Abstract
The present study suggests that standardized methodology, careful site selection, and stratigraphy are essential for investigating ancient ecosystems in order to evaluate biodiversity and DNA-based time series. Based on specific keywords, this investigation reviewed 146 publications using the SCOPUS, Web of Science (WoS), PUBMED, and Google Scholar databases. Results indicate that environmental deoxyribose nucleic acid (eDNA) can be pivotal for assessing and conserving ecosystems. Our review revealed that in the last 12 years (January 2008–July 2021), 63% of the studies based on eDNA have been reported from aquatic ecosystems, 25% from marine habitats, and 12% from terrestrial environments. Out of studies conducted in aquatic systems using the environmental DNA (eDNA) technique, 63% of the investigations have been reported from freshwater ecosystems, with an utmost focus on fish diversity (40%). Further analysis of the literature reveals that during the same period, 24% of the investigations using the environmental DNA technique were carried out on invertebrates, 8% on mammals, 7% on plants, 6% on reptiles, and 5% on birds. The results obtained clearly indicate that the environmental DNA technique has a clear-cut edge over other biodiversity monitoring methods. Furthermore, we also found that eDNA, in conjunction with different dating techniques, can provide better insight into deciphering eco-evolutionary feedback. Therefore, an attempt has been made to offer extensive information on the application of dating methods for different taxa present in diverse ecosystems. Last, we provide suggestions and elucidations on how to overcome the caveats and delineate some of the research avenues that will likely shape this field in the near future. This paper aims to identify the gaps in environmental DNA (eDNA) investigations to help researchers, ecologists, and decision-makers to develop a holistic understanding of environmental DNA (eDNA) and its utility as a palaeoenvironmental contrivance.
1. Introduction
The greatest challenge in the 21st century is the incessant diminution of Earth’s biodiversity, as the populations of world biota are being depleted at a greater rate than in pre-human periods [1,2,3,4,5,6]. Despite the fact that information on biodiversity is limited or even non-existent for many species and geographical locations, there is a worldwide political consensus to prevent the present biodiversity loss [7,8]. Smaller disintegrated populations are at increased risk of extinction due to habitat destruction by enhanced demographic, genetic, and environmental haphazardness. Though, such smaller natural populations vulnerable to extinction, as established by experimental investigations, are finite [9]. However, biological assessment and monitoring to acquire accurate species data apportionment on an ecological and political timescale becomes an essential conservation exertion to save rapidly diminishing biodiversity [10].
Visual surveys and morphological characterization of species have been the conventional method of choice for biological surveillance over a period of time. Although these methods are highly reliable when species are evident and abundant, reliability is compromised when organisms are present in low densities, thereby inflating error and biased parameter estimates [11,12]. Due to morphological plasticity and closely related species with extremely similar appearances in the juvenile stages, these methodologies may fall short of executing efficient and uniform surveys in certain circumstances. As a result, there are instances of species databases including inaccuracies. Furthermore, conventional monitoring approaches, such as marine surveys that rely on extremely destructive procedures, have occasionally proved to be invasive on the species or environment under investigation [12]. In many cases, what has been noticed is a flawed species database with errors in morphological identification, considerably highlighting the importance of taxonomic expertise, which is often not of the highest quality or is in expeditious decay currently. Therefore, there is a need for alternative and innovative methods to overcome the limitations of traditional biodiversity surveillance techniques.
Critical development for accelerated research efforts is periodically stimulated by scientific advancements considerably altering scientific thought. One such phenomenon of cardinal prominence is the discovery of eDNA, i.e., genetic material obtained directly from environmental samples (soil, sediment, water, etc.) to inscribe ecological questions [13]. Environmental DNA, a prime component of the ecologist’s and environmental manager’s toolbox, is composite genetic DNA originating from the environmental specimen with no self-explanatory signs of biological acquired material [14,15] for the discovery of the most all-encompassing DNA-based taxonomic or functional information for the ecosystem under consideration [16]. Environmental DNA has been a novel technique for biodiversity monitoring, palaeoenvironmental reconstruction [17], and spatio-temporal shifts in ecosystem diversity [18]. Moreover, eDNA has widespread utility in species biomonitoring in a wide variety of habitats such as desert springs [19], lagoons [20], arctic [21], seawater [22], forests [23], ponds [24], estuarine sediments [25], and permafrost [26]. Studies have also reported that eDNA surveys provide complementary information, particularly for the management of important inland fisheries [27]. Investigations have also revealed that eDNA metabarcoding detects more species than traditional survey methods such as gillnet surveys [28]. Similarly, it is a fast and broad biodiversity survey method that can dispense an exhaustive review of species in extremely heterogeneous tropical marine coral reefs [29]. The scope of environmental DNA is limited by DNA degradation, affecting the inference of fine-scale species and community’s spatio-temporal trends. However, despite these shortcomings, environmental DNA has tremendous potential in conservation, biodiversity monitoring, and ecosystem assessment, provided the procedure is optimized, standardized, and unified, thereby integrating taxonomy and molecular methods for any ecological study.
Even if methodological improvements are still needed [14,15], an environmental sample may be acquired in a highly consistent way across locales in a particular kind of habitat. This is more challenging using conventional approaches since outcomes are often dependent on the taxonomic expertise and experience of survey employees. eDNA is a completely non-invasive technology that does not harm the animals or environments being studied. Even high-quality taxonomic competence is sometimes insufficient in cases where the species of conservation interest have cryptic life patterns or requires the study of juvenile life stages that are difficult to distinguish from closely related species. In this case, eDNA approaches may outperform conventional methods in detecting species. It would be incorrect to generalize that eDNA is less expensive than conventional approaches since this depends on the target species. Nonetheless, multiple studies show that utilizing eDNA saves time and money when compared to conventional monitoring procedures [11,12]. As the cost of sequencing each base pair continues to fall exponentially, eDNA will likely outperform some existing approaches, particularly when adopting a metabarcoding approach. Traditional surveys are problematic for numerous species (e.g., amphibians) outside of certain seasons or weather circumstances when adult vocal activity is at its highest. eDNA, on the other hand, may persist in their ecosystem outside of these high-activity times (e.g., from juveniles), increasing the duration for monitoring. Harsh weather conditions may limit the use of conventional fishing equipment for other species (such as fish) but not eDNA sampling. The advancement of DNA sequencing technology has substantially extended the applications of eDNA and is predicted to continue further improvements in the future. Finally, we seek the most complete method of using eDNA for the benefit of our planet and all of its people. Environmental DNA will essentially be a tool for monitoring biodiversity, providing quick and efficient insights into species distribution, abundance estimates, and, eventually, population numbers, all of which will serve as the foundation for proper conservation activities. As a result, it will never directly address the biodiversity catastrophe, which is a more challenging issue that requires, particularly, political will, commitment, and action.
The concept of eDNA not only finds its applicability in monitoring the health and eminence of modern ecosystems but also provides a fascinating way to reconstruct past environments to provide a complete overview of ancient ecosystems. For future conservation planning, such reconstructed ecosystems can be utilized for backward testing of climate change models, emergence tracking of invasive species, and the valuation of anthropogenic impacts on ancient biodiversity and landscape [30].
In this review, we highlight the primary outcomes of the eDNA technique for specifying high-resolution community alignment for biodiversity surveillance and its nexus with ancient environment reconstruction via dating techniques that can be used with environmental DNA to explicate more knowledge for better management and conservation of global biodiversity. This review attempts to develop a better understanding of the concept of eDNA as a novel tool for biodiversity monitoring, its limitations, and possible solutions to achieve desirable and accurate results.
2. Methodology
2.1. Literature Search
To highlight the prominence and application of environmental DNA, an extensive literature review was undertaken using diverse scientific databases (PubMed, Web of Science, Scopus, and Google Scholar) by adopting a keyword-based search (Table 1). Keywords used include eDNA, environmental DNA, palaeoenvironmental reconstructions, biodiversity monitoring, and conservation. The article search was limited to the past 12 years (January 2008–July 2021) to extract and explore the recent progressions and findings in eDNA research. These searches were filtered using a thorough study of their abstracts, results, and conclusions to achieve the objective of the review.

Table 1.
Literature search based on specific keywords utilizing scientific databases.
2.2. PRISMA Analysis
We followed the PRISMA protocol for this systemic review, as shown in Figure 1B. Furthermore, we mostly focused on themes such as the emergence of environmental DNA, sampling design optimization, eDNA, palaeoenvironmental reconstructions, problems associated with environmental DNA techniques, and possible elucidations. After rigorous literature searches and tediously following the protocol, we finally shortlisted 146 publications that fulfilled the above-mentioned aim and themes we framed for this review.
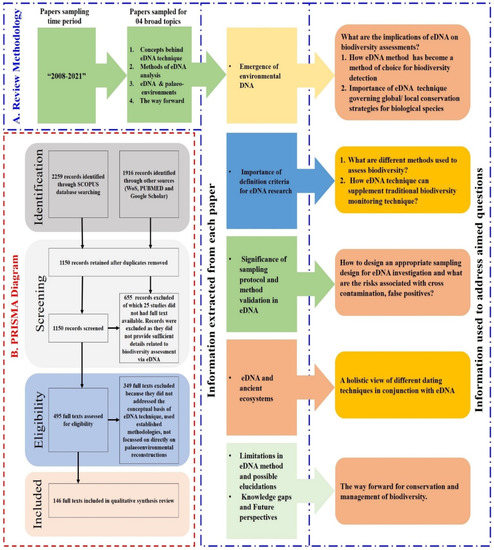
Figure 1.
Overview of the review methodology. (A) Literature review design, information collected, and research questions. (B) PRISMA methodology flow chart showing the selection and rejection of papers. Of the original 4175 articles, 495 were evaluated for eligibility based on the relevant questions, aims, and objectives.
3. Sampling Design Optimization and Method Validation
For any ecological investigation, an appropriate sampling design is crucial. Due to the dearth of a universally trusted perspective, the sampling strategy should be adjusted as per the type of environment, the scientific question, and financial, logistical, and statistical considerations [15]. Pilot study results eventually become very important from the sampling point of view. For eDNA analyses, the exact procedure should be followed. In case of any ambiguity regarding experimental problems arising from eDNA analysis, it is better to carry out pilot experiments, which can help in structuring a reliable experiment to vanquish the errors that may arise during sampling, eDNA extraction, PCR sequencing, and data interpretation. This setup will provide errorless results [15].
The standardization of the procedure for minimum sample degradation is another facet to bear in mind while designing the sampling strategy for eDNA studies. Biological replicates are crucial for every study so that the estimation of variability introduced during sampling can be interpreted correctly [31]. Prior knowledge about the origin, fate, and transport of eDNA is also an essential consideration to be kept in mind while structuring the sampling strategy [32]. One should decide whether the sampling should be stratified or pooled as it can give variable results even in the same habitat. So, to fully ascertain the complete diversity in heterogeneous environments, samples should be gathered in different sections. One should also decide whether sieving or filtering of the sample is needed or not [33,34]. In sifting soil or sediment soil, for example, there will be a greater proportion of microorganisms and propagules (pupas, pollens, or seeds), producing a bulk sample enriched in meiofauna and root tissues providing extortionate quality DNA as most inorganic and humic substances are excluded as reported for benthic meiofauna [35] or plants [36]. An imperative aspect to consider during sampling is the appropriate magnitude of the investigation area and its resolution for capturing the environmental heterogeneity of the area under consideration. It will help in describing patterns, estimating diversity parameters, or detecting species.
3.1. Delineating the Sample Strategy
There are different sampling designs that can be employed in different environmental settings. Systematic, random, and stratified sampling designs can be employed on soil samples. When the studied area displays linear environmental gradients, the appropriate approach that can be used is systematic sampling [37,38]. If the objective of the investigation is to analyze the distance decay (as the distance between samples increases, the level of similarity decreases), a systematic model with a logarithmic spacing of representative units is most appropriate. Another benefit of the systematic strategy is that it can be employed on transect (linear or belt). Ramirez et al. (2014) utilized the technique of random sampling for ecological investigation using the eDNA technique. Stratified sampling is applied to heterogeneous environments entailing discrete entities, allowing improved sample depiction by reducing the sampling error.
Contemporary scientific inquiries have also defined the sampling strategy based on climatic or biological space modeling, which has proven more proficient in delineating the biological patterns of interest [39,40]. Sampling stratum with an equal quantity of samples in habitats with high contrasted diversity and heterogeneity might not be appropriate, so in such cases, the sampling representativeness in each habitat has to be maximized [41]. It is critical that the same effort and related inferential pitfalls must apply to eDNA as they do to traditional species survey methods [42]. In case of collection of water per sample, the best recommendation is to collect water from the proposed sample site and determine how much can be pushed through filters of various pore sizes or how much water can be centrifuged. It has been reported that a 2 L water sample with glass microfiber with a pore size of 1.5 μm worked best in the search for Asian carp [43]. Similarly, 500 mL samples with a 0.22-μm filter capsule worked for the detection of white sharks in southern California [44].
