Spirulina platensis Immobilized Alginate Beads for Removal of Pb(II) from Aqueous Solutions
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Immobilization of Spirulina platensis in Sodium Alginate
2.3. Characterization of Spirulina platensis and S.P@Ca-SA
2.4. Batch Experiments
3. Results and Discussion
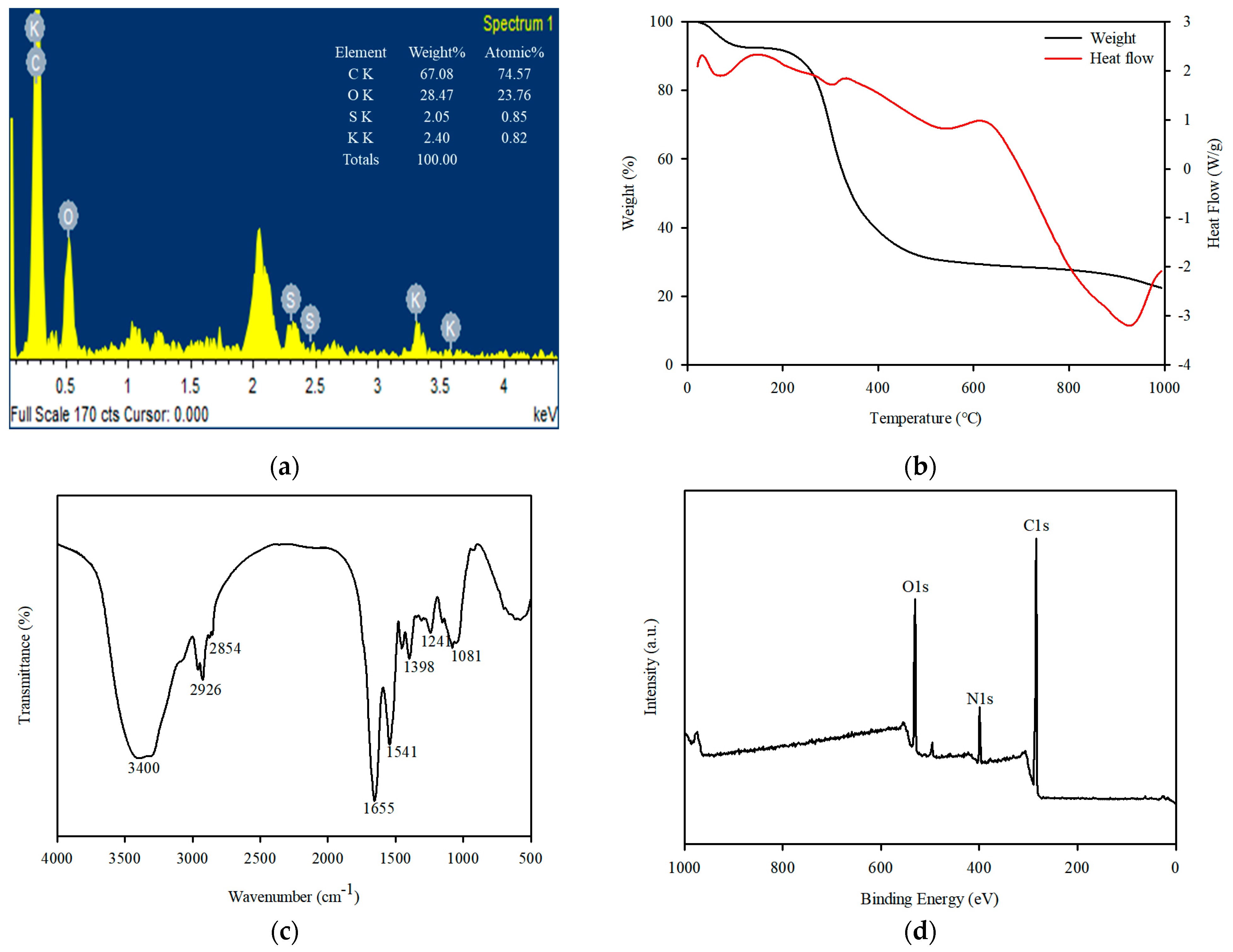
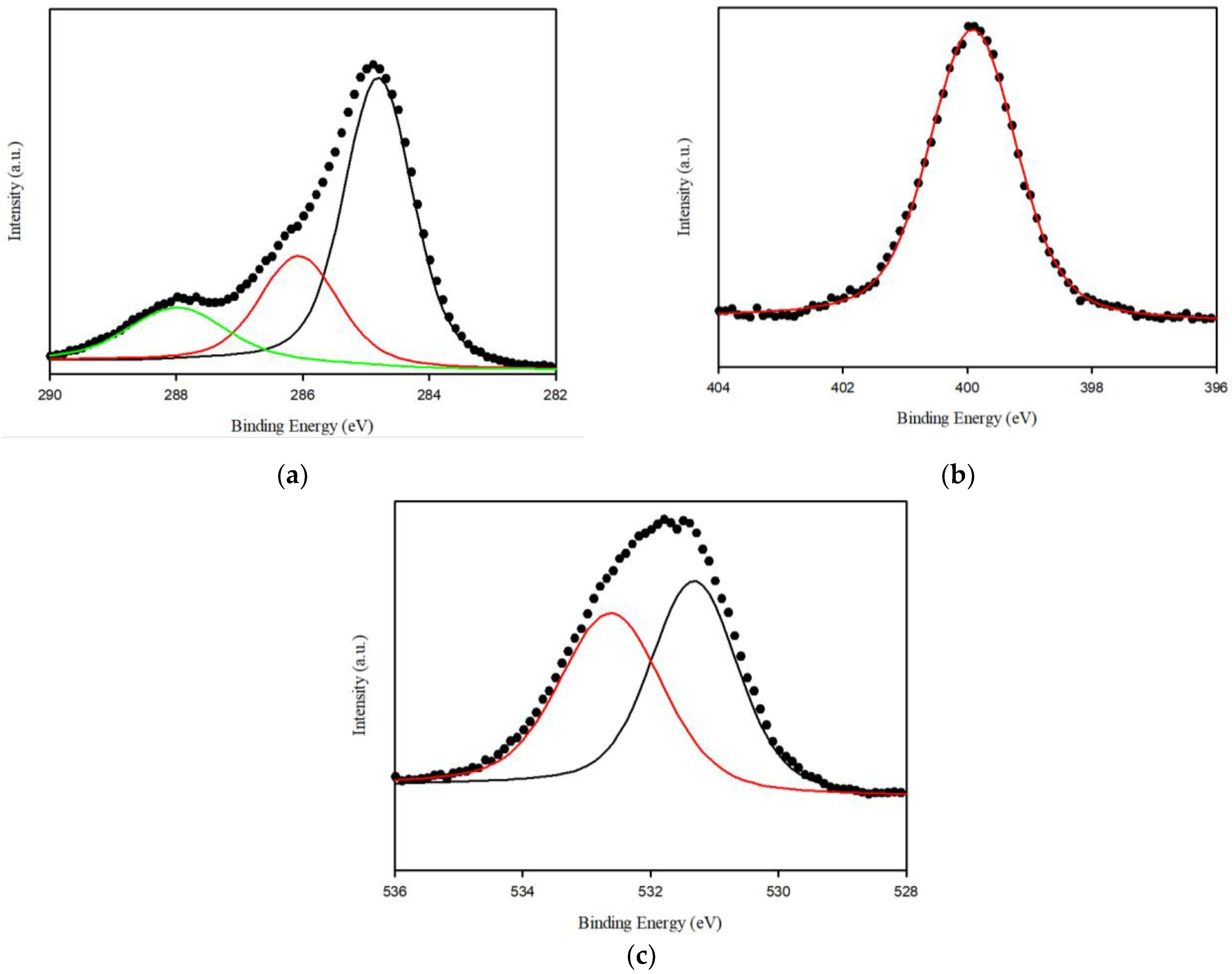
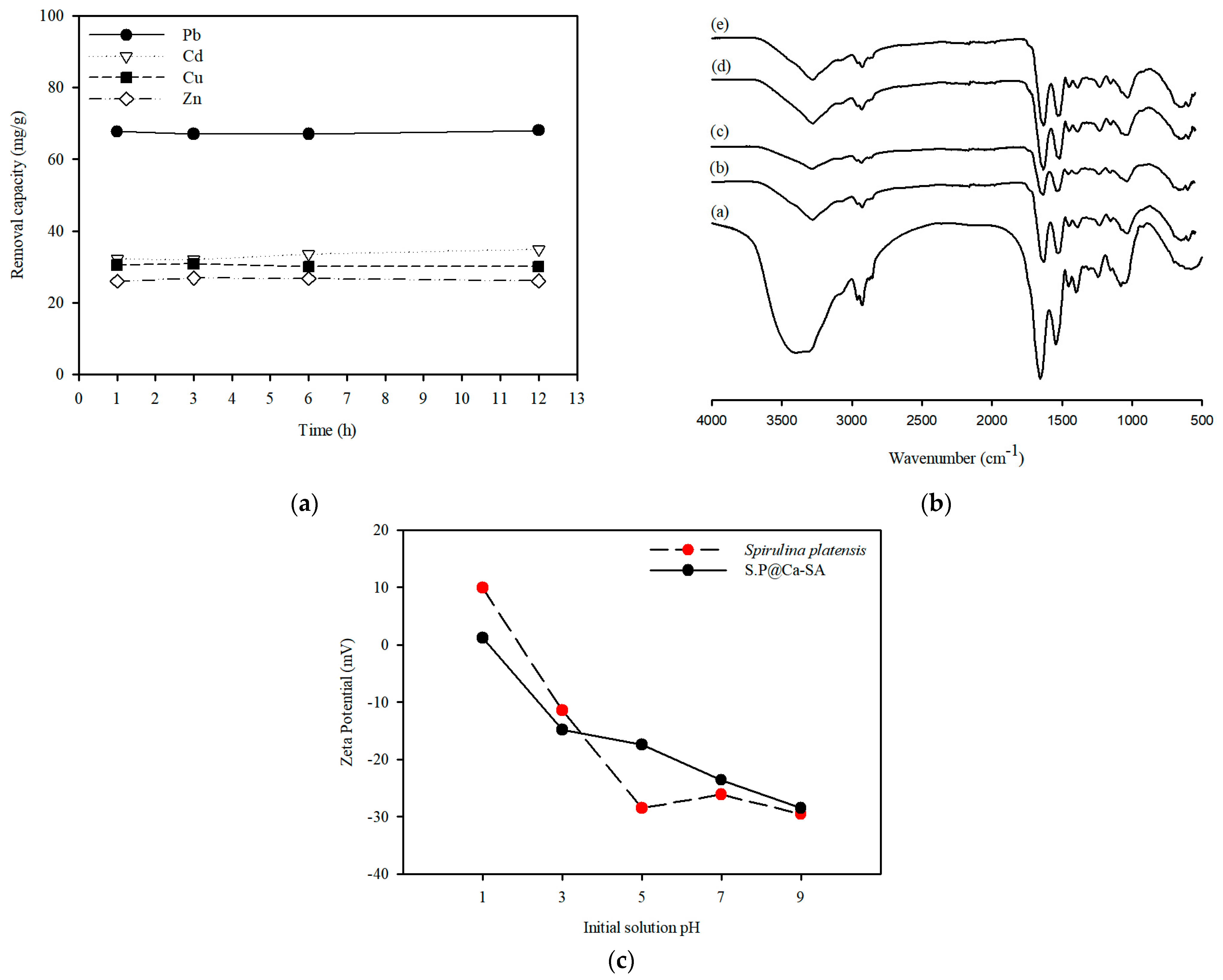
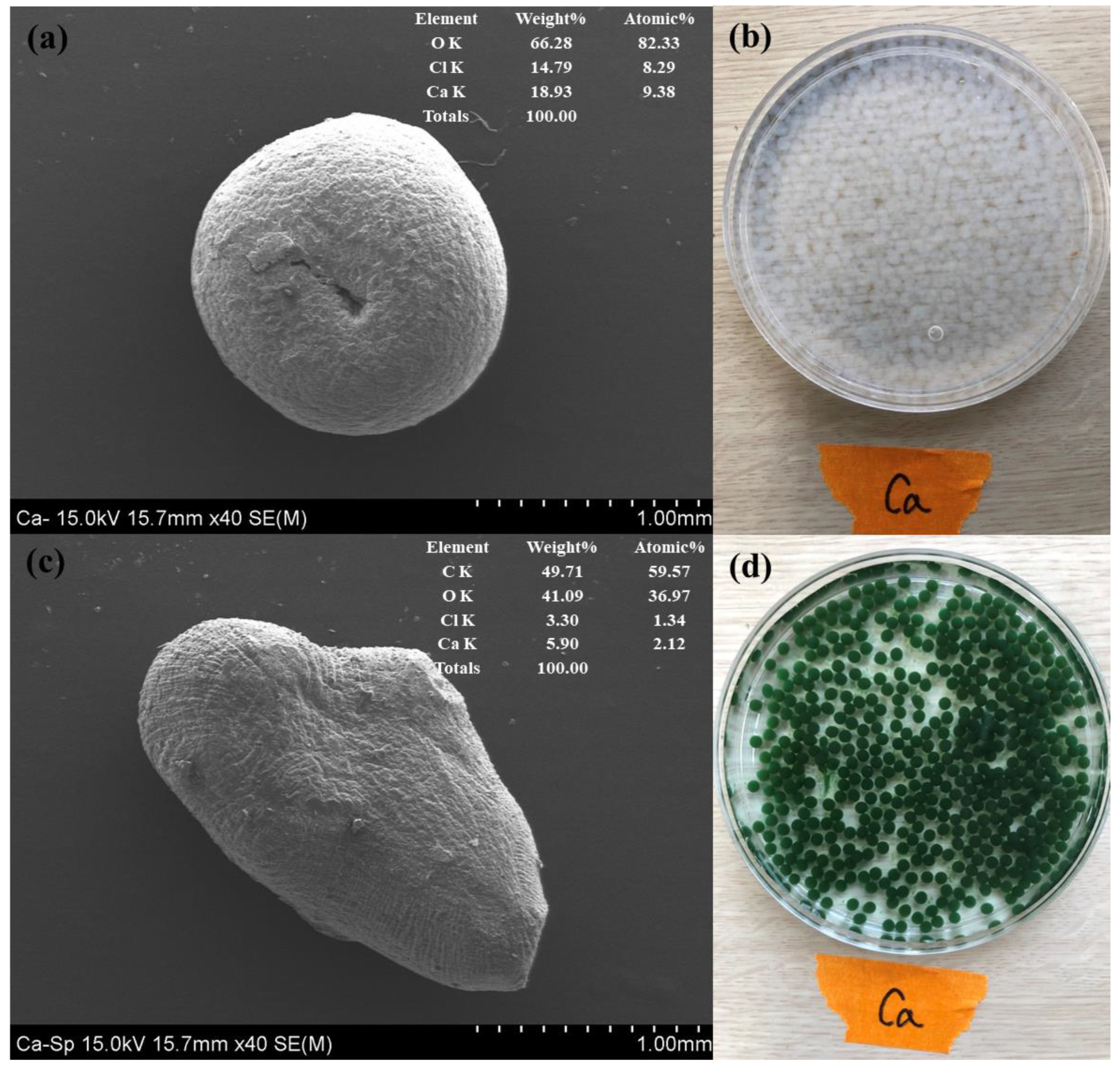
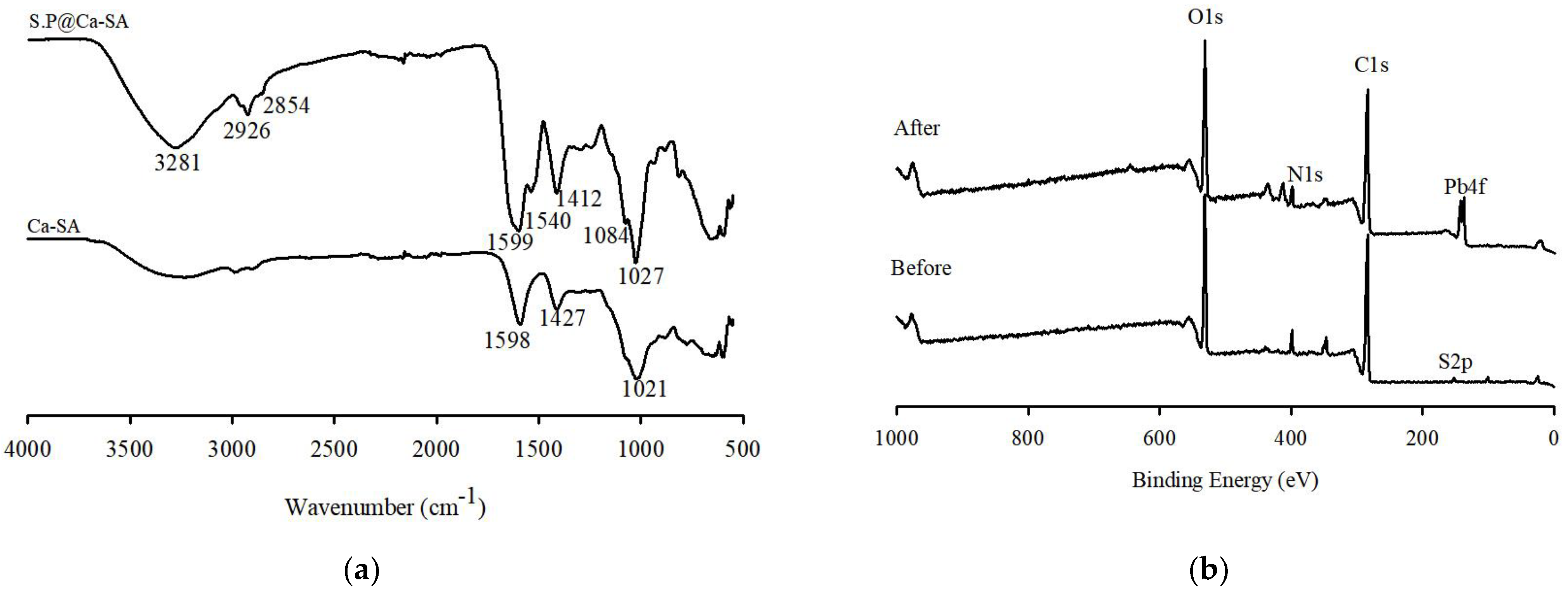
3.1. Characterization of Spirulina platensis
3.2. Characterization of SP@Ca-SA
3.3. Adsorption Experiments
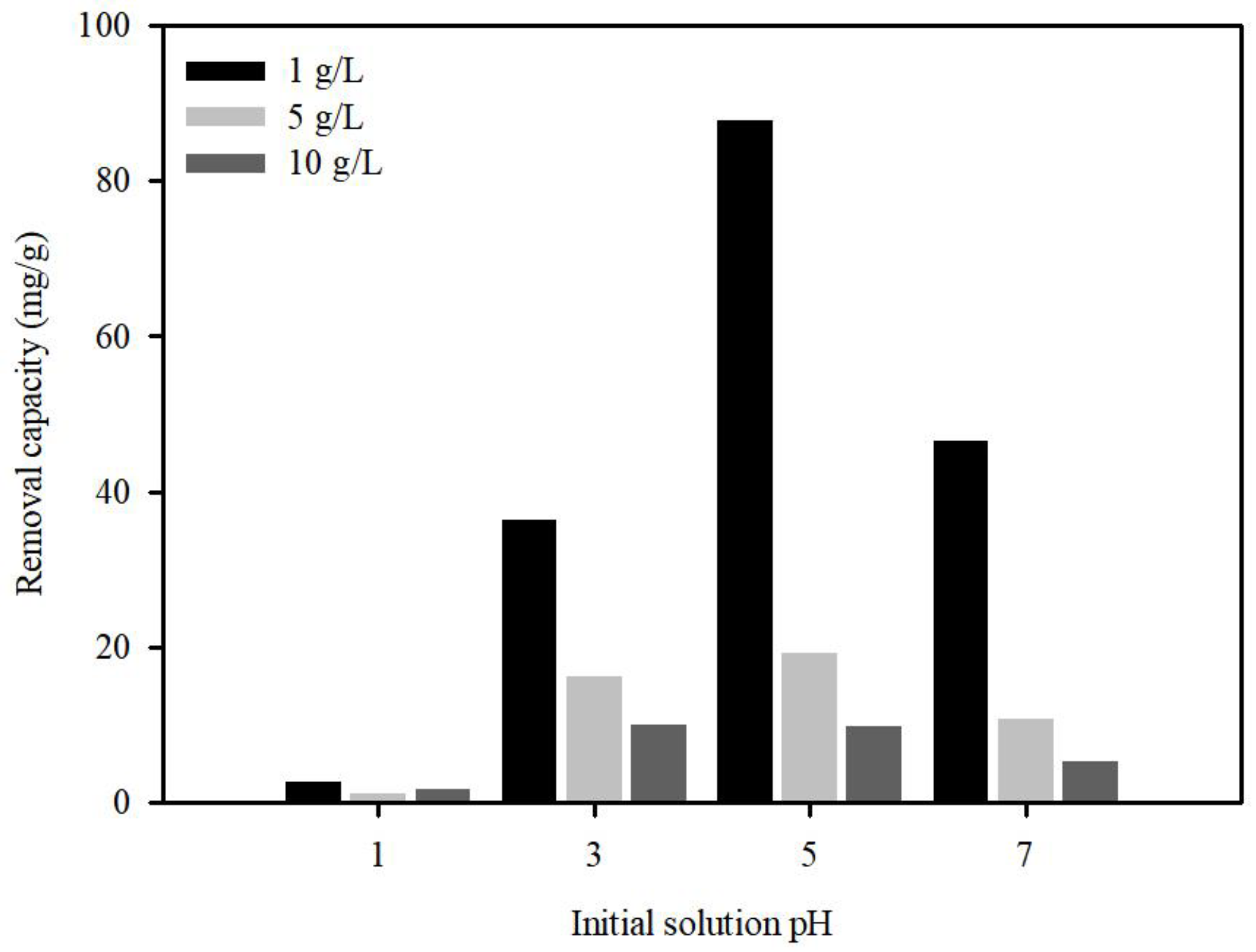
3.3.1. Effect of Initial pH on the Adsorption
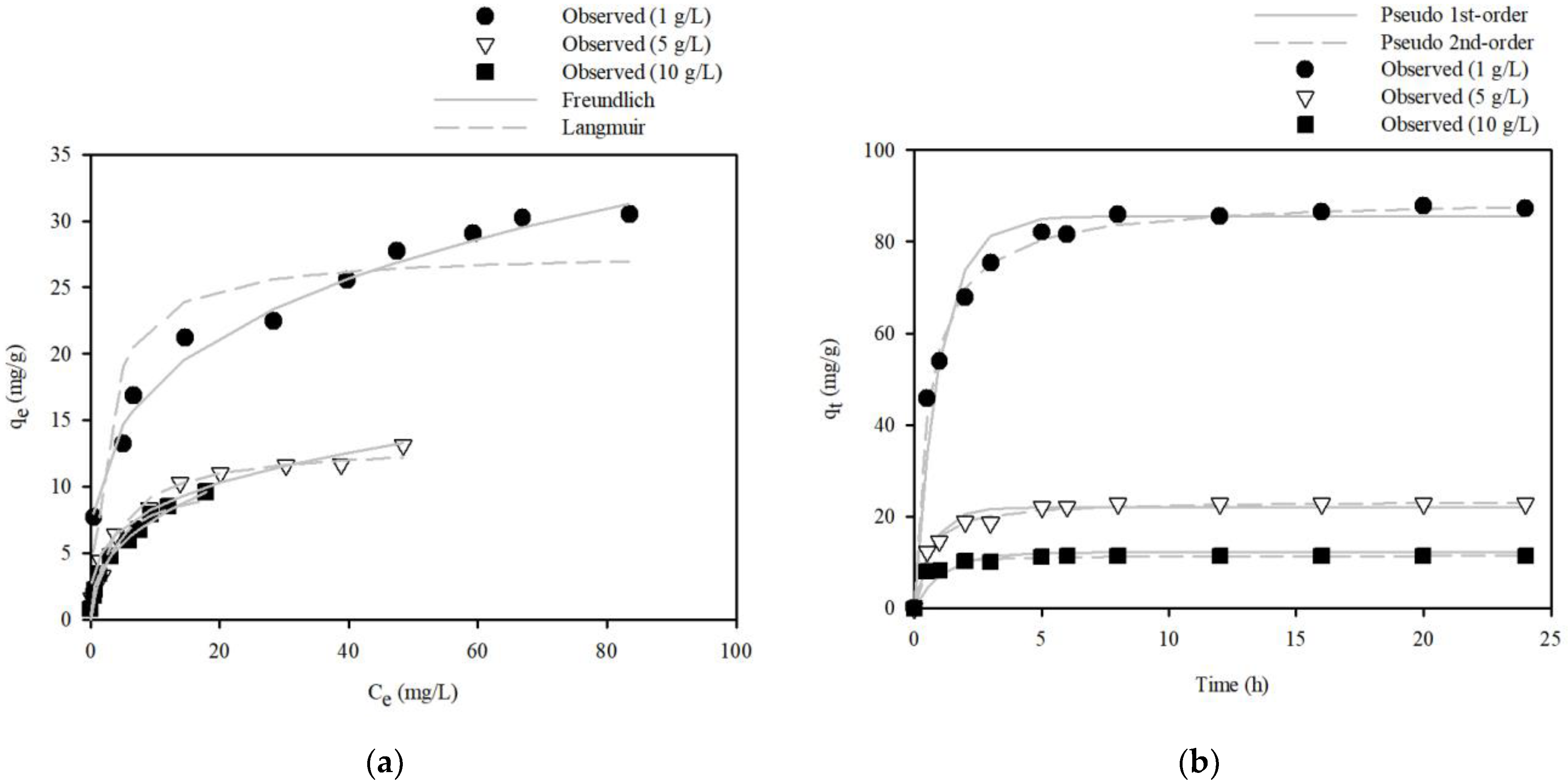
3.3.2. Equilibrium Isotherms and Kinetic Model Analyses
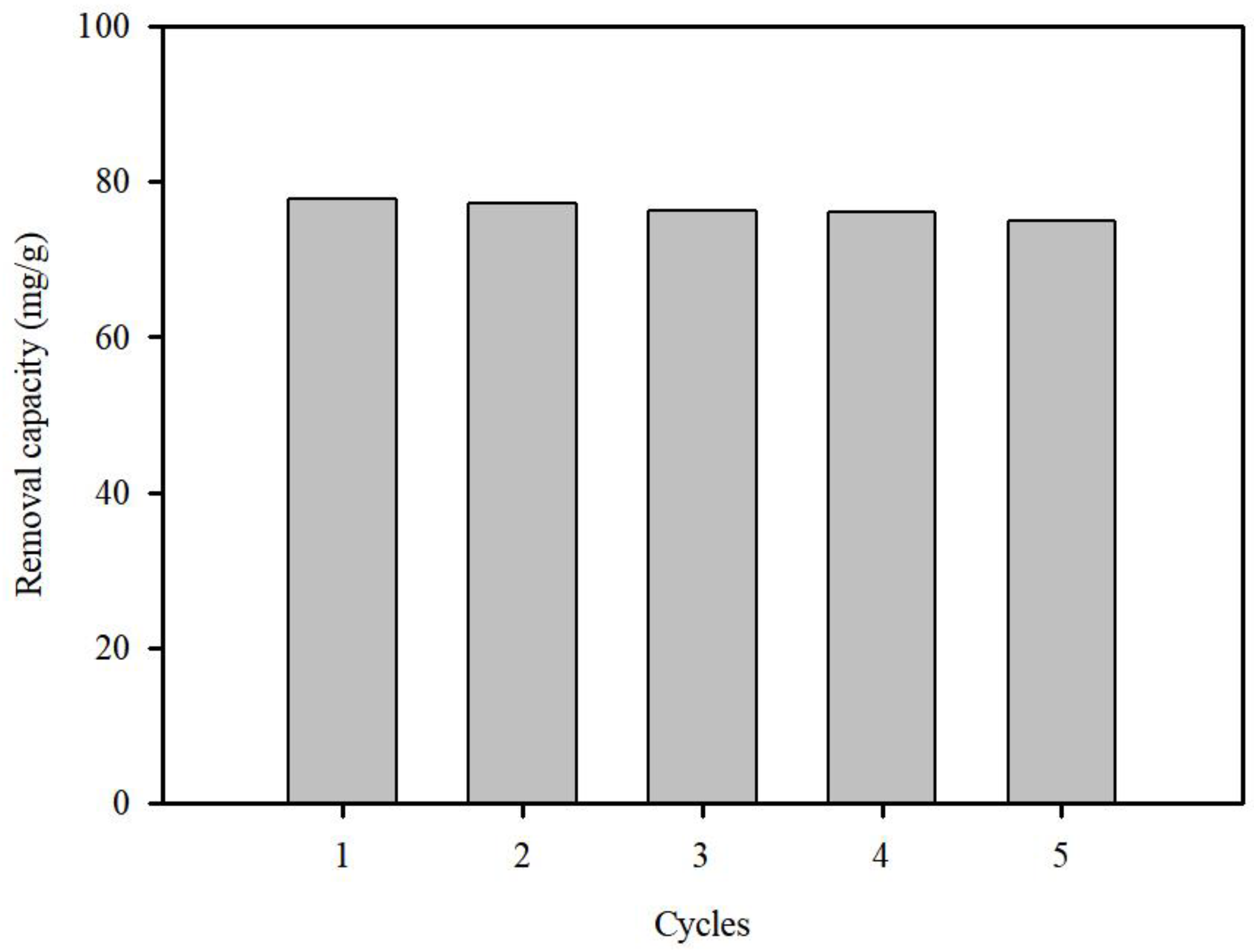
3.3.3. Recyclability of SP@Ca-SA
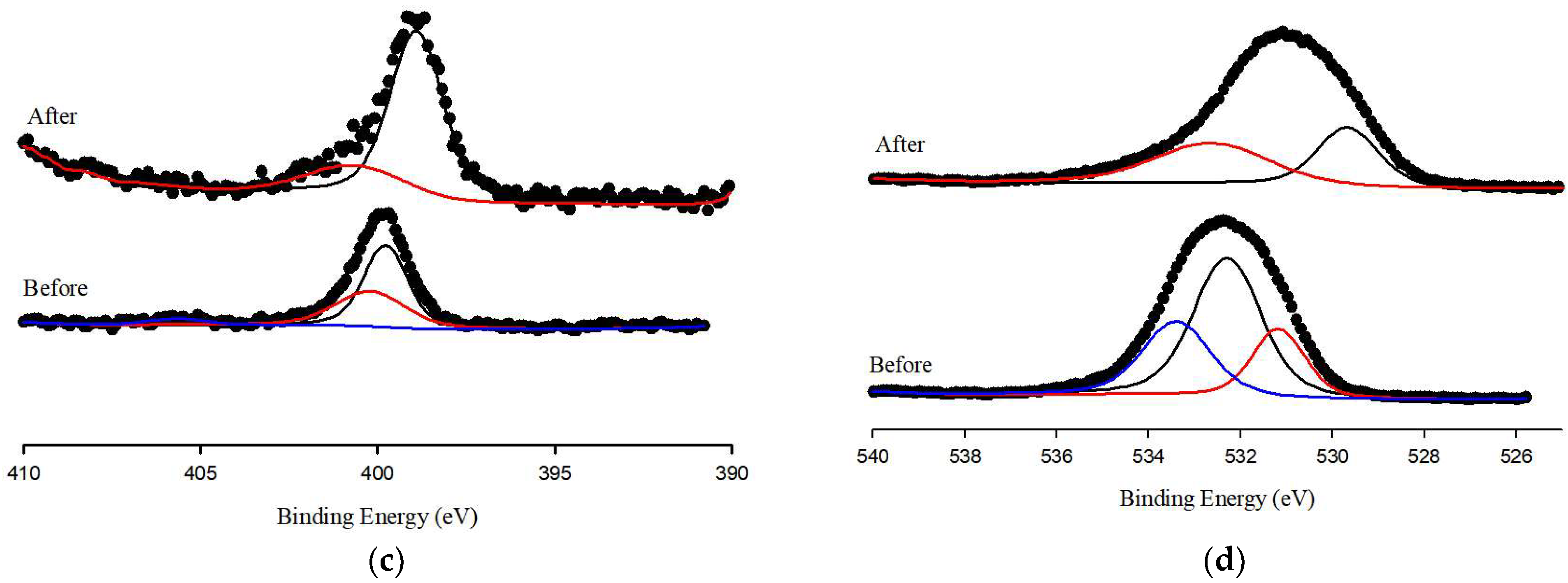
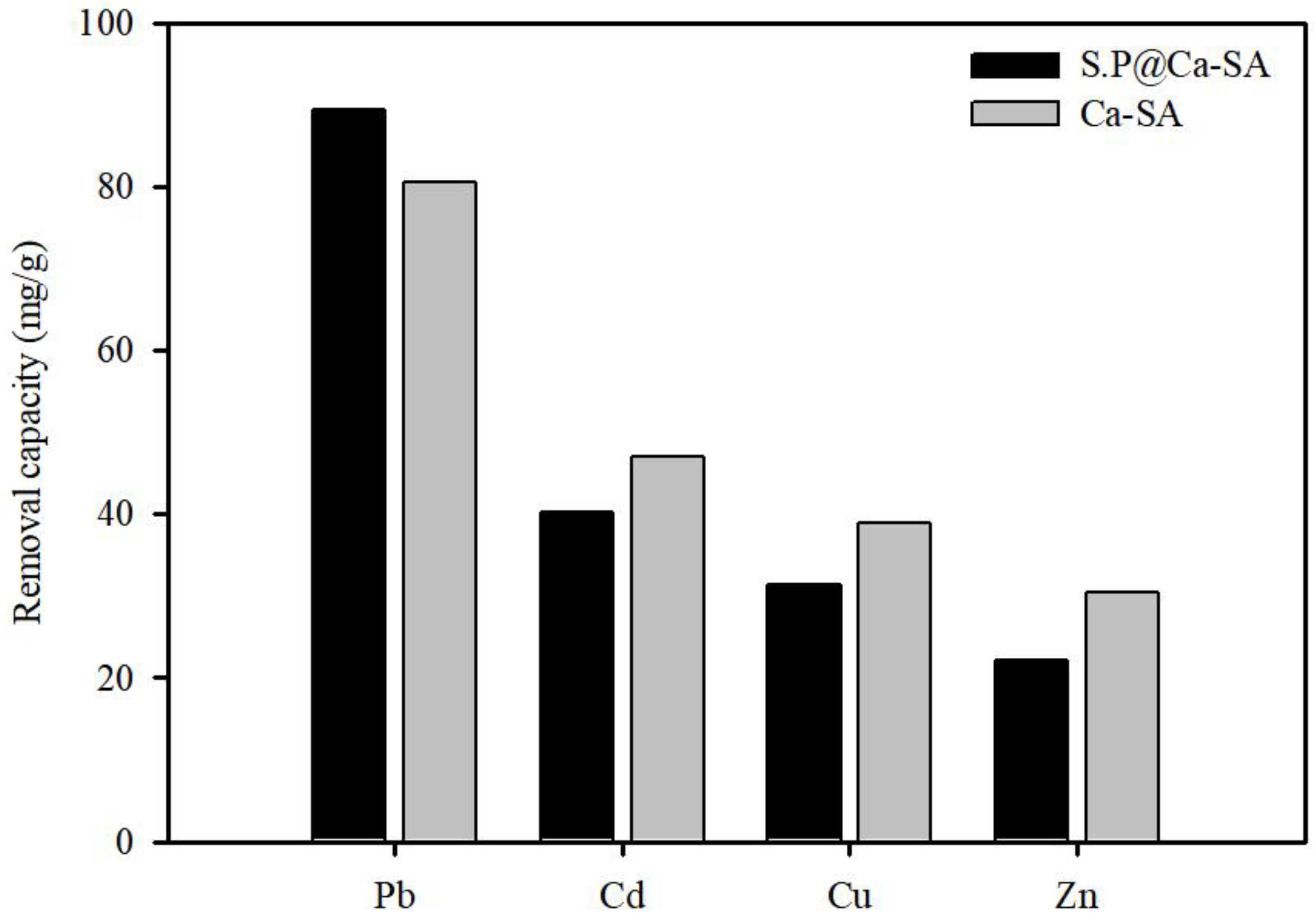
3.4. Adsorption Mechanisms
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ye, Z.; Yin, X.; Chen, L.; He, X.; Lin, Z.; Liu, C.; Ning, S.; Wang, X.; Wei, Y. An integrated process for removal and recovery of Cr(VI) from electroplating wastewater by ion exchange and reduction–precipitation based on a silica-supported pyridine resin. J. Clean. Prod. 2019, 236, 117631. [Google Scholar] [CrossRef]
- Wen, J.; Jiang, T.; Wang, J.; Gao, H.; Lu, L. An efficient utilization of high chromium vanadium slag: Extraction of vanadium based on manganese carbonate roasting and detoxification processing of chromium-containing tailings. J. Hazard. Mater. 2019, 378, 120733. [Google Scholar] [CrossRef]
- Król, A.; Mizerna, K.; Bożym, M. An assessment of pH-dependent release and mobility of heavy metals from metallurgical slag. J. Hazard. Mater. 2019, 384, 121502. [Google Scholar] [CrossRef]
- Collin, M.S.; Venkatraman, S.K.; Vijayakumar, N.; Kanimozhi, V.; Arbaaz, S.M.; Stacey, R.G.S.; Anusha, J.; Choudhary, R.; Lvov, V.; Tovar, G.I.; et al. Bioaccumulation of lead (Pb) and its effects on human: A review. J. Hazard. Mater. Adv. 2022, 7, 100094. [Google Scholar] [CrossRef]
- Bai, C.; Wang, L.; Zhu, Z. Adsorption of Cr(III) and Pb(II) by graphene oxide/alginate hydrogel membrane: Characterization, adsorption kinetics, isotherm and thermodynamics studies. Int. J. Biol. Macromol. 2020, 147, 898–910. [Google Scholar] [CrossRef]
