In Utero Exposure to Caffeine and Acetaminophen, the Gut Microbiome, and Neurodevelopmental Outcomes: A Prospective Birth Cohort Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Participants
2.2. Exposure Assessment
2.3. Microbiome Assessment
2.4. Neurodevelopmental Assessment
2.5. Statistical Methods
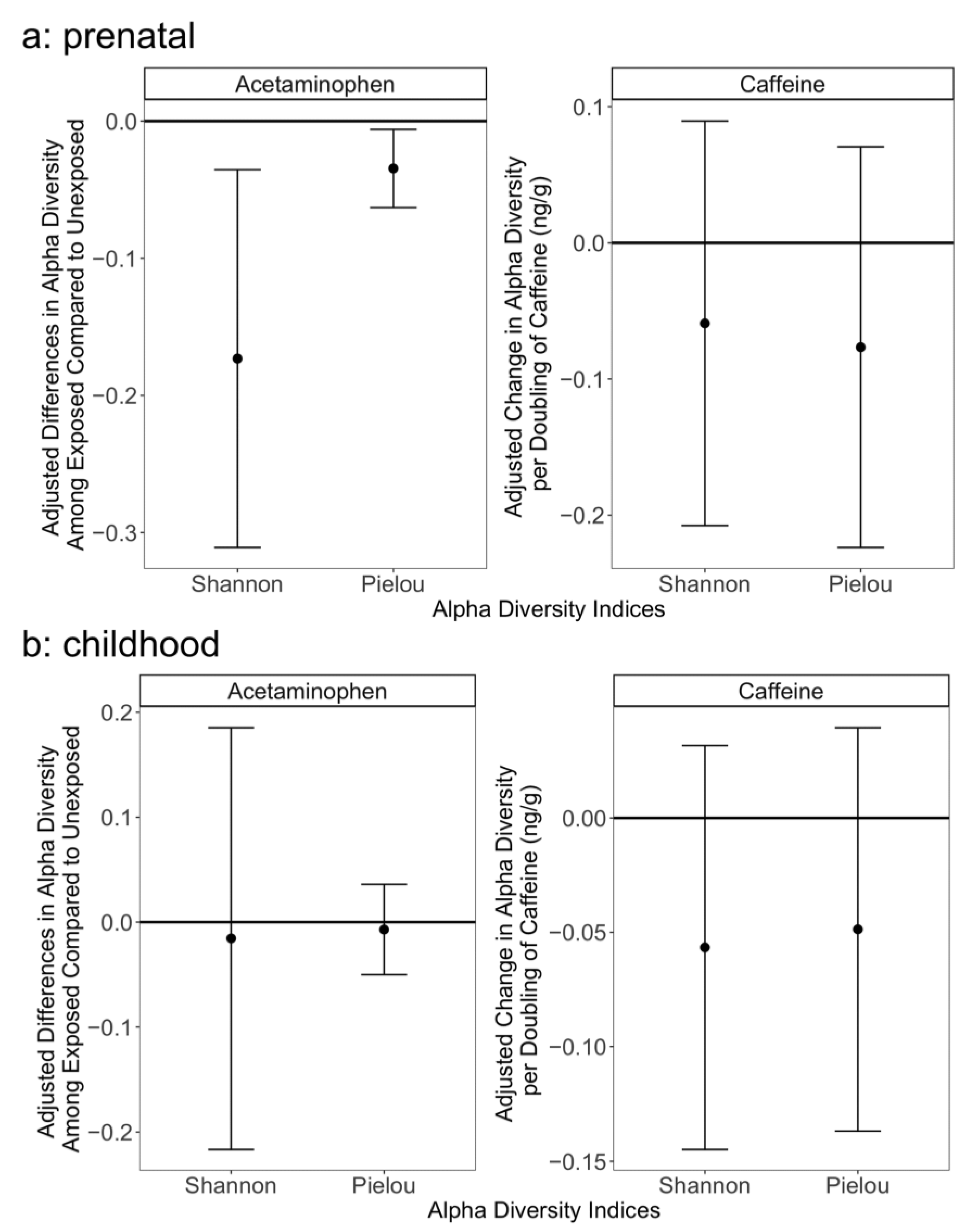
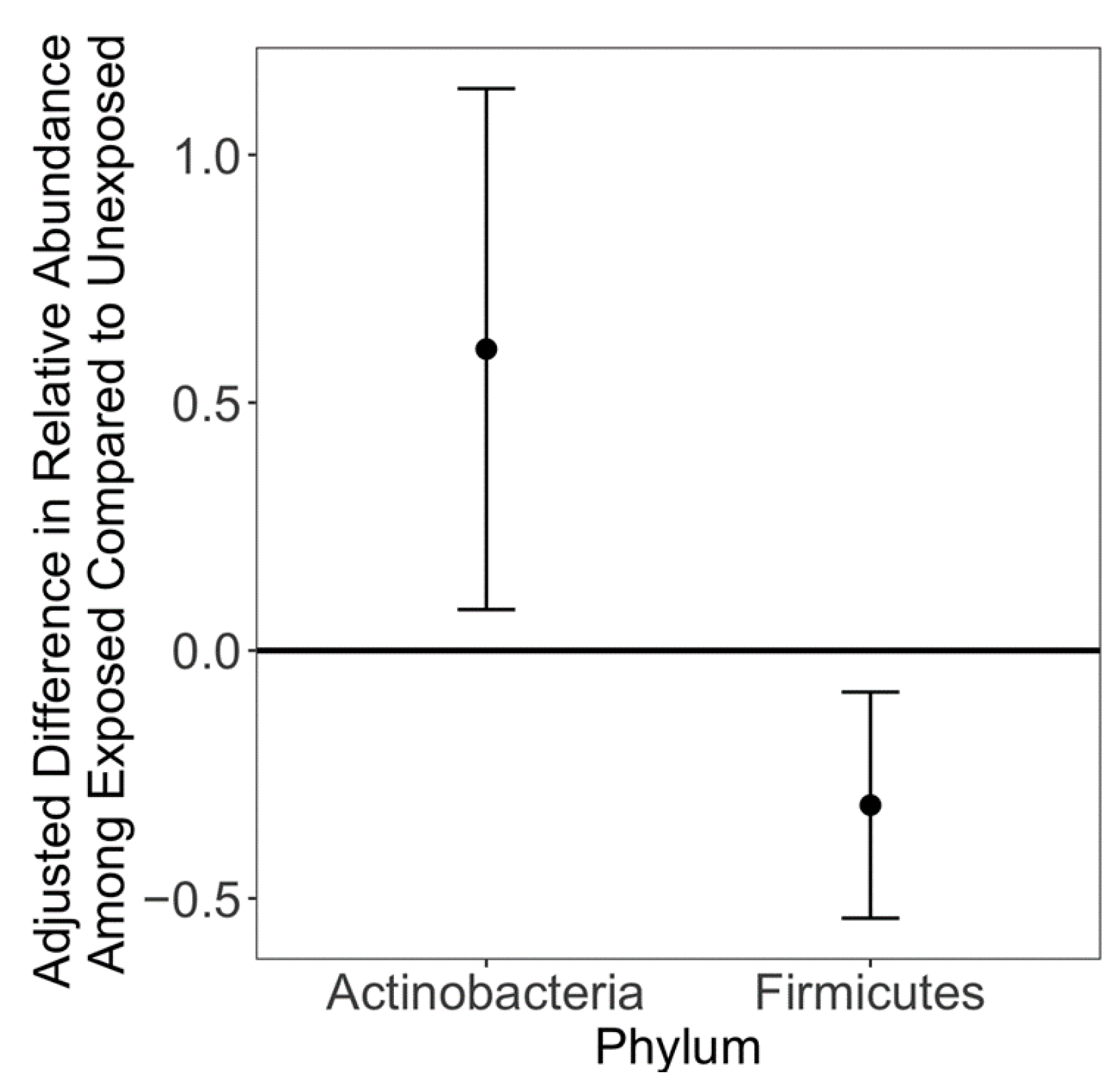
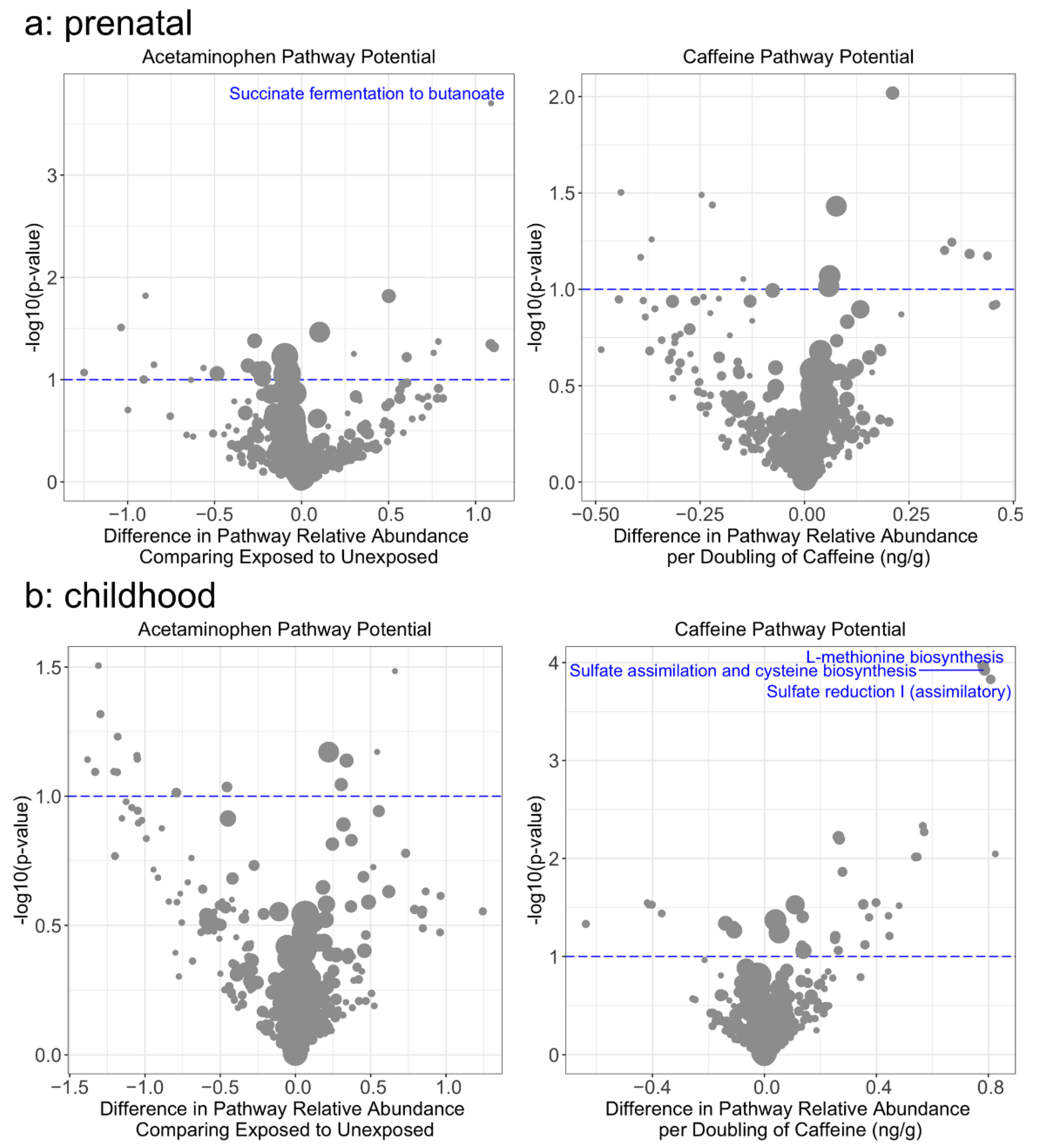
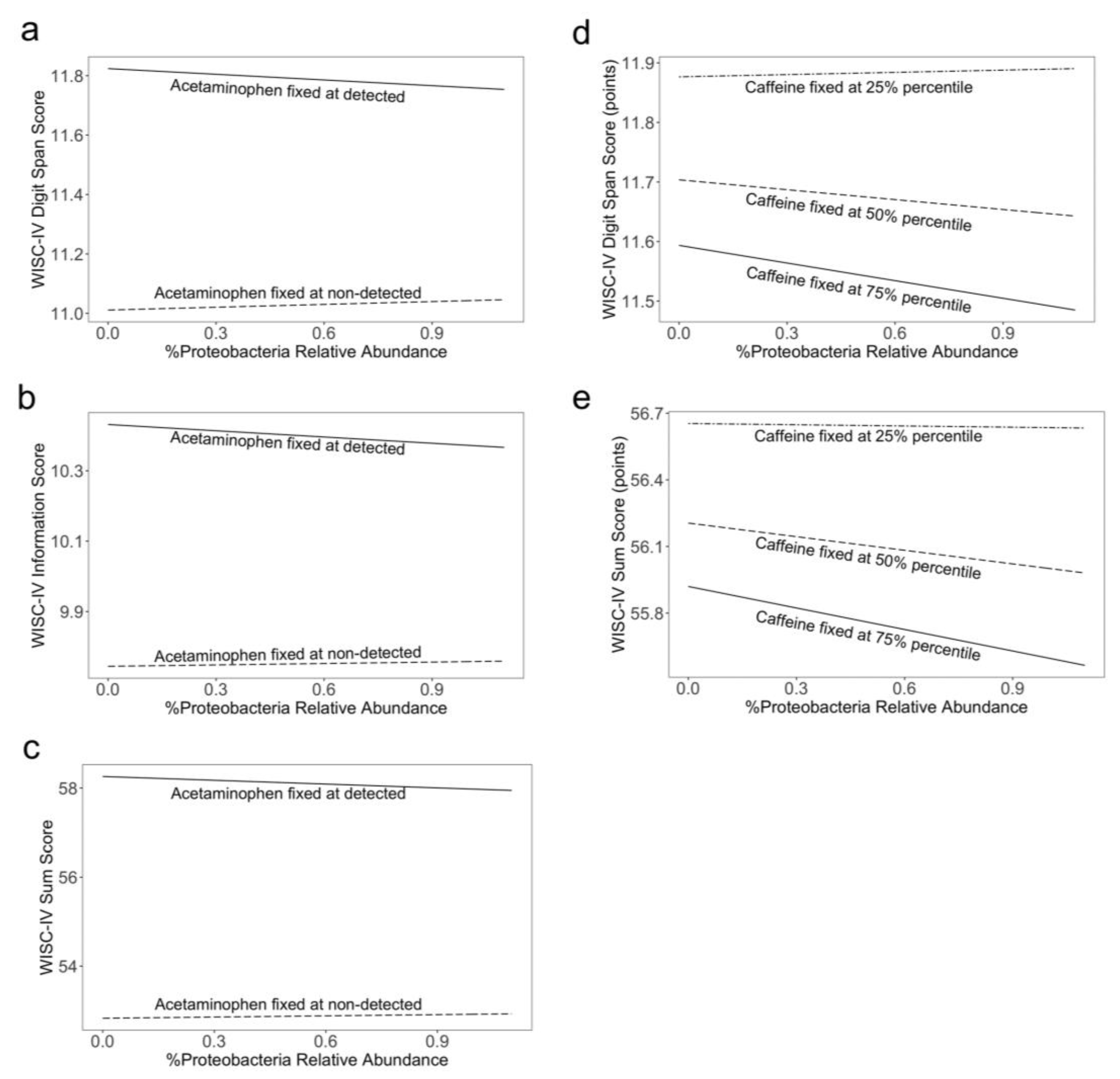
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- McKenna, L.; McIntyre, M. What over-the-counter preparations are pregnant women taking? A literature review. J. Adv. Nurs. 2006, 56, 636–645. [Google Scholar] [CrossRef]
- Werler, M.M.; Mitchell, A.A.; Hernandez-Diaz, S.; Honein, M.A. Use of over-the-counter medications during pregnancy. Am. J. Obstet. Gynecol. 2005, 193, 771–777. [Google Scholar] [CrossRef]
- Vernacchio, L.; Kelly, J.P.; Kaufman, D.W.; Mitchell, A.A. Medication Use Among Children <12 Years of Age in the United States: Results From the Slone Survey. Pediatrics 2009, 124, 446–454. [Google Scholar] [CrossRef]
- Baker, B.H.; Lugo-Candelas, C.; Wu, H.; Laue, H.E.; Boivin, A.; Gillet, V.; Aw, N.; Rahman, T.; Lepage, J.-F.; Whittingstall, K. Association of prenatal acetaminophen exposure measured in meconium with risk of attention-deficit/hyperactivity disorder mediated by frontoparietal network brain connectivity. JAMA Pediatrics 2020, 174, 1073–1081. [Google Scholar] [CrossRef]
- Bauer, A.Z.; Kriebel, D.; Herbert, M.R.; Bornehag, C.-G.; Swan, S.H. Prenatal paracetamol exposure and child neurodevelopment: A review. Horm. Behav. 2018, 101, 125–147. [Google Scholar] [CrossRef]
- Laue, H.E.; Cassoulet, R.; Abdelouahab, N.; Serme-Gbedo, Y.K.; Desautels, A.-S.; Brennan, K.J.; Bellenger, J.-P.; Burris, H.H.; Coull, B.A.; Weisskopf, M.G.; et al. Association between meconium acetaminophen and childhood neurocognitive development in GESTE, a Canadian cohort study. Toxicol. Sci. 2019, 167, 138–144. [Google Scholar] [CrossRef]
- Bertoldi, A.D.; Rifas-Shiman, S.L.; Boing, A.C.; Silva Dal Pizzol, T.; Miranda, V.I.A.; Silveira, M.P.T.; Freitas Silveira, M.; Domingues, M.R.; Santos, I.S.; Bassani, D.G.; et al. Associations of acetaminophen use during pregnancy and the first year of life with neurodevelopment in early childhood. Paediatr. Perinat. Epidemiol. 2020, 34, 267–277. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO Recommendations on Antenatal Care for a Positive Pregnancy Experience; WHO Press: Geneva, Switzerland, 2016. [Google Scholar]