3.2. Sediment Coring
Similarly, while dealing with deep sediments, sediment cores serve as an excellent setup for demonstrating the sedimentation rate, the history of pollutant additives to the water systems, and as inventories of contaminants that could prove vital to the comprehension of species dynamics and surveillance. In the last decades, extensive demand for sediment coring has ensued rapid progression of sampling techniques. Studies have revealed that the sediment core is subjected to various disturbances and may not always elucidate the in situ sediment characteristics [45]. Different corers utilized for sample extraction can eventually be employed for contaminant history inputs to preserve the sediment structural integrity and ambient (in situ) conditions. The extent of exogenous contamination penetration in the palaeoenvironmental retrieved core can be determined using genetic tracers when coring, as indicated by many palaeoenvironmental investigations [26,46,47]. Genetic tracers have been widely utilized for monitoring contamination during the coring process by introducing the laboratory strains of Serratia marcescens bacteria around the drilling apparatus [48]. Similarly, scientific investigations have employed perfluorocarbon tracers during the drilling process to test the cores on board for coring and sampling contamination [49]. Fluorescent plastic microspheres and Pseudomonas putida genetically tagged with green fluorescent protein production have been used to mimic potential microbial contamination of permafrost cores [50].
Incision of at least 1 m of the sampling surface should ideally be carried out to lessen the possibility of recent DNA deposition [51]. Intra-site and inter-site variation can be examined by employing parallel sampling, allowing multiple-sample analysis from the same deposit, ensuring palaeoecological regenerations are not distorted [52]. While investigating sediment cores, an outside portion (1–3 cm) should be removed owing to its exposure to exogenous contaminates during the coring process [51]. Sub-samples from the center, base, and top should be selected to elude contaminations on older layers by the younger DNA.
3.3. Inclusion of Appropriate Controls and Sample Preservation
To detect very low levels of DNA, most molecular ecologists include negative controls in PCR reactions. Negative controls typically account only for contamination in the laboratory. However, accounting for contamination in the field may be more important and challenging. In order to gain insight into the potential contamination, field equipment should be sampled through swabbing. Similarly, sample media and containers can also be tested. The use of negative controls in the laboratory stages of analysis is crucial to maintain sample integrity. During PCR, the low affinity between the primer and primer-binding sites results in false negative detections. To minimize PCR biases, thorough in silico and in vivo evaluation of universal primers is needed to test their biodiversity coverage before their use. Therefore, the DNA metabarcoding protocol should be fitted to geographical regions and taxonomic interest groups. After the sample collection, DNA degradation occurs or the microbial community changes over time, so it is better to extract DNA as quickly as possible after sampling. On the other hand, it is quite difficult to achieve this in the field, so blocking all biological activities as quickly as possible becomes mandatory in eDNA investigations. This can be achieved by freezing the sample in liquid nitrogen or a freezer. However, it might impact the DNA extraction process by targeting extracellular DNA, as cell lysis can occur during the storage process. On the other hand, if total DNA is the target, then the freezing/unfreezing process can facilitate cell lysis and subsequent DNA extraction.
4. Results and Discussion
4.1. Conceptual Background and Emergence of eDNA
The first report on the eDNA extraction protocol was published in 1987 by Ogram et al. (1987), where lake sediments were used to investigate microbial DNA (Figure 2). Within a short span of 3 years after the first DNA metabarcoding research investigation was disseminated and analyzed, the diversity of the 16S rRNA gene in bacterioplankton sampled from the Sargasso Sea using PCR amid cloning was published in the year 1990 [53]. For the synthesis of bioactive molecules in uncultivated microorganisms, the metagenomics technique was used by Handelsman et al. (1998), where cloning and sequencing of soil eDNA fragments were carried out to discern new pathways. A noteworthy study on DNA metabarcoding was published in 2003, which elucidated the retrieval of megafaunal (mammoth, Bison horse) and ancient plant DNA from permafrost and DNA of extinct ratite moa from cave sediments [48]. The expensive and time-consuming cloning step was made superfluous by the tumult of next-generation sequencing (NGS) after 2005 [54]. By 2010, DNA barcoding was stretched out to macroorganisms for diet analysis [55,56] and then for water and soil eDNA studies [57,58]). Recently there have been numerous publications on freshwater microorganisms concerning single species detection [24,59,60,61,62]. Over the years, different methods have been applied for eDNA analysis, for targeting single species, and standard or quantitative PCR for detecting all taxa from a given taxonomic group. PCR-based assays are vital, such as for bacteria [34,63], fungi [64,65], plants [58,66,67], eukaryotes [25,68,69], fish [62,70,71,72,73], etc.
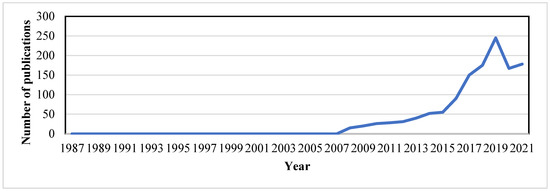
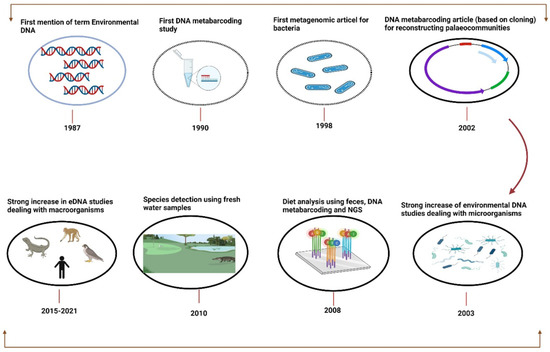
Figure 2.
Overview and emergence of environmental DNA as a tool for biodiversity monitoring from “2008–2021”.
In contemporary times, while analyzing the literature, it has been found that environmental DNA is a tool of wide application for studying freshwater ecosystems (63%) followed by marine (25%) and terrestrial habitats (12%). From these habitats, fish diversity has been widely studied, followed by invertebrates and mammals (Figure 3). Studies aiming to distinguish multiple taxa through metagenomics and metabarcoding have also been reported [22,72] and have proved to be an attractive approach, gaining insight into the past communities typically after 2014 [74,75,76]. Notwithstanding a very innovative approach at the bench and bioinformatics level, some other technical aspects remain highly challenging.
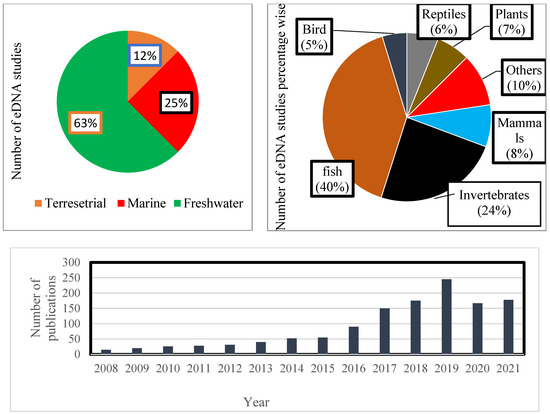
Figure 3.
Using keywords ‘environmental DNA’, ‘eDNA’, ‘environmental DNA’, and ‘palaeoenvironmental reconstruction’: the number of studies that contained one of these terms in their title, the keywords, or the abstract recovered from a literature search during the period between 1 January 2008 to July 2021.
4.2. Species Biomonitoring through Environmental DNA
Spear et al. (2021) conducted a study on the freshwater ecosystem for biodiversity detection and revealed that the environmental DNA technique can dispense knowledge that might be key for the management and conservation of lakes. The correlation observed in this study is considered second to none, as, in this study, it was demonstrated that eDNA and population abundance has a strong relationship compared to traditional species surveys [27]. Comparison of the detection sensitivity with traditional surveillance methods is fundamental to making eDNA study a concrete biodiversity monitoring tool. Afzali et al. (2021) applied the concept of environmental DNA to an estuarine ecosystem in Canada. This study utilized a traditional probing assessment of the demersal fish community to compare the result with eDNA metabarcoding. This study revealed about 53% concurrences in the estimation of species detection concerning eDNA metabarcoding in combination with trawl surveys. The combination of these methods showed a significant correlation in estimating relative abundance; however, the relationship was affected by environmental factors (temperature, depth, salinity, and oxygen) [77]. Overall, it has been established that eDNA can deliver more sensitive surveillance outcomes that are improved in comparison to traditional surveys. A similar type of study was reported from Rupert River, Canada, where it was reported that eDNA metabarcoding detected more fish species [28]. In addition, the environmental DNA technique detects a diverse range of taxonomic groups as compared to traditional surveys, as indicated by the investigation conducted by Polanco Fernadez et al. (2021) in Colombia. This study indicated that eDNA provides inclusive knowledge about the fish diversity in highly mixed tropical coral reefs. A study by Thalinger et al. (2021) revealed the spatio-temporal shifts in riverine ecosystem diversity as seasonal changes affect the eDNA distribution. To make a valid and accurate inference in both time and space for the studied species, it is highly pertinent to understand the behavior of DNA in the environment. This is because the detection process by eDNA varies seasonally in lotic environments, making parameters such as hydrological conditions and traits of species extremely important to make inferences in time. Similarly, in desert springs, the eDNA method provides better insight into species recovery, as indicated by the investigation by Mejia et al. (2021).
Various studies have been conducted in different habitats such as lagoons [20], arctic [21], pelagic diversity in marine ecosystems [78,79,80], stream biodiversity [81], seawater [22], lake sediments [82,83], and permafrost [84]. All these investigations have indicated that eDNA can be a method of choice for species identification. Such a technique has greater reliability and reflects more information and species resolution. Forest and pond ecosystems have also been studied for the mammalian diversity and distribution of invasive species. The investigation carried out by Calvignae et al. (2013) utilized Caryion fly-derived DNA to detect mammalian diversity. Similarly, invasive species presence has also been studied using the eDNA technique by Takahara et al. (2013). Dejean et al. (2012), in their study on the American bullfrog, indicated that the environmental DNA technique has a better sensitivity and reduces sampling time and effort. Similarly, Ficetola et al. (2008), while applying the eDNA method on Rana catesbeiana, a frog species, also revealed higher species-specific detection from environmental samples. An increasing number of studies have showcased the tremendous potential of eDNA technology for providing new insights into the species ecology, moving beyond species detection occurrence and community description [85]. While going through the literature review on eDNA, it can be stated that it also has notable usefulness as a practical tool for studying fish reproductive biology [86]. Some selected studies (January 2008–July 2021) enlightening the applicability of environmental DNA as a biodiversity monitoring tool are summarized below with their respective sub-titles (Table 2).
The above studies reveal that eDNA has a clear edge over other biodiversity monitoring methods. These studies indicate that environmental DNA analysis can be a powerful non-invasive detection method, posing higher reliability in the diversity of systems and across the branches of the tree of life [87,88,89,90]. Progression of eDNA as a tool to measure broad species diversity and population has also been investigated in various contributions. The applicability of eDNA as a novel tool to evaluate the efficiency of restoration and/or management strategies is emerging at an impressive amplitude to provide faster and potentially reliable monitoring [91,92,93]. The coupled approach of eDNA detection and conventional/traditional survey methods can have several complementary advantages. However, due to the lack of standardization guidelines, those advantages are sometimes undermined. The requisite guidelines, if adopted, will allow greater precision and accuracy in data comparison from multiple monitoring sites at varying points in time.

Table 2.
A sample of research publications that focused on environmental DNA as a species monitoring technique.
Table 2.
A sample of research publications that focused on environmental DNA as a species monitoring technique.
