Abstract
This study provides data on variation in the content of metals and metalloids measured in the lichens (Xanthoria calcicola Oxner) collected in the Syracusan petrochemical complex (Sicily, Italy) which is considered one of the largest in Europe. Concentrations of eighteen trace elements measured in the lichens that were collected from 49 different points were analyzed using an inductively coupled plasma (ICP-MS) device. The concentrations of the typical elements of industrial emissions (As, Cr, Ni, and V) highlight the environmental criticality that exists in the study area. The interpretation of the data in terms of multi-element statistical analysis (FA) and enrichment factor (EFs) proved to be particularly useful in identifying several sources that contribute to the presence of trace elements in the atmospheric particulate between anthropogenic emissions and geogenic emissions. The results of this study reveal the versatility of the lichen species Xanthoria calcicola Oxner in the search for trace elements in highly anthropized environments, so the approach followed in this study can also be applied to other industrial contexts.
1. Introduction
Trace elements emitted into the atmosphere from various anthropogenic sources are significant due to their threat to the environment and human health. Extensive research has been conducted in recent decades to determine the distribution, sources, and toxicity of atmospheric trace elements in population centers, and industrial and economic areas around the world [1,2,3,4]. Metal pollutants are toxic and are considered important due to their nature of rapid dispersion, persistence, and bioaccumulation [5,6]. Several kinds of research have revealed that exposure to high concentrations of metal pollutants causes damage to the central and peripheral nervous systems, lungs, kidneys, and liver and can further lead to death. However, prolonged exposure to even low concentrations of metal pollutants irritates the nose and throat, and can lead to cough, dyspnea, and asthma [7,8].
The monitoring of environmental quality by cosmopolitan organisms has developed considerably in recent decades [9,10,11]. The types of organisms used can react, respond, or adapt to changes in environmental quality, both as an individual and as a community. Over the past 50 years, biomonitoring studies using lichens as a biological indicator have increased and expanded in terms of various parameters, monitoring techniques, and sampling areas [12,13,14,15,16,17,18,19,20]. Since then, lichens have been the most studied biological indicator [13,21] and have been defined as “permanent control systems” for assessing air pollution. Biomonitoring with lichens has several advantages over conventional techniques, such as low cost, easy sampling, and the ability to monitor large areas [22]. Lichens are organisms formed by a symbiotic relationship between a fungus (mycobiont) and one or more photosynthetic partners, algae, or cyanobacteria (photobiont). Lichens are slow-growing and long-lived organisms and depend on nutrients, from wet and dry atmospheric deposition for their growth and metabolism [23]. Their morphology does not change seasonally. Lichens can accumulate and retain trace elements at levels above their physiological needs [24] without showing morphological changes. Some lichen species found around industrial areas, power plants, and highways can be used to detect heavy metal pollution in the air. Since lichens accumulate elements from the atmosphere directly in their tissues, there are several studies on the evaluation of heavy metal pollution in the air through lichens [25,26,27].
Over time, the concentrations of trace elements in the lichen thallus reach an equilibrium with the average levels of air pollution [28], and the chemical composition of the lichens reflects the availability of trace elements in the environment [29]. In this way, the accumulation of air pollutants in lichen thalli can be used to evaluate the patterns of spatial and temporal deposition [30].
This study aims to examine the spatial distribution of the concentrations of elements through the analysis of lichens in highly anthropized environments of the Syracusan petrochemical complex, defined by the Italian government as an area at “high risk of environmental crisis”. Therefore, we also aimed to (1) evaluate the atmospheric deposition of trace elements at different distances and directions from the industrial plants; (2) identify common sources of trace elements in the area; (3) distinguish natural from anthropogenic sources of trace elements through multivariate statistical analysis.
The results presented here belong to a larger research project on the dispersion of heavy metals and metalloids in various matrices (vegetation, soil, atmospheric particulate, and groundwater) in the industrial districts of Sicily (Italy) and the definition of regional background and baseline levels.
2. Materials and Methods
2.1. Description of the Study Area
The study area falls within the Syracusan petrochemical complex which is considered one of the largest in Europe. The main activity of the settlement is the refining of oil, the transformation of its derivatives, and energy production companies. This industrialized coastal area of eastern Sicily includes the municipalities of Augusta (about 36,000 inhabitants), Priolo Gargallo (about 11,400 inhabitants), and Melilli (about 13,000 inhabitants), covering an area of about 300 km2 and reaching the gates of Syracuse.
Industrial activities began in the 1950s and developed rapidly until the 1980s, making the site the most important hub in Europe. Subsequently, some industries have closed, while others are still active. Since the 1980s, several epidemiological studies have found a strong incidence of lung tumors in the male gender but, above all, a high incidence of births of malformed children is reported [31,32]. Only in 1990 was this area defined as an area at “elevated risk of environmental crisis” by the Italian Government (Italian Law n. 349/1986, [33]). Since 1998, Augusta, Priolo Gargallo, and Melilli were included among the 57 Italian Polluted Sites (IPSs) of national interest for environmental remediation (Italian Law n. 426/1998, [34]).
The outcropping rocks in the study area are mainly of sedimentary origin characterized by alluvial and fluvial deposits together with coarse sands, and limestone dating back to the Upper Cretaceous, with sporadic outcrop of basaltic rocks [35] (Figure 1).
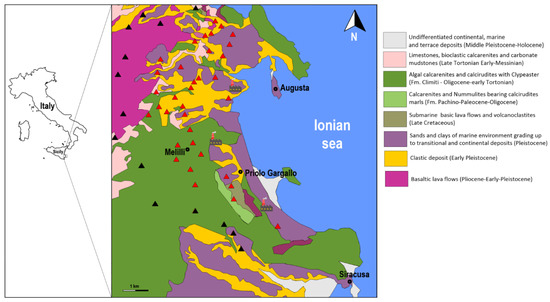
Figure 1.
Geological sketch map of the study area with the location of the sampling sites (modified from [35]). Control samples (SUBIND) collected in rural environment (black triangle); lichen samples (IND) collected near the Syracusan petrochemical complex (red triangle).
The climate classification of the study area is Csa type (Mediterranean climate with hot summer) as established by Köppen and Geiger. The study area is characterized by a period of greater rainfall in the winter months than in the summer (Figure 2). The average annual temperature is 17.8 °C. In general, the predominant winds in the area under consideration are those coming from the southern quadrants; among these the most representative is the sirocco. Due to the different heating of the sea and the land, land and sea breeze is determined: the first occurs during the night and the second during the day [36].
