Abstract
Heavy metals are toxic, persistent, and non-degradable. After sedimentation and adsorption, they accumulate in water sediments. The aim of this study was to assess the extent of heavy metal pollution of Qinjiang River sediments and its effects on the ecological environment and apportioning sources. The mean total concentrations of Mn, Zn, Cr, Cu, and Pb are 3.14, 2.33, 1.39, 5.79, and 1.33 times higher than the background values, respectively. Co, Ni, and Cd concentrations are lower than the background values. Fe, Co, Ni, Cd, Cr, Cu, and Pb are all primarily in the residual state, while Mn and Zn are primarily in the acid-soluble and oxidizable states, respectively. Igeo, RI, SQGs, and RAC together indicate that the pollution status and ecological risk of heavy metals in Qinjiang River sediments are generally moderate; among them, Fe, Co, Ni, Cd, Cr, and Pb are not harmful to the ecological environment of the Qinjiang River. Cu is not readily released because of its higher residual composition, suggesting that Cu is less harmful to the ecological environment. Mn and Zn, as the primary pollution factors of the Qinjiang River, are harmful to the ecological environment. This heavy metal pollution in surface sediments of the Qinjiang River primarily comes from manganese and zinc ore mining. Manganese carbonate and its weathered secondary manganese oxide are frequently associated with a significant amount of residual copper and Cd, as a higher pH is suitable for the deposition and enrichment of these heavy metals. Lead–zinc ore and its weathering products form organic compounds with residual Fe, Co, Cr, and Ni, and their content is related to salinity. The risk assessment results of heavy metals in sediments provide an important theoretical basis for the prevention and control of heavy metal pollution in Qinjiang River.
1. Introduction
Sediments are a crucial part of rivers and lakes. During several physical and chemical processes, suspended solids and different ions in the water are adsorbed and enriched in sediments in river channels, reducing the contamination in water sediments [1,2,3]. This can objectively show the area’s water quality. Unlike other pollutants in water bodies, heavy metal contaminants cannot be efficiently eliminated by natural decomposition processes and instead accumulate in sediments in different ways [4,5,6]. In most cases, more than 95% of heavy metals in water bodies are eliminated and stored in sediments in various forms [7,8,9]. Thus, there is a continuous accumulation process of heavy metals in sediments. The sediments in the water body are the “sinks” of heavy metal pollutants in the water body [10,11]. When the environmental medium conditions (such as pH, Eh) change, the heavy metals in the sediments can be released into the water body and become the water body’s “secondary pollution source” [12,13,14]. Heavy metals in the bottom sediments of water bodies have extensive sources, simple accumulation, and long residual time and are difficult to detect after pollution [15,16]. Generally, heavy metals in sediments are present in various fractions (acid-soluble, reducible, oxidizable, and residual) [17,18], and the fraction content influences the heavy metals’ bioavailability; for example, the residual is more stable and less likely to be released. Thus, the total concentrations of heavy metals in sediments do not exactly show the environmental pollution status. Additionally, it must be determined in combination with the geochemical fractions of heavy metals in sediments [19,20].
Qinzhou City is located in the southernmost part of the Qinhang metallogenic belt [21]. There are several ilmenite, manganese, and lead–zinc ore fields in Qinzhou. Medium-sized and large metal ores include Xinhua lead–zinc ore in Pubei, Huarong-Dadong manganese ore, and Nahualing manganese ore in Qinnan District [22]. With the implementation of regional economic development policies, including the Belt and Road Initiative, free trade zones, and world-class petrochemical industrial parks, Qinzhou’s economy has rapidly developed, and the degree of industrialization and urbanization has been continuously promoted, bringing extensive pressure on environmental quality [23,24,25]. Recently, the water quality of monitored sections of the Qinjiang River has typically been classified as inferior V, indicating serious pollution. However, few studies have focused on the ecological risks of sediment pollution in the Qinjiang River; therefore, it is urgent and crucial to perform research work related to heavy metal pollution in Qinjiang River sediments.