| References | Habitat/Ecosystem | Representative Species | Use | Major Findings |
|---|---|---|---|---|
| Spear et al. (2021) [27] | Freshwater | Sander vitreus | Assessing population abundance | eDNA monitoring can appropriately dispense lakes to real world management categories for early warning for at-risk lakes in need of attention. |
| Afzali et al. (2021) [77] | Estuary | Demersal fish communities | Monitoring species biodiversity | eDNA metabarcoding out-competes traditional survey methods by enabling detection of rare and endangered taxa. |
| Boivin-Delisle et al. (2021) [28] | Freshwater | Sander vitreus | Species-specific biomonitoring | eDNA technique based on species-specific primers can provide insightful cognizance on fish biodiversity. |
| Polanco Fernández et al. (2021) [29] | Tropical marine coral reefs | Actinopterygii and Elasmobranchii | Species-specific biomonitoring | eDNA approach can provide an inclusive outline of fish composition in highly assorted coral reefs. |
| Capo et al. (2021) [17] | Lake sediments | Aquatic community | Biodiversity monitoring and palaeoenvironmental reconstructions | Despite a lack of clear and concise guidelines regarding sediment ancient DNA (SedaDNA), future SedaDNA research will provide more robust and result-oriented information about palaeoenvironments. |
| Thalinger et al. (2021) [18] | Riverine | Phoxinus phoxinus Salmo trutta Oncorhynchus mykiss Salvelinus fontinalis | Spatio-temporal shifts in ecosystem biodiversity | Seasonal discharge conditions prompt deep lateral and longitudinal changes in eDNA distribution. |
| Tsuji & Shibata (2021) [86] | Freshwater | Oryzias latipes Oryzias sakaizumii | Reproductive biology | Spawning events spike eDNA concentration, which offers the prospect to monitor and comprehend spawning timings with less effort than traditional methods. |
| Mejia et al. (2021) [19] | Desert springs | Plant and animal | Species recovery | eDNA is a promising supplemental tool to traditional approaches for biodiversity monitoring in desert springs. |
| Oka et al. (2021) [20] | Lagoon | Enneapterygius philippinus Spratelloides delicatulus Rhabdoblennius nitidus Enneapterygius similis | Biodiversity monitoring | For estimation of species diversity in tropical and subtropical areas, eDNA is a useful, rapid, and cost-effective method. |
| Székely et al. (2021) [21] | Arctic | Balaena mysticetus | Genetic diversity | Cetacean footprints are a promising cradle of genomic DNA. |
| Agerbo Rasmussen et al. (2021) [80] | Experimental vineyard | Fungi and arthropods | Species biomonitoring | eDNA offers a context for diversity assessment in vineyards to make more universal conclusions. |
| Shu, Ludwig, & Peng (2020) [73] | Freshwater | Freshwater fish Misgurnis anguillicaudates Cyprinus carpio Salvelinus fontinalis | Quantification | Despite its methodological obstacles, eDNA remains a promising and powerful contrivance for fish monitoring and conservation. |
| Zhang et al. (2020) [79] | Marine | Bacteria and marine mammals | Pelagic diversity | eDNA-based metabarcoding has the potential for successful multiple biodiversity surveillance, offering technical support and knowledge for future ecosystem protection and resource reservation. |
| Jeunen et al. (2019) [78] | Marine | Multi-specific | Species-specific biodiversity | The DNA extraction protocols when corrected and optimized provide clear illustration of eDNA monitoring in the marine environment. |
| Li et al. (2018) [85] | Freshwater | Invertebrates and human-induced contamination | Ecological monitoring | eDNA is not only applied for biodiversity monitoring but can be promising tool for understanding the impact of human-induced contamination in river ecosystems. |
| Ushio et al. (2018) [87] | Freshwater | Bird communities | Avian biodiversity patterns | eDNA metabarcoding method can serve as an essential alternative for taking a snapshot of bird diversity and potentially can be effective for ecosystem conservation and management. |
| Ramírez et al. (2018) [83] | Sediments | 16S rRNA extracellular genes | Biomonitoring | Extracellular 16S rRNA genes do not greatly influence the overall composition, abundance, and community richness. |
| Sansom & Sassoubre, (2017) [88] | Freshwater | Lampsilis siliquoidea | Quantification | eDNA approach holds tremendous potential for biomonitoring of species and can act as a complementary tool to protect the biodiversity. |
| Apothéloz-Perret-Gentil et al. (2017) [81] | Freshwater and streams | Epilithic samples | Benthic diatoms index | Taxonomy free molecular index can potentially extend its gauge and frequency to compliment current morphology-based methods for environmental biomonitoring. |
| Rees et al. (2017) [89] | Freshwater | Triturus cristatus | Species-specific identification | Environmental DNA has great proficiency and reproducibility in species-specific detection. |
| Klymus et al. (2017) [90] | Freshwater | Invasive species and native species | Biodiversity monitoring | The technique of eDNA can enhance identification and conservation efforts of native species and eradicating invasive species. |
| Deiner et al. (2016) [14] | Freshwater | Metazoan eukaryotes microinvertebrates | Biodiversity patterns | eDNA evaluates the biodiversity and ecological data over an entire landscape. |
| Guardiola et al. (2016) [33] | Marine | Deep-sea communities | Spatio-temporal biodiversity monitoring | eDNA can be a cornerstone for biomonitoring of deep-sea communities. |
| Valentini et al. (2016) [72] | Freshwater Marine | Amphibians bony fish | Aquatic biodiversity monitoring | For rare and secretive species, eDNA metabarcoding is the most proficient tool. Such an approach is crucial to address the fundamental and applied research question in ecology. |
| Thomsen et al. (2016) [22] | Sea water | Fish | Biodiversity monitoring | Application of eDNA for biodiversity assessment can be potentially beneficial not only for marine fish biomonitoring but also for science, society, and the global economy. |
| Davy et al. (2015) [91] | Freshwater | Sympatric turtles | Biomonitoring of threatened species | eDNA approach could provide a rapid and cost-effective alternative for the detection of freshwater turtles. |
| Willerslev et al. (2014) [94] | Arctic | Circumpolar plant diversity Nematode diversity | Arctic vegetation history by SedaDNA | eDNA in conjunction with dating methods can reflect information about the vegetation response to glacial climates. |
| Calvignac-Spencer et al. (2013) [23] | Forest | Mammalian diversity | Species biomonitoring | Caryion fly-derived DNA can be used to address the research questions pertaining to mammalian biodiversity. |
| Takahara et al. (2013) [92] | Ponds | Lepomis macrochirus | Distribution of invasive species | Distribution or presence of invasive species can be estimated more precisely based on eDNA as compared to traditional methods. |
| Taberlet et al. (2012) [93] | Soil Water | Multi-specific | Biodiversity assessment | Environmental DNA metabarcoding has massive potential to increase data acquisition in biodiversity exploration. |
| Dejean et al. (2012) [24] | Pond | American bullfrog | Species detection | eDNA method is valuable for species detection and surpasses survey methods in terms of sensitivity and sampling effort. |
| Darling & Mahon (2011) [42] | Freshwater | Invasive Asian carp | Biological invasion | eDNA technique is highly effective for the monitoring of aquatic invasive species. |
| Chariton et al. (2010) [25] | Estuarine sediments | Eukaryote ribosomal DNA | Ecological assessment | Next-generation pyrosequencing has the ability to identify and enumerate eukaryote species assemblages. |
| Hebsgaard et al. (2009) [26] | Permafrost | Dirt DNA | Archaeological context | Ancient DNA (aDNA) preserved in sediments can provide insights about the palaeoenvironmental conditions. |
| Ficetola et al. (2008) [57] | Freshwater | Frog (Rana catesbeiana) | Species-specific detection | Development of eDNA contrivance has opened new perspectives for biodiversity monitoring from environmental samples. |
4.3. Relation between eDNA and Palaeoenvironments
Palaeoenvironmental DNA is expanding as a dynamic and prominent method in quaternary and archaeological research for reconstructing ancient environments (Figure 4) [95]. Although the first inquiry on palaeoenvironmental DNA was available in 1998 [96], some key matters regarding the diverse origin(s) of DNA and its state affecting eDNA extraction efficacy, stratigraphic consistency, and degradation of DNA for the reconstruction of palaeoenvironments remain a point of concern. The concept of eDNA, in conjunction with dating, is a fascinating method for reconstructing historical environments, be it paleoecology or archaeology [97]. However, to chronicle the palaeoenvironment, applying the notion of eDNA is not as straightforward as it seems.
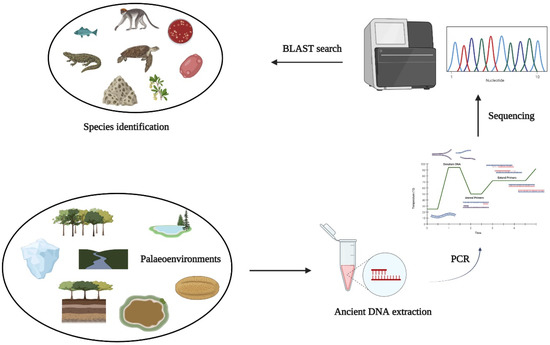
Figure 4.
Processes involved in palaeoenvironmental reconstruction and species identification through eDNA.
Utilizing eDNA with dating techniques, marine and lacustrine sediment signatures have an inordinate perspective about past evolutionary events. Different dating techniques can be employed on palaeoenvironmental DNA samples to validate the chronological overview for understanding the response of diverse flora, fauna, and microbes to natural and human influences over time (Table 3 and Table 4). Table 3 indicates the different dating methods, age range, and materials on which these can be carried out. Table 4 gives information on different taxa that can be dated in different ecosystems/habitats.

Table 3.
An overview of dating techniques that can be used in tandem with environmental DNA.

Table 4.
A sample of research publications that focused on the congruency between eDNA and palaeoecology.
To reconstruct palaeoenvironments and evaluate ecosystem change through time, bacteria, animals, and plants are utilized in palaeoecological research utilizing palaeoenvironmental DNA: silt-rich ice (dated 450–800 ka) of Central Greenland was investigated by [47], reporting the existence of mixed flora and fauna during a key ice retreat phase before it was subsequently sheltered by ice. Similarly, palaeoenvironmental DNA has been extracted from frozen plant material (dated 4500–5200 cal a BP) in southeastern Peru by [159]. The outcomes revealed ice-free vegetation before the climatic condition in the area altered in the Mid-Holocene. This analysis further indicated that pre-glacial vegetation was distinctive of wetland settings. The investigation carried out by D Costa et al. (2011) isolated and characterized genes from past bacterial DNA from ice-covered sediments aged 30,000 cal (approximately) BP (before present) to confer that antibiotic resistance is a regular occurrence in such ecosystems. Palaeoenvironmental DNA of coprolites and their preserved gut matter has been studied by [52,175,176,177] to reconstruct palaeo diets of extinct fauna. This research further revealed that it is feasible to map parasite abundance geographically.
4.3.1. Lake Sediments
In the coming years, lake sediments will epitomize the utmost utilized material for capturing past events. Capo et al. (2021) and Parducci et al. (2017) have revealed that lake sediments provide an all-inclusive and comprehensive understanding of past diversity over a range of spatio-temporal scales. Similarly, an investigation undertaken by Garcia- Rodriguez et al. (2021) and Giguet-Covex et al. (2015) indicated that sediments obtained from different depths provide favorable settings for DNA-based time series. Over a period of time, lake sediments have been affected by anthropogenic climate change, resulting in prominent changes in species composition, as indicated by the studies of Stein et al. (2020) and Yaccoz et al. (2012). Different studies have been carried out over a period of time to evaluate the efficacy of pollen analysis and ancient sediment DNA (SedaDNA). One such study was carried out by Wilmshurst et al. (2014) in a northern New Zealand offshore island, where sediment cores were retrieved. The investigation confirmed that ancient DNA (aDNA) divulged a better dataset about the indigenous occurrence of certain taxa. Taberlet et al. (2012) and Giguet-Covex et al. (2011) studied ancient plant and mammalian diversity in lake Anterne in northern France using species-specific primers via eDNA. These investigations revealed that the amalgamation of environmental DNA and dating techniques can be used to reconstruct different plant communities over time. Results from such studies have confirmed that lake sediments can dispense eDNA-based time series covering not only plant communities but also domestic animals. Application of environmental DNA on lake sediments followed by pollen analysis using species-specific primers should be utilized to distinguish most all-encompassing signals from the environment [178,179]. As pollen analysis suffers from production and dispersal variables, the investigation by Wood et al. (2008) confirmed that such bias could be augmented by employing proxies such as geographical dispersal statistics and historic vegetation checklists. Very few studies have compared sediment DNA with macrofossils as it is limited by the nature and conditions of the sampling sites, taxonomic group, and the amount of biomass produced [180]. However, in recent years, due to rapid advancement in reference libraries and improved molecular methods, the detection of taxa in ancient sediment DNA has greatly increased with higher taxonomic overlap between DNA and pollen. As per the study conducted by Zinger et al. (2019), the success of recovering the targeted ancient DNA (aDNA) from the sedimentary archives is influenced by the taphonomy and preservation of DNA molecules and DNA extraction, sequencing, and taxonomic identification. Therefore, careful consideration should be kept in mind while performing ancient DNA (SedaDNA) analysis.
4.3.2. Permafrosts and Midden Material
Permafrosts have also been used as representative samples for environmental reconstruction. However, to properly reconstruct the palaeoenvironment in permafrost, it is pertinent to merge many permafrost samples containing a tiny segment of adjacent biodiversity [84]. The first investigation regarding the usage of permafrost samples for evaluating the evolution of plant communities over the course of the past 50,000 years was carried out in the Arctic by [94]. Radiocarbon dating was employed to date around 242 permafrost samples, accompanied by DNA extraction and amplification using plant metabarcodes. For taxonomic recognition of three families, Poaceae, Asteraceae, and Cyperaceae, the universal primer Sper01 pair targeting Spermatophyta and complementary primers Poac01, Aste01, and Cype01 were used. This study revealed that flora was quite diversified prior to the last glacial maximum, revealing 109 plant molecular taxonomic units (MOTUs) and decreasing to about 45 MOTUs. However, with the temperature upsurge during the post-glacial period, 73 MOTUs were observed, with a shift towards graminoids (Poaceae and Cyperaceae) and shrubs. Non-graminoids herbaceous plants (forbs) expectedly dominated about 10,000 years ago for the considered periods. Apart from this, the megafaunal diets of four woolly mammoths, based on their intestinal/stomach contents and coprolites, have also been investigated. It was found that all samples were mostly dominated by forbs. This indicates that forbs were an essential component of their diet. Similarly, Roy-Leveillee (2015) confirmed that under absolute conditions, the theoretical limit of aDNA is ca (approximately) 1 million years in permafrost and ice. Willerslev et al. (2007) reported that for permafrost and ice palaeoenvironmental DNA, the existing practical maximum is up to 400–800 ka. As DNA conservancy is locale-restricted and profoundly affected by the thermal account of material, ref. [181] revealed that specimens from the ice-covered area have the maximum ascendancy rate for palaeoenvironmental DNA isolation.
It has also been found that midden material from archaeological sites epitomizes an imperative cradle of information to assess the food habits of primordial plant communities [182]. It dispenses knowledge about biodiversity by employing humans as biodiversity samplers, as evident from investigations utilizing leeches [183] or carrion flies [23] as an indirect provenance of mammalian DNA [184].
5. Uncertainties Associated with eDNA Analysis and Potential Elucidations
The single problem of principal prominence that confronts the approval of eDNA investigations is the ambiguity concerning false positives (detect species when no target species eDNA is present in the sample) and false negatives (fails to detect species when target species DNA is present in the sample). For investigations targeting invasive, rare, or endangered species, false negatives are a notable obstacle while false positives are reflective of sample contaminations, also create ambiguities during the various steps of sequencing assignments [185]. Therefore, to counteract such limitations, this paper has listed a few possible elucidations and solutions to optimize the eDNA technique for wide-spectrum detection of biodiversity. Table 5 shows the different limitations of environmental DNA techniques with their possible solutions and elucidations.