- Chiew, C.S.C.; Yeoh, H.K.; Pasbakhsh, P.; Krishnaiah, K.; Poh, P.E.; Tey, B.T.; Chan, E.S. Halloysite/alginate nanocomposite beads: Kinetics, equilibrium and mechanism for lead adsorption. Appl. Clay Sci. 2016, 119, 301–310. [Google Scholar] [CrossRef]
- Wu, D.; Hu, L.; Wang, Y.; Wei, Q.; Yan, L.; Yan, T.; Li, Y.; Du, B. EDTA modified β-cyclodextrin/chitosan for rapid removal of Pb(II) and acid red from aqueous solution. J. Colloid Interface Sci. 2018, 523, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Li, Y.; Pei, H. Algal–bacterial consortia for bioproduct generation and wastewater treatment. Renew. Sustain. Energy Rev. 2021, 149, 111395. [Google Scholar] [CrossRef]
- Qu, P.; Li, Y.; Huang, H.; Chen, J.; Yu, Z.; Huang, J.; Wang, H.; Gao, B. Urea formaldehyde modified alginate beads with improved stability and enhanced removal of Pb2+, Cd2+, and Cu2+. J. Hazard. Mater. 2020, 396, 122664. [Google Scholar] [CrossRef] [PubMed]
- Long, Y.; Yu, G.; Dong, L.; Xu, Y.; Lin, H.; Deng, Y.; You, X.; Yang, L.; Liao, B.-Q. Synergistic fouling behaviors and mechanisms of calcium ions and polyaluminum chloride associated with alginate solution in coagulation-ultrafiltration (UF) process. Water Res. 2021, 189, 116665. [Google Scholar] [CrossRef]
- Ahmad, Z.; Li, Y.; Huang, C.; Gou, X.; Fan, Y.; Chen, J. Underwater suspended bifunctionalized polyethyleneimine-based sponge for selective removal of anionic pollutants from aqueous solution. J. Hazard. Mater. 2021, 412, 125284. [Google Scholar] [CrossRef] [PubMed]
- Mo, Y.; Vincent, T.; Faur, C.; Guibal, E. Se(VI) sorption from aqueous solution using alginate/polyethylenimine membranes: Sorption performance and mechanism. Int. J. Biol. Macromol. 2020, 147, 832–843. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Wang, B.; Tang, H.; Jin, Z.; Mao, Y.; Huang, T. Removal of phosphate from wastewater by modified bentonite entrapped in Ca-alginate beads. J. Environ. Manag. 2020, 260, 110130. [Google Scholar] [CrossRef]
- Pap, S.; Bezanovic, V.; Radonic, J.; Babic, A.; Saric, S.; Adamovic, D.; Turk Sekulic, M. Synthesis of highly-efficient functionalized biochars from fruit industry waste biomass for the removal of chromium and lead. J. Mol. Liq. 2018, 268, 315–325. [Google Scholar] [CrossRef]