- Nehlig, A.; Debry, G. Potential teratogenic and neurodevelopmental consequences of coffee and caffeine exposure: A review on human and animal data. Neurotoxicology Teratol. 1994, 16, 531–543. [Google Scholar] [CrossRef]
- Ma, Z.-l.; Qin, Y.; Wang, G.; Li, X.-d.; He, R.-r.; Chuai, M.; Kurihara, H.; Yang, X. Exploring the Caffeine-Induced Teratogenicity on Neurodevelopment Using Early Chick Embryo. PLoS ONE 2012, 7, e34278. [Google Scholar] [CrossRef]
- Sobotka, T.J. Neurobehavioral Effects of Prenatal Caffeine. Ann. N. Y. Acad. Sci. 1989, 562, 327–339. [Google Scholar] [CrossRef]
- Del-Ponte, B.; Santos, I.S.; Tovo-Rodrigues, L.; Anselmi, L.; Munhoz, T.N.; Matijasevich, A. Caffeine consumption during pregnancy and ADHD at the age of 11 years: A birth cohort study. BMJ Open 2016, 6, e012749. [Google Scholar] [CrossRef] [PubMed]
- Loomans, E.M.; Hofland, L.; van der Stelt, O.; van der Wal, M.F.; Koot, H.M.; Van den Bergh, B.R.; Vrijkotte, T.G. Caffeine intake during pregnancy and risk of problem behavior in 5- to 6-year-old children. Pediatrics 2012, 130, e305–e313. [Google Scholar] [CrossRef] [PubMed]
- Morgan, S.; Koren, G.; Bozzo, P. Is caffeine consumption safe during pregnancy? Can. Fam. Physician 2013, 59, 361–362. [Google Scholar] [PubMed]
- Castellanos, F.X.; Rapoport, J.L. Effects of caffeine on development and behavior in infancy and childhood: A review of the published literature. Food Chem. Toxicol. 2002, 40, 1235–1242. [Google Scholar] [CrossRef][Green Version]
- Turnbaugh, P.J.; Ley, R.E.; Hamady, M.; Fraser-Liggett, C.M.; Knight, R.; Gordon, J.I. The Human Microbiome Project. Nature 2007, 449, 804–810. [Google Scholar] [CrossRef] [PubMed]
- Malfatti, M.A.; Kuhn, E.A.; Murugesh, D.K.; Mendez, M.E.; Hum, N.; Thissen, J.B.; Jaing, C.J.; Loots, G.G. Manipulation of the Gut Microbiome Alters Acetaminophen Biodisposition in Mice. Sci. Rep. 2020, 10, 4571. [Google Scholar] [CrossRef] [PubMed]
- Laue, H.E.; Brennan, K.J.M.; Gillet, V.; Abdelouahab, N.; Coull, B.A.; Weisskopf, M.G.; Burris, H.H.; Zhang, W.; Takser, L.; Baccarelli, A.A. Associations of prenatal exposure to polybrominated diphenyl ethers and polychlorinated biphenyls with long-term gut microbiome structure: A pilot study. Environ. Epidemiol. 2019, 3, e039. [Google Scholar] [CrossRef] [PubMed]
- Laue, H.E.; Moroishi, Y.; Jackson, B.P.; Palys, T.J.; Madan, J.C.; Karagas, M.R. Nutrient-toxic element mixtures and the early postnatal gut microbiome in a United States longitudinal birth cohort. Environ. Int. 2020, 138, 105613. [Google Scholar] [CrossRef]
- Chiu, K.; Warner, G.; Nowak, R.A.; Flaws, J.A.; Mei, W. The Impact of Environmental Chemicals on the Gut Microbiome. Toxicol. Sci. 2020, 176, 253–284. [Google Scholar] [CrossRef]
- Goldstein, A.; Warren, R. Passage of caffeine into human gonadal and fetal tissue. Biochem. Pharmacol. 1962, 11, 166–168. [Google Scholar] [CrossRef]
- Levy, G.; Garrettson, L.K.; Soda, D.M. Letter: Evidence of placental transfer of acetaminophen. Pediatrics 1975, 55, 895. [Google Scholar] [CrossRef] [PubMed]
- Nitsche, J.F.; Patil, A.S.; Langman, L.J.; Penn, H.J.; Derleth, D.; Watson, W.J.; Brost, B.C. Transplacental Passage of Acetaminophen in Term Pregnancy. Am. J. Perinatol. 2017, 34, 541–543. [Google Scholar] [CrossRef] [PubMed]
- Mian, P.; Allegaert, K.; Conings, S.; Annaert, P.; Tibboel, D.; Pfister, M.; van Calsteren, K.; van den Anker, J.N.; Dallmann, A. Integration of Placental Transfer in a Fetal-Maternal Physiologically Based Pharmacokinetic Model to Characterize Acetaminophen Exposure and Metabolic Clearance in the Fetus. Clin. Pharmacokinet. 2020, 59, 911–925. [Google Scholar] [CrossRef] [PubMed]
- Grosso, L.M.; Bracken, M.B. Caffeine Metabolism, Genetics, and Perinatal Outcomes: A Review of Exposure Assessment Considerations during Pregnancy. Ann. Epidemiol. 2005, 15, 460–466. [Google Scholar] [CrossRef]
- Yassour, M.; Vatanen, T.; Siljander, H.; Hämäläinen, A.-M.; Härkönen, T.; Ryhänen, S.J.; Franzosa, E.A.; Vlamakis, H.; Huttenhower, C.; Gevers, D.; et al. Natural history of the infant gut microbiome and impact of antibiotic treatment on bacterial strain diversity and stability. Sci. Transl. Med. 2016, 8, 343ra381. [Google Scholar] [CrossRef] [PubMed]
- Cowan, T.E.; Palmnäs, M.S.A.; Yang, J.; Bomhof, M.R.; Ardell, K.L.; Reimer, R.A.; Vogel, H.J.; Shearer, J. Chronic coffee consumption in the diet-induced obese rat: Impact on gut microbiota and serum metabolomics. J. Nutr. Biochem. 2014, 25, 489–495. [Google Scholar] [CrossRef] [PubMed]
- Scorza, C.; Piccini, C.; Martinez Busi, M.; Abin Carriquiry, J.A.; Zunino, P. Alterations in the Gut Microbiota of Rats Chronically Exposed to Volatilized Cocaine and Its Active Adulterants Caffeine and Phenacetin. Neurotox. Res. 2018. [Google Scholar] [CrossRef]
- Clayton, T.A.; Baker, D.; Lindon, J.C.; Everett, J.R.; Nicholson, J.K. Pharmacometabonomic identification of a significant host-microbiome metabolic interaction affecting human drug metabolism. Proc. Natl. Acad. Sci. USA 2009, 106, 14728–14733. [Google Scholar] [CrossRef]