Table 5.
Problems associated with eDNA-based species identification and potential possible solutions.
6. The Way Forward
For the eDNA technique to truly take off, the present methods employed for ecological assessments would be needed to be adopted to the eDNA metabarcoding framework. As exploration encompassing eDNA investigations is increasing, it can be anticipated that primers for further species will be available. There will be a tremendous build-up of knowledge on eDNA influenced by perseverance and its dispersion in diverse environments, helping to understand DNA quantities and densities in organisms as sampling protocols improve. eDNA can be an essential technique for the surveillance of endangered species. Utilizing three-way approaches viz the traditional approach, taxonomic, and ecological proficiency approach with the dominant potential of eDNA can deliver the results that could be fruitful for efficient and quick species preservation and monitoring. eDNA is vital for the provenance of temporal ecological data. While eDNA is progressively prompted as a method for monitoring, its worth for hypothesis-driven temporal ecological research can prove just as consequential. Similarly, the notion of eDNA can expedite the depiction of the environment–organism interactions. Deterioration of bee species globally and colony collapse are disadvantageous to universal food production. Utilizing eDNA with NGS can deliver a simple, accurate, fast, and robust tactic to ascertain the geographical foundation of plant DNA existing in honey samples. Similarly, spatio-temporal variation in the feeding behavior of species (diet analysis) has not yet been investigated regularly, possibly due to logistic constraints. However, the eDNA approach has been utilized for analyzing the prey content of nearly 2000 fecal samples in France [195]. This investigation reported that much diversity in species diet is contrary to what has been stated in the literature. This is a prime illustration of how eDNA technology can enhance our understanding of elusive species. Beyond diet characterization, eDNA can provide more exhaustive characterization than other methods. The feces and stomach contents can further be explored for investigating resource portioning, planning conservation measures, range and habitat use, ecological impressions of varying populations, and human dimensions.
Research on environmental DNA has seen a serious progression and expansion over the years, and with time, now the scientific community is applying the concept of eRNA alongside eDNA. Researchers during the COVID-19 pandemic have utilized eRNA analysis to track large-scale outbreaks of diseases in wastewater. Such detection of SARS-CoV-2 prior to medical detection of human outbreaks amplified promptly, providing progressive warning of a surge in infected individuals. With such advancing technology, pivotal and limited medical resources can be provisioned in areas of most need. The advantages of eDNA are not limited to the uncovering of human pathogens. In the immediate future, such a technique will benefit in understanding the behavior of pathogens that creel biodiversity conservation efforts (e.g., turtle-specific DNA virus).
Environmental DNA has a wider application in citizen science, where citizens can be engaged in the know-how of commercially available sampling kits. This can help involve people in biodiversity sampling in a way that could complement existing traditional monitoring methods. As such, a more user-friendly method of data analysis is needed. In the near future, it may be possible to automate eDNA sampling, but it needs further research to investigate the temporal longevity and spatial dispersal of eDNA in diverse ecosystems. Therefore, further assessments are needed to evaluate the factors that affect eDNA presence, perseverance, and dispersal in dissimilar environments.
We want to underline that eDNA techniques will supplement conventional monitoring rather than replace it. This is obvious from the sediment literature, where macrofossil, pollen, and traditional flora surveys supplement SedaDNA (sediment ancient DNA), and eDNA has a lower detection probability for certain freshwater species. Furthermore, the last procedure of eDNA studies (which is difficult to standardize) is the interpretation of the data, and the need for well-trained taxonomists and ecologists to effectively interpret results for recommending future actions. The future of environmental DNA technology is bright with its immensely exceeding potential in comparison to traditional biodiversity monitoring methods. With the advancement of technology, the eDNA method may become a commonplace method for biodiversity monitoring, but there is a definite need to let such a technique mature as it is still a developing field.
7. Conclusions
The findings of the present review indicate that environmental DNA is quickly emerging as an important apparatus for expeditious research in ecosystem management.
Analysis of the studies carried out in the last decade reveals that compared to the traditional methods, the detection probabilities employing eDNA exertion are considerably higher, predominantly when targeting rare and secretive species.
The present study reveals that the environmental DNA technique has found a wider application in aquatic systems followed by marine and terrestrial ecosystems to fishes followed by invertebrates.
Environmental DNA can be significantly inexpensive and consumes less time. As out taxonomic understanding is becoming more meager, the data reveals that specific primers utilized for species documentation could provide better taxonomic tenacity than conventional methods.
The current study has demonstrated that the environmental DNA procedure has an incredible prospective for approximating population abundance, which is essential, particularly for surveying invasion by harmful, rare, and threatened species.
This review further establishes that careful site selection, sampling design, and reliable stratigraphy become critical for palaeoenvironmental reconstructions as post-depositional reworking and DNA leaching can diminish its efficacy as a palaeoecological contrivance.
Furthermore, this paper summarized and discussed the different dating techniques that can be applied with environmental DNA to provide more insight into past ecosystems. However, issues that ensue during its application need further attention to streamline and authenticate the methodologies to remove ambiguities during the various phases of eDNA application.
Author Contributions
S.H., Z.K. and S. led the development and design of the manuscript and writing under the supervision of B.A.G. The author B.S.B. contributed the dating part. R.Z.S., P.P. and M.Z. helped in writing, reviewing, and editing the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
Open Access funding provided by University of Helsinki, Helsinki, Finland.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors are indebted to the Former Director Centre of Research for Development, University of Kashmir, B.A Ganai for rousing our interest in environmental DNA contrivance. The author Shahnawaz Hassan acknowledges the financial support from UGC-MANF for Ph.D. research in environmental DNA (MANF UGC beneficiary Code: BININ01669533). The authors also thank Department of Environmental Science, University of Kashmir for providing facilities which together could make the present study possible. The authors are also thankful to Mohammad Jalil, Aarif Yaseen and Muzafar Zaman for their valuable suggestions for overall improvement of this manuscript. Open Access funding provided by University of Helsinki, Helsinki, Finland.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Barnosky, A.D.; Matzke, N.; Tomiya, S.; Wogan, G.O.; Swartz, B.; Quental, T.B.; Marshall, C.; McGuire, J.L.; Lindsey, E.L.; Maguire, K.C. Has the Earth’s sixth mass extinction already arrived? Nature 2011, 471, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Dirzo, R.; Young, H.S.; Galetti, M.; Ceballos, G.; Isaac, N.J.; Collen, B. Defaunation in the Anthropocene. Science 2014, 345, 401–406. [Google Scholar] [CrossRef] [PubMed]
- Pimm, S.; Russell, G.; Gittleman, J.; Brooks, T. Futures. Biodivers. Sci. 1995, 269, 347–350. [Google Scholar]
- Ceballos, G.; Ehrlich, P.R.; Dirzo, R. Biological annihilation via the ongoing sixth mass extinction signaled by vertebrate population losses and declines. Proc. Natl. Acad. Sci. USA 2017, 114, E6089–E6096. [Google Scholar] [CrossRef] [PubMed]
- Brondizio, E.S.; Settele, J.; Díaz, S.; Ngo, H.T. Global Assessment Report on Biodiversity and Ecosystem Services of the Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services; IPBES Secretariat: Bonn, Germany, 2019. [Google Scholar]
- Maron, M.; Juffe-Bignoli, D.; Krueger, L.; Kiesecker, J.; Kümpel, N.F.; ten Kate, K.; Milner-Gulland, E.; Arlidge, W.N.; Booth, H.; Bull, J.W. Setting robust biodiversity goals. Conserv. Lett. 2021, 14, e12816. [Google Scholar] [CrossRef]
- Vié, J.-C.; Hilton-Taylor, C.; Stuart, S.N. Wildlife in a Changing World: An Analysis of the 2008 IUCN Red List of Threatened Species; IUCN: Fontainebleau, France, 2009. [Google Scholar]
- Abegão, J.L.R. Human Overpopulation Atlas. 2018. Available online: https://www.overpopulationatlas.com/ (accessed on 5 February 2020).
- Morita, K.; Sahashi, G.; Miya, M.; Kamada, S.; Kanbe, T.; Araki, H. Ongoing localized extinctions of stream-dwelling white-spotted charr populations in small dammed-off habitats of Hokkaido Island, Japan. Hydrobiologia 2019, 840, 207–213. [Google Scholar] [CrossRef]
- Niesenbaum, R.A. The integration of conservation, biodiversity, and sustainability. Sustainability 2019, 11, 4676. [Google Scholar] [CrossRef]
- Gu, W.; Swihart, R.K. Absent or undetected? Effects of non-detection of species occurrence on wildlife–habitat models. Biol. Conserv. 2004, 116, 195–203. [Google Scholar] [CrossRef]
- MacKenzie, D.I.; Nichols, J.D.; Royle, J.A.; Pollock, K.H.; Bailey, L.; Hines, J.E. Occupancy Estimation and Modeling: Inferring Patterns and Dynamics of Species Occurrence; Elsevier: Burlington, MA, USA, 2017. [Google Scholar]
- Pawlowski, J.; Apothéloz-Perret-Gentil, L.; Altermatt, F. Environmental DNA: What’s behind the term? Clarifying the terminology and recommendations for its future use in biomonitoring. Mol. Ecol. 2020, 29, 4258–4264. [Google Scholar] [CrossRef]
- Deiner, K.; Bik, H.M.; Mächler, E.; Seymour, M.; Lacoursière-Roussel, A.; Altermatt, F.; Creer, S.; Bista, I.; Lodge, D.M.; De Vere, N. Environmental DNA metabarcoding: Transforming how we survey animal and plant communities. Mol. Ecol. 2017, 26, 5872–5895. [Google Scholar] [CrossRef] [PubMed]
- Taberlet, P.; Bonin, A.; Coissac, E.; Zinger, L. Environmental DNA: For Biodiversity Research and Monitoring; Oxford University Press: Oxford, UK, 2018. [Google Scholar]
- Cordier, T.; Alonso-Sáez, L.; Apothéloz-Perret-Gentil, L.; Aylagas, E.; Bohan, D.A.; Bouchez, A.; Chariton, A.; Creer, S.; Frühe, L.; Keck, F. Ecosystems monitoring powered by environmental genomics: A review of current strategies with an implementation roadmap. Mol. Ecol. 2021, 30, 2937–2958. [Google Scholar] [CrossRef]