- Li, Z.; Wang, L.; Wu, J.; Xu, Y.; Wang, F.; Tang, X.; Xu, J.; Ok, Y.S.; Meng, J.; Liu, X. Zeolite-supported nanoscale zero-valent iron for immobilization of cadmium, lead, and arsenic in farmland soils: Encapsulation mechanisms and indigenous microbial responses. Environ. Pollut. 2020, 260, 114098. [Google Scholar] [CrossRef] [PubMed]
- Acosta, R.; Fierro, V.; Martinez de Yuso, A.; Nabarlatz, D.; Celzard, A. Tetracycline adsorption onto activated carbons produced by KOH activation of tyre pyrolysis char. Chemosphere 2016, 149, 168–176. [Google Scholar] [CrossRef] [PubMed]
- Law, X.N.; Cheah, W.Y.; Chew, K.W.; Ibrahim, M.F.; Park, Y.-K.; Ho, S.-H.; Show, P.L. Microalgal-based biochar in wastewater remediation: Its synthesis, characterization and applications. Environ. Res. 2022, 204, 111966. [Google Scholar] [CrossRef]
- Bhadra, B.N.; Jhung, S.H. Adsorptive removal of nitrogenous compounds from microalgae-derived bio-oil using metal-organic frameworks with an amino group. Chem. Eng. J. 2020, 388, 124195. [Google Scholar] [CrossRef]
- Xiong, C.; Xue, C.; Huang, L.; Hu, P.; Fan, P.; Wang, S.; Zhou, X.; Yang, Z.; Wang, Y.; Ji, H. Enhanced selective removal of Pb(II) by modification low-cost bio-sorbent: Experiment and theoretical calculations. J. Clean. Prod. 2021, 316, 128372. [Google Scholar] [CrossRef]
- Jaiswal, K.K.; Kumar, V.; Verma, R.; Verma, M.; Kumar, A.; Vlaskin, M.S.; Nanda, M.; Kim, H. Graphitic bio-char and bio-oil synthesis via hydrothermal carbonization-co-liquefaction of microalgae biomass (oiled/de-oiled) and multiple heavy metals remediations. J. Hazard. Mater. 2021, 409, 124987. [Google Scholar] [CrossRef]
- Huang, R.; Huo, G.; Song, S.; Li, Y.; Xia, L.; Gaillard, J.-F. Immobilization of mercury using high-phosphate culture-modified microalgae. Environ. Pollut. 2019, 254, 112966. [Google Scholar] [CrossRef] [PubMed]
- Sayadi, M.H.; Rashki, O.; Shahri, E. Application of modified Spirulina platensis and Chlorella vulgaris powder on the adsorption of heavy metals from aqueous solutions. J. Environ. Chem. Eng. 2019, 7, 103169. [Google Scholar] [CrossRef]
- Rai, A.; Sirotiya, V.; Mourya, M.; Khan, M.J.; Ahirwar, A.; Sharma, A.K.; Kawatra, R.; Marchand, J.; Schoefs, B.; Varjani, S.; et al. Sustainable treatment of dye wastewater by recycling microalgal and diatom biogenic materials: Biorefinery perspectives. Chemosphere 2022, 305, 135371. [Google Scholar] [CrossRef] [PubMed]