This study primarily addresses the environmental pollution levels and feasible sources of heavy metals (Fe, Mn, Zn, Co, Ni, Cd, Cr, Cu, and Pb) in the sediments of the Qinjiang River. The heavy metals were categorized into four different fractions using the improved BCR sequential extraction method, and then the ecological risk was evaluated using geo-accumulation index (Igeo), potential ecological risk index (RI), sediment quality guidelines (SQGs), and risk assessment code (RAC), and finally, the sources of heavy metals were examined using principal component analysis and geological tracing of metallic ore. The combination of statistical analysis and each index can offer a comprehensive understanding of the heavy metal risks of Qinjiang River sediments and can be employed to provide a scientific basis for environmental management and environmental legislation, including pollution control of Qinjiang River water bodies, substrate dredging, etc., so that relevant managers can make targeted adjustments to the regional industrial structure and formulate environmental protection methods that are more suitable to the Qinzhou City’s development stage and the Qinjiang River’s functional needs.
2. Materials and Methods
2.1. Study Area
The Qinjiang River is located in Qinzhou City, Guangxi Zhuang Autonomous Region, and belongs to the Pearl River system. It originates from Bainiuling at the eastern foot of Dongshan Mountain, Pingshan Town, Lingshan County, flows through more than half of Qinzhou City, and finally flows into the Maowei Sea from Shajing. With a total length of 195.26 km and a catchment area of 2391.34 km2, it is the largest river flowing into the Maowei Sea area of Qinzhou Bay. It is a crucial water source for industrial and agricultural production and life in Qinzhou City. The study area is situated in the subtropical monsoon climate zone, with abundant rainfall and usual floods and droughts.
2.2. Sample Collection and Pre-Treatment
A total of 19 stations were chosen from the upstream to the Qinjiang estuary along the Qinjiang River Basin (marked as S1 to S19) (Figure 1). The surface sediments (approximately 0–10 cm in depth) were obtained in December 2021 using a gravity sampler. All the surface sediments in contact with the sampler were cleaned with a plastic scraper to reduce the disturbance of sediment samples. Three parallel subsamples were obtained from each station. All samples were subsequently transported back to the laboratory in labeled polyethylene sealable bags and freeze-dried using a freeze drier (CHRIST Alpha2-4LSC basic). The frozen samples were then sequentially dried in natural air, dried at high temperature, ground, and passed through a 200-mesh nylon sieve to obtain the sample to be examined [26].
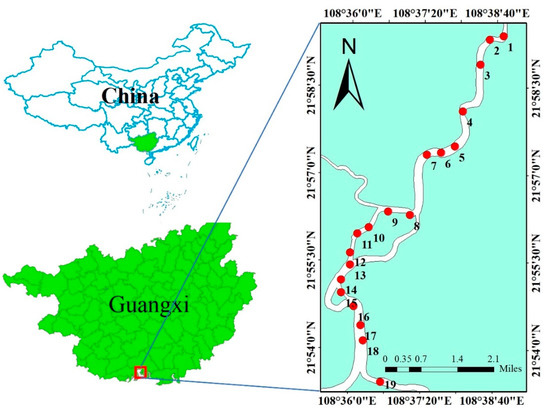
Figure 1.
Location of the study area and sample sites.
2.3. Physicochemical Analysis of Sediments
Fresh samples were mixed at a water-to-soil ratio of 1:2.5 [27] and centrifuged at 3500 rpm for 10 min; subsequently, the sediment pH and salinity were measured using a multiparameter analyzer (DZC-708). The total organic carbon (TOC) was determined by the loss-on-ignition [28]. The pretreated samples were roasted in a high-temperature muffle furnace (HT16/17, Nabertherm), and the content of organic matter (OM) in the samples was calculated according to the mass difference before and after, and the TOC content was finally converted using OM.
2.4. Microwave-Assisted Acid Digestion and Determination of Metals
Aliquots of ~0.1 g of sediments were placed in digestion tanks containing a 5:4:2 mixture of HNO3 + HF + HClO4. The samples were subsequently heated in microwave digestion apparatus (CEM/MARS6) for the following cycle. At 1600 W of power, the temperature was raised to 170 °C for 30 min and maintained for 20 min, followed by 210 °C for 40 min maintained for 30 min [29]. Subsequently, the digested samples were inserted into an acid purifier at 150 °C raising the acid to 1 mL. After cooling, the samples were diluted to 50 mL with ultrapure water and filtered through a 0.45 μm filter membrane, and finally, a 50 mL centrifuge tube was used to perform the test.
The concentrations of Fe, Mn, Zn, Co, Ni, Cd, Cr, Cu, and Pb in samples extracted from sequential extraction and microwave digestion were measured using ICP-OES (PE Optima8000, Crystