Does Exercise Training Improve Cardiac-Parasympathetic Nervous System Activity in Sedentary People? A Systematic Review with Meta-Analysis
Abstract
1. Introduction
2. Method
2.1. Data Search and Sources
2.2. Study Selection
2.3. Data Extraction, Coding Study Characteristics and Potential Moderator Variables
2.4. Methodological Quality Assessment
2.5. Computation of Effect Size and Statistical Analyses
3. Results
3.1. Methodological Quality Assessment
3.2. Outcome Measures
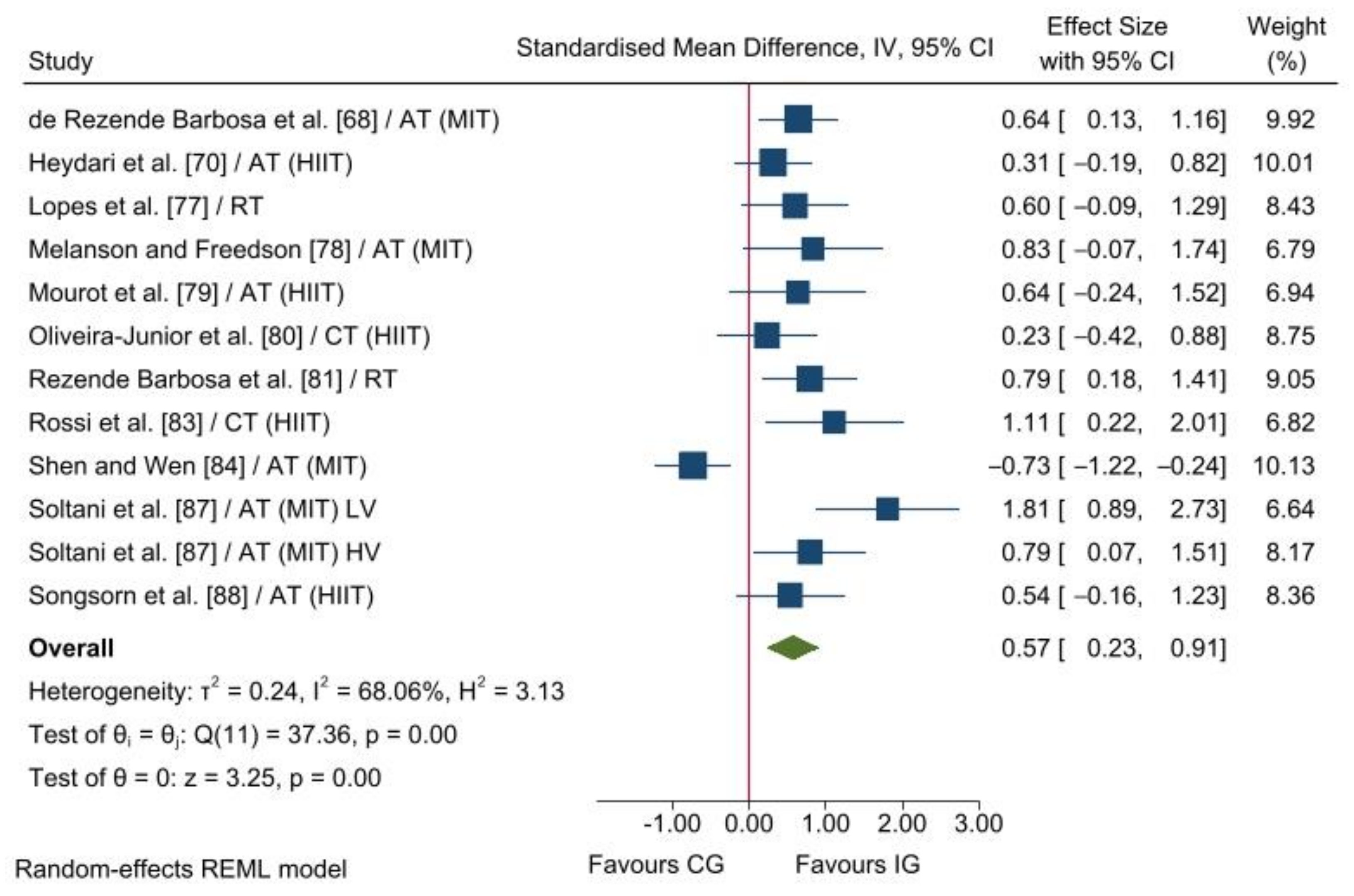
3.2.1. Parasympathetic Nervous System Modulation
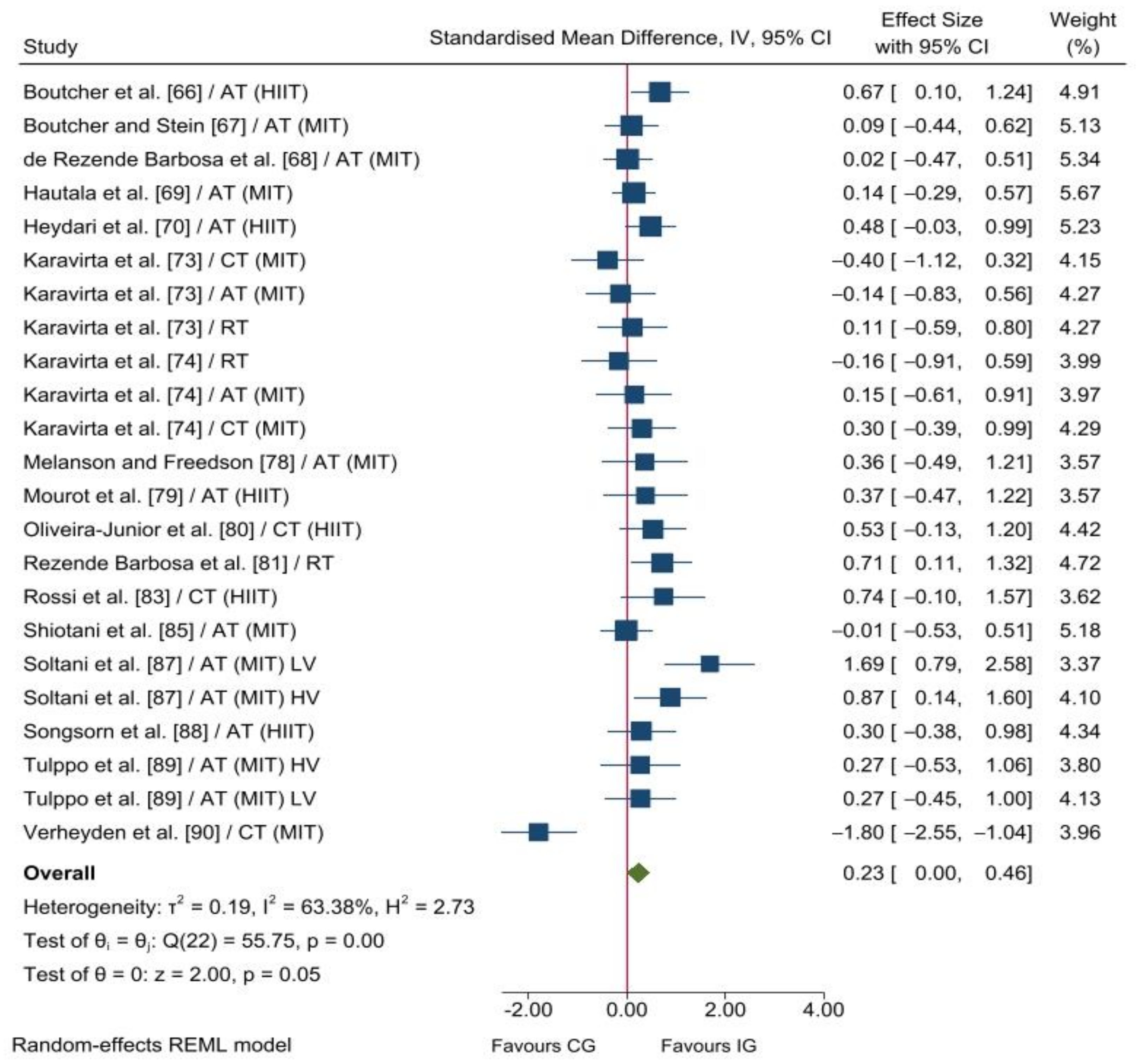
3.2.2. Parasympathetic Nervous System Reactivation
4. Discussion
4.1. Parasympathetic Nervous System Modulation
4.2. Parasympathetic Nervous System Reactivation
4.3. Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lahiri, M.K.; Kannankeril, P.J.; Goldberger, J.J. Assessment of autonomic function in cardiovascular disease: Physiological basis and prognostic implications. J. Am. Coll. Cardiol. 2008, 51, 1725–1733. [Google Scholar] [CrossRef] [PubMed]
- Thayer, J.F.; Sternberg, E. Beyond heart rate variability: Vagal regulation of allostatic systems. Ann. N. Y. Acad. Sci. 2006, 1088, 361–372. [Google Scholar] [CrossRef] [PubMed]
- Araujo, C.G.; Nobrega, A.C.; Castro, C.L. Heart rate responses to deep breathing and 4-seconds of exercise before and after pharmacological blockade with atropine and propranolol. Clin. Auton. Res. 1992, 2, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Montano, N.; Ruscone, T.G.; Porta, A.; Lombardi, F.; Pagani, M.; Malliani, A. Power spectrum analysis of heart rate variability to assess the changes in sympathovagal balance during graded orthostatic tilt. Circulation 1994, 90, 1826–1831. [Google Scholar] [CrossRef] [PubMed]
- Perini, R.; Orizio, C.; Gamba, A.; Veicsteinas, A. Kinetics of heart rate and catecholamines during exercise in humans. The effect of heart denervation. Eur. J. Appl. Physiol. Occup. Physiol 1993, 66, 500–506. [Google Scholar] [CrossRef]
- Tulppo, M.P.; Mäkikallio, T.H.; Takala, T.E.; Seppänen, T.; Huikuri, H.V. Quantitative beat-to-beat analysis of heart rate dynamics during exercise. Am. J. Physiol. 1996, 271, H244–H252. [Google Scholar] [CrossRef]
- Goldberger, J.J.; Le, F.K.; Lahiri, M.; Kannankeril, P.J.; Ng, J.; Kadish, A.H. Assessment of parasympathetic reactivation after exercise. Am. J. Physiol. Heart Circ. Physiol. 2006, 290, H2446–H2452. [Google Scholar] [CrossRef]
- Al Haddad, H.; Laursen, P.B.; Chollet, D.; Lemaitre, F.; Ahmaidi, S.; Buchheit, M. Effect of cold or thermoneutral water immersion on post-exercise heart rate recovery and heart rate variability indices. Auton. Neurosci. 2010, 156, 111–116. [Google Scholar] [CrossRef]
- Buchheit, M.; Papelier, Y.; Laursen, P.B.; Ahmaidi, S. Noninvasive assessment of cardiac parasympathetic function: Postexercise heart rate recovery or heart rate variability? Am. J. Physiol. Heart Circ. Physiol. 2007, 293, H8–H10. [Google Scholar] [CrossRef]
- Kleiger, R.E.; Stein, P.K.; Bigger, J.T., Jr. Heart rate variability: Measurement and clinical utility. Ann. Noninvasive Electrocardiol. 2005, 10, 88–101. [Google Scholar] [CrossRef]
- Shaffer, F.; McCraty, R.; Zerr, C.L. A healthy heart is not a metronome: An integrative review of the heart’s anatomy and heart rate variability. Front. Psychol. 2014, 5, 1040. [Google Scholar] [CrossRef]
- Buchheit, M. Monitoring training status with HR measures: Do all roads lead to Rome? Front. Physiol. 2014, 5, 73. [Google Scholar] [CrossRef]
- Plews, D.J.; Laursen, P.B.; Stanley, J.; Kilding, A.E.; Buchheit, M. Training adaptation and heart rate variability in elite endurance athletes: Opening the door to effective monitoring. Sport. Med. 2013, 43, 773–781. [Google Scholar] [CrossRef]
- Peçanha, T.; Silva-Júnior, N.D.; Forjaz, C.L. Heart rate recovery: Autonomic determinants, methods of assessment and association with mortality and cardiovascular diseases. Clin. Physiol. Funct. Imaging 2014, 34, 327–339. [Google Scholar] [CrossRef]
- Imai, K.; Sato, H.; Hori, M.; Kusuoka, H.; Ozaki, H.; Yokoyama, H.; Takeda, H.; Inoue, M.; Kamada, T. Vagally mediated heart rate recovery after exercise is accelerated in athletes but blunted in patients with chronic heart failure. J. Am. Coll. Cardiol. 1994, 24, 1529–1535. [Google Scholar] [CrossRef]
- Dewland, T.A.; Androne, A.S.; Lee, F.A.; Lampert, R.J.; Katz, S.D. Effect of acetylcholinesterase inhibition with pyridostigmine on cardiac parasympathetic function in sedentary adults and trained athletes. Am. J. Physiol. Heart Circ. Physiol. 2007, 293, H86–H92. [Google Scholar] [CrossRef]
- Shaffer, F.; Ginsberg, J.P. An Overview of Heart Rate Variability Metrics and Norms. Front. Public Health 2017, 5, 258. [Google Scholar] [CrossRef]
- Esco, M.R.; Flatt, A.A. Ultra-short-term heart rate variability indexes at rest and post-exercise in athletes: Evaluating the agreement with accepted recommendations. J. Sport. Sci. Med. 2014, 13, 535–541. [Google Scholar]
- Pereira, L.A.; Flatt, A.A.; Ramirez-Campillo, R.; Loturco, I.; Nakamura, F.Y. Assessing Shortened Field-Based Heart-Rate-Variability-Data Acquisition in Team-Sport Athletes. Int. J. Sport. Physiol. Perform. 2016, 11, 154–158. [Google Scholar] [CrossRef]