- Leong, Y.-K.; Chang, J.-S. Bioremediation of heavy metals using microalgae: Recent advances and mechanisms. Bioresour. Technol. 2020, 303, 122886. [Google Scholar] [CrossRef]
- Leng, L.; Wei, L.; Xiong, Q.; Xu, S.; Li, W.; Lv, S.; Lu, Q.; Wan, L.; Wen, Z.; Zhou, W. Use of microalgae based technology for the removal of antibiotics from wastewater: A review. Chemosphere 2020, 238, 124680. [Google Scholar] [CrossRef]
- Peres, E.C.; Cunha, J.M.; Dortzbacher, G.F.; Pavan, F.A.; Lima, É.C.; Foletto, E.L.; Dotto, G.L. Treatment of leachates containing cobalt by adsorption on Spirulina sp. and activated charcoal. J. Environ. Chem. Eng. 2018, 6, 677–685. [Google Scholar] [CrossRef]
- Nithya, K.; Sathish, A.; Pradeep, K.; Kiran Baalaji, S. Algal biomass waste residues of Spirulina platensis for chromium adsorption and modeling studies. J. Environ. Chem. Eng. 2019, 7, 103273. [Google Scholar] [CrossRef]
- Pez Jaeschke, D.; Rocha Teixeira, I.; Ferreira Marczak, L.D.; Mercali, G.D. Phycocyanin from Spirulina: A review of extraction methods and stability. Food Res. Int. 2021, 143, 110314. [Google Scholar] [CrossRef]
- Chakraborty, I.; Bhowmick, G.D.; Ghosh, D.; Dubey, B.K.; Pradhan, D.; Ghangrekar, M.M. Novel low-cost activated algal biochar as a cathode catalyst for improving performance of microbial fuel cell. Sustain. Energy Technol. Assess. 2020, 42, 100808. [Google Scholar] [CrossRef]
- Cho, D.-W.; Yoon, K.; Kwon, E.E.; Biswas, J.K.; Song, H. Fabrication of magnetic biochar as a treatment medium for As(V) via pyrolysis of FeCl3-pretreated spent coffee ground. Environ. Pollut. 2017, 229, 942–949. [Google Scholar] [CrossRef]
- Liu, Y.; Xu, J.; Cao, Z.; Fu, R.; Zhou, C.; Wang, Z.; Xu, X. Adsorption behavior and mechanism of Pb(II) and complex Cu(II) species by biowaste-derived char with amino functionalization. J. Colloid Interface Sci. 2020, 559, 215–225. [Google Scholar] [CrossRef]
- Ma, C.; Zhao, Y.; Chen, H.; Liu, Y.; Huang, R.; Pan, J. Biochars derived from by-products of microalgae pyrolysis for sorption of gaseous H2S. J. Environ. Chem. Eng. 2022, 10, 107310. [Google Scholar] [CrossRef]
- Nateras-Ramírez, O.; Martínez-Macias, M.R.; Sánchez-Machado, D.I.; López-Cervantes, J.; Aguilar-Ruiz, R.J. An overview of microalgae for Cd2+ and Pb2+ biosorption from wastewater. Bioresour. Technol. Rep. 2022, 17, 100932. [Google Scholar] [CrossRef]