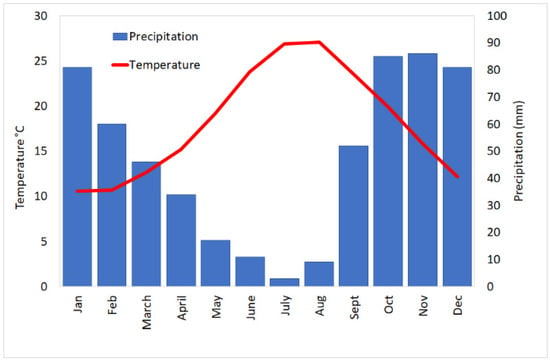
Figure 2.
Weather data from meteorological station of Augusta. Mean monthly precipitation (mm) and mean monthly temperature (°C).
2.2. Sampling and Analytical Method
A total of 49 composite samples of lichens, Xanthoria calcicola Oxner, were collected in November 2019 in the Syracusan petrochemical complex and its surroundings, from an irregular sample grid. The locations depended on the availability of lichens randomly distributed near the petrochemical plant (IND-35 samples) and distant from the polluting sources that can be considered as control samples (SUBIND-14 samples). Samples were taken from tree trunks and rocky substrate by scraping with a plastic spatula and carried to the laboratory in a plastic bag.
The non-washed samples were dried for 24 h at 40 °C and then ground to a fine powder using an agate mortar. Powdered lichens (~0.500 g) were digested with a mixture of 5 mL of 65% HNO3 (Suprapur, Merck, Germany) and 2 mL of 30% H2O2 (Suprapur, Sigma Aldrich, St. Louis, MO, USA). The digest was diluted to 50 mL using deionized water. In each sample, 16 trace elements (Al, As, Ba, Co, Cr, Cu, Fe, Mn, Mo, Pb, Rb, Sb, Sr, U, V, Zn) were determined using an inductive coupled plasma mass spectrometer (Elan 6100 DRC-e, Perkin Elmer, Waltham, MA, USA) at the Dept. Scienze della Terra e del Mare, University of Palermo, after the addition of Re–Sc–Y as an internal standard. For As, Cr, Fe, and V the ICP-MS was operated in DRC (Dynamic Reaction Cell) mode with CH4 as reaction gas. All standard solutions were prepared with 18 MΩ·cm deionized water, the ICP Multi-Element Standard Solutions XXI CertiPUR (Merck, Kenilworth, NJ, USA), and the Mo and Sb CertiPUR standards (Merck). Calibration curves ranging from 0.05 μg L−1 to 500 μg L−1 were constructed. To minimize matrix effects, the standard addition technique was used for all metal determinations. Sample blanks were also analyzed, and the operational limit of detection (LOD) for each element was calculated as 3 times the standard deviation of the analyte concentration in blank samples. To validate the analytical procedure, the standard reference material NIST SRM 1515 Apple Leaves was analyzed for corresponding elements. The metal recovery rates resulted in good agreement with the certified concentrations, ranging between 89% and 102%.
Analytical precision, estimated from triplicate analyses of every tenth sample, was in the range of 3–11% for all analyzed elements.
2.3. Statistical Analysis and Spatial Distribution
Data were analyzed statistically by the STATISTICA program (Tulsa, OK, USA), StatSoft version 6.0. All the tests in this study were considered significant at p < 0.05. Shapiro-Wilk test, with a level of significance set at p < 0.05, was used to verify the normality of data distribution. The non-parametric Mann-Whitney test at p < 0.05 was also used to verify the statistical significance of observed differences between IND and SUBIND samples. The Spearman correlation matrix was used to measure the association between variables in terms of rank. To identify possible common sources of pollutants, we evaluated the relationships between trace elements by factor analyses.
To spatially represent pollutant patterns, we elaborated distribution maps for the same trace element using the program SURFER v. 8.05 (Golden, CO, USA). The maps were based on a grid created by the Kriging interpolation method [37].
2.4. Geochemical Indices for Evaluating Trace Element Pollution
The Contamination Factor (CF) is called the pollution index, which expresses the quotient obtained by dividing the metal concentration in the investigated site by the background values [38]. The CF was calculated using Equation (1:)
where Cm is the concentration of an element measured in lichen species and Cb is the background level of the element in the lichen. The lichen sample collected distant from the polluting sources can be considered for extracting the background values of metals and metalloids. The contamination factor of each metal was classified as follows [39,40]:
CF = Cm/Cb
- CF 0–1 No contamination
- CF > 1–2 Suspected contamination
- CF > 2–3.5 Slight contamination
- CF > 3.5–8 Moderate contamination
- CF > 8–27 Severe contamination
- CF > 27 Extreme contamination
The Pollution Load Index (PLI) originally proposed by Tomlinson et al. (1980) was also used to evaluate the overall air pollution load, as follows: PLI = (CF1 × CF2 × CF3 × CF4 ×…× CFn)1/n, where CF is a contamination factor and n is number of studied elements. A PLI value less than 0. 9 indicates an unpolluted area, a PLI approaching 1 indicates an air pollution load close to the background level [41], and a value higher than 1 indicates low pollution (1.1 < PLI < 1.5), moderate pollution (1.5 ≤ PLI < 2.0), or very high pollution (PLI ≥ 2.5).
The Ecological risk index (ERI) is used for quantitatively expressing the potential ecological risk of investigated metals and is calculated as shown in Equation (2) [38,42]:
where Tri is the toxic response factor for the metals/metalloids and CF is the contamination factor. The Tri values of metals/metalloids (As = 10, Cr = 2, Cu = 5, Ni = 5, Pb = 5, V = 2, Zn = 1) were reported by Hakanson [38], Qing et al. [43], and Wu et al. [44]. The ERI values can be classified as per the following:
ERI = Tri × CF
- ERI < 5 Low risk
- 5 ≤ ERI < 10 Moderate risk
- 10 ≤ ERI < 20 Considerable risk
- 20 ≤ ERI < 40 High risk
- ERI ≥ 40 Very high risk
Although risk indices were initially used as a diagnostic tool to control water pollution, in recent years they have also been widely used to assess the quality of sediments and soils [45], and recently in biomonitoring [46].