- Heart rate variability. Standards of measurement, physiological interpretation, and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Eur. Heart J. 1996, 17, 354–381. [CrossRef]
- Buchheit, M.; Laursen, P.B.; Al Haddad, H.; Ahmaidi, S. Exercise-induced plasma volume expansion and post-exercise parasympathetic reactivation. Eur. J. Appl. Physiol. 2009, 105, 471–481. [Google Scholar] [CrossRef]
- Catai, A.M.; Pastre, C.M.; Godoy, M.F.; Silva, E.D.; Takahashi, A.C.M.; Vanderlei, L.C.M. Heart rate variability: Are you using it properly? Standardisation checklist of procedures. Braz. J. Phys. Ther. 2020, 24, 91–102. [Google Scholar] [CrossRef]
- Al Haddad, H.; Laursen, P.B.; Chollet, D.; Ahmaidi, S.; Buchheit, M. Reliability of resting and postexercise heart rate measures. Int. J. Sport. Med. 2011, 32, 598–605. [Google Scholar] [CrossRef]
- Sanderson, J.E.; Yeung, L.Y.; Yeung, D.T.; Kay, R.L.; Tomlinson, B.; Critchley, J.A.; Woo, K.S.; Bernardi, L. Impact of changes in respiratory frequency and posture on power spectral analysis of heart rate and systolic blood pressure variability in normal subjects and patients with heart failure. Clin. Sci. 1996, 91, 35–43. [Google Scholar] [CrossRef]
- Goldberger, J.J.; Challapalli, S.; Tung, R.; Parker, M.A.; Kadish, A.H. Relationship of heart rate variability to parasympathetic effect. Circulation 2001, 103, 1977–1983. [Google Scholar] [CrossRef]
- Kiviniemi, A.M.; Hautala, A.J.; Mäkikallio, T.H.; Seppänen, T.; Huikuri, H.V.; Tulppo, M.P. Cardiac vagal outflow after aerobic training by analysis of high-frequency oscillation of the R-R interval. Eur. J. Appl. Physiol. 2006, 96, 686–692. [Google Scholar] [CrossRef] [PubMed]
- Buchheit, M.; Chivot, A.; Parouty, J.; Mercier, D.; Al Haddad, H.; Laursen, P.B.; Ahmaidi, S. Monitoring endurance running performance using cardiac parasympathetic function. Eur. J. Appl. Physiol. 2010, 108, 1153–1167. [Google Scholar] [CrossRef]
- Plews, D.J.; Laursen, P.B.; Kilding, A.E.; Buchheit, M. Heart rate variability in elite triathletes, is variation in variability the key to effective training? A case comparison. Eur. J. Appl. Physiol. 2012, 112, 3729–3741. [Google Scholar] [CrossRef]
- Plews, D.J.; Laursen, P.B.; Kilding, A.E.; Buchheit, M. Evaluating training adaptation with heart-rate measures: A methodological comparison. Int. J. Sport. Physiol. Perform. 2013, 8, 688–691. [Google Scholar] [CrossRef]
- Plews, D.J.; Laursen, P.B.; Le Meur, Y.; Hausswirth, C.; Kilding, A.E.; Buchheit, M. Monitoring training with heart rate-variability: How much compliance is needed for valid assessment? Int. J. Sport. Physiol. Perform. 2014, 9, 783–790. [Google Scholar] [CrossRef]
- Carter, R., 3rd; Watenpaugh, D.E.; Wasmund, W.L.; Wasmund, S.L.; Smith, M.L. Muscle pump and central command during recovery from exercise in humans. J. Appl. Physiol. 1999, 87, 1463–1469. [Google Scholar] [CrossRef] [PubMed]
- Shibasaki, M.; Sakai, M.; Oda, M.; Crandall, C.G. Muscle mechanoreceptor modulation of sweat rate during recovery from moderate exercise. J. Appl. Physiol. 2004, 96, 2115–2119. [Google Scholar] [CrossRef] [PubMed]
- Anderson, N.B.; Bulatao, R.A.; Cohen, B.; National Research Council (US) Panel on Race, Ethnicity and Health in Later Life (Eds.) Critical Perspectives on Racial and Ethnic Differences in Health in Late Life. A Neurovisceral Integration Model of Health Disparities in Aging. In Critical Perspectives on Racial and Ethnic Differences in Health in Late Life; National Academies Press: Washington, DC, USA, 2004; ISBN 0-309-53200-0. [Google Scholar]
- Brook, R.D.; Julius, S. Autonomic imbalance, hypertension, and cardiovascular risk. Am. J. Hypertens. 2000, 13, 112s–122s. [Google Scholar] [CrossRef]
- Chen, X.J.; Barywani, S.B.; Hansson, P.O.; Östgärd Thunström, E.; Rosengren, A.; Ergatoudes, C.; Mandalenakis, Z.; Caidahl, K.; Fu, M.L. Impact of changes in heart rate with age on all-cause death and cardiovascular events in 50-year-old men from the general population. Open Heart 2019, 6, e000856. [Google Scholar] [CrossRef]
- Lachman, S.; Terbraak, M.S.; Limpens, J.; Jorstad, H.; Lucas, C.; Scholte Op Reimer, W.; Boekholdt, S.M.; Ter Riet, G.; Peters, R.J.G. The prognostic value of heart rate recovery in patients with coronary artery disease: A systematic review and meta-analysis. Am. Heart J. 2018, 199, 163–169. [Google Scholar] [CrossRef]
- Xu, T.; Zhan, Y.; Xiong, J.; Lu, N.; He, Z.; Su, X.; Tan, X. The relationship between heart rate and mortality of patients with acute coronary syndromes in the coronary intervention era: Meta-analysis. Medicine 2016, 95, e5371. [Google Scholar] [CrossRef]
- Blair, S.N. Physical inactivity: The biggest public health problem of the 21st century. Br. J. Sport. Med. 2009, 43, 1–2. [Google Scholar]
- Danaei, G.; Ding, E.L.; Mozaffarian, D.; Taylor, B.; Rehm, J.; Murray, C.J.; Ezzati, M. The preventable causes of death in the United States: Comparative risk assessment of dietary, lifestyle, and metabolic risk factors. PLoS Med. 2009, 6, e1000058. [Google Scholar] [CrossRef]
- Morris, J.N.; Heady, J.A.; Raffle, P.A.; Roberts, C.G.; Parks, J.W. Coronary heart-disease and physical activity of work. Lancet 1953, 262, 1053–1057. [Google Scholar] [CrossRef]