- Maier, L.; Pruteanu, M.; Kuhn, M.; Zeller, G.; Telzerow, A.; Anderson, E.E.; Brochado, A.R.; Fernandez, K.C.; Dose, H.; Mori, H.; et al. Extensive impact of non-antibiotic drugs on human gut bacteria. Nature 2018, 555, 623. [Google Scholar] [CrossRef]
- Haroune, L.; Cassoulet, R.; Lafontaine, M.P.; Belisle, M.; Garant, D.; Pelletier, F.; Cabana, H.; Bellenger, J.P. Liquid chromatography-tandem mass spectrometry determination for multiclass pesticides from insect samples by microwave-assisted solvent extraction followed by a salt-out effect and micro-dispersion purification. Anal. Chim. Acta 2015, 891, 160–170. [Google Scholar] [CrossRef]
- Shen, Y.; Laue, H.E.; Shrubsole, M.J.; Wu, H.; Bloomquist, T.R.; Larouche, A.; Zhao, K.; Gao, F.; Boivin, A.; Prada, D.; et al. Associations of Childhood and Perinatal Blood Metals with Children’s Gut Microbiomes in a Canadian Gestation Cohort. Environ. Health Perspect. 2022, 130, 017007. [Google Scholar] [CrossRef] [PubMed]
- Caspi, R.; Altman, T.; Billington, R.; Dreher, K.; Foerster, H.; Fulcher, C.A.; Holland, T.A.; Keseler, I.M.; Kothari, A.; Kubo, A.; et al. The MetaCyc database of metabolic pathways and enzymes and the BioCyc collection of Pathway/Genome Databases. Nucleic Acids Res. 2013, 42, D459–D471. [Google Scholar] [CrossRef] [PubMed]
- Franzosa, E.A.; McIver, L.J.; Rahnavard, G.; Thompson, L.R.; Schirmer, M.; Weingart, G.; Lipson, K.S.; Knight, R.; Caporaso, J.G.; Segata, N. Species-level functional profiling of metagenomes and metatranscriptomes. Nat. Methods 2018, 15, 962–968. [Google Scholar] [CrossRef] [PubMed]
- Shannon, C.E. A mathematical theory of communication. Bell Syst. Tech. J. 1948, 27, 379–423. [Google Scholar] [CrossRef]
- Pielou, E.C. The measurement of diversity in different types of biological collections. J. Theor. Biol. 1966, 13, 131–144. [Google Scholar] [CrossRef]
- McMurdie, P.J.; Holmes, S. phyloseq: An R package for reproducible interactive analysis and graphics of microbiome census data. PLoS ONE 2013, 8, e61217. [Google Scholar] [CrossRef] [PubMed]
- Lozupone, C.; Lladser, M.E.; Knights, D.; Stombaugh, J.; Knight, R. UniFrac: An effective distance metric for microbial community comparison. ISME J. 2011, 5, 169–172. [Google Scholar] [CrossRef] [PubMed]
- Bray, J.R.; Curtis, J.T. An Ordination of the Upland Forest Communities of Southern Wisconsin. Ecol. Monogr. 1957, 27, 325–349. [Google Scholar] [CrossRef]
- Jaccard, P. The Distribution of the Flora in the Alpine Zone. New Phytol. 1912, 11, 37–50. [Google Scholar] [CrossRef]
- Martini, R.; St-Pierre, M.F.; Wilson, B.N. French Canadian cross-cultural adaptation of the Developmental Coordination Disorder Questionnaire ′07: DCDQ-FC. Can. J. Occup. Ther. 2011, 78, 318–327. [Google Scholar] [CrossRef]
- Wechsler, D. Wechsler Intelligence Scale for Children-WISC-IV.; Psychological Corporation: San Antonio, TX, USA, 2003. [Google Scholar]
- Benjamini, Y.; Hochberg, Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J. R. Stat. Soc. Ser. B 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Oksanen, J.; Kindt, R.; Legendre, P.; O’Hara, B.; Stevens, M.H.H.; Oksanen, M.J.; Suggests, M. The vegan package. Community Ecol. Package 2007, 10, 719. [Google Scholar]
- Mallick, H.; Rahnavard, A.; McIver, L.J.; Ma, S.; Zhang, Y.; Nguyen, L.H.; Tickle, T.L.; Weingart, G.; Ren, B.; Schwager, E.H.; et al. Multivariable association discovery in population-scale meta-omics studies. PLOS Comput. Biol. 2021, 17, e1009442. [Google Scholar] [CrossRef]
- Lozupone, C.A.; Stombaugh, J.I.; Gordon, J.I.; Jansson, J.K.; Knight, R. Diversity, stability and resilience of the human gut microbiota. Nature 2012, 489, 220–230. [Google Scholar] [CrossRef] [PubMed]
- Vatanen, T.; Franzosa, E.A.; Schwager, R.; Tripathi, S.; Arthur, T.D.; Vehik, K.; Lernmark, Å.; Hagopian, W.A.; Rewers, M.J.; She, J.X.; et al. The human gut microbiome in early-onset type 1 diabetes from the TEDDY study. Nature 2018, 562, 589–594. [Google Scholar] [CrossRef] [PubMed]
- Ling, Y.; Gong, T.; Zhang, J.; Gu, Q.; Gao, X.; Weng, X.; Liu, J.; Sun, J. Gut Microbiome Signatures Are Biomarkers for Cognitive Impairment in Patients With Ischemic Stroke. Front. Aging Neurosci. 2020, 12, 511562. [Google Scholar] [CrossRef]
- Liu, P.; Wu, L.; Peng, G.; Han, Y.; Tang, R.; Ge, J.; Zhang, L.; Jia, L.; Yue, S.; Zhou, K.; et al. Altered microbiomes distinguish Alzheimer’s disease from amnestic mild cognitive impairment and health in a Chinese cohort. Brain Behav. Immun. 2019, 80, 633–643. [Google Scholar] [CrossRef] [PubMed]
- Manderino, L.; Carroll, I.; Azcarate-Peril, M.A.; Rochette, A.; Heinberg, L.; Peat, C.; Steffen, K.; Mitchell, J.; Gunstad, J. Preliminary Evidence for an Association Between the Composition of the Gut Microbiome and Cognitive Function in Neurologically Healthy Older Adults. J. Int. Neuropsychol. Soc. 2017, 23, 700–705. [Google Scholar] [CrossRef]
- Tamana, S.K.; Tun, H.M.; Konya, T.; Chari, R.S.; Field, C.J.; Guttman, D.S.; Becker, A.B.; Moraes, T.J.; Turvey, S.E.; Subbarao, P.; et al. Bacteroides-dominant gut microbiome of late infancy is associated with enhanced neurodevelopment. Gut Microbes 2021, 13, 1930875. [Google Scholar] [CrossRef]