The Enrichment Factor (EF) is defined as EF = (X/Ref)sample/(X/Ref)soil, where X is the element of interest and Ref is the reference element; the index sample indicates the analyzed sample (lichen, airborne particulate matter, vegetation, soil, etc.) whereas the soil index is relative to the average concentration of X and Ref in the local parent material. There are no universal fixed rules for the choice of reference element, except that it must be immobile and almost exclusively of crustal origin. In calculating the enrichment factor, Al, Fe, Sc, or Ti are the most used elements as reference. In this study, we used aluminum as a reference element based on these considerations: (1) it is of high natural abundance, (2) it is easily determined by conventional techniques, and (3) it may be assumed as derived wholly from soil sources, and (4) has a limited metabolic value in lichens [47].
3. Results and Discussion
3.1. Descriptive Statistics
The main statistical parameters of the analyzed elements in lichens samples collected near the Syracusan petrochemical complex (IND) and its surroundings (SUBIND) are reported in Table 1, which also shows the data grouped as a single dataset (TOT). Most of the elements analyzed, based on the test Shapiro-Wilk test (p < 0.05) show asymmetric distribution, and only Al and Ba show a normal distribution. In the total database, median concentrations of the most abundant elements ranged from 8200 μg g−1 (Al) to 600 μg g−1 (Ti) with the following order of abundance: Al > Fe > Ti. The elements Mn > Zn > Ba > V > Sr > Pb > Ni > Cr, Rb > Cu, according to the following order of abundance, showed median concentrations in the range of 85–14 μg g−1. The remaining elements showed a concentration below 3 μg g−1 (Co > As > Mo, Sb > U).

Table 1.
Mean and median concentrations for 18 trace elements analyzed in lichen samples. TOT: total database; IND: samples near the industrial plant; SUBIND: control samples. Test U: Mann-Whitney test (p < 0.05). The level with statistical significance is indicated in italics.
The relations among the elements were evaluated by the Spearman correlation matrix. The elements showing significant correlation with each other have common sources and were exposed to similar atmospheric conditions. The correlations between the elements are provided in Table 2.

Table 2.
Spearman correlation matrix of variables measured at the lichen samples from the Syracusan petrochemical complex. The significant cases are indicated in italics. Upper critical value: 0.29.
A strong association between elements of crustal origin Al and Ti was identified with R = 0.91 (p < 0.05). Various elements of typical geogenic contributions such as Ba, Co, Rb, and Sr reveal good correlations (R = 0.65–0.72) highlighting the heterogeneity of the type of outcropping rocks. For Sr, it is not possible to exclude an anthropic contribution, considering the good correlations with Cr and Mn (RCr = 0.71; RMn = 0.67, p < 0.05). Furthermore, good correlations have also been found between Cr, Fe, and Mn (RCr-Fe = 0.66; RCr-Mn = 0.64; RMn-Fe = 0.63). The link between these elements may be crustal origin [48]. The Spearman correlation coefficients between V, Ni, Sb, Pb, and Zn were calculated within the range of 0.68–0.87 (p < 0.05). These elements are mostly emitted from anthropogenic activity such as fossil fuel combustion and refineries [48,49].
From the comparison of the median values in the two groups (IND-SUBIND) reported in Table 1, it is observed that the abundance orders in the total database are generally respected except for Zn > Mn, Pb > Sr, and Rb > Cr, Cu in the IND database. Furthermore, from the comparison of medians between groups (IND-SUBIND), concentration differences are observed in some trace elements (Al, As, Fe, Mn, Ni, Pb, Ti, V, and Zn). A non-parametric Mann-Whitney test (at significance level p < 0.05) was applied to verify the significant differences in metal and metalloid concentrations in lichen samples between IND and SUBIND. The results reported in Table 1 show that Al, As, Cr, Fe, Mn, Ni, Pb, Sb, Ti, V, and Zn were statistically significant differences.
Figure 3 shows the metal profiles of chemical elements in lichen groupIND and groupSUBIND. Al, Co, Fe, Rb, Sr, Ti, and U are considered typical crustal elements, even if a minority anthropogenic component cannot be excluded for iron and titanium. The elements of typical anthropogenic sources (As, Cr, Cu, Mn, Mo, Ni, Pb, Sb, V, and Zn) show higher concentrations in industrial sites than in control samples.
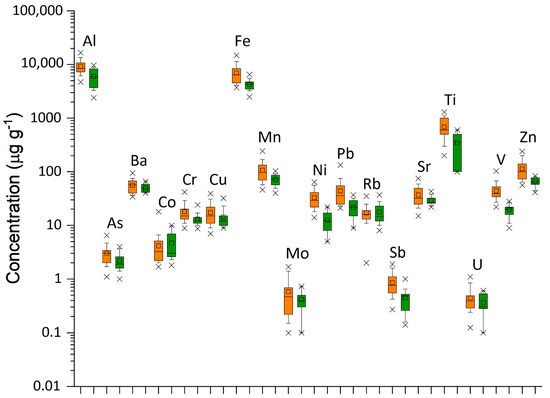
Figure 3.
Box plot of chemical elements in the analyzed lichen samples. Boxes delineate the interquartile range (25–75%) with the indication of the median (dark line); a small square inside the box marks the mean value; whiskers indicate the 10–90% range; points outside the box are minimum and maximum values. Data are given in μg g−1. Lichen samples IND (orange box); Lichen samples SUBIND (green box).
The statically different elements were used in Factor Analysis (FA) to identify relationships between variables and possible sources of air pollution. The FA model used in this study was applied to elemental raw data. The input variables were the concentrations of 11 selected elements in lichen samples. The raw calculated factor loading coefficients were rotated by Kaiser’s varimax rotation scheme [50].
The obtained factor loadings for the three-factor model, together with communalities, are given in Table 3. The loadings indicate the influence a variable has on the formation of each factor; communality indicates the total variance of a variable explained by the combination of the three common factors. FA indicates that approximately 79% of the variance may be explained by the first three factors. Factor 1, showing high positive loadings on elements Ni, Pb, Sb, V, and Zn, accounts for 32% of the total variance in the database. These are typical elements associated with fuel burning; this factor is named the “petrochemical factor”.

Table 3.
Factor loadings (Varimax rotation) for the lichen samples from the Syracusan petrochemical complex (p < 0.05).