- Cornelissen, V.A.; Fagard, R.H. Effects of endurance training on blood pressure, blood pressure-regulating mechanisms, and cardiovascular risk factors. Hypertension 2005, 46, 667–675. [Google Scholar] [CrossRef]
- Esler, M.; Rumantir, M.; Kaye, D.; Jennings, G.; Hastings, J.; Socratous, F.; Lambert, G. Sympathetic nerve biology in essential hypertension. Clin. Exp. Pharmacol. Physiol. 2001, 28, 986–989. [Google Scholar] [CrossRef]
- Fagard, R.H.; Cornelissen, V.A. Effect of exercise on blood pressure control in hypertensive patients. Eur. J. Cardiovasc. Prev. Rehabil. 2007, 14, 12–17. [Google Scholar] [CrossRef]
- Guyenet, P.G. The sympathetic control of blood pressure. Nat. Rev. Neurosci. 2006, 7, 335–346. [Google Scholar] [CrossRef]
- Julius, S.; Valentini, M. Consequences of the increased autonomic nervous drive in hypertension, heart failure and diabetes. Blood Press. Suppl. 1998, 3, 5–13. [Google Scholar] [CrossRef]
- Schlaich, M.P.; Lambert, E.; Kaye, D.M.; Krozowski, Z.; Campbell, D.J.; Lambert, G.; Hastings, J.; Aggarwal, A.; Esler, M.D. Sympathetic augmentation in hypertension: Role of nerve firing, norepinephrine reuptake, and Angiotensin neuromodulation. Hypertension 2004, 43, 169–175. [Google Scholar] [CrossRef]
- Straznicky, N.E.; Lambert, E.A.; Lambert, G.W.; Masuo, K.; Esler, M.D.; Nestel, P.J. Effects of dietary weight loss on sympathetic activity and cardiac risk factors associated with the metabolic syndrome. J. Clin. Endocrinol. Metab. 2005, 90, 5998–6005. [Google Scholar] [CrossRef]
- Fisher, J.P.; Paton, J.F. The sympathetic nervous system and blood pressure in humans: Implications for hypertension. J. Hum. Hypertens. 2012, 26, 463–475. [Google Scholar] [CrossRef]
- Fisher, J.P.; Young, C.N.; Fadel, P.J. Central sympathetic overactivity: Maladies and mechanisms. Auton. Neurosci. 2009, 148, 5–15. [Google Scholar] [CrossRef]
- Kang, J.; Chang, Y.; Kim, Y.; Shin, H.; Ryu, S. Ten-Second Heart Rate Variability, Its Changes Over Time, and the Development of Hypertension. Hypertension 2022, 79, 1308–1318. [Google Scholar] [CrossRef]
- Schaarup, J.; Christensen, M.S.; Hulman, A.; Hansen, C.S.; Vistisen, D.; Tabak, A.G.; Witte, D.R.; Bjerg, L. Autonomic dysfunction is associated with the development of arterial stiffness: The Whitehall II cohort. medRxiv 2022. [Google Scholar] [CrossRef]
- Sandercock, G.R.; Bromley, P.D.; Brodie, D.A. Effects of exercise on heart rate variability: Inferences from meta-analysis. Med Sci. Sport. Exerc. 2005, 37, 433–439. [Google Scholar] [CrossRef] [PubMed]
- Manresa-Rocamora, A.; Ribeiro, F.; Casanova-Lizón, A.; Flatt, A.A.; Sarabia, J.M.; Moya-Ramón, M. Cardiac Rehabilitation Improves Endothelial Function in Coronary Artery Disease Patients. Int. J. Sport. Med. 2022, 43, 905–920. [Google Scholar] [CrossRef]
- Pearson, M.J.; Smart, N.A. Effect of exercise training on endothelial function in heart failure patients: A systematic review meta-analysis. Int. J. Cardiol. 2017, 231, 234–243. [Google Scholar] [CrossRef]
- Pearson, M.J.; Smart, N.A. Aerobic Training Intensity for Improved Endothelial Function in Heart Failure Patients: A Systematic Review and Meta-Analysis. Cardiol. Res. Pract. 2017, 2017, 2450202. [Google Scholar] [CrossRef]
- Manresa-Rocamora, A.; Ribeiro, F.; Sarabia, J.M.; Íbias, J.; Oliveira, N.L.; Vera-García, F.J.; Moya-Ramón, M. Exercise-based cardiac rehabilitation and parasympathetic function in patients with coronary artery disease: A systematic review and meta-analysis. Clin. Auton. Res. 2021, 31, 187–203. [Google Scholar] [CrossRef] [PubMed]
- Helgerud, J.; Høydal, K.; Wang, E.; Karlsen, T.; Berg, P.; Bjerkaas, M.; Simonsen, T.; Helgesen, C.; Hjorth, N.; Bach, R.; et al. Aerobic high-intensity intervals improve VO2max more than moderate training. Med. Sci. Sport. Exerc. 2007, 39, 665–671. [Google Scholar] [CrossRef] [PubMed]
- Pattyn, N.; Beulque, R.; Cornelissen, V. Aerobic Interval vs. Continuous Training in Patients with Coronary Artery Disease or Heart Failure: An Updated Systematic Review and Meta-Analysis with a Focus on Secondary Outcomes. Sport. Med. 2018, 48, 1189–1205. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. J. Clin. Epidemiol. 2021, 134, 178–189. [Google Scholar] [CrossRef]
- Smart, N.A.; Waldron, M.; Ismail, H.; Giallauria, F.; Vigorito, C.; Cornelissen, V.; Dieberg, G. Validation of a new tool for the assessment of study quality and reporting in exercise training studies: TESTEX. Int. J. Evid. Based Healthc. 2015, 13, 9–18. [Google Scholar] [CrossRef]
- Hedges, L.V.; Olkin, I. Statistical Methods for Meta-Analysis; Academic Press: Cambridge, UK, 2014; ISBN 0-12-336380-2. [Google Scholar]
- Higgins, J.P.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. Cochrane Handbook for Systematic Reviews of Interventions, 2nd ed.; John Wiley & Sons: Chichester, UK, 2019; ISBN 9781119536628. [Google Scholar]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences; Academic Press: Cambridge, UK, 2013; ISBN 9780203771587. [Google Scholar]
- Egger, M.; Davey Smith, G.; Schneider, M.; Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997, 315, 629–634. [Google Scholar] [CrossRef]