- Shi, H.; Ge, X.; Ma, X.; Zheng, M.; Cui, X.; Pan, W.; Zheng, P.; Yang, X.; Zhang, P.; Hu, M.; et al. A fiber-deprived diet causes cognitive impairment and hippocampal microglia-mediated synaptic loss through the gut microbiota and metabolites. Microbiome 2021, 9, 223. [Google Scholar] [CrossRef]
- Shin, N.R.; Whon, T.W.; Bae, J.W. Proteobacteria: Microbial signature of dysbiosis in gut microbiota. Trends Biotechnol. 2015, 33, 496–503. [Google Scholar] [CrossRef] [PubMed]
- Jetten, M.J.A.; Gaj, S.; Ruiz-Aracama, A.; de Kok, T.M.; van Delft, J.H.M.; Lommen, A.; van Someren, E.P.; Jennen, D.G.J.; Claessen, S.M.; Peijnenburg, A.A.C.M.; et al. Omics analysis of low dose acetaminophen intake demonstrates novel response pathways in humans. Toxicol. Appl. Pharmacol. 2012, 259, 320–328. [Google Scholar] [CrossRef] [PubMed]
- Al Reef, T.; Ghanem, E. Caffeine: Well-known as psychotropic substance, but little as immunomodulator. Immunobiology 2018, 223, 818–825. [Google Scholar] [CrossRef]
- Mukhopadhya, I.; Hansen, R.; El-Omar, E.M.; Hold, G.L. IBD—what role do Proteobacteria play? Nat. Rev. Gastroenterol. Hepatol. 2012, 9, 219–230. [Google Scholar] [CrossRef] [PubMed]
- Park, B.Y.; Lee, B.K. Use of meconium in perinatal epidemiology: Potential benefits and pitfalls. Ann. Epidemiol. 2014, 24, 878–881. [Google Scholar] [CrossRef] [PubMed]
- Heaton, K.W.; Radvan, J.; Cripps, H.; Mountford, R.A.; Braddon, F.E.; Hughes, A.O. Defecation frequency and timing, and stool form in the general population: A prospective study. Gut 1992, 33, 818–824. [Google Scholar] [CrossRef] [PubMed]
- Nehlig, A. Interindividual Differences in Caffeine Metabolism and Factors Driving Caffeine Consumption. Pharmacol. Rev. 2018, 70, 384. [Google Scholar] [CrossRef] [PubMed]
- Hines, R.N. The ontogeny of drug metabolism enzymes and implications for adverse drug events. Pharmacol. Ther. 2008, 118, 250–267. [Google Scholar] [CrossRef] [PubMed]
- Solazzo, G.; Wu, H.; Laue, H.E.; Brennan, K.; Knox, J.M.; Gillet, V.; Bovin, A.; Abdelouahab, N.; Posner, J.; Raffanello, E.; et al. The association between prenatal concentrations of polybrominated diphenyl ether and child cognitive and psychomotor function. Environ Epidemiol. 2021, 5, e156. [Google Scholar] [CrossRef]
- Sussman, T.J.; Baker, B.H.; Wakhloo, A.J.; Gillet, V.; Abdelouahab, N.; Whittingstall, K.; Lepage, J.-F.; St-Cyr, L.; Boivin, A.; Gagnon, A.; et al. The relationship between persistent organic pollutants and Attention Deficit Hyperactivity Disorder phenotypes: Evidence from task-based neural activity in an observational study of a community sample of Canadian mother-child dyads. Environ Res. 2022, 206, 112593. [Google Scholar] [CrossRef]
- Abdelouahab, N.; Langlois, M.F.; Lavoie, L.; Corbin, F.; Pasquier, J.C.; Takser, L. Maternal and cord-blood thyroid hormone levels and exposure to polybrominated diphenyl ethers and polychlorinated biphenyls during early pregnancy. Am. J. Epidemiol. 2013, 178, 701–713. [Google Scholar] [CrossRef]
- Raven, J.; Baker, B.H.; Wakhloo, A.J.; Gillet, V.; Abdelouahab, N.; Whittingstall, K.; Lepage, J.-F.; St-Cyr, L.; Boivin, A.; Gagnon, A.; et al. Raven Progressive Matrices. In Handbook of nonverbal assessment; Kluwer Academic/Plenum Publishers: New York, NY, USA, 2003; pp. 223–237. [Google Scholar]
| Full Cohort (n = 365) | Population with Meconium (n = 197) | Meconium Analytic Population (n = 49) | Cross-Sectional Analytic Population (n = 85) | |
|---|---|---|---|---|
| Family Characteristics | ||||
| Maternal Age at Recruitment (years) | 29.1 ± 4.44 | 29.4 ± 4.45 | 29.1 ± 3.85 | 28.9 ± 4.09 |
| Maternal Pre-pregnancy Body Mass Index (kg/m2) | ||||
| Available | 25.3 ± 5.95 | 25.4 ± 5.85 | 25.5 ± 6.30 | 25.2 ± 6.01 |
| Missing | 68 (18.6) | 13 (6.6) | 12 (24.5) | 16 (18.8) |
| Family Income (CAD a) | ||||
| Available | 70,800 ± 41,200 | 71,600 ± 47,300 | 69,600 ± 34,600 | 93,900 ± 47,100 b |
| Missing | 20 (5.5) | 14 (7.1) | 4 (8.2) | 6 (7.1) b |
| Parity | ||||
| Nulliparous | 205 (56.2) | 113 (57.4) | 26 (53.1) | 46 (54.1) |
| Parous | 158 (43.3) | 83 (42.1) | 23 (46.9) | 39 (45.9) |
| Missing | 2 (0.5) | 1 (0.5) | 0 (0) | 0 (0) |
| Birth Characteristics | ||||
| Gestational Age (weeks) | 39.1 ± 1.43 | 39.1 ± 1.44 | 39.4 ± 1.09 | 39.4 ± 1.18 |
| Birth Mode | ||||
| Vaginal | 299 (81.9) | 162 (82.2) | 40 (81.6) | 68 (80) |
| Caesarean Section | 66 (18.1) | 35 (17.8) | 9 (18.4) | 17 (20) |
| Child Sex | ||||
| Male | 199 (54.5) | 107 (54.3) | 25 (51.0) | 44 (51.8) |
| Female | 166 (45.5) | 90 (45.7) | 24 (49.0) | 41 (48.2) |
| Child Birthweight (g) | 3400 ± 486 | 3370 ± 484 | 3440 ± 419 | 3460 ± 445 |
| Breast Feeding Status | ||||
| Ever breastfed | 284 (77.8) | 159 (80.7) | 43 (87.8) | 68 (80.0) |
| Never breastfed | 65 (17.8) | 30 (15.2) | 4 (8.2) | 14 (16.5) |
| Missing | 16 (4.4) | 8 (4.1) | 2 (4.1) | 3 (3.5) |
| Neurological Outcomes | ||||
| WISC-IV c: Block Design | 9.54 ± 2.92 | 9.43 ± 2.88 | 10.4 ± 2.99 | 10.3 ± 2.98 |
| WISC-IV: Coding | 10.5 ± 2.94 | 10.5 ± 2.92 | 10.8 ± 2.56 | 11.1 ± 2.67 |
| WISC-IV: Digit Span | 9.40 ± 2.58 | 9.39 ± 2.61 | 10.0 ± 2.26 | 9.81 ± 2.18 |