- Sun, X.; Huang, H.; Zhao, D.; Lin, J.; Gao, P.; Yao, L. Adsorption of Pb2+ onto freeze-dried microalgae and environmental risk assessment. J. Environ. Manag. 2020, 265, 110472. [Google Scholar] [CrossRef] [PubMed]
- Binda, G.; Spanu, D.; Bettinetti, R.; Magagnin, L.; Pozzi, A.; Dossi, C. Comprehensive comparison of microalgae-derived biochar from different feedstocks: A prospective study for future environmental applications. Algal Res. 2020, 52, 102103. [Google Scholar] [CrossRef]
- Akbarbaglu, Z.; Ayaseh, A.; Ghanbarzadeh, B.; Sarabandi, K. Techno-functional, biological and structural properties of Spirulina platensis peptides from different proteases. Algal Res. 2022, 66, 102755. [Google Scholar] [CrossRef]
- Çelekli, A.; Yavuzatmaca, M.; Bozkurt, H. An eco-friendly process: Predictive modelling of copper adsorption from aqueous solution on Spirulina platensis. J. Hazard. Mater. 2010, 173, 123–129. [Google Scholar] [CrossRef]
- Choi, Y.-K.; Choi, T.-R.; Gurav, R.; Bhatia, S.K.; Park, Y.-L.; Kim, H.J.; Kan, E.; Yang, Y.-H. Adsorption behavior of tetracycline onto Spirulina sp. (microalgae)-derived biochars produced at different temperatures. Sci. Total Environ. 2020, 710, 136282. [Google Scholar] [CrossRef]
- Guo, D.; Wang, Y.; Gao, Y.; Lyu, Y.; Lin, Y.; Pan, Y.; Zhu, L.; Zhu, Y. Nitrogen migration in products during the microwave-assisted hydrothermal carbonization of spirulina platensis. Bioresour. Technol. 2022, 351, 126968. [Google Scholar] [CrossRef]
- Ferreira, L.S.; Rodrigues, M.S.; de Carvalho, J.C.M.; Lodi, A.; Finocchio, E.; Perego, P.; Converti, A. Adsorption of Ni2+, Zn2+ and Pb2+ onto dry biomass of Arthrospira (Spirulina) platensis and Chlorella vulgaris. I. Single metal systems. Chem. Eng. J. 2011, 173, 326–333. [Google Scholar] [CrossRef]
- Fila, D.; Hubicki, Z.; Kołodyńska, D. Applicability of new sustainable and efficient alginate-based composites for critical raw materials recovery: General composites fabrication optimization and adsorption performance evaluation. Chem. Eng. J. 2022, 446, 137245. [Google Scholar] [CrossRef]
- Jiang, X.; Wang, H.; Wang, Q.; Hu, E.; Duan, Y. Immobilizing amino-functionalized mesoporous silica into sodium alginate for efficiently removing low concentrations of uranium. J. Clean. Prod. 2020, 247, 119162. [Google Scholar] [CrossRef]