- Audette, J.F.; Jin, Y.S.; Newcomer, R.; Stein, L.; Duncan, G.; Frontera, W.R. Tai Chi versus brisk walking in elderly women. Age Ageing 2006, 35, 388–393. [Google Scholar] [CrossRef]
- Boutcher, S.H.; Park, Y.; Dunn, S.L.; Boutcher, Y.N. The relationship between cardiac autonomic function and maximal oxygen uptake response to high-intensity intermittent-exercise training. J. Sport. Sci. 2013, 31, 1024–1029. [Google Scholar] [CrossRef]
- Boutcher, S.H.; Stein, P. Association between heart rate variability and training response in sedentary middle-aged men. Eur. J. Appl. Physiol. Occup. Physiol. 1995, 70, 75–80. [Google Scholar] [CrossRef]
- De Rezende Barbosa, M.P.; Vanderlei, L.C.M.; Neves, L.M.; Takahashi, C.; Torquato, P.; Fortaleza, A.C.S.; Freitas Júnior, I.F.; Sorpreso, I.C.E.; Abreu, L.C.; Pérez Riera, A.R. Impact of functional training on geometric indices and fractal correlation property of heart rate variability in postmenopausal women. Ann. Noninvasive Electrocardiol. 2018, 23, e12469. [Google Scholar] [CrossRef]
- Hautala, A.J.; Rankinen, T.; Kiviniemi, A.M.; Mäkikallio, T.H.; Huikuri, H.V.; Bouchard, C.; Tulppo, M.P. Heart rate recovery after maximal exercise is associated with acetylcholine receptor M2 (CHRM2) gene polymorphism. Am. J. Physiol. Heart Circ. Physiol. 2006, 291, H459–H466. [Google Scholar] [CrossRef]
- Heydari, M.; Boutcher, Y.N.; Boutcher, S.H. High-intensity intermittent exercise and cardiovascular and autonomic function. Clin. Auton. Res. 2013, 23, 57–65. [Google Scholar] [CrossRef]
- Jelinek, H.F.; Karmakar, C.; Kiviniemi, A.M.; Hautala, A.J.; Tulppo, M.P.; Mäkikallio, T.H.; Huikuri, H.V.; Khandoker, A.H.; Palaniswami, M. Temporal dynamics of the circadian heart rate following low and high volume exercise training in sedentary male subjects. Eur. J. Appl. Physiol. 2015, 115, 2069–2080. [Google Scholar] [CrossRef]
- Kanegusuku, H.; Queiroz, A.C.; Silva, V.J.; de Mello, M.T.; Ugrinowitsch, C.; Forjaz, C.L. High-Intensity Progressive Resistance Training Increases Strength With No Change in Cardiovascular Function and Autonomic Neural Regulation in Older Adults. J. Aging Phys. Act. 2015, 23, 339–345. [Google Scholar] [CrossRef]
- Karavirta, L.; Costa, M.D.; Goldberger, A.L.; Tulppo, M.P.; Laaksonen, D.E.; Nyman, K.; Keskitalo, M.; Häkkinen, A.; Häkkinen, K. Heart rate dynamics after combined strength and endurance training in middle-aged women: Heterogeneity of responses. PLoS ONE 2013, 8, e72664. [Google Scholar] [CrossRef]
- Karavirta, L.; Tulppo, M.P.; Laaksonen, D.E.; Nyman, K.; Laukkanen, R.T.; Kinnunen, H.; Häkkinen, A.; Häkkinen, K. Heart rate dynamics after combined endurance and strength training in older men. Med. Sci. Sport. Exerc. 2009, 41, 1436–1443. [Google Scholar] [CrossRef]
- Kim, C.S.; Kim, M.K.; Jung, H.Y.; Kim, M.J. Effects of exercise training intensity on cardiac autonomic regulation in habitual smokers. Ann. Noninvasive Electrocardiol. 2017, 22, e12434. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.M.; Wood, R.H.; Welsch, M.A. Influence of short-term endurance exercise training on heart rate variability. Med. Sci. Sport. Exerc. 2003, 35, 961–969. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lopes, F.L.; Pereira, F.M.; Reboredo, M.M.; Castro, T.M.; Vianna, J.M.; Novo, J.M., Jr.; Silva, L.P. Reduction of heart rate variability in middle-aged individuals and the effect of strength training. Braz. J. Phys. Ther. 2007, 11, 113–119. [Google Scholar] [CrossRef]
- Melanson, E.L.; Freedson, P.S. The effect of endurance training on resting heart rate variability in sedentary adult males. Eur. J. Appl. Physiol. 2001, 85, 442–449. [Google Scholar] [CrossRef] [PubMed]
- Mourot, L.; Tordi, N.; Perrey, S.; Bouhaddi, M.; Rouillon, J.D.; Regnard, J. Overall increase in heart rate variability after the Square-Wave Endurance Exercise Test training. Sport Sci. 2005, 20, 83–90. [Google Scholar] [CrossRef]
- Oliveira-Junior, S.A.; Boullosa, D.; Mendonça, M.L.M.; Vieira, L.F.C.; Mattos, W.W.; Amaral, B.O.C.; Lima-Borges, D.S.; Reis, F.A.; Cezar, M.D.M.; Vanderlei, L.C.M.; et al. Effects of Circuit Weight-Interval Training on Physical Fitness, Cardiac Autonomic Control, and Quality of Life in Sedentary Workers. Int. J. Environ. Res. Public Health 2021, 18, 4606. [Google Scholar] [CrossRef]
- Rezende Barbosa, M.P.; Netto Júnior, J.; Cassemiro, B.M.; de Souza, N.M.; Bernardo, A.F.; da Silva, A.K.; Pastre, C.M.; Vanderlei, L.C. Impact of functional training on cardiac autonomic modulation, cardiopulmonary parameters and quality of life in healthy women. Clin. Physiol. Funct. Imaging 2016, 36, 318–325. [Google Scholar] [CrossRef]
- Romagnoli, M.; Alis, R.; Sanchis-Gomar, F.; Lippi, G.; Arduini, A. An Eighteen-Minute Submaximal Exercise Test to Assess Cardiac Fitness in Response to Aerobic Training. J. Strength Cond. Res. 2018, 32, 2846–2852. [Google Scholar] [CrossRef]
- Rossi, F.E.; Ricci-Vitor, A.L.; Buonani, C.S.; Vanderlei, L.C.; Freitas Junior, I.F. The effects of combined aerobic and resistance training on heart rate variability in postmenopausal women. Medicina 2013, 46, 171–177. [Google Scholar]
- Shen, T.W.; Wen, H.J. Aerobic exercise affects T-wave alternans and heart rate variability in postmenopausal women. Int. J. Sport. Med. 2013, 34, 1099–1105. [Google Scholar] [CrossRef]