The dominant elements in Factor 2 are As, Cr, Fe, and Mn (24% of total variance). This factor is mainly attributed to the influence of different manufacturing processes present in the investigated area. The profile of Factor 3, which accounts for a further 23% of the remaining variance, is determined by elements such as Al, and Ti. Due to the dominance of elements of typical crustal origin, this factor confirms the fundamental role of resuspension of soil particles in determining the total quantity of particulate matter on lichen samples.
Figure 4 shows the spatial variations of accumulation of some trace elements representative of the main factor extracted from the FA. The highest concentrations of Ni, Pb, V, and Zn are generally detected in samples collected near petrochemical plants.
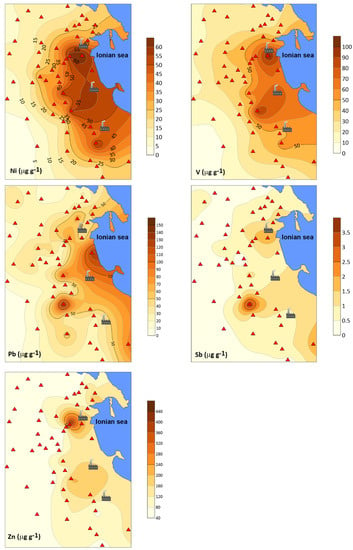
Figure 4.
Distribution maps of some trace elements in lichens from the survey area. Concentrations are given in μg g−1.
3.2. Enrichment Factor and Geochemical Indices
A serious problem in the interpretation of biogeochemical data is being able to distinguish between the origin of elements that are derived from soil and those arising from anthropogenic sources. To better understand the datasets, enrichment factors (EFs) for all elements were calculated, to differentiate natural and anthropogenic inputs in lichens. On the basis of the considerations by Hernandez et al. [51], EF values ranging between 0.5 and 2 can be considered in the range of natural variability, whereas ratios greater than 2 indicate some enrichment corresponding mainly to anthropogenic inputs. The calculated EF values between 0.5 and 2 are indicative of a geogenic input. Trace elements such as As, Cr, Cu, Ni, Pb, Sb, V and Zn had EF > 2, indicating a certain enrichment corresponding mainly to anthropogenic inputs (Figure 5). To know the level of distribution and absorption of heavy metals, the contamination factor (CF) was calculated for the trace elements that were found to be enriched by the EF. The selected elements are classified in the following sequence: Ni > V>Pb > Sb > Zn > As, Cr, Cu (Table 4), indicating that these metals and metalloids contribute to slight pollution the study area. Using the same elements, the pollutant load index (PLI) of the studied area was calculated to equal 1.76. The obtained value indicates moderate overall trace element contamination in the study area. In terms of the ecological risk posed by metals, As, Ni and Pb represent a considerable risk. Furthermore, Cu and V impose a moderate ecological risk in the order of V < Cu. Both CF and ERI calculations suggested that the environmental state of the study area is primarily threatened by As, Ni, Pb, V, and Cu. These metals can derive from various anthropogenic activities but are mainly emitted by industrial plants. The CF and ERI values are reported in Table 4.
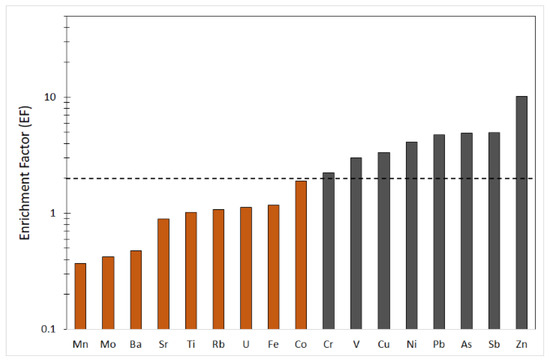
Figure 5.
Average enrichment factors (EFs) for the analyzed elements in lichen samples from the survey area. The dashed line indicates the boundary between enriched and non-enriched elements.

Table 4.
Contamination Factor (CF), Ecological Risk Index (ERI), and Pollution Load Index (PLI) of selected trace elements.
3.3. Pollution Assessment
Some elements traced by the factor analyses and enrichment factors are listed as potentially toxic to humans. These elements do not play an essential role, but several studies have emphasized that their presence in the human body can induce disease. The International Agency for Research on Cancer (IARC) has classified vanadium pentoxide as possibly carcinogenic to humans [52]. Although there is some evidence that vanadium is an essential nutrient, its functional role in humans has not been established [53]. It is well-known that the most important source of vanadium in the atmosphere is fossil fuel combustion [54] In this study, the highest concentrations of vanadium in lichens were found in those samples collected close to the industrial zone, and this was also true for a nickel. The analyses revealed high contents of nickel, corresponding to high contents of vanadium, with a correlation coefficient of 0.87 (Figure 6).
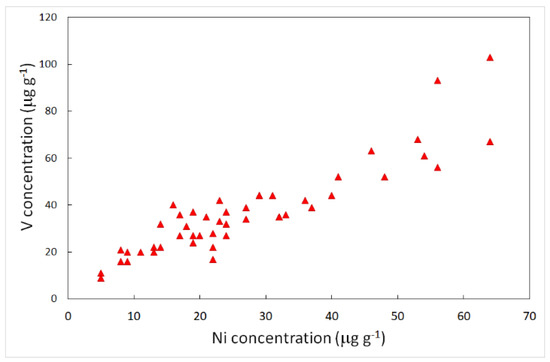
Figure 6.
Relationships among Ni and V concentrations in lichen samples. Data are given in μg g−1.
The industrial origin is further supported by the comparison of the V/Ni ratio in pet-coke: 1.4 [55], with the same ratio calculated in the IND group (1.41) compared to the average value observed in SUBIND lichens (1.69). Human exposure to high nickel concentrations in the environment may cause a variety of pathological effects [56,57,58]. Accumulation of nickel and nickel compounds in the body through chronic exposure may be responsible for a variety of adverse effects on the health of human beings, such as lung fibrosis, kidney and cardiovascular diseases, and cancer of the respiratory tract [59]. A high incidence of nasal and lung cancer in workers exposed to nickel and nickel compounds was observed [60]. Arsenic is widely distributed in the environment, especially in its organic and inorganic forms [61]. The main anthropogenic sources that play an important role in the dispersion of arsenic into the environment are mining, and the use of pesticides or petrochemical plants [62]. In 2012 the Agency for Toxic Substances and Disease Registry (ATSDR) ranked arsenic at the top of the list of dangerous substances [63]. In addition, the International Agency for Research on Cancer (IARC) classified arsenic as a human carcinogen. Antimony may be emitted into the environment through natural and anthropogenic sources. Human activities include smelting, fuel combustion, waste incineration, production of plastics and textiles, and brake wear [64]. US Environmental Protection Agency (USEPA) has listed antimony as a priority pollutant that can cause adverse effects on human health, with impacts on the skin, eyes, gastrointestinal tract, and respiratory system [65]. The IARC has classified antimony trioxide as a possible human carcinogen. The toxicity of Sb(III) is due to its greater affinity for red cells and thiol groups of cell constituents [66]. Lead is considered the most commonly studied element in various environmental compartments and its toxicity to humans has been known for over 2000 years. Human exposure to lead and its compounds can occur from various sources such as industrial processes, coal burning, ceramics, etc., although the main source of lead during the twentieth century was attributable to the use of gasoline containing lead, totally banned in 2002. Once absorbed by the body, lead accumulates in the blood and bones, as well as in organs such as the liver, kidneys, brain, and skin. Its negative health effects can be both acute and chronic because the human body fails to excrete lead. Accumulation of lead has been shown to affect the reproductive, hepatic, endocrine, immune, and gastrointestinal systems [67].
Anthropogenic elements identified in this research are compatible with various studies on different environmental matrices, both biotic and abiotic, carried out in the Syracusan petrochemical area. A study carried out by Mudu et al. [68] pointed out that the resident population in the last few decades has been exposed to various pollutants through multiple exposure scenarios involving inhalation and ingestion (drinking water, fish products, agricultural and zootechnical products), emphasizing that the concentrations determined exceeded the legislative threshold limits by several orders [68]. Nicotra et al. [69] showed the overcoming of Pb and Cd in various fish products (fish and shellfish). Di Bella et al. [70] show a strong accumulation of As and Pb in fish products; instead high concentrations of zinc have been found in beef and pork animal products. A study conducted on hair samples taken from adolescents residing in the same area showed an accumulation of Fe, V, and Zn [71].
To better understand the degree of pollution in the study area, the concentrations of the typical elements of industrial emissions (As, Cr, Ni, and V) detected in the samples of lichens taken near the industrial plant (IND) were compared with other industrial areas. The levels of As, Ni, and V determined in our samples were higher than those found in Huelva [72], San Paulo [73], Livorno [25], and Kocaeli [22]. The chromium level was consistent with concentrations measured in Huela and San Paulo [72,73], and higher than those found in Livorno [25]. This comparison highlights the environmental criticality that exists in the study area.
4. Conclusions
The results of this study reveal the versatility of the lichen species Xanthoria calcicola Oxner when searching for trace elements. Although it is impossible to establish a quantitative relationship between the concentration of a trace element in the lichen thallus and its concentration in the atmosphere, the data obtained show that the area around the Syracusan petrochemical complex is heavily affected by industrial emissions of metals and metalloids that can represent a potential danger for the local population. Although anemological conditions can favor the transport and dispersion of aerosol particles away from the source, the highest concentrations of pollutants were found near industrial plants. However, the variations in the content of a lichen species depend on various factors, such as the geological and geographical context of the growing environment and the presence of anthropogenic sources. The results of this study reveal high concentrations of Ni, Pb, V, As, and Cr compared to other European industrial situations. The interpretation of the data in terms of multivariate statistical analysis (FA) and enrichment factor (EFs) proved to be particularly useful. In particular, the Factor analysis has identified several sources that contribute to the presence of trace elements in the atmospheric particulate between anthropogenic emissions and geogenic emissions. By calculating the enrichment factor, the elements were classified into: geogenic elements, derived from the local crustal material (Ba, Sr, Co, Ti, Rb, U, Fe, Mo, Mn), and enriched elements (Zn, Sb, As, Pb, Ni, Cu, V, Cr), coming mainly from anthropogenic sources traceable in the various industrial sectors persisting in the area.
The calculation of the geochemical indices has established that the study area is certainly classified as an area where moderate pollution persists, which implies a broader study in the Syracusan area that also involves other environmental matrices. Lichen analysis turns out to be an interesting, useful, economical, and fast method for monitoring atmospheric deposition of trace elements. However, even if the lichens provide information on the long-term impact of even low levels of pollution, they do not accurately characterize the level of a certain pollutant or the total degree of pollution of a study area, therefore we suggest that the biomonitoring data are associated with continuous environmental monitoring of the chemical composition of the finest fraction of airborne particulate. In general, it is hoped that both geogenic and anthropogenic sources should be considered in the planning of environmental controls, since in municipalities close to industrial areas, in addition to emissions related to human activity, natural emissions are added.
Author Contributions
Conceptualization, D.V. and M.G.A.; Methodology, D.V., F.L.M. and M.G.A.; Data curation, D.V. and M.G.A.; Formal analysis, M.G.A., F.L.M. and D.V.; Writing–original draft, D.V. and M.G.A. All authors have read and agreed to the published version of the manuscript.
Funding
This study was jointly funded by FFR2020 (D.V. and M.G.A.).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Moreno, T.; Querol, X.; Alastuey, A.; Reche, C.; Cusack, M.; Amato, F.; Pandolfi, M.; Pey, J.; Richard, A.; Prévôt, A.S.H.; et al. Variations in time and space of trace metal aerosol concentrations in urban areas and their surroundings. Atmos. Chem. Phys. 2011, 11, 9415–9430. [Google Scholar] [CrossRef] [Green Version]
- Fernández-Camacho, R.; Rodríguez, S.; de la Rosa, J.; Sánchez de la Campa, A.M.; Alastuey, A.; Querol, X.; González-Castanedo, Y.; Garcia-Orellana, I.; Nava, S. Ultrafine particle and fine trace metal (As, Cd, Cu, Pb and Zn) pollution episodes induced by industrial emissions in Huelva, SW Spain. Atmos. Environ. 2012, 61, 507–517. [Google Scholar] [CrossRef] [Green Version]
- Pan, Y.; Tian, S.; Li, X.; Sun, Y.; Li, Y.; Wentworth, G.R.; Wang, Y. Trace elements in particulate matter from metropolitan regions of Northern China: Sources, concentrations and size distributions. Sci. Total Environ. 2015, 537, 9–22. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.P.; Wang, Y.S. Atmospheric wet and dry deposition of trace elements at 10 sites in northern China. Atmos. Chem. Phys. 2015, 15, 951–972. [Google Scholar] [CrossRef] [Green Version]
- Koz, B.; Cevik, U.; Akbulut, S. Heavy metal analysis around Murgul (Artvin) copper mining area of Turkey using moss and soil. Ecol. Indic. 2012, 20, 17–23. [Google Scholar] [CrossRef]
- Turkyilmaz, A.; Sevik, H.; Cetin, M.; Ahmaida Saleh, E.A. Changes in heavy metal accumulation depending on traffic density in some landscape plants. Pol. J. Environ. Stud. 2018, 27, 2277–2284. [Google Scholar] [CrossRef]
- Koedrith, P.; Kim, H.; Weon, J.I.; Seo, Y.R. Toxicogenomic approaches for understanding molecular mechanisms of heavy metal mutagenicity and carcinogenicity. Int. J. Hyg. Environ. Health 2013, 216, 587–598. [Google Scholar] [CrossRef]
- Caballero-Segura, B.; Ávila-Pérez, P.; Barrera Díaz, C.E.; Ramírez García, J.J.; Zarazúa, G.; Soria, R.; Ortiz-Oliveros, H.B. Metal content in mosses from the Metropolitan Area of the Toluca Valley: A comparative study between inductively coupled plasma optical emission spectrometry (ICP-OES) and total reflection X-ray fluorescence spectrometry (TXRF). Int. J. Environ. Anal. Chem. 2014, 94, 1288. [Google Scholar] [CrossRef]
- Conti, M.; Cecchetti, G. Biological monitoring: Lichens as bioindicators of air pollution assessment—A review. Environ. Pollut. 2001, 114, 471–492. [Google Scholar] [CrossRef]
- Bing, H.; Wu, Y.; Li, J.; Xiang, Z.; Luo, X.; Zhou, J.; Sun, H.; Zhang, G. Biomonitoring trace element contamination impacted by atmospheric deposition in China’s remote mountains. Atmos. Res. 2019, 224, 30–41. [Google Scholar] [CrossRef]
- Abas, A.; Sulaiman, N.; Adnan, N.R.; Aziz, S.A.; Nawang, W.N.S.W. Using lichen (Dirinaria sp.) as bio-indicator for airborne heavy metal at selected industrial areas in Malaysia. Environ. Asia 2019, 12, 85–90. [Google Scholar]
- Oksanen, J.; Laara, E.; Zobel, K. Statistical analysis of bioindicator value of epiphytic lichens. Lichenologist 1991, 23, 167–180. [Google Scholar] [CrossRef]
- Nimis, P.; Scheidegger, C.; Wolseley, P. Monitoring with Lichens—Monitoring Lichens; Springer: Dordrecht, The Netherlands, 2002. [Google Scholar]
- Spagnuolo, V.; Zampella, M.; Giordano, S.; Adamo, P. Cytological stress and element uptake in moss and lichen exposed in bags in urban area. Ecotoxicol. Environ. Saf. 2011, 74, 1434–1443. [Google Scholar] [CrossRef]
- Malaspina, P.; Giordani, P.; Modenesi, P.; Abelmoschi, M.L.; Magi, E.; Soggia, F. Bioaccumulation capacity of two chemical varieties of the lichen Pseudevernia furfuracea. Ecol. Indic. 2014, 45, 605–610. [Google Scholar] [CrossRef]
- Sujetoviene, G.; Galinyte, V. Effects of the urban environmental conditions on the physiology of lichen and moss. Atmos. Pollut. Res. 2016, 7, 611–618. [Google Scholar] [CrossRef]
- De La Cruz, A.R.H.; De La Cruz, J.K.H.; Tolentino, A.; Gioda, A. Trace element biomonitoring in the Peruvian andes metropolitan region using Flavoparmelia caperata lichen. Chemosphere 2018, 210, 849–858. [Google Scholar] [CrossRef] [PubMed]
- Ndlovu, N.B.; Frontasyeva, M.V.; Newman, R.T.; Maleka, P.P. Active biomonitoring of atmospheric pollution in the Western Cape (South Africa) using INAA and ICP-MS. J. Radioanal. Nucl. Chem. 2019, 322, 1549–1559. [Google Scholar] [CrossRef]
- Ramić, E.; Huremović, J.; Muhić-Šarac, T.; Đug, S.; Žero, S.; Olovčić, A. Biomonitoring of air pollution in bosnia and herzegovina using epiphytic lichen Hypogymnia physodes. Bull. Environ. Contam. Toxicol. 2019, 102, 763–769. [Google Scholar] [CrossRef]
- Rola, K.; Osyczka, P. Temporal changes in accumulation of trace metals in vegetative and generative parts of Xanthoria parietina lichen thalli and their implications for biomonitoring studies. Ecol. Indic. 2019, 96, 293–302. [Google Scholar] [CrossRef]
- Boonpeng, C.; Sriviboon, C.; Polyiam, W.; Sangiamdee, D.; Watthana, S.; Boonpragob, K. Assessing atmospheric pollution in a petrochemical industrial district using a lichen-air quality index (LiAQI). Ecol. Indic. 2018, 95, 589–594. [Google Scholar] [CrossRef]
- Doğrul-Demiray, A.; Yolcubal, I.; Hakan-Akyol, N.; Çobanoğlu, G. Biomonitoring of airborne metals using the Lichen Xanthoria parietina in Kocaeli Province, Turkey. Ecol. Indic. 2012, 18, 632–643. [Google Scholar]
- Loppi, S.; Pirintsos, S.A. Epiphytic lichens as sentinels for heavy metal pollution at forest ecosystems (central Italy). Environ. Poll. 2003, 121, 327–332. [Google Scholar] [CrossRef]
- Bačkor, M.; Loppi, S. Interactions of lichens with heavy metals. Biol. Plant. 2009, 53, 214–222. [Google Scholar]
- Scerbo, R.; Possenti, L.; Lampugnani, L.; Ristori, T.; Barale, R.; Barghigiani, C. Lichen (Xanthoria parietina) biomonitoring of trace element contamination and air quality assessment in Livorno Province (Tuscany, Italy). Sci. Total Environ. 1999, 241, 91–106. [Google Scholar] [CrossRef]
- Policnik, H.; Simoncic, P.; Batic, F. Monitoring air quality with lichens: A comparison between mapping in forest sites and in open areas. Environ. Pollut. 2008, 151, 395–400. [Google Scholar] [CrossRef] [PubMed]
- Gerdol, R.; Marchesini, R.; Iacumin, P.; Brancaleoni, L. Monitoring temporal trends of air pollution in an urban area using mosses and lichens as biomonitors. Chemistry 2014, 108, 388–395. [Google Scholar] [CrossRef]
- Kularatne, K.I.A.; De Freitas, C.R. Epiphytic lichens as biomonitors of airborne heavy metal pollution. Environ. Exp. Bot. 2013, 88, 24–32. [Google Scholar] [CrossRef]
- Bargagli, R.; Mikhailova, I. Accumulation of inorganic contaminants. In Monitoring with Lichens−Monitoring Lichens; NATO Science Series; Nimis, P.L., Scheidegger, C., Wolseley, P.A., Eds.; Springer: Berlin/Heidelberg, Germany, 2002; Volume 7, pp. 65–84. [Google Scholar]
- Paoli, L.; Munzi, S.; Guttová, A.; Senko, D.; Sardella, G.; Loppi, S. Lichens as suitable indicators of the bio- logical effects of atmospheric pollutants around a municipal solid waste incinerator (S Italy). Ecol. Indic. 2015, 52, 362–370. [Google Scholar] [CrossRef]
- Fano, V.; Cernigliaro, A.; Scondotto, S.; Addario, S.P.; Caruso, S.; Mira, A.; Forastiere, F.; Perucci, C.A. Mortality (1995−2000) and hospital admissions (2001–2003) in the industrial area of Gela. Epidemiol. Prev. 2006, 30, 27–32. [Google Scholar]
- Bianchi, F.; Bianca, S.; Barone, C.; Pierini, A. Updating of the prevalence of congenital anomalies among resident births in the Municipality of Gela (Southern Italy). Epidemiol. Prev. 2014, 38, 219–226. [Google Scholar]
- Italian Law 349/1986 Istituzione del Ministero Dell’ambiente e Norme in Materia di Danno Ambientale. (GU n.162 del 15-7-1986—Suppl. Ordinario n. 59). Available online: https://www.normattiva.it/uri-res/N2Ls?urn:nir:stato:legge:1986-07-08;349!vig= (accessed on 1 March 2022).
- Italian Law 426/1998. Nuovi Interventi in Campo Ambientale. (GU n.291 del 14-12-1998). Available online: https://www.normattiva.it/uri-res/N2Ls?urn:nir:stato:legge:1998;426 (accessed on 1 March 2022).
- Carbone, S. Note Illustrative Della Carta Geologica d’Italia Scala 1:50,000, Foglio 641 Augusta Ed. ISPRA—Regione Siciliana, 2011; ISBN 978-88-240-2965-0. [Google Scholar]
- Agenzia Regionale per la Protezione per L’ambiente (ARPA, Sicilia). Rapporto Sulla Qualità Dell’aria nel Comprensorio Dell’area ad Elevato Rischio di Crisi Ambientale di Siracusa. 2018. Available online: http://www.provincia.siracusa.it/rapp_aria_2018.pdf (accessed on 1 March 2022).
- Cressie, N.A.C. Statistics for Spatial Data; John Wiley and Sons, Inc.: New York, NY, USA, 1991; p. 900. [Google Scholar]
- Rutkowski, P.; Diatta, J.; Konatowska, M.; Andrzejewska, A.; Tyburski, Ł.; Przybylski, P. Geochemical referencing of natural forest contamination in Poland. Forests 2020, 11, 157. [Google Scholar] [CrossRef] [Green Version]
- Fernández, J.A.; Carballeira, A.A. comparison of indigenous mosses and topsoils for use in monitoring atmospheric heavy metal deposition in Galicia (northwest Spain). Environ. Pollut. 2001, 114, 431–441. [Google Scholar] [CrossRef]
- Shakya, K.; Chettri, M.K.; Sawidis, T. Use of mosses for the survey of heavy metal deposition in ambient air of the Kathmandu valley applying active monitoring technique. Ecoprint 2012, 19, 17–29. [Google Scholar] [CrossRef] [Green Version]
- Salo, H.; Bućko, M.S.; Vaahtovuo, E.; Limo, J.; Mäkinen, J.; Pesonen, L.J. Biomonitoring of air pollution in SW Finland by magnetic and chemical measurements of moss bags and lichens. J. Geochem. Explor. 2012, 115, 69–81. [Google Scholar] [CrossRef]
- Hakanson, L. An ecological risk index for aquatic pollution control. A sedimentological approach. Water Res. 1980, 14, 975–1001. [Google Scholar] [CrossRef]
- Qing, X.; Yutong, Z.; Shenggao, L. Assessment of heavy metal pollution and human health risk in urban soils of steel industrial city (Anshan), Liaoning, Northeast China. Ecotoxicol. Environ. Saf. 2015, 120, 377–385. [Google Scholar] [CrossRef]
- Wu, S.; Peng, S.; Zhang, X.; Wu, D.; Luo, W.; Zhang, T.; Zhou, S.; Yang, G.; Wan, H.; Wu, L. Levels and health risk assessments of heavy metals in urban soils in Dongguan, China. J. Geochem. Explor. 2015, 148, 71–78. [Google Scholar] [CrossRef]
- Liu, C.; Zhou, P.; Fang, Y. Monitoring Airborne Heavy Metal Using Mosses in the City of Xuzhou, China. Bull. Environ. Contam. Toxicol. 2016, 96, 638–644. [Google Scholar] [CrossRef]
- Hussain, S.; Hoque, R.R. Biomonitoring of metallic air pollutants in unique habitations of the Brahmaputra Valley using moss species—Atrichum angustatum: Spatiotemporal deposition patterns and sources. Environ. Sci. Pollut. Res. 2022, 29, 10617–10634. [Google Scholar] [CrossRef]
- Bargagli, R. Trace Elements in Terrestrial Plants. An Ecophysiological Approach to Biomonitoring and Biorecovery; Springer: Berlin/Heidelberg, Germany, 1998; p. 324. [Google Scholar]
- Hsu, C.Y.; Chiang, H.C.; Lin, S.L.; Chen, M.J.; Lin, T.Y.; Chen, Y.C. Elemental characterization and source apportionment of PM10 and PM2.5 in the western coastal area of central Taiwan. Sci. Total Environ. 2016, 541, 1139–1150. [Google Scholar] [CrossRef]
- Brunialti, G.; Frati, L. Biomonitoring of nine elements by the lichen Xanthoria parietina in Adriatic Italy: A retrospective study over a 7-year time span. Sci. Total Environ. 2007, 387, 289–300. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, H.F. The varimax criteria for analytical rotation in factor analysis. Psychometrika 1958, 23, 187–200. [Google Scholar] [CrossRef]
- Hernandez, L.; Probst, A.; Probst, J.L.; Ulrich, E. Heavy metal distribution in some French forest soils: Evidence for atmospheric contamination. Sci. Total Environ. 2003, 312, 195–219. [Google Scholar] [CrossRef] [Green Version]
- ATSDR. Toxicological Profile for Vanadium. U.S. Department of Health and Human Services. Public Health Service; Agency for Toxic Substances and Disease Registry: Atlanta, GA, USA, 2012. [Google Scholar]
- Mehri, A. Trace Elements in Human Nutrition (II)—An Update. Int. J. Prev. Med. 2020, 11, 2. [Google Scholar] [CrossRef] [PubMed]
- Duce, R.A.; Hoffman, G.L. Atmospheric vanadium transport to the ocean. Atmos. Environ. 1976, 10, 989–996. [Google Scholar] [CrossRef]
- Bosco, M.L.; Dongarrà, G.; Varrica, D. Case study: Inorganic pollutants associated with particulate matter from an area near a petrochemical plant. Environ. Res. 2005, 99, 18–30. [Google Scholar] [CrossRef]
- Genchi, G.; Carocci, A.; Lauria, G.; Sinicropi, M.S.; Catalano, A. Nickel: Human Health and Environmental Toxicology. Int. J. Environ. Res. Public Health 2020, 17, 679. [Google Scholar] [CrossRef] [Green Version]
- Zhao, J.; Shi, X.; Castranova, V.; Ding, M. Occupational toxicology of nickel and nickel compounds. J. Environ. Pathol. Toxicol. Oncol. 2009, 28, 177–208. [Google Scholar] [CrossRef]
- Zambelli, B.; Ciurli, S. Nickel and human health. Met. Ions Life Sci. 2013, 13, 321–357. [Google Scholar]
- McGregor, D.B.; Baan, R.A.; Partensky, C.; Rice, J.M.; Wilbourn, J.D. Evaluation of the carcinogenic risks to humans associated with surgical implants and other foreign bodies—A report of an IARC Monographs Programme Meeting. International Agency for Research on Cancer. Eur. J. Cancer 2000, 36, 307–313. [Google Scholar] [CrossRef]
- Zambelli, B.; Uversky, V.N.; Ciurli, S. Nickel impact on human health: An intrinsic disorder perspective. BBA Proteins Proteom. 2016, 1864, 1714–1731. [Google Scholar] [CrossRef] [PubMed]
- Ferrante, M.; Napoli, S.; Grasso, A.; Zuccarello, P.; Cristaldi, A.; Copat, C. Systematic review of arsenic in fresh seafood from the Mediterranean Sea and European Atlantic coasts: A health risk assessment. Food Chem. Toxicol. 2019, 126, 322–331. [Google Scholar] [CrossRef]
- Chio, C.-P.; Yuan, T.-H.; Shie, R.-H.; Chan, C.-C. Assessing vanadium and arsenic exposure of people living near a petrochemical complex with two-stage dispersion models. J. Hazard. Mater. 2014, 271, 98–107. [Google Scholar] [CrossRef] [PubMed]
- ATDSR. ATSDR’s Substance Priority List. 2019. Available online: https://www.atsdr.cdc.gov/spl/index.html (accessed on 1 April 2022).
- Yan, G.; Mao, L.; Jiang, B.; Chen, X.; Gao, Y.; Chen, C.; Li, F.; Chen, L. The source apportionment, pollution characteristic and mobility of Sb in roadside soils affected by traffic and industrial activities. J. Hazard. Mater. 2020, 384, 121352. [Google Scholar] [CrossRef]
- Jiang, J.; Wu, Y.; Sun, G.; Zhang, L.; Li, Z.; Sommar, J.; Yao, H.; Feng, X. Characteristics, Accumulation, and Potential Health Risks of Antimony in Atmospheric Particulate Matter. ACS Omega 2021, 6, 9460–9470. [Google Scholar] [CrossRef] [PubMed]
- Boreiko, C.J.; Rossman, T.G. Antimony and its compounds: Health impacts related to pulmonary toxicity, cancer, and genotoxicity. Toxicol. Appl. Pharmacol. 2020, 403, 115156. [Google Scholar] [CrossRef]
- Charkiewicz, A.E.; Backstrand, J.R. Lead Toxicity and Pollution in Poland. Int. J. Environ. Res. Public Health 2020, 17, 4385. [Google Scholar] [CrossRef]
- Mudu, P.; Terracini, B.; Martuzzi, M. Human Health in Areas with Industrial Contamination; WHO Regional Office for Europe: Copenhagen, Denmark, 2014. [Google Scholar]
- Nicotra, M.; Brundo, M.; Carpinteri, G.; Sciacca, S. Metalli pesanti e malformazioni nei pesci. Inquinamento 2007, 92, 56–60. [Google Scholar]
- Di Bella, C.; Traina, A.; Giosuè, C.; Carpintieri, D.; Lo Dico, G.M.; Bellante, A.; Del Core, M.; Falco, F.; Gherardi, S.; Uccello, M.M.; et al. Heavy Metals and PAHs in Meat, Milk, and Seafood From Augusta Area (Southern Italy): Contamination Levels, Dietary Intake, and Human Exposure Assessment. Front. Public Health 2020, 8, 273. [Google Scholar] [CrossRef]
- Varrica, D.; Tamburo, E.; Alaimo, M.G. Levels of trace elements in human hair samples of adolescents living near petrochemical plants. Environ. Geochem. Health 2021. [Google Scholar] [CrossRef]
- Parviainen, A.; Casares-Porcel, M.; Marchesi, C.; Garrido, C.J. Lichens as a spatial record of metal air pollution in the industrialized city of Huelva (SW Spain). Environ. Pollut. 2019, 253, 918–929. [Google Scholar] [CrossRef] [PubMed]
- Fuga, A.; Saiki, M.; Marcelli, M.P.; Saldiva, P.H.N. Atmospheric pollutants monitoring by analysis of epilitic lichens. Environ. Pollut. 2008, 151, 334–340. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).