- Hu, C.; Lu, W.; Mata, A.; Nishinari, K.; Fang, Y. Ions-induced gelation of alginate: Mechanisms and applications. Int. J. Biol. Macromol. 2021, 177, 578–588. [Google Scholar] [CrossRef]
- Yang, K.; Lou, Z.; Fu, R.; Zhou, J.; Xu, J.; Baig, S.A.; Xu, X. Multiwalled carbon nanotubes incorporated with or without amino groups for aqueous Pb(II) removal: Comparison and mechanism study. J. Mol. Liq. 2018, 260, 149–158. [Google Scholar] [CrossRef]
- Luo, Z.; Chen, H.; Wu, S.; Yang, C.; Cheng, J. Enhanced removal of bisphenol A from aqueous solution by aluminum-based MOF/sodium alginate-chitosan composite beads. Chemosphere 2019, 237, 124493. [Google Scholar] [CrossRef]
- Liu, Q.; Wu, H.; Chen, J.; Guo, B.; Zhao, X.; Lin, H.; Li, W.; Zhao, X.; Lv, S.; Huang, C. Adsorption mechanism of trace heavy metals on microplastics and simulating their effect on microalgae in river. Environ. Res. 2022, 214, 113777. [Google Scholar] [CrossRef] [PubMed]
- Gong, D.; Li, B.; Celi, N.; Cai, J.; Zhang, D. Efficient Removal of Pb(II) from Aqueous Systems Using Spirulina-Based Biohybrid Magnetic Helical Microrobots. ACS Appl. Mater. Interfaces 2021, 13, 53131–53142. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, R.; Liu, H.; Li, M.; Chen, T.; Chen, D.; Zou, X.; Frost, R.L. Green preparation of magnetic biochar for the effective accumulation of Pb(II): Performance and mechanism. Chem. Eng. J. 2019, 375, 122011. [Google Scholar] [CrossRef]
- Esfandiar, N.; Suri, R.; McKenzie, E.R. Competitive sorption of Cd, Cr, Cu, Ni, Pb and Zn from stormwater runoff by five low-cost sorbents; Effects of co-contaminants, humic acid, salinity and pH. J. Hazard. Mater. 2022, 423, 126938. [Google Scholar] [CrossRef]
- Tang, S.; Lin, L.; Wang, X.; Feng, A.; Yu, A. Pb(II) uptake onto nylon microplastics: Interaction mechanism and adsorption performance. J. Hazard. Mater. 2020, 386, 121960. [Google Scholar] [CrossRef]
- Ge, Y.; Qin, L.; Li, Z. Lignin microspheres: An effective and recyclable natural polymer-based adsorbent for lead ion removal. Mater. Des. 2016, 95, 141–147. [Google Scholar] [CrossRef]