- Capo, E.; Giguet-Covex, C.; Rouillard, A.; Nota, K.; Heintzman, P.D.; Vuillemin, A.; Ariztegui, D.; Arnaud, F.; Belle, S.; Bertilsson, S. Lake sedimentary DNA research on past terrestrial and aquatic biodiversity: Overview and recommendations. Quaternary 2021, 4, 6. [Google Scholar] [CrossRef]
- Thalinger, B.; Kirschner, D.; Pütz, Y.; Moritz, C.; Schwarzenberger, R.; Wanzenböck, J.; Traugott, M. Lateral and longitudinal fish environmental DNA distribution in dynamic riverine habitats. Environ. DNA 2021, 3, 305–318. [Google Scholar] [CrossRef]
- Palacios Mejia, M.; Curd, E.; Edalati, K.; Renshaw, M.A.; Dunn, R.; Potter, D.; Fraga, N.; Moore, J.; Saiz, J.; Wayne, R. The utility of environmental DNA from sediment and water samples for recovery of observed plant and animal species from four Mojave Desert springs. Environ. DNA 2021, 3, 214–230. [Google Scholar] [CrossRef]
- Oka, S.I.; Doi, H.; Miyamoto, K.; Hanahara, N.; Sado, T.; Miya, M. Environmental DNA metabarcoding for biodiversity monitoring of a highly diverse tropical fish community in a coral reef lagoon: Estimation of species richness and detection of habitat segregation. Environ. DNA 2021, 3, 55–69. [Google Scholar] [CrossRef]
- Székely, D.; Corfixen, N.L.; Mørch, L.L.; Knudsen, S.W.; McCarthy, M.L.; Teilmann, J.; Heide-Jørgensen, M.P.; Olsen, M.T. Environmental DNA captures the genetic diversity of bowhead whales (Balaena mysticetus) in West Greenland. Environ. DNA 2021, 3, 248–260. [Google Scholar] [CrossRef]
- Thomsen, P.F.; Møller, P.R.; Sigsgaard, E.E.; Knudsen, S.W.; Jørgensen, O.A.; Willerslev, E. Environmental DNA from seawater samples correlate with trawl catches of subarctic, deepwater fishes. PLoS ONE 2016, 11, e0165252. [Google Scholar] [CrossRef]
- Calvignac-Spencer, S.; Merkel, K.; Kutzner, N.; Kühl, H.; Boesch, C.; Kappeler, P.M.; Metzger, S.; Schubert, G.; Leendertz, F.H. Carrion fly-derived DNA as a tool for comprehensive and cost-effective assessment of mammalian biodiversity. Mol. Ecol. 2013, 22, 915–924. [Google Scholar] [CrossRef]
- Dejean, T.; Valentini, A.; Miquel, C.; Taberlet, P.; Bellemain, E.; Miaud, C. Improved detection of an alien invasive species through environmental DNA barcoding: The example of the American bullfrog Lithobates catesbeianus. J. Appl. Ecol. 2012, 49, 953–959. [Google Scholar] [CrossRef]
- Chariton, A.A.; Court, L.N.; Hartley, D.M.; Colloff, M.J.; Hardy, C.M. Ecological assessment of estuarine sediments by pyrosequencing eukaryotic ribosomal DNA. Front. Ecol. Environ. 2010, 8, 233–238. [Google Scholar] [CrossRef]
- Hebsgaard, M.B.; Gilbert, M.T.P.; Arneborg, J.; Heyn, P.; Allentoft, M.E.; Bunce, M.; Munch, K.; Schweger, C.; Willerslev, E. ‘The Farm Beneath the Sand’—An archaeological case study on ancient ‘dirt’DNA. Antiquity 2009, 83, 430–444. [Google Scholar] [CrossRef]
- Spear, M.J.; Embke, H.S.; Krysan, P.J.; Vander Zanden, M.J. Application of eDNA as a tool for assessing fish population abundance. Environ. DNA 2021, 3, 83–91. [Google Scholar] [CrossRef]
- Boivin-Delisle, D.; Laporte, M.; Burton, F.; Dion, R.; Normandeau, E.; Bernatchez, L. Using environmental DNA for biomonitoring of freshwater fish communities: Comparison with established gillnet surveys in a boreal hydroelectric impoundment. Environ. DNA 2021, 3, 105–120. [Google Scholar] [CrossRef]
- Polanco Fernández, A.; Marques, V.; Fopp, F.; Juhel, J.B.; Borrero-Pérez, G.H.; Cheutin, M.C.; Dejean, T.; González Corredor, J.D.; Acosta-Chaparro, A.; Hocdé, R. Comparing environmental DNA metabarcoding and underwater visual census to monitor tropical reef fishes. Environ. DNA 2021, 3, 142–156. [Google Scholar] [CrossRef]
- Ellegaard, M.; Clokie, M.R.; Czypionka, T.; Frisch, D.; Godhe, A.; Kremp, A.; Letarov, A.; McGenity, T.J.; Ribeiro, S.; John Anderson, N. Dead or alive: Sediment DNA archives as tools for tracking aquatic evolution and adaptation. Commun. Biol. 2020, 3, 169. [Google Scholar] [CrossRef]
- Prosser, J.I. Replicate or lie. Environ. Microbiol. 2010, 12, 1806–1810. [Google Scholar] [CrossRef]
- Barnes, M.A.; Turner, C.R. The ecology of environmental DNA and implications for conservation genetics. Conserv. Genet. 2016, 17, 1–17. [Google Scholar] [CrossRef]
- Guardiola, M.; Uriz, M.J.; Taberlet, P.; Coissac, E.; Wangensteen, O.S.; Turon, X. Deep-sea, deep-sequencing: Metabarcoding extracellular DNA from sediments of marine canyons. PLoS ONE 2015, 10, e0139633. [Google Scholar] [CrossRef] [PubMed]
- Sogin, M.L.; Morrison, H.G.; Huber, J.A.; Welch, D.M.; Huse, S.M.; Neal, P.R.; Arrieta, J.M.; Herndl, G.J. Microbial diversity in the deep sea and the underexplored “rare biosphere”. Proc. Natl. Acad. Sci. USA 2006, 103, 12115–12120. [Google Scholar] [CrossRef]
- Leray, M.; Knowlton, N. DNA barcoding and metabarcoding of standardized samples reveal patterns of marine benthic diversity. Proc. Natl. Acad. Sci. USA 2015, 112, 2076–2081. [Google Scholar] [CrossRef] [PubMed]
- Hiiesalu, I.; Oepik, M.; Metsis, M.; Lilje, L.; Davison, J.; Vasar, M.; Moora, M.; Zobel, M.; Wilson, S.D.; Paertel, M. Plant species richness belowground: Higher richness and new patterns revealed by next-generation sequencing. Mol. Ecol. 2012, 21, 2004–2016. [Google Scholar] [CrossRef] [PubMed]
- Barberán, A.; McGuire, K.L.; Wolf, J.A.; Jones, F.A.; Wright, S.J.; Turner, B.L.; Essene, A.; Hubbell, S.P.; Faircloth, B.C.; Fierer, N. Relating belowground microbial composition to the taxonomic, phylogenetic, and functional trait distributions of trees in a tropical forest. Ecol. Lett. 2015, 18, 1397–1405. [Google Scholar] [CrossRef] [PubMed]
- Philippot, L.; Bru, D.; Saby, N.P.; Čuhel, J.; Arrouays, D.; Šimek, M.; Hallin, S. Spatial patterns of bacterial taxa in nature reflect ecological traits of deep branches of the 16S rRNA bacterial tree. Environ. Microbiol. 2009, 11, 3096–3104. [Google Scholar] [CrossRef]
- Albert, C.H.; Yoccoz, N.G.; Edwards, T.C., Jr.; Graham, C.H.; Zimmermann, N.E.; Thuiller, W. Sampling in ecology and evolution–bridging the gap between theory and practice. Ecography 2010, 33, 1028–1037. [Google Scholar] [CrossRef]
- Manel, S.; Albert, C.H.; Yoccoz, N.G. Sampling in landscape genomics. In Data Production and Analysis in Population Genomics; Springer: New York, NY, USA, 2012; pp. 3–12. [Google Scholar]
- Cao, Y.; Williams, D.D.; Larsen, D.P. Comparison of ecological communities: The problem of sample representativeness. Ecol. Monogr. 2002, 72, 41–56. [Google Scholar] [CrossRef]
- Darling, J.A.; Mahon, A.R. From molecules to management: Adopting DNA-based methods for monitoring biological invasions in aquatic environments. Environ. Res. 2011, 111, 978–988. [Google Scholar] [CrossRef] [PubMed]
- Jerde, C.L.; Chadderton, W.L.; Mahon, A.R.; Renshaw, M.A.; Corush, J.; Budny, M.L.; Mysorekar, S.; Lodge, D.M. Detection of Asian carp DNA as part of a Great Lakes basin-wide surveillance program. Can. J. Fish. Aquat. Sci. 2013, 70, 522–526. [Google Scholar] [CrossRef]
- Lafferty, K.D.; Benesh, K.C.; Mahon, A.R.; Jerde, C.L.; Lowe, C.G. Detecting southern California’s white sharks with environmental DNA. Front. Mar. Sci. 2018, 5, 355. [Google Scholar] [CrossRef]
- Jutzeler, M.; White, J.D.; Talling, P.J.; McCanta, M.; Morgan, S.; Le Friant, A.; Ishizuka, O. Coring disturbances in IODP piston cores with implications for offshore record of volcanic events and the Missoula megafloods. Geochem. Geophys. Geosyst. 2014, 15, 3572–3590. [Google Scholar] [CrossRef]
- D’Costa, V.M.; King, C.E.; Kalan, L.; Morar, M.; Sung, W.W.; Schwarz, C.; Froese, D.; Zazula, G.; Calmels, F.; Debruyne, R. Antibiotic resistance is ancient. Nature 2011, 477, 457–461. [Google Scholar] [CrossRef]
- Willerslev, E.; Cappellini, E.; Boomsma, W.; Nielsen, R.; Hebsgaard, M.B.; Brand, T.B.; Hofreiter, M.; Bunce, M.; Poinar, H.N.; Dahl-Jensen, D. Ancient biomolecules from deep ice cores reveal a forested southern Greenland. Science 2007, 317, 111–114. [Google Scholar] [CrossRef] [PubMed]
- Willerslev, E.; Hansen, A.J.; Binladen, J.; Brand, T.B.; Gilbert, M.T.P.; Shapiro, B.; Bunce, M.; Wiuf, C.; Gilichinsky, D.A.; Cooper, A. Diverse plant and animal genetic records from Holocene and Pleistocene sediments. Science 2003, 300, 791–795. [Google Scholar] [CrossRef]
- Kirkpatrick, J.B.; Walsh, E.A.; D’Hondt, S. Fossil DNA persistence and decay in marine sediment over hundred-thousand-year to million-year time scales. Geology 2016, 44, 615–618. [Google Scholar] [CrossRef]
- Bang-Andreasen, T.; Schostag, M.; Priemé, A.; Elberling, B.; Jacobsen, C.S. Potential microbial contamination during sampling of permafrost soil assessed by tracers. Sci. Rep. 2017, 7, 43338. [Google Scholar] [CrossRef] [PubMed]
- Willerslev, E.; Hansen, A.J.; Poinar, H.N. Isolation of nucleic acids and cultures from fossil ice and permafrost. Trends Ecol. Evol. 2004, 19, 141–147. [Google Scholar] [CrossRef]
- Wood, J.R.; Wilmshurst, J.M.; Wagstaff, S.J.; Worthy, T.H.; Rawlence, N.J.; Cooper, A. High-resolution coproecology: Using coprolites to reconstruct the habits and habitats of New Zealand’s extinct upland moa (Megalapteryx didinus). PLoS ONE 2012, 7, e40025. [Google Scholar] [CrossRef] [PubMed]
- Giovannoni, S.J.; Britschgi, T.B.; Moyer, C.L.; Field, K.G. Genetic diversity in Sargasso Sea bacterioplankton. Nature 1990, 345, 60–63. [Google Scholar] [CrossRef] [PubMed]
- Shendure, J.; Ji, H. Next-generation DNA sequencing. Nat. Biotechnol. 2008, 26, 1135–1145. [Google Scholar] [CrossRef]
- Deagle, B.E.; Chiaradia, A.; McInnes, J.; Jarman, S.N. Pyrosequencing faecal DNA to determine diet of little penguins: Is what goes in what comes out? Conserv. Genet. 2010, 11, 2039–2048. [Google Scholar] [CrossRef]
- Pompanon, F.; Deagle, B.E.; Symondson, W.O.; Brown, D.S.; Jarman, S.N.; Taberlet, P. Who is eating what: Diet assessment using next generation sequencing. Mol. Ecol. 2012, 21, 1931–1950. [Google Scholar] [CrossRef] [PubMed]
- Ficetola, G.F.; Miaud, C.; Pompanon, F.; Taberlet, P. Species detection using environmental DNA from water samples. Biol. Lett. 2008, 4, 423–425. [Google Scholar] [CrossRef] [PubMed]
- Sønstebø, J.; Gielly, L.; Brysting, A.; Elven, R.; Edwards, M.; Haile, J.; Willerslev, E.; Coissac, E.; Rioux, D.; Sannier, J. Using next-generation sequencing for molecular reconstruction of past Arctic vegetation and climate. Mol. Ecol. Resour. 2010, 10, 1009–1018. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, C.S.; Pilliod, D.S.; Arkle, R.S.; Waits, L.P. Molecular detection of vertebrates in stream water: A demonstration using Rocky Mountain tailed frogs and Idaho giant salamanders. PLoS ONE 2011, 6, e22746. [Google Scholar] [CrossRef] [PubMed]
- Jerde, C.L.; Mahon, A.R.; Chadderton, W.L.; Lodge, D.M. “Sight-unseen” detection of rare aquatic species using environmental DNA. Conserv. Lett. 2011, 4, 150–157. [Google Scholar] [CrossRef]
- Koziol, A.; Stat, M.; Simpson, T.; Jarman, S.; DiBattista, J.D.; Harvey, E.S.; Marnane, M.; McDonald, J.; Bunce, M. Environmental DNA metabarcoding studies are critically affected by substrate selection. Mol. Ecol. Resour. 2019, 19, 366–376. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, S.; Takahara, T.; Doi, H.; Shibata, N.; Yamanaka, H. The detection of aquatic macroorganisms using environmental DNA analysis—A review of methods for collection, extraction, and detection. Environ. DNA 2019, 1, 99–108. [Google Scholar] [CrossRef]
- Tringe, S.G.; Von Mering, C.; Kobayashi, A.; Salamov, A.A.; Chen, K.; Chang, H.W.; Podar, M.; Short, J.M.; Mathur, E.J.; Detter, J.C. Comparative metagenomics of microbial communities. Science 2005, 308, 554–557. [Google Scholar] [CrossRef] [PubMed]
- Blaalid, R.; Carlsen, T.; Kumar, S.; Halvorsen, R.; Ugland, K.I.; Fontana, G.; Kauserud, H. Changes in the root-associated fungal communities along a primary succession gradient analysed by 454 pyrosequencing. Mol. Ecol. 2012, 21, 1897–1908. [Google Scholar] [CrossRef] [PubMed]
- Tedersoo, L.; Bahram, M.; Põlme, S.; Kõljalg, U.; Yorou, N.S.; Wijesundera, R.; Ruiz, L.V.; Vasco-Palacios, A.M.; Thu, P.Q.; Suija, A.; et al. Global diversity and geography of soil fungi. Science 2014, 346, 6213. [Google Scholar] [CrossRef] [PubMed]
- Kartzinel, T.R.; Chen, P.A.; Coverdale, T.C.; Erickson, D.L.; Kress, W.J.; Kuzmina, M.L.; Rubenstein, D.I.; Wang, W.; Pringle, R.M. DNA metabarcoding illuminates dietary niche partitioning by African large herbivores. Proc. Natl. Acad. Sci. USA 2015, 112, 8019–8024. [Google Scholar] [CrossRef] [PubMed]
- Yoccoz, N.G.; Bråthen, K.A.; Gielly, L.; Haile, J.; Edwards, M.E.; Goslar, T.; von Stedingk, H.; Brysting, A.; Coissac, E.; Pompanon, F.; et al. DNA from soil mirrors plant taxonomic and growth form diversity. Mol. Ecol. 2012, 21, 3647–3655. [Google Scholar] [CrossRef] [PubMed]
- Baldwin, D.S.; Colloff, M.J.; Rees, G.N.; Chariton, A.A.; Watson, G.O.; Court, L.N.; Hartley, D.M.; Morgan, M.J.; King, A.J.; Wilson, J.S. Impacts of inundation and drought on eukaryote biodiversity in semi-arid floodplain soils. Mol. Ecol. 2013, 22, 1746–1758. [Google Scholar] [CrossRef] [PubMed]
- De Vargas, C.; Audic, S.; Henry, N.; Decelle, J.; Mahé, F.; Logares, R.; Lara, E.; Berney, C.; Le Bescot, N.; Probert, I. Eukaryotic plankton diversity in the sunlit ocean. Science 2015, 348, 6237. [Google Scholar] [CrossRef] [PubMed]
- Kelly, R.P.; Port, J.A.; Yamahara, K.M.; Crowder, L.B. Using environmental DNA to census marine fishes in a large mesocosm. PLoS ONE 2014, 9, e86175. [Google Scholar] [CrossRef]
- Thomsen, P.F.; Kielgast, J.; Iversen, L.L.; Møller, P.R.; Rasmussen, M.; Willerslev, E. Detection of a diverse marine fish fauna using environmental DNA from seawater samples. PLoS ONE 2012, 7, e41732. [Google Scholar] [CrossRef] [PubMed]
- Valentini, A.; Taberlet, P.; Miaud, C.; Civade, R.; Herder, J.; Thomsen, P.F.; Bellemain, E.; Besnard, A.; Coissac, E.; Boyer, F. Next-generation monitoring of aquatic biodiversity using environmental DNA metabarcoding. Mol. Ecol. 2016, 25, 929–942. [Google Scholar] [CrossRef] [PubMed]
- Shu, L.; Ludwig, A.; Peng, Z. Standards for methods utilizing environmental DNA for detection of fish species. Genes 2020, 11, 296. [Google Scholar] [CrossRef] [PubMed]
- Epp, L.S.; Gussarova, G.; Boessenkool, S.; Olsen, J.; Haile, J.; Schrøder-Nielsen, A.; Ludikova, A.; Hassel, K.; Stenøien, H.K.; Funder, S. Lake sediment multi-taxon DNA from North Greenland records early post-glacial appearance of vascular plants and accurately tracks environmental changes. Quat. Sci. Rev. 2015, 117, 152–163. [Google Scholar] [CrossRef]
- Giguet-Covex, C.; Pansu, J.; Arnaud, F.; Rey, P.-J.; Griggo, C.; Gielly, L.; Domaizon, I.; Coissac, E.; David, F.; Choler, P.; et al. Long livestock farming history and human landscape shaping revealed by lake sediment DNA. Nat. Commun. 2014, 5, 3211. [Google Scholar] [CrossRef] [PubMed]
- Pansu, J.; Giguet-Covex, C.; Ficetola, G.F.; Gielly, L.; Boyer, F.; Zinger, L.; Arnaud, F.; Poulenard, J.; Taberlet, P.; Choler, P. Reconstructing long-term human impacts on plant communities: An ecological approach based on lake sediment DNA. Mol. Ecol. 2015, 24, 1485–1498. [Google Scholar] [CrossRef] [PubMed]
- Afzali, S.F.; Bourdages, H.; Laporte, M.; Mérot, C.; Normandeau, E.; Audet, C.; Bernatchez, L. Comparing environmental metabarcoding and trawling survey of demersal fish communities in the Gulf of St. Lawrence, Canada. Environ. DNA 2021, 3, 22–42. [Google Scholar] [CrossRef]
- Jeunen, G.J.; Knapp, M.; Spencer, H.G.; Lamare, M.D.; Taylor, H.R.; Stat, M.; Bunce, M.; Gemmell, N.J. Environmental DNA (eDNA) metabarcoding reveals strong discrimination among diverse marine habitats connected by water movement. Mol. Ecol. Resour. 2019, 19, 426–438. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Zhao, J.; Yao, M. A comprehensive and comparative evaluation of primers for metabarcoding eDNA from fish. Methods Ecol. Evol. 2020, 11, 1609–1625. [Google Scholar] [CrossRef]
- Agerbo Rasmussen, J.; Nielsen, M.; Mak, S.S.; Döring, J.; Klincke, F.; Gopalakrishnan, S.; Gilbert, M.T.P. eDNA-based biomonitoring at an experimental German vineyard to characterize how management regimes shape ecosystem diversity. Environ. DNA 2021, 3, 70–82. [Google Scholar] [CrossRef]
- Apothéloz-Perret-Gentil, L.; Cordonier, A.; Straub, F.; Iseli, J.; Esling, P.; Pawlowski, J. Taxonomy-free molecular diatom index for high-throughput eDNA biomonitoring. Mol. Ecol. Resour. 2017, 17, 1231–1242. [Google Scholar] [CrossRef]
- Capo, E.; Spong, G.; Koizumi, S.; Puts, I.; Olajos, F.; Königsson, H.; Karlsson, J.; Byström, P. Droplet digital PCR applied to environmental DNA, a promising method to estimate fish population abundance from humic-rich aquatic ecosystems. Environ. DNA 2021, 3, 343–352. [Google Scholar] [CrossRef]
- Ramírez, G.A.; Jørgensen, S.L.; Zhao, R.; D’Hondt, S. Minimal influence of extracellular DNA on molecular surveys of marine sedimentary communities. Front. Microbiol. 2018, 9, 2969. [Google Scholar] [CrossRef]
- Liang, R.; Li, Z.; Lau Vetter, M.C.; Vishnivetskaya, T.A.; Zanina, O.G.; Lloyd, K.G.; Pfiffner, S.M.; Rivkina, E.M.; Wang, W.; Wiggins, J.; et al. Genomic reconstruction of fossil and living microorganisms in ancient Siberian permafrost. Microbiome 2021, 9, 110. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Peng, Y.; Fang, W.; Altermatt, F.; Xie, Y.; Yang, J.; Zhang, X. Application of environmental DNA metabarcoding for predicting anthropogenic pollution in rivers. Environ. Sci. Technol. 2018, 52, 11708–11719. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, S.; Shibata, N. Identifying spawning events in fish by observing a spike in environmental DNA concentration after spawning. Environ. DNA 2021, 3, 190–199. [Google Scholar] [CrossRef]
- Ushio, M.; Murata, K.; Sado, T.; Nishiumi, I.; Takeshita, M.; Iwasaki, W.; Miya, M. Demonstration of the potential of environmental DNA as a tool for the detection of avian species. Sci. Rep. 2018, 8, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Sansom, B.J.; Sassoubre, L.M. Environmental DNA (eDNA) shedding and decay rates to model freshwater mussel eDNA transport in a river. Environ. Sci. Technol. 2017, 51, 14244–14253. [Google Scholar] [CrossRef] [PubMed]
- Rees, H.C.; Baker, C.A.; Gardner, D.S.; Maddison, B.C.; Gough, K.C. The detection of great crested newts year round via environmental DNA analysis. BMC Res. Notes 2017, 10, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Klymus, K.E.; Marshall, N.T.; Stepien, C.A. Environmental DNA (eDNA) metabarcoding assays to detect invasive invertebrate species in the Great Lakes. PLoS ONE 2017, 12, e0177643. [Google Scholar] [CrossRef] [PubMed]
- Davy, C.M.; Kidd, A.G.; Wilson, C.C. Development and validation of environmental DNA (eDNA) markers for detection of freshwater turtles. PLoS ONE 2015, 10, e0130965. [Google Scholar] [CrossRef]
- Takahara, T.; Minamoto, T.; Doi, H. Using environmental DNA to estimate the distribution of an invasive fish species in ponds. PLoS ONE 2013, 8, e56584. [Google Scholar]
- Taberlet, P.; Coissac, E.; Pompanon, F.; Brochmann, C.; Willerslev, E. Towards next-generation biodiversity assessment using DNA metabarcoding. Mol. Ecol. 2012, 21, 2045–2050. [Google Scholar] [CrossRef]
- Willerslev, E.; Davison, J.; Moora, M.; Zobel, M.; Coissac, E.; Edwards, M.E.; Lorenzen, E.D.; Vestergård, M.; Gussarova, G.; Haile, J. Fifty thousand years of Arctic vegetation and megafaunal diet. Nature 2014, 506, 47–51. [Google Scholar] [CrossRef] [PubMed]
- Rawlence, N.J.; Lowe, D.J.; Wood, J.R.; Young, J.M.; Churchman, G.J.; Huang, Y.T.; Cooper, A. Using palaeoenvironmental DNA to reconstruct past environments: Progress and prospects. J. Quat. Sci. 2014, 29, 610–626. [Google Scholar] [CrossRef]
- Poinar, H.N.; Hofreiter, M.; Spaulding, W.G.; Martin, P.S.; Stankiewicz, B.A.; Bland, H.; Evershed, R.P.; Possnert, G.; Pääbo, S. Molecular coproscopy: Dung and diet of the extinct ground sloth Nothrotheriops shastensis. Science 1998, 281, 402–406. [Google Scholar] [CrossRef]
- Murchie, T.J. Ancient Environmental DNA as a Means of Understanding Ecological Restructuring during the Pleistocene-Holocene Transition in Yukon, Canada. Ph.D. Thesis, McMaster University, Hamilton, ON, Canada, 2021. [Google Scholar]
- Jones, G.; Jull, A.T.; Linick, T.; Donahue, D.J. Radiocarbon dating of deep-sea sediments: A comparison of accelerator mass spectrometer and beta-decay methods. Radiocarbon 1989, 31, 105–116. [Google Scholar] [CrossRef][Green Version]
- Stuiver, M. Tree ring, varve and carbon-14 chronologies. Nature 1970, 228, 454–455. [Google Scholar] [CrossRef] [PubMed]
- Jones, N. Carbon dating, the archaeological workhorse, is getting a major reboot. Nature 2020. [Google Scholar] [CrossRef] [PubMed]
- Ku, T.-L. The uranium-series methods of age determination. Annu. Rev. Earth Planet. Sci. 1976, 4, 347–379. [Google Scholar] [CrossRef]
- Chen, T.; Li, S.; Zhao, J.; Feng, Y. Uranium-thorium dating of coral mortality and community shift in a highly disturbed inshore reef (Weizhou Island, northern South China Sea). Sci. Total Environ. 2021, 752, 141866. [Google Scholar] [CrossRef]
- Berger, G.W. Dating Quaternary events by luminescence. In Dating Quaternary Sediments; Geological Society of America Boulder: Boulder, CO, USA, 1988; Volume 227, pp. 13–50. [Google Scholar]
- Hong, S.; Lee, M.K.; Seong, Y.B.; Owen, L.A.; Rhee, H.H.; Lee, J.I.; Yoo, K.-C. Holocene sea-level history and tectonic implications derived from luminescence dating of raised beaches in Terra Nova Bay, Antarctica. Geosci. J. 2021, 25, 283–298. [Google Scholar] [CrossRef]
- Aitken, M.J. Introduction to Optical Dating: The Dating of Quaternary Sediments by the Use of Photon-Stimulated Luminescence; Clarendon Press: Oxford, UK, 1998. [Google Scholar]
- Nian, X.; Zhang, W.; Wang, Z.; Sun, Q.; Chen, Z. Inter-comparison of optically stimulated luminescence (OSL) ages between different fractions of Holocene deposits from the Yangtze delta and its environmental implications. Mar. Geol. 2021, 432, 106401. [Google Scholar] [CrossRef]
- Nishiizumi, K.; Winterer, E.; Kohl, C.; Klein, J.; Middleton, R.; Lal, D.; Arnold, J. Cosmic ray production rates of 10Be and 26Al in quartz from glacially polished rocks. J. Geophys. Res. Solid Earth 1989, 94, 17907–17915. [Google Scholar] [CrossRef]
- Charreau, J.; Lave, J.; France-Lanord, C.; Puchol, N.; Blard, P.H.; Pik, R.; Gajurel, A.P.; ASTER Team. A 6 Ma record of palaeodenudation in the central Himalayas from in situ cosmogenic 10Be in the Surai section. Basin Res. 2021, 33, 1218–1239. [Google Scholar] [CrossRef]
- Cerling, T.E.; Craig, H. Geomorphology and in-situ cosmogenic isotopes. Annu. Rev. Earth Planet. Sci. 1994, 22, 273–317. [Google Scholar] [CrossRef]
- Yans, J.; Verhaert, M.; Gautheron, C.; Antoine, P.-O.; Moussi, B.; Dekoninck, A.; Decrée, S.; Chaftar, H.-R.; Hatira, N.; Dupuis, C. (U-Th)/He Dating of Supergene Iron (Oxyhydr-) Oxides of the Nefza-Sejnane District (Tunisia): New Insights into Mineralization and Mammalian Biostratigraphy. Minerals 2021, 11, 260. [Google Scholar] [CrossRef]
- Phillips, F.M.; Leavy, B.D.; Jannik, N.O.; Elmore, D.; Kubik, P.W. The accumulation of cosmogenic chlorine-36 in rocks: A method for surface exposure dating. Science 1986, 231, 41–43. [Google Scholar] [CrossRef] [PubMed]
- Ram, R.; Purtschert, R.; Adar, E.M.; Bishof, M.; Jiang, W.; Lu, Z.-T.; Mueller, P.; Sy, A.; Vockenhuber, C.; Yechieli, Y.; et al. Controls on the 36Cl/Cl input ratio of paleo-groundwater in arid environments: New evidence from 81Kr/Kr data. Sci. Total Environ. 2021, 762, 144106. [Google Scholar] [CrossRef] [PubMed]
- Sarna-Wojcicki, A.; Lajoie, K.; Meyer, C.; Adam, D.; Rieck, H.; Morrison, R. Tephrochronologic correlation of upper Neogene sediments along the Pacific margin, conterminous United States. In Quaternary Nonglacial Geology; Geological Society of America, Inc.: Boulder, CO, USA, 1991; Volume 2, pp. 117–140. [Google Scholar]
- Westgate, J.A.; Gorton, M.P. Correlation techniques in tephra studies. In Tephra Studies; Springer: Dordrecht, The Netherlands, 1981; pp. 73–94. [Google Scholar]
- Smith, K.P.; Ólafsson, G.; Pálsdóttir, A.H. Ritual responses to catastrophic volcanism in Viking Age Iceland: Reconsidering Surtshellir Cave through Bayesian analyses of AMS dates, tephrochronology, and texts. J. Archaeol. Sci. 2021, 126, 105316. [Google Scholar] [CrossRef]
- Bada, J.L. The dating of fossil bones using the racemization of isoleucine. Earth Planet. Sci. Lett. 1972, 15, 223–231. [Google Scholar] [CrossRef]
- Bada, J.L.; Luyendyk, B.P.; Maynard, J.B. Marine sediments: Dating by the racemization of amino acids. Science 1970, 170, 730–732. [Google Scholar] [CrossRef]
- Wehmiller, J.F.; Belknap, D.F.; Boutin, B.S.; Mirecki, J.E.; Rahaim, S.D.; York, L.L. A review of the aminostratigraphy of Quaternary mollusks from United States Atlantic Coastal Plain sites. Geol. Soc. Am. Spec. Pap. 1988, 227, 69–110. [Google Scholar]
- Murray-Wallace, C.V.; Cann, J.H.; Yokoyama, Y.; Nicholas, W.A.; Lachlan, T.J.; Pan, T.-Y.; Dosseto, A.; Belperio, A.P.; Gostin, V.A. Late Pleistocene interstadial sea-levels (MIS 5a) in Gulf St Vincent, southern Australia, constrained by amino acid racemization dating of the benthic foraminifer Elphidium macelliforme. Quat. Sci. Rev. 2021, 259, 106899. [Google Scholar] [CrossRef]
- Cox, A.; Doell, R.R.; Dalrymple, G.B. Reversals of the earth’s magnetic field. Science 1964, 144, 1537–1543. [Google Scholar] [CrossRef]
- Rampino, M.R.; Shen, S.-Z. The end-Guadalupian (259.8 Ma) biodiversity crisis: The sixth major mass extinction? Hist. Biol. 2021, 33, 716–722. [Google Scholar] [CrossRef]
- Castelli, M.A.; Georges, A.; Cherryh, C.; Rosauer, D.F.; Sarre, S.D.; Contador-Kelsall, I.; Holleley, C.E. Evolving thermal thresholds explain the distribution of temperature sex reversal in an Australian dragon lizard. Divers. Distrib. 2021, 27, 427–438. [Google Scholar] [CrossRef]
- Creer, K. The dispersion of the geomagnetic field due to secular variation and its determination for remote times from paleomagnetic data. J. Geophys. Res. 1962, 67, 3461–3476. [Google Scholar] [CrossRef]
- Lund, S.P. A comparison of Holocene paleomagnetic secular variation records from North America. J. Geophys. Res. Solid Earth 1996, 101, 8007–8024. [Google Scholar] [CrossRef]
- Bieber, A.; St-Onge, G.; Feuillet, N.; Carlut, J.; Moreno, E.; Michel, E. Regional chronostratigraphy in the eastern Lesser Antilles quaternary fore-arc and accretionary wedge sediments: Relative paleointensity, oxygen isotopes and reversals. Quat. Geochronol. 2021, 65, 101179. [Google Scholar] [CrossRef]
- Fritts, H. Tree Rings and Climate; Academic Press: New York, NY, USA, 1976; p. 567. [Google Scholar]
- Jacoby, G.C.; Sheppard, P.R.; Sieh, K.E. Irregular recurrence of large earthquakes along the San Andreas fault: Evidence from trees. Science 1988, 241, 196–199. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, D.K.; Hoblitt, R.P. Tree-ring dating of pre-1980 volcanic flowage deposits at Mount St. Helens, Washington. Geol. Soc. Am. Bull. 1995, 107, 1077–1093. [Google Scholar] [CrossRef]
- Robinson, E.; Bocinsky, R.K.; Bird, D.; Freeman, J.; Kelly, R.L. Dendrochronological dates confirm a Late Prehistoric population decline in the American Southwest derived from radiocarbon dates. Philos. Trans. R. Soc. B 2021, 376, 20190718. [Google Scholar] [CrossRef]
- Suyama, Y.; Gunnarsson, U.; Parducci, L. Analysis of short DNA fragments from Holocene peatmoss samples. Holocene 2008, 18, 1003–1006. [Google Scholar] [CrossRef]
- Suyama, Y.; Kawamuro, K.; Kinoshita, I.; Yoshimura, K.; Tsumura, Y.; Takahara, H. DNA sequence from a fossil pollen of Abies spp. from Pleistocene peat. Genes Genet. Syst. 1996, 71, 145–149. [Google Scholar] [CrossRef] [PubMed]
- Shao, K.; Zhang, J.; He, K.; Wang, C.; Lu, H. Impacts of the Wetland Environment on Demographic Development during the Neolithic in the Lower Yangtze Region—Based on Peat and Archaeological Dates. Front. Earth Sci. 2021, 9, 59. [Google Scholar] [CrossRef]
- Arnold, L.J.; Roberts, R.G.; MacPhee, R.D.; Haile, J.S.; Brock, F.; Möller, P.; Froese, D.G.; Tikhonov, A.N.; Chivas, A.R.; Gilbert, M.T.P. Paper II–dirt, dates and DNA: OSL and radiocarbon chronologies of perennially frozen sediments in Siberia, and their implications for sedimentary ancient DNA studies. Boreas 2011, 40, 417–445. [Google Scholar] [CrossRef]
- Johnson, S.S.; Hebsgaard, M.B.; Christensen, T.R.; Mastepanov, M.; Nielsen, R.; Munch, K.; Brand, T.; Gilbert, M.T.P.; Zuber, M.T.; Bunce, M. Ancient bacteria show evidence of DNA repair. Proc. Natl. Acad. Sci. USA 2007, 104, 14401–14405. [Google Scholar] [CrossRef] [PubMed]
- Shi, T.; Reeves, R.; Gilichinsky, D.; Friedmann, E. Characterization of viable bacteria from Siberian permafrost by 16S rDNA sequencing. Microb. Ecol. 1997, 33, 169–179. [Google Scholar] [CrossRef]
- Vishnivetskaya, T.A.; Petrova, M.A.; Urbance, J.; Ponder, M.; Moyer, C.L.; Gilichinsky, D.A.; Tiedje, J.M. Bacterial community in ancient Siberian permafrost as characterized by culture and culture-independent methods. Astrobiology 2006, 6, 400–414. [Google Scholar] [CrossRef] [PubMed]
- Massicotte, P.; Amon, R.M.; Antoine, D.; Archambault, P.; Balzano, S.; Bélanger, S.; Benner, R.; Boeuf, D.; Bricaud, A.; Bruyant, F. The MALINA oceanographic expedition: How do changes in ice cover, permafrost and UV radiation impact biodiversity and biogeochemical fluxes in the Arctic Ocean? Earth Syst. Sci. Data 2021, 13, 1561–1592. [Google Scholar] [CrossRef]
- Murchie, T.J.; Kuch, M.; Duggan, A.T.; Ledger, M.L.; Roche, K.; Klunk, J.; Karpinski, E.; Hackenberger, D.; Sadoway, T.; MacPhee, R. Optimizing extraction and targeted capture of ancient environmental DNA for reconstructing past environments using the PalaeoChip Arctic-1.0 bait-set. Quat. Res. 2021, 99, 305–328. [Google Scholar] [CrossRef]
- Varotto, C.; Pindo, M.; Bertoni, E.; Casarotto, C.; Camin, F.; Girardi, M.; Maggi, V.; Cristofori, A. A pilot study of eDNA metabarcoding to estimate plant biodiversity by an alpine glacier core (Adamello Glacier, North Italy). Sci. Rep. 2021, 11, 1208. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.-J.; Rogers, S.O.; Catranis, C.M.; Starmer, W.T. Detection and characterization of ancient fungi entrapped in glacial ice. Mycologia 2000, 92, 286–295. [Google Scholar] [CrossRef]
- Tian, Y.; Spicer, R.A.; Huang, J.; Zhou, Z.; Su, T.; Widdowson, M.; Jia, L.; Li, S.; Wu, W.; Xue, L. New early oligocene zircon U-Pb dates for the ‘Miocene’ Wenshan Basin, Yunnan, China: Biodiversity and paleoenvironment. Earth Planet. Sci. Lett. 2021, 565, 116929. [Google Scholar] [CrossRef]
- Ingels, J.; Aronson, R.B.; Smith, C.R.; Baco, A.; Bik, H.M.; Blake, J.A.; Brandt, A.; Cape, M.; Demaster, D.; Dolan, E. Antarctic ecosystem responses following ice-shelf collapse and iceberg calving: Science review and future research. Wiley Interdiscip. Rev. Clim. Chang. 2021, 12, e682. [Google Scholar] [CrossRef]
- Bennett, K.D.; Parducci, L. DNA from pollen: Principles and potential. Holocene 2006, 16, 1031–1034. [Google Scholar] [CrossRef]
- Gugerli, F.; Alvarez, N.; Tinner, W. A deep dig—Hindsight on Holocene vegetation composition from ancient environmental DNA. Mol. Ecol. 2013, 22, 3433–3436. [Google Scholar] [CrossRef] [PubMed]
- Parducci, L.; Suyama, Y.; Lascoux, M.; Bennett, K.D. Ancient DNA from pollen: A genetic record of population history in Scots pine. Mol. Ecol. 2005, 14, 2873–2882. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, M.W.; Ginolhac, A.; Orlando, L.; Olsen, J.; Andersen, K.; Holm, J.; Funder, S.; Willerslev, E.; Kjær, K.H. A comparative study of ancient environmental DNA to pollen and macrofossils from lake sediments reveals taxonomic overlap and additional plant taxa. Quat. Sci. Rev. 2013, 75, 161–168. [Google Scholar] [CrossRef]
- Xu, Z.-H.; Jiang, X.-D.; Wang, G.-Z.; He, J.-F.; Cai, M.-H.; Wu, L.-S.; Jiang, J.-L.; Chen, X.-L. DNA extraction, amplification and analysis of the 28S rRNA portion in sediment-buried copepod DNA in the Great Wall Bay and Xihu Lake, Antarctica. J. Plankton Res. 2011, 33, 917–925. [Google Scholar] [CrossRef][Green Version]
- Rudaya, N.; Nazarova, L.; Frolova, L.; Palagushkina, O.; Soenov, V.; Cao, X.; Syrykh, L.; Grekov, I.; Otgonbayar, D.; Bayarkhuu, B. The link between climate change and biodiversity of lacustrine inhabitants and terrestrial plant communities of the Uvs Nuur Basin (Mongolia) during the last three millennia. Holocene 2021, 31, 09596836211019093. [Google Scholar] [CrossRef]
- Zhang, H.; Huo, S.; Yeager, K.M.; Wu, F. Sedimentary DNA record of eukaryotic algal and cyanobacterial communities in a shallow Lake driven by human activities and climate change. Sci. Total Environ. 2021, 753, 141985. [Google Scholar] [CrossRef]
- Thomsen, P.F.; Elias, S.; Gilbert, M.T.P.; Haile, J.; Munch, K.; Kuzmina, S.; Froese, D.G.; Sher, A.; Holdaway, R.N.; Willerslev, E. Non-destructive sampling of ancient insect DNA. PLoS ONE 2009, 4, e5048. [Google Scholar] [CrossRef]
- Lazagabaster, I.A.; Ullman, M.; Porat, R.; Halevi, R.; Porat, N.; Davidovich, U.; Marom, N. Changes in the large carnivore community structure of the Judean Desert in connection to Holocene human settlement dynamics. Sci. Rep. 2021, 11, 3548. [Google Scholar] [CrossRef] [PubMed]
- David, B.; Arnold, L.J.; Delannoy, J.-J.; Fresløv, J.; Urwin, C.; Petchey, F.; McDowell, M.C.; Mullett, R.; Land, G.; Mialanes, J. Late survival of megafauna refuted for Cloggs Cave, SE Australia: Implications for the Australian Late Pleistocene megafauna extinction debate. Quat. Sci. Rev. 2021, 253, 106781. [Google Scholar] [CrossRef]
- Caldeira, A.T.; Schiavon, N.; Mauran, G.; Salvador, C.; Rosado, T.; Mirão, J.; Candeias, A. On the Biodiversity and Biodeteriogenic Activity of Microbial Communities Present in the Hypogenic Environment of the Escoural Cave, Alentejo, Portugal. Coatings 2021, 11, 209. [Google Scholar] [CrossRef]
- Paffetti, D.; Vettori, C.; Caramelli, D.; Vernesi, C.; Lari, M.; Paganelli, A.; Paule, L.; Giannini, R. Unexpected presence of Fagus orientalis complex in Italy as inferred from 45,000-year-old DNA pollen samples from Venice lagoon. BMC Evol. Biol. 2007, 7, S6. [Google Scholar] [CrossRef] [PubMed]
- Albano, P.G.; Steger, J.; Bošnjak, M.; Dunne, B.; Guifarro, Z.; Turapova, E.; Hua, Q.; Kaufman, D.S.; Rilov, G.; Zuschin, M. Native biodiversity collapse in the eastern Mediterranean. Proc. R. Soc. B 2021, 288, 20202469. [Google Scholar] [CrossRef] [PubMed]
- Alves, E.Q.; Macario, K.D.; Spotorno, P.; Oliveira, F.M.; Muniz, M.C.; Fallon, S.; Souza, R.; Salvador, A.; Eschner, A.; Ramsey, C.B. Nineteenth-century expeditions and the radiocarbon marine reservoir effect on the Brazilian coast. Geochim. Cosmochim. Acta 2021, 297, 276–287. [Google Scholar] [CrossRef]
- Quarta, G.; Maruccio, L.; D’Elia, M.; Calcagnile, L. Radiocarbon Dating of Marine Samples: Methodological Aspects, Applications and Case Studies. Water 2021, 13, 986. [Google Scholar] [CrossRef]
- Vieira, C.; Antoine De Ramon, N.Y.; Rasoamanendrika, F.A.; D’Hondt, S.; Tran, L.-A.T.; Van den Spiegel, D.; Kawai, H.; De Clerck, O. Marine macroalgal biodiversity of northern Madagascar: Morpho-genetic systematics and implications of anthropic impacts for conservation. Biodivers. Conserv. 2021, 30, 1501–1546. [Google Scholar] [CrossRef]
- Gould, B.A.; León, B.; Buffen, A.M.; Thompson, L.G. Evidence of a high-Andean, mid-Holocene plant community: An ancient DNA analysis of glacially preserved remains. Am. J. Bot. 2010, 97, 1579–1584. [Google Scholar] [CrossRef]
- Schlütz, F.; Lehmkuhl, F. Holocene climatic change and the nomadic Anthropocene in Eastern Tibet: Palynological and geomorphological results from the Nianbaoyeze Mountains. Quat. Sci. Rev. 2009, 28, 1449–1471. [Google Scholar] [CrossRef]
- Oliva, M.; Ruiz-Fernández, J. Late Quaternary environmental dynamics in Lenin Peak area (Pamir Mountains, Kyrgyzstan). Sci. Total Environ. 2018, 645, 603–614. [Google Scholar] [CrossRef] [PubMed]
- Falardeau, J.; de Vernal, A.; Seidenkrantz, M.-S.; Fritz, M. Benthic foraminifer and ostracod assemblages in the Beaufort Sea continental shelf over the last millennia: Evidence of unprecedented changes in the last two centuries. In Proceedings of the PALEOARC 2021—2nd International Conference on ‘Processes and Palaeo-Environmental Changes in the Arctic from Past to Present’, Online, 25–28 May 2021. [Google Scholar]
- Jørgensen, T.; Kjaer, K.H.; Haile, J.; Rasmussen, M.; Boessenkool, S.; Andersen, K.; Coissac, E.; Taberlet, P.; Brochmann, C.; Orlando, L. Islands in the ice: Detecting past vegetation on Greenlandic nunataks using historical records and sedimentary ancient DNA Meta-barcoding. Mol. Ecol. 2012, 21, 1980–1988. [Google Scholar] [CrossRef] [PubMed]
- Wilmshurst, J.M.; Moar, N.T.; Wood, J.R.; Bellingham, P.J.; Findlater, A.M.; Robinson, J.J.; Stone, C. Use of pollen and ancient DNA as conservation baselines for offshore islands in New Zealand. Conserv. Biol. 2014, 28, 202–212. [Google Scholar] [CrossRef] [PubMed]
- Fan, K.; Delgado-Baquerizo, M.; Guo, X.; Wang, D.; Zhu, Y.-G.; Chu, H. Biodiversity of key-stone phylotypes determines crop production in a 4-decade fertilization experiment. ISME J. 2021, 15, 550–561. [Google Scholar] [CrossRef] [PubMed]
- Guerra, C.A.; Bardgett, R.D.; Caon, L.; Crowther, T.W.; Delgado-Baquerizo, M.; Montanarella, L.; Navarro, L.M.; Orgiazzi, A.; Singh, B.K.; Tedersoo, L. Tracking, targeting, and conserving soil biodiversity. Science 2021, 371, 239–241. [Google Scholar] [CrossRef]
- Jou, R.M.; Macario, K.D.; Pessenda, L.C.; Pereira, M.G.; Lorente, F.L.; Pedrosa, R.; da Silva Neto, E.C.; Fallon, S.; Muniz, M.C.; Cardoso, R.P. The use of carbon isotopes (13C, 14C) in different soil types and vegetation coverage in a montane atlantic forest region, Southeast Brazil. Quat. Geochronol. 2021, 61, 101133. [Google Scholar] [CrossRef]
- Kuch, M.; Poinar, H. Extraction of DNA from paleofeces. In Ancient DNA; Springer: New York, NY, USA, 2012; pp. 37–42. [Google Scholar]
- Merklinger, F.F.; Böhnert, T.; Arakaki, M.; Weigend, M.; Quandt, D.; Luebert, F. Quaternary diversification of a columnar cactus in the driest place on earth. Am. J. Bot. 2021, 108, 184–199. [Google Scholar] [CrossRef] [PubMed]
- Oswald, J.A.; Terrill, R.S.; Stucky, B.J.; LeFebvre, M.J.; Steadman, D.W.; Guralnick, R.P.; Allen, J.M. Ancient DNA from the extinct Haitian cave-rail (Nesotrochis steganinos) suggests a biogeographic connection between the Caribbean and Old World. Biol. Lett. 2021, 17, 20200760. [Google Scholar] [CrossRef]
- Rabinow, S.; Giovas, C. A systematic review of agouti (Dasyproctidae: Dasyprocta) records from the pre-1492 Lesser Antilles: New perspectives on an introduced commensal. Int. J. Osteoarchaeol. 2021, 31, 758–769. [Google Scholar] [CrossRef]
- Witt, K.E.; Yarlagadda, K.; Allen, J.M.; Bader, A.C.; Simon, M.L.; Kuehn, S.R.; Swanson, K.S.; Cross, T.-W.L.; Hedman, K.M.; Ambrose, S.H.; et al. Integrative analysis of DNA, macroscopic remains and stable isotopes of dog coprolites to reconstruct community diet. Sci. Rep. 2021, 11, 3113. [Google Scholar] [CrossRef] [PubMed]
- Leung, T.L. Parasites of Fossil Vertebrates: What We Know and What Can We Expect from the Fossil Record? In The Evolution and Fossil Record of Parasitism: Identification and Macroevolution of Parasites; Springer: Cham, Switzerland, 2021. [Google Scholar]
- Rummy, P.; Halaclar, K.; Chen, H. The first record of exceptionally-preserved spiral coprolites from the Tsagan-Tsab formation (lower cretaceous), Tatal, western Mongolia. Sci. Rep. 2021, 11, 7891. [Google Scholar] [CrossRef] [PubMed]
- Van Geel, B.; Aptroot, A.; Baittinger, C.; Birks, H.H.; Bull, I.D.; Cross, H.B.; Evershed, R.P.; Gravendeel, B.; Kompanje, E.J.; Kuperus, P. The ecological implications of a Yakutian mammoth’s last meal. Quat. Res. 2008, 69, 361–376. [Google Scholar] [CrossRef]
- Wood, J.R.; Rawlence, N.J.; Rogers, G.M.; Austin, J.J.; Worthy, T.H.; Cooper, A. Coprolite deposits reveal the diet and ecology of the extinct New Zealand megaherbivore moa (Aves, Dinornithiformes). Quat. Sci. Rev. 2008, 27, 2593–2602. [Google Scholar] [CrossRef]
- Wood, J.R.; Wilmshurst, J.M.; Richardson, S.J.; Rawlence, N.J.; Wagstaff, S.J.; Worthy, T.H.; Cooper, A. Resolving lost herbivore community structure using coprolites of four sympatric moa species (Aves: Dinornithiformes). Proc. Natl. Acad. Sci. USA 2013, 110, 16910–16915. [Google Scholar] [CrossRef] [PubMed]
- Parducci, L.; Matetovici, I.; Fontana, S.L.; Bennett, K.D.; Suyama, Y.; Haile, J.; Kjær, K.H.; Larsen, N.K.; Drouzas, A.D.; Willerslev, E. Molecular-and pollen-based vegetation analysis in lake sediments from central Scandinavia. Mol. Ecol. 2013, 22, 3511–3524. [Google Scholar] [CrossRef]
- Ruppert, K.M.; Kline, R.J.; Rahman, M.S. Past, present, and future perspectives of environmental DNA (eDNA) metabarcoding: A systematic review in methods, monitoring, and applications of global eDNA. Glob. Ecol. Conserv. 2019, 17, e00547. [Google Scholar] [CrossRef]
- Edwards, M.E. The maturing relationship between Quaternary paleoecology and ancient sedimentary DNA. Quat. Res. 2020, 96, 39–47. [Google Scholar] [CrossRef]
- Sawyer, S.; Krause, J.; Guschanski, K.; Savolainen, V.; Pääbo, S. Temporal patterns of nucleotide misincorporations and DNA fragmentation in ancient DNA. PLoS ONE 2012, 7, e34131. [Google Scholar]
- Richards, G. Human Evolution: An Introduction for the Behavioural Sciences; Routledge: Oxford, UK, 2019. [Google Scholar]
- Schnell, I.B.; Thomsen, P.F.; Wilkinson, N.; Rasmussen, M.; Jensen, L.R.; Willerslev, E.; Bertelsen, M.F.; Gilbert, M.T.P. Screening mammal biodiversity using DNA from leeches. Curr. Biol. 2012, 22, R262–R263. [Google Scholar] [CrossRef] [PubMed]
- Seersholm, F.V.; Cole, T.L.; Grealy, A.; Rawlence, N.J.; Greig, K.; Knapp, M.; Stat, M.; Hansen, A.J.; Easton, L.J.; Shepherd, L. Subsistence practices, past biodiversity, and anthropogenic impacts revealed by New Zealand-wide ancient DNA survey. Proc. Natl. Acad. Sci. USA 2018, 115, 7771–7776. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, C.S.; Sepulveda, A.; Ray, A.; Baumgardt, J.; Waits, L.P. Environmental DNA as a new method for early detection of New Zealand mudsnails (Potamopyrgus antipodarum). Freshw. Sci. 2013, 32, 792–800. [Google Scholar] [CrossRef]
- Clare, E.L.; Chain, F.J.; Littlefair, J.E.; Cristescu, M.E. The effects of parameter choice on defining molecular operational taxonomic units and resulting ecological analyses of metabarcoding data. Genome 2016, 59, 981–990. [Google Scholar] [CrossRef] [PubMed]
- Creer, S.; Deiner, K.; Frey, S.; Porazinska, D.; Taberlet, P.; Thomas, W.K.; Potter, C.; Bik, H.M. The ecologist’s field guide to sequence-based identification of biodiversity. Methods Ecol. Evol. 2016, 7, 1008–1018. [Google Scholar] [CrossRef]
- Piggott, M.P. Evaluating the effects of laboratory protocols on eDNA detection probability for an endangered freshwater fish. Ecol. Evol. 2016, 6, 2739–2750. [Google Scholar] [CrossRef] [PubMed]
- Scott, R.; Zhan, A.; Brown, E.A.; Chain, F.J.; Cristescu, M.E.; Gras, R.; MacIsaac, H.J. Optimization and performance testing of a sequence processing pipeline applied to detection of nonindigenous species. Evol. Appl. 2018, 11, 891–905. [Google Scholar] [CrossRef]
- Coissac, E.; Riaz, T.; Puillandre, N. Bioinformatic challenges for DNA metabarcoding of plants and animals. Mol. Ecol. 2012, 21, 1834–1847. [Google Scholar] [CrossRef]
- Roussel, J.M.; Paillisson, J.M.; Treguier, A.; Petit, E. The downside of eDNA as a survey tool in water bodies. J. Appl. Ecol. 2015, 52, 823–826. [Google Scholar] [CrossRef]
- Cristescu, M.E. From barcoding single individuals to metabarcoding biological communities: Towards an integrative approach to the study of global biodiversity. Trends Ecol. Evol. 2014, 29, 566–571. [Google Scholar] [CrossRef] [PubMed]
- Kraaijeveld, K.; De Weger, L.A.; Ventayol García, M.; Buermans, H.; Frank, J.; Hiemstra, P.S.; Den Dunnen, J.T. Efficient and sensitive identification and quantification of airborne pollen using next-generation DNA sequencing. Mol. Ecol. Resour. 2015, 15, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Cordier, T.; Esling, P.; Lejzerowicz, F.; Visco, J.; Ouadahi, A.; Martins, C.; Cedhagen, T.; Pawlowski, J. Predicting the ecological quality status of marine environments from eDNA metabarcoding data using supervised machine learning. Environ. Sci. Technol. 2017, 51, 9118–9126. [Google Scholar] [CrossRef] [PubMed]
- Tournayre, O.; Leuchtmann, M.; Galan, M.; Trillat, M.; Piry, S.; Pinaud, D.; Filippi-Codaccioni, O.; Pontier, D.; Charbonnel, N. eDNA metabarcoding reveals a core and secondary diets of the greater horseshoe bat with strong spatio-temporal plasticity. Environ. DNA 2021, 3, 277–296. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).