| WISC-IV: Information | 9.55 ± 2.29 | 9.37 ± 2.28 | 9.45 ± 2.17 | 9.81 ± 2.23 |
| WISC-IV: Vocabulary | 10.4 ± 2.70 | 10.1 ± 2.76 | 10.3 ± 3.29 | 10.6 ± 2.97 |
| WISC-IV Summary Score | 49.4 ± 8.36 | 48.9 ± 8.17 | 51.0 ± 7.70 | 51.7 ± 7.86 |
| QTAC d | 61.0 ± 8.91 | 61.6 ± 8.32 | 62.2 ± 8.20 | 62.1 ± 8.11 |
| Maternal Intelligence Quotient | ||||
| >95 percentile | 30 (61.2) | |||
| ≤95 percentile | 19 (38.8) | |||
| Exposure of Interest | ||||
| Caffeine (median [25th percentile, 75th percentile]; ng/g meconium) | - | 399 [2.82, 5170] | 390 [15.3, 3110] | 24.7 [0.184, 231] e |
| Acetaminophen | ||||
| Detected | - | 100 (50.8) | 20 (40.8) | 7 (8.2) b |
| Not Detected | - | 97 (49.2) | 29 (59.2) | 78 (91.8) b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Laue, H.E.; Shen, Y.; Bloomquist, T.R.; Wu, H.; Brennan, K.J.M.; Cassoulet, R.; Wilkie, E.; Gillet, V.; Desautels, A.-S.; Abdelouahab, N.; et al. In Utero Exposure to Caffeine and Acetaminophen, the Gut Microbiome, and Neurodevelopmental Outcomes: A Prospective Birth Cohort Study. Int. J. Environ. Res. Public Health 2022, 19, 9357. https://doi.org/10.3390/ijerph19159357
Laue HE, Shen Y, Bloomquist TR, Wu H, Brennan KJM, Cassoulet R, Wilkie E, Gillet V, Desautels A-S, Abdelouahab N, et al. In Utero Exposure to Caffeine and Acetaminophen, the Gut Microbiome, and Neurodevelopmental Outcomes: A Prospective Birth Cohort Study. International Journal of Environmental Research and Public Health. 2022; 19(15):9357. https://doi.org/10.3390/ijerph19159357
Chicago/Turabian StyleLaue, Hannah E., Yike Shen, Tessa R. Bloomquist, Haotian Wu, Kasey J. M. Brennan, Raphael Cassoulet, Erin Wilkie, Virginie Gillet, Anne-Sandrine Desautels, Nadia Abdelouahab, and et al. 2022. "In Utero Exposure to Caffeine and Acetaminophen, the Gut Microbiome, and Neurodevelopmental Outcomes: A Prospective Birth Cohort Study" International Journal of Environmental Research and Public Health 19, no. 15: 9357. https://doi.org/10.3390/ijerph19159357
APA StyleLaue, H. E., Shen, Y., Bloomquist, T. R., Wu, H., Brennan, K. J. M., Cassoulet, R., Wilkie, E., Gillet, V., Desautels, A.-S., Abdelouahab, N., Bellenger, J. P., Burris, H. H., Coull, B. A., Weisskopf, M. G., Zhang, W., Takser, L., & Baccarelli, A. A. (2022). In Utero Exposure to Caffeine and Acetaminophen, the Gut Microbiome, and Neurodevelopmental Outcomes: A Prospective Birth Cohort Study. International Journal of Environmental Research and Public Health, 19(15), 9357. https://doi.org/10.3390/ijerph19159357