- Bartczak, P.; Klapiszewski, Ł.; Wysokowski, M.; Majchrzak, I.; Czernicka, W.; Piasecki, A.; Ehrlich, H.; Jesionowski, T. Treatment of model solutions and wastewater containing selected hazardous metal ions using a chitin/lignin hybrid material as an effective sorbent. J. Environ. Manag. 2017, 204, 300–310. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhu, H.; Qin, C.; Zhou, J.; Zhao, J.R.; Wang, S. Adsorption of heavy metal ion from aqueous single metal solution by aminated epoxy-lignin. BioResources 2013, 8, 2257–2269. [Google Scholar] [CrossRef]
- Ge, Y.; Li, Z.; Kong, Y.; Song, Q.; Wang, K. Heavy metal ions retention by bi-functionalized lignin: Synthesis, applications, and adsorption mechanisms. J. Ind. Eng. Chem. 2014, 20, 4429–4436. [Google Scholar] [CrossRef]
- Jin, C.; Zhang, X.; Xin, J.; Liu, G.; Wu, G.; Kong, Z.; Zhang, J. Clickable synthesis of 1, 2, 4-triazole modified lignin-based adsorbent for the selective removal of Cd(II). ACS Sustain. Chem. Eng. 2017, 5, 4086–4093. [Google Scholar] [CrossRef]
- Alakhras, F.; Al-Shahrani, H.; Al-Abbad, E.; Al-Rimawi, F.; Ouerfelli, N. Removal of Pb(II) Metal Ions from Aqueous Solutions Using Chitosan-Vanillin Derivatives of Chelating Polymers. Pol. J. Environ. Stud. 2019, 28, 1523–1534. [Google Scholar] [CrossRef]
- Zhang, M.; Yin, Q.; Ji, X.; Wang, F.; Gao, X.; Zhao, M. High and fast adsorption of Cd(II) and Pb(II) ions from aqueous solutions by a waste biomass based hydrogel. Sci. Rep. 2020, 10, 3285. [Google Scholar] [CrossRef]
- Ma, Y.; Deng, Z.; Li, Z.; Lin, Q.; Wu, Y.; Dou, W. Adsorption characteristics and mechanism for K2Ti4O9 whiskers removal of Pb(II), Cd(II), and Cu(II) cations in wastewater. J. Environ. Chem. Eng. 2021, 9, 106236. [Google Scholar] [CrossRef]
- Bo, S.; Luo, J.; An, Q.; Xiao, Z.; Wang, H.; Cai, W.; Zhai, S.; Li, Z. Efficiently selective adsorption of Pb(II) with functionalized alginate-based adsorbent in batch/column systems: Mechanism and application simulation. J. Clean. Prod. 2020, 250, 119585. [Google Scholar] [CrossRef]

| Species | Initial Metal Concentration (mg/L) | Type of Wastewater | Removal Capacity (mg/g) |
|---|---|---|---|
| Chaetoceros sp. | 20 | Synthetic | 46.94 |
| Chlorella sp. | 50 | Synthetic | 20.95 |
| Spirogyra | 500 | Real | 94.34 |
| Cyanothece sp. | 500 | Synthetic | 28.57 |
| Phormidium sp. | 10 | Synthetic | 5.15 |
| Rhizoclonium hookeri | 1000 | Synthetic | 81.7 |
| Spirulina platensis | 100 | Synthetic | 68.0 |
| Adsorbent Dose (g/L) | Freundlich Model | ||||
| KF (L/g) | 1/n | R2 | SSE | ||