- Shiotani, H.; Umegaki, Y.; Tanaka, M.; Kimura, M.; Ando, H. Effects of aerobic exercise on the circadian rhythm of heart rate and blood pressure. Chronobiol. Int. 2009, 26, 1636–1646. [Google Scholar] [CrossRef]
- Sloan, R.P.; Shapiro, P.A.; Lauriola, V.; McIntyre, K.; Pavlicova, M.; Choi, C.J.; Choo, T.H.; Scodes, J.M. The Impact of Aerobic Training on Cardiovascular Reactivity to and Recovery From Psychological and Orthostatic Challenge. Psychosom. Med. 2021, 83, 125–137. [Google Scholar] [CrossRef]
- Soltani, M.; Baluchi, M.J.; Boullosa, D.; Daraei, A.; Doyle-Baker, P.K.; Saeidi, A.; Knechtle, B.; Dehbaghi, K.M.; Mollabashi, S.S.; VanDusseldorp, T.A.; et al. Effect of Intensity on Changes in Cardiac Autonomic Control of Heart Rate and Arterial Stiffness After Equated Continuous Running Training Programs. Front. Physiol. 2021, 12, 758299. [Google Scholar] [CrossRef]
- Songsorn, P.; Somnarin, K.; Jaitan, S.; Kupradit, A. The effect of whole-body high-intensity interval training on heart rate variability in insufficiently active adults. J. Exerc. Sci. Fit. 2022, 20, 48–53. [Google Scholar] [CrossRef]
- Tulppo, M.P.; Hautala, A.J.; Mäkikallio, T.H.; Laukkanen, R.T.; Nissilä, S.; Hughson, R.L.; Huikuri, H.V. Effects of aerobic training on heart rate dynamics in sedentary subjects. J. Appl. Physiol. 2003, 95, 364–372. [Google Scholar] [CrossRef]
- Verheyden, B.; Eijnde, B.O.; Beckers, F.; Vanhees, L.; Aubert, A.E. Low-dose exercise training does not influence cardiac autonomic control in healthy sedentary men aged 55–75 years. J. Sport. Sci. 2006, 24, 1137–1147. [Google Scholar] [CrossRef]
- Pearson, M.J.; Smart, N.A. Exercise therapy and autonomic function in heart failure patients: A systematic review and meta-analysis. Heart Fail. Rev. 2018, 23, 91–108. [Google Scholar] [CrossRef]
- Picard, M.; Tauveron, I.; Magdasy, S.; Benichou, T.; Bagheri, R.; Ugbolue, U.C.; Navel, V.; Dutheil, F. Effect of exercise training on heart rate variability in type 2 diabetes mellitus patients: A systematic review and meta-analysis. PLoS ONE 2021, 16, e0251863. [Google Scholar] [CrossRef]
- Goulopoulou, S.; Baynard, T.; Franklin, R.M.; Fernhall, B.; Carhart, R., Jr.; Weinstock, R.; Kanaley, J.A. Exercise training improves cardiovascular autonomic modulation in response to glucose ingestion in obese adults with and without type 2 diabetes mellitus. Metabolism 2010, 59, 901–910. [Google Scholar] [CrossRef][Green Version]
- Von Haaren, B.; Ottenbacher, J.; Muenz, J.; Neumann, R.; Boes, K.; Ebner-Priemer, U. Does a 20-week aerobic exercise training programme increase our capabilities to buffer real-life stressors? A randomized, controlled trial using ambulatory assessment. Eur. J. Appl. Physiol. 2016, 116, 383–394. [Google Scholar] [CrossRef]
- Chowdhary, S.; Townend, J.N. Role of nitric oxide in the regulation of cardiovascular autonomic control. Clin. Sci. 1999, 97, 5–17. [Google Scholar] [CrossRef]
- Tian, Y.; Huang, C.; He, Z.; Hong, P.; Zhao, J. Autonomic function responses to training: Correlation with body composition changes. Physiol. Behav. 2015, 151, 308–313. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, B.; Lira, F.S.; Consolim-Colombo, F.M.; Rocha, J.A.; Caperuto, E.C.; De Angelis, K.; Irigoyen, M.C. Role of exercise training on autonomic changes and inflammatory profile induced by myocardial infarction. Mediat. Inflamm. 2014, 2014, 702473. [Google Scholar] [CrossRef] [PubMed]
- Polli, A.; Van Oosterwijck, J.; Nijs, J.; Marusic, U.; De Wandele, I.; Paul, L.; Meeus, M.; Moorkens, G.; Lambrecht, L.; Ickmans, K. Relationship Between Exercise-induced Oxidative Stress Changes and Parasympathetic Activity in Chronic Fatigue Syndrome: An Observational Study in Patients and Healthy Subjects. Clin. Ther. 2019, 41, 641–655. [Google Scholar] [CrossRef] [PubMed]
- Collier, S.R.; Sandberg, K.; Moody, A.M.; Frechette, V.; Curry, C.D.; Ji, H.; Gowdar, R.; Chaudhuri, D.; Meucci, M. Reduction of plasma aldosterone and arterial stiffness in obese pre- and stage1 hypertensive subjects after aerobic exercise. J. Hum. Hypertens. 2015, 29, 53–57. [Google Scholar] [CrossRef]
- Saavedra, M.J.; Romero, F.; Roa, J.; Rodríguez-Núñez, I. Exercise training to reduce sympathetic nerve activity in heart failure patients. A systematic review and meta-analysis. Braz. J. Phys. Ther. 2018, 22, 97–104. [Google Scholar] [CrossRef]
- Bhati, P.; Moiz, J.A.; Menon, G.R.; Hussain, M.E. Does resistance training modulate cardiac autonomic control? A systematic review and meta-analysis. Clin. Auton. Res. 2019, 29, 75–103. [Google Scholar] [CrossRef]
- Collier, S.R.; Kanaley, J.A.; Carhart, R., Jr.; Frechette, V.; Tobin, M.M.; Bennett, N.; Luckenbaugh, A.N.; Fernhall, B. Cardiac autonomic function and baroreflex changes following 4 weeks of resistance versus aerobic training in individuals with pre-hypertension. Acta Physiol. 2009, 195, 339–348. [Google Scholar] [CrossRef]
- Grant, C.C.; Murray, C.; Janse van Rensburg, D.C.; Fletcher, L. A comparison between heart rate and heart rate variability as indicators of cardiac health and fitness. Front. Physiol. 2013, 4, 337. [Google Scholar] [CrossRef]
- Plaza-Florido, A.; Migueles, J.H.; Mora-Gonzalez, J.; Molina-Garcia, P.; Rodriguez-Ayllon, M.; Cadenas-Sanchez, C.; Esteban-Cornejo, I.; Solis-Urra, P.; de Teresa, C.; Gutiérrez, Á.; et al. Heart Rate Is a Better Predictor of Cardiorespiratory Fitness Than Heart Rate Variability in Overweight/Obese Children: The ActiveBrains Project. Front. Physiol. 2019, 10, 510. [Google Scholar] [CrossRef]
- Besnier, F.; Labrunée, M.; Richard, L.; Faggianelli, F.; Kerros, H.; Soukarié, L.; Bousquet, M.; Garcia, J.L.; Pathak, A.; Gales, C.; et al. Short-term effects of a 3-week interval training program on heart rate variability in chronic heart failure. A randomised controlled trial. Ann. Phys. Rehabil. Med. 2019, 62, 321–328. [Google Scholar] [CrossRef]
- Fyfe, J.J.; Bishop, D.J.; Stepto, N.K. Interference between concurrent resistance and endurance exercise: Molecular bases and the role of individual training variables. Sport. Med. 2014, 44, 743–762. [Google Scholar] [CrossRef]
- Marin, J.M.S.; Rocamora, A.M.; Oliveira, J.; Ramon, M.M. Influence of the exercise frequency, intensity, time and type according to different training modalities on the cardiac rehabilitation programs. Eur. J. Hum. Mov. 2018, 41, 49–72. [Google Scholar]
- Stanley, J.; Peake, J.M.; Buchheit, M. Cardiac parasympathetic reactivation following exercise: Implications for training prescription. Sport. Med. 2013, 43, 1259–1277. [Google Scholar] [CrossRef]
- Suchomel, T.J.; Nimphius, S.; Bellon, C.R.; Stone, M.H. The Importance of Muscular Strength: Training Considerations. Sport. Med. 2018, 48, 765–785. [Google Scholar] [CrossRef]
- Manresa-Rocamora, A.; Flatt, A.A.; Casanova-Lizón, A.; Ballester-Ferrer, J.A.; Sarabia, J.M.; Vera-Garcia, F.J.; Moya-Ramón, M. Heart rate-based indices to detect parasympathetic hyperactivity in functionally overreached athletes. A meta-analysis. Scand. J. Med. Sci. Sport. 2021, 31, 1164–1182. [Google Scholar] [CrossRef]
- Flatt, A.A.; Howells, D. Effects of varying training load on heart rate variability and running performance among an Olympic rugby sevens team. J. Sci. Med. Sport 2019, 22, 222–226. [Google Scholar] [CrossRef]
- Flatt, A.A.; Esco, M.R. Evaluating Individual Training Adaptation With Smartphone-Derived Heart Rate Variability in a Collegiate Female Soccer Team. J. Strength Cond. Res. 2016, 30, 378–385. [Google Scholar] [CrossRef]
- González-Fimbres, R.A.; Hernández-Cruz, G.; Flatt, A.A. Ultrashort Versus Criterion Heart Rate Variability Among International-Level Girls’ Field Hockey Players. Int. J. Sport. Physiol. Perform. 2021, 16, 985–992. [Google Scholar] [CrossRef]
- Johansson, J.K.; Niiranen, T.J.; Puukka, P.J.; Jula, A.M. Prognostic value of the variability in home-measured blood pressure and heart rate: The Finn-Home Study. Hypertension 2012, 59, 212–218. [Google Scholar] [CrossRef]
- Kiviniemi, A.M.; Hautala, A.J.; Seppänen, T.; Mäkikallio, T.H.; Huikuri, H.V.; Tulppo, M.P. Saturation of high-frequency oscillations of R-R intervals in healthy subjects and patients after acute myocardial infarction during ambulatory conditions. Am. J. Physiol. Heart Circ. Physiol. 2004, 287, H1921–H1927. [Google Scholar] [CrossRef] [PubMed]
- Aubert, A.E.; Seps, B.; Beckers, F. Heart rate variability in athletes. Sport. Med. 2003, 33, 889–919. [Google Scholar] [CrossRef] [PubMed]
- Carnethon, M.R.; Jacobs, D.R., Jr.; Sidney, S.; Sternfeld, B.; Gidding, S.S.; Shoushtari, C.; Liu, K. A longitudinal study of physical activity and heart rate recovery: CARDIA, 1987-1993. Med. Sci. Sport. Exerc. 2005, 37, 606–612. [Google Scholar] [CrossRef] [PubMed]
- Otsuki, T.; Maeda, S.; Iemitsu, M.; Saito, Y.; Tanimura, Y.; Sugawara, J.; Ajisaka, R.; Miyauchi, T. Postexercise heart rate recovery accelerates in strength-trained athletes. Med. Sci. Sport. Exerc. 2007, 39, 365–370. [Google Scholar] [CrossRef] [PubMed]
- Bonnemeier, H.; Richardt, G.; Potratz, J.; Wiegand, U.K.; Brandes, A.; Kluge, N.; Katus, H.A. Circadian profile of cardiac autonomic nervous modulation in healthy subjects: Differing effects of aging and gender on heart rate variability. J. Cardiovasc. Electrophysiol. 2003, 14, 791–799. [Google Scholar] [CrossRef]
- Carter, J.B.; Banister, E.W.; Blaber, A.P. Effect of endurance exercise on autonomic control of heart rate. Sport. Med. 2003, 33, 33–46. [Google Scholar] [CrossRef]
- Goldsmith, R.L.; Bigger, J.T., Jr.; Bloomfield, D.M.; Steinman, R.C. Physical fitness as a determinant of vagal modulation. Med. Sci. Sport. Exerc. 1997, 29, 812–817. [Google Scholar] [CrossRef]
- Da Costa, B.R.; Juni, P. Systematic reviews and meta-analyses of randomized trials: Principles and pitfalls. Eur. Heart J. 2014, 35, 3336–3345. [Google Scholar] [CrossRef]
- Cole, C.R.; Blackstone, E.H.; Pashkow, F.J.; Snader, C.E.; Lauer, M.S. Heart-rate recovery immediately after exercise as a predictor of mortality. N. Engl. J. Med. 1999, 341, 1351–1357. [Google Scholar] [CrossRef]
- Bellenger, C.R.; Fuller, J.T.; Thomson, R.L.; Davison, K.; Robertson, E.Y.; Buckley, J.D. Monitoring Athletic Training Status Through Autonomic Heart Rate Regulation: A Systematic Review and Meta-Analysis. Sport. Med. 2016, 46, 1461–1486. [Google Scholar] [CrossRef]
- Buchheit, M.; Millet, G.P.; Parisy, A.; Pourchez, S.; Laursen, P.B.; Ahmaidi, S. Supramaximal training and postexercise parasympathetic reactivation in adolescents. Med. Sci. Sport. Exerc. 2008, 40, 362–371. [Google Scholar] [CrossRef]
- Buchheit, M.; Laursen, P.B.; Ahmaidi, S. Parasympathetic reactivation after repeated sprint exercise. Am. J. Physiol. Heart Circ. Physiol. 2007, 293, H133–H141. [Google Scholar] [CrossRef]
- Peçanha, T.; Bartels, R.; Brito, L.C.; Paula-Ribeiro, M.; Oliveira, R.S.; Goldberger, J.J. Methods of assessment of the post-exercise cardiac autonomic recovery: A methodological review. Int. J. Cardiol. 2017, 227, 795–802. [Google Scholar] [CrossRef]
- Le Meur, Y.; Buchheit, M.; Aubry, A.; Coutts, A.J.; Hausswirth, C. Assessing Overreaching With Heart-Rate Recovery: What Is the Minimal Exercise Intensity Required? Int. J. Sport. Physiol. Perform. 2017, 12, 569–573. [Google Scholar] [CrossRef]
| Study (Author, Year) | Group; Exercise Type (Aerobic Method) | Study Characteristics | Patient Characteristics | |
|---|---|---|---|---|
| Country; Study Design | Sex; Final Sample Size; Male Percentage; Age; Body Weight; Body Mass Index | Resting HR; VO2 Peak; | ||
| Audette et al. [65] 2006 * | IG; AT (MIT) | USA; non-randomised | Female; 8; 0%; 71.3 ± 4.4 years; 71.2 ± 9.5 kg; NR | NR; 23.7 ± 4.7 mL·kg−1·min−1 |
| CG; NA | Female; 8; 0%; 73.5 ± 5.7 years; 73.0 ± 11.3 kg; NR | NR; 26.8 ± 8.3 mL·kg−1·min−1 | ||
| Boutcher et al. [66] 2013 | IG; AT (HIIT) | Australia; non-randomised | Female; 16; 0%; 23.0 ± 4.0 years; 74.1 ± 12.0 kg; 27.7 ± 3.2 | 68.0 ± 10.4 bpm; 27.3 ± 5.2 mL·kg−1·min−1 |
| CG; NA | Female; 16; 0%; 21.1 ± 2.4 years; 71.3 ± 10.8 kg; 26.0 ± 2.4 | 70.0 ± 6.0 bpm; 29.3 ± 5.2 mL·kg−1·min−1 | ||
| Lopes et al. [77] 2007 | IG; RT (NA) | Brazil; non-randomised | Male; 12; 100%; 50.0 ± 4.3 years; NR; NR | 68.0 ± 6.9 bpm; NR |
| CG; NA | Male; 10; 100%; 48.5 ± 6.4 years; NR; NR | 72.9 ± 6.2 bpm; NR | ||
| Melanson and Freedson [78] 2001 | IG; AT (MIT) | USA; non-randomised | Male; 11; 100%; 36.6 ± 1.7 years; 87.2 ± 4.3 kg; 28.4 ± 1.5 | 64.9 ± 3.2 bpm; 38.9 ± 9.6 mL·kg−1·min−1 |
| CG; NA | Male; 5; 100%; 36.6 ± 4.2 years; 83.8 ± 6.6 kg; 24.9 ± 1.8 | 61.0 ± 4.7 bpm; 40.8 ± 7.0 mL·kg−1·min−1 | ||
| Mourot et al. [79] 2005 | IG; AT (HIIT) | France; non-randomised | Female; 7; 0%; 22.1 ± 3.4 years; 61.0 ± 2.9 kg; 22.2 ± NR | 67.5 ± 8.7 bpm; 40.4 ± 4.8 mL·kg−1·min−1 |
| CG; NA | Female; 7; 0%; 22.1 ± 2.3 years; 59.6 ± 8.6 kg; 21.8 ± NR | 61.5 ± 11.5 bpm; 41.5 ± 6.0 mL·kg−1·min−1 | ||
| Oliveira-Junior et al. [80] 2021 | IG; CT (HIIT) | Brazil; non-randomised | Both; 19; 42.1%; 36.1 ± 8.7 years; 76.5 ± 15.8 kg; 27.2 ± 4.4 | NR; 34.0 ± 5.4 mL·kg−1·min−1 |
| CG; NA | Both; 8; 62.5%; 38.7 ± 10.6 years; 78.9 ± 14.7 kg; 27.7 ± 3.9 | NR; 33.9 ± 11.5 mL·kg−1·min−1 | ||
| Boutcher and Stein [67] 1995 | IG; AT (MIT) | Australia; randomised | Male; 19; 100%; 46.2 ± 6.1 years; 87.1 ± 6.0 kg; NR | 71.3 ± 10.0 bpm; 34.3 ± 9.8 mL·kg−1·min−1 |
| CG; NA | Male; 15; 100%; 45.0 ± 5.4 years; 83.9 ± 9.3 kg; NR | 73.1 ± 8.1 bpm; 34.2 ± 9.4 mL·kg−1·min−1 | ||
| de Rezende Barbosa et al. [68] 2018 | IG; AT (MIT) | Brazil; randomised | Female; 19; 0%; 60.0 ± 4.5 years; 67.6 ± 11.6 kg; 27.3 ± 4.2 | 71.3 ± 8.6 bpm; NR |
| CG; NA | Female; 20; 0%; 58.5 ± 4.9 years; 66.9 ± 13.2 kg; 27.6 ± 4.8 | 64.5 ± 8.1 bpm; NR | ||
| Hautala et al. [69] 2006 | IG; AT (MIT) | Finland; randomised | Both; 80; 45%; 41.0 ± 5.0 years; NR; 25.0 ± 3.0 | 59.0 ± 7.0 bpm; 2.43 ± 0.7 l·min−1 |
| CG; NA | Both; 15; 60%; 41.0 ± 5.0 years; NR; 25.0 ± 2.0 | 57.0 ± 11.0 bpm; 2.66 ± 0.7 l·min−1 | ||
| Heydari et al. [70] 2013 | IG; AT (HIIT) | Australia; randomised | Male; 20; 100%; NR; 87.8 ± 11.7 kg; 28.4 ± 2.4 | 67.4 ± 9.7 bpm; 34.2 ± 4.4 mL·kg−1·min−1 |
| CG; NA | Male; 18; 100%; NR; 89.0 ± 12.4 kg; 29.0 ± 3.9 | 68.9 ± 7.7 bpm; 29.0 ± 5.5 mL·kg−1·min−1 | ||
| Jelinek et al. [71] 2015 * | IG; AT (MIT) HV | Australia; randomised | Male; 15; 100%; NR; NR; NR; | NR; NR |
| IG; AT (MIT) LV | Male; 19; 100%; NR; NR; NR | NR; NR | ||
| CG; NA | Male; 16; 100%; NR; NR; NR | NR; NR | ||
| Kanegusuku et al. [72] 2015 * | IG; RT (NA) | Brazil; randomised | Both; 12; 41.7%; 64.0 ± 4.0 years; 68.3 ± 15.1 kg; 25.7 ± 4.2 kg | 70.0 ± 9.0 bpm; 22.6 ± 4.9 mL·kg−1·min−1 |
| CG; NA | Both; 13; 15.4%; 63.0 ± 4.0 years; 68.1 ± 10.5 kg; 26.8 ± 4.7 | 66.0 ± 11.0 bpm; 23.3 ± 3.9 mL·kg−1·min−1 | ||
| Karavirta et al. [73] 2013 | IG; AT (MIT) | Finland; randomised | Female; 26; 0%; 52.0 ± 7.0 years; 66.9 ± 9.7 kg; 25.1 ± 2.7 | 63.0 ± 6.5 bpm; 25.3 ± 5.2 mL·kg−1·min−1 |
| IG; RT (NA) | Female; 26; 0%; 52.0 ± 8.0 years; 66.3 ± 9.7 kg; 24.7 ± 3.0 | 62.0 ± 6.5 bpm; 25.9 ± 5.4 mL·kg−1·min- | ||
| IG; CT (MIT) | Female; 21; 0%; 49.0 ± 6.0 years; 66.2 ± 9.1 kg; 24.7 ± 3.3 | 62.0 ± 7.0 bpm; 27.7 ± 4.6 mL·kg−1·min−1 | ||
| CG; NA | Female; 17; 0%; 52.0 ± 8.0 years; 66.5 ± 7.5 kg; 24.1 ± 2.4 | 65.0 ± 5.3 bpm; 26.1 ± 5.8 mL·kg−1·min−1 | ||
| Karavirta et al. [74] 2009 | IG; AT (MIT) | Finland; randomised | Male; 23; 100%; 54.0 ± 8.0 years; 78.1 ± 10.0 kg; 25.1 ± 3.1 kg | 61.0 ± 10.0 bpm; 32.9 ± 7.2 mL·kg−1·min−1 |
| IG; RT (NA) | Male; 25; 100%; 56.0 ± 6.0 years; 84.8 ± 10.0 kg; 26.8 ± 3.1 | 59.0 ± 9.0 bpm; 33.2 ± 6.2 mL·kg−1·min−1 | ||
| IG; CT (MIT) | Male; 29; 100%; 56.0 ± 7.0 years; 83.4 ± 11.9 kg; 26.4 ± 3.1 | 58.0 ± 8.0 bpm; 32.4 ± 4.2 mL·kg−1·min−1 | ||
| CG; NA | Male; 16; 100%; 54.0 ± 8.0 years; 78.0 ± 6.2 kg; 25.1 ± 1.6 | 54.0 ± 5.0 bpm; 35.3 ± 5.6 mL·kg−1·min−1 | ||
| Kim et al. [75] 2017 * | IG; AT (MIT) 1 | South Korea; randomised | Male; 12; 100%; 23.3 ± 2.2 years; NR; 24.2 ± 2.8 | 70.4 ± 6.6 bpm; 46.5 ± 5.0 mL·kg−1·min−1 |
| IG; AT (MIT) 2 | Male; 12; 100%; 24.1 ± 2.2 years; NR; 23.2 ± 4.5 | 64.5 ± 5.7 bpm; 47.7 ± 5.4 mL·kg−1·min−1 | ||
| CG; NA | Male; 10; 100%; 23.3 ± 1.5 years; NR; 23.0 ± 3.4 | 70.3 ± 6.6 bpm; 46.2 ± 4.8 mL·kg−1·min−1 | ||
| Lee et al. [76] 2003 * | IG; AT (MIT) | USA; randomised | Male; 12; 100%; 23.1 ± 3.0 years; 82.6 ± 16.0 kg; 26.4 ± NR | NR; 33.5 ± 3.7 mL·kg−1·min−1 |
| CG; NA | Male; 12; 100%; 23.1 ± 4.0 years; 79.1 ± 13.0 kg; 25.7 ± NR | NR; 33.9 ± 3.0 mL·kg−1·min−1 | ||
| Rezende Barbosa et al. [81] 2016 | IG; RT (NA) | Brazil; randomised | Female; 13; 0%; 23.0 ± 2.5 years; 58.3 ± 8.7 kg; 21.9 ± 2.8 | NR; NR |
| CG; NA | Female; 16; 0%; 20.6 ± 1.0 years; 58.4 ± 10.4 kg; 22.1 ± 3.9 | NR; NR | ||
| Romagnoli et al. [82] 2018 * | IG; AT (MIT) | Spain; randomised | Both; 10; 50%; 29.7 ± 6.3 years; 67.6 ± 11.3 kg; 23.2 ± 2.7 | NR; 35.4 ± 6.0 mL·kg−1·min−1 |
| CG; NA | Both; 10; 50%; 32.3 ± 5.7 years; 69.9 ± 13.7 kg; 23.7 ± 2.5 | NR; 33.2 ± 3.5 mL·kg−1·min−1 | ||
| Rossi et al. [83] 2013 | IG; CT (HIIT) | Brazil; randomised | Female; 11; 0%; 62.1 ± 6.6 years; 62.4 ± 8.2 kg; NR | 68.1 ± 6.4 bpm; NR |
| CG; NA | Female; 6; 0%; 58.5 ± 5.1 years; 72.8 ± 17.4 kg; NR | 60.7 ± 5.9 bpm; NR | ||
| Shen and Wen [84] 2013 | IG; AT (MIT) | Taiwan; randomised | Female; 22; 0%; 57.9 ± 3.0 years; 55.0 ± 5.6 kg; 22.7 ± 2.2 | 70.9 ± 7.8 bpm; 21.5 ± 5.5 mL·kg−1·min−1 |
| CG; NA | Female; 22; 0%; 59.1 ± 3.9 years; 58.1± 7.7 kg; 23.7 ± 3.1 | 67.9 ± 8.0 bpm; 20.8 ± 6.4 mL·kg−1·min−1 | ||
| Shiotani et al. [85] 2009 | IG; AT (MIT) | Japan; randomised | Both; 16; 37.5%; 21.6 ± 1.7 years; NR; 20.6 ± 2.3 | 58.2 ± 6.5 bpm; NR |
| CG; NA | Both; 19; 36.8%; 22.9 ± 1.1 years; NR; 19.7 ± 1.8 | 59.8 ± 7.2 bpm; NR | ||
| Sloan et al. [86] 2021 * | IG; AT (MIT) | USA; randomised | Both; 45; 46.7%; 31.2 ± 5.7 years; NR; 24.9 ± 3.8 | 65.3 ± 7.8 bpm; NR |
| CG; NA | Both; 58; 47.5%; 31.4 ± 6.2 years; NR; 24.9 ± 3.8 | 67.2 ± 9.2 bpm; NR | ||
| Soltani et al. [87] 2021 | IG; AT (MIT) HV | Switzerland; randomised | Male; 15; 100%; 42.5 ± 6.2 years; 73.5 ± 5.5 kg; 24.0 ± 1.3 | 79.2 ± 7.6 bpm; 33.3 ± 4.5 mL·kg−1·min−1 |
| IG; AT (MIT) LV | Male; 15; 100%; 42.2 ± 5.3 years; 73.4 ± 6.0 kg; 23.3 ± 1.5 | 80.1 ± 7.7 bpm; 35.2 ± 4.1 mL·kg−1·min−1 | ||
| CG; NA | Male; 15; 100%; 41.5 ± 5.6 years; 74.9 ± 6.8 kg; 24.4 ± 1.4 | 79.5 ± 7.7 bpm; 33.5 ± 5.0 mL·kg−1·min−1 | ||
| Songsorn et al. [88] 2022 | IG; AT (HIIT) | Thailand; randomised | Both; 10; 80%; 22.0 ± 0.8 years; 52.7 ± 5.9 kg; 19.5 ± 1.0 | 73.9 ± 13.2 bpm; NR |
| CG; NA | Both; 11; 57.1%; 21.7 ± 0.8 years; 51.6 ± 5.9 kg; 19.8 ± 0.9 | 65.7 ± 8.9 bpm; NR | ||
| Tulppo et al. [89] 2003 | IG; AT (MIT) HV | Finland; randomised | Male; 16; 100%; 35.0 ± 10.0 years; 79.0 ± 9.0 kg; 25.0 ± 2.0 | 54.0 ± 4.0 bpm; 42.0 ± 5.0 mL·kg−1·min−1 |
| IG; AT (MIT) LV | Male; 19; 100%; 35.0 ± 10.0 years; 82.0 ± 11.0 kg; 25.0 ± 3.0 | 55.0 ± 7.0 bpm; 41.0 ± 4.0 mL·kg−1·min−1 | ||
| CG; NA | Male; 11; 100%; 36.0 ± 11.0 years; 81.0 ± 9.0; 25.0 ± 3.0 | 53.0 ± 5.0 bpm; 41.0 ± 4.0 mL·kg−1·min−1 | ||
| Verheyden et al. [90] 2006 | IG; CT (MIT) | Belgium; randomised | Male; 14; 100%; 62.4 ± 6.1 years; 81.5 ± 3.4 kg; 26.2 ± 2.5 | 65.0 ± 3.0 bpm; 26.4 ± 4.5 mL·kg−1·min−1 |
| CG; NA | Male; 15; 100%; 64.2 ± 6.5 years; 79.8 ± 4.0 kg; 26.4 ± 3.0 | 66.0 ± 2.0 bpm; NR | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Casanova-Lizón, A.; Manresa-Rocamora, A.; Flatt, A.A.; Sarabia, J.M.; Moya-Ramón, M. Does Exercise Training Improve Cardiac-Parasympathetic Nervous System Activity in Sedentary People? A Systematic Review with Meta-Analysis. Int. J. Environ. Res. Public Health 2022, 19, 13899. https://doi.org/10.3390/ijerph192113899
Casanova-Lizón A, Manresa-Rocamora A, Flatt AA, Sarabia JM, Moya-Ramón M. Does Exercise Training Improve Cardiac-Parasympathetic Nervous System Activity in Sedentary People? A Systematic Review with Meta-Analysis. International Journal of Environmental Research and Public Health. 2022; 19(21):13899. https://doi.org/10.3390/ijerph192113899
Chicago/Turabian StyleCasanova-Lizón, Antonio, Agustín Manresa-Rocamora, Andrew A. Flatt, José Manuel Sarabia, and Manuel Moya-Ramón. 2022. "Does Exercise Training Improve Cardiac-Parasympathetic Nervous System Activity in Sedentary People? A Systematic Review with Meta-Analysis" International Journal of Environmental Research and Public Health 19, no. 21: 13899. https://doi.org/10.3390/ijerph192113899
APA StyleCasanova-Lizón, A., Manresa-Rocamora, A., Flatt, A. A., Sarabia, J. M., & Moya-Ramón, M. (2022). Does Exercise Training Improve Cardiac-Parasympathetic Nervous System Activity in Sedentary People? A Systematic Review with Meta-Analysis. International Journal of Environmental Research and Public Health, 19(21), 13899. https://doi.org/10.3390/ijerph192113899







