerph19159748
Carbonell-Hernandez L, Ballester-Ferrer JA, Sitges-Macia E, Bonete-Lopez B, Roldan A, Cervello E, Pastor D. Different Exercise Types Produce the Same Acute Inhibitory Control Improvements When the Subjective Intensity Is Equal. International Journal of Environmental Research and Public Health. 2022; 19(15):9748. https://doi.org/10.3390/ijerph19159748
Chicago/Turabian StyleCarbonell-Hernandez, Laura, Juan Arturo Ballester-Ferrer, Esther Sitges-Macia, Beatriz Bonete-Lopez, Alba Roldan, Eduardo Cervello, and Diego Pastor. 2022. "Different Exercise Types Produce the Same Acute Inhibitory Control Improvements When the Subjective Intensity Is Equal" International Journal of Environmental Research and Public Health 19, no. 15: 9748. https://doi.org/10.3390/ijerph19159748
APA StyleCarbonell-Hernandez, L., Ballester-Ferrer, J. A., Sitges-Macia, E., Bonete-Lopez, B., Roldan, A., Cervello, E., & Pastor, D. (2022). Different Exercise Types Produce the Same Acute Inhibitory Control Improvements When the Subjective Intensity Is Equal. International Journal of Environmental Research and Public Health, 19(15), 9748. https://doi.org/10.3390/ijerph19159748