| 1 | 9.48 | 0.27 | 0.98 | 0.49 | 8.42 |
| 5 | 4.32 | 0.29 | 0.96 | 1.37 | 6.33 |
| 10 | 2.99 | 0.40 | 0.99 | 0.90 | 2.51 |
| Adsorbent Dose (g/L) | Langmuir Model | ||||
| Qm (mg/g) | KL (L/mg) | R2 | SSE | ||
| 1 | 27.7 | 0.43 | 0.81 | 5.64 | 29.3 |
| 5 | 13.2 | 0.25 | 0.98 | 1.83 | 4.61 |
| 10 | 10.8 | 0.28 | 0.98 | 47.4 | 4.93 |
| Adsorbent Dose (g/L) | Pseudo First-Order Model | ||||
| qe (mg/g) | k1 (1/h) | R2 | SSE | ||
| 1 | 75.5 | 1.03 | 0.92 | 3.48 | 37.2 |
| 5 | 21.4 | 0.87 | 0.90 | 1.79 | 11.7 |
| 10 | 11.4 | 1.25 | 0.84 | 1.53 | 4.07 |
| Adsorbent Dose (g/L) | Pseudo Second-Order Model | ||||
| qe (mg/g) | k2 (g/mg/h) | R2 | SSE | ||
| 1 | 80.3 | 0.01 | 0.99 | 0.56 | 13.3 |
| 5 | 22.8 | 0.06 | 0.97 | 0.36 | 5.24 |
| 10 | 11.6 | 0.39 | 0.89 | 0.25 | 2.88 |
| Adsorbent | Removal Capacity (mg/g) | Reference |
|---|---|---|
| Lignin microspheres | 33.9 | [51] |
| Lignin-chitin hybrid material | 75.7 | [52] |
| Aminated epoxy-lignin | 72.4 | [53] |
| Bi-functionlized lignin | 53.8 | [54] |
| 1, 2, 4-triazole modified lignin | 42 | [55] |
| Chitosan-vanillin derivatives of chelating polymers | 23.3 | [56] |
| S.P@Ca-SA | 87.9 | This study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Purev, O.; Park, C.; Kim, H.; Myung, E.; Choi, N.; Cho, K. Spirulina platensis Immobilized Alginate Beads for Removal of Pb(II) from Aqueous Solutions. Int. J. Environ. Res. Public Health 2023, 20, 1106. https://doi.org/10.3390/ijerph20021106
Purev O, Park C, Kim H, Myung E, Choi N, Cho K. Spirulina platensis Immobilized Alginate Beads for Removal of Pb(II) from Aqueous Solutions. International Journal of Environmental Research and Public Health. 2023; 20(2):1106. https://doi.org/10.3390/ijerph20021106
Chicago/Turabian StylePurev, Oyunbileg, Chulhyun Park, Hyunsoo Kim, Eunji Myung, Nagchoul Choi, and Kanghee Cho. 2023. "Spirulina platensis Immobilized Alginate Beads for Removal of Pb(II) from Aqueous Solutions" International Journal of Environmental Research and Public Health 20, no. 2: 1106. https://doi.org/10.3390/ijerph20021106
APA StylePurev, O., Park, C., Kim, H., Myung, E., Choi, N., & Cho, K. (2023). Spirulina platensis Immobilized Alginate Beads for Removal of Pb(II) from Aqueous Solutions. International Journal of Environmental Research and Public Health, 20(2), 1106. https://doi.org/10.3390/ijerph20021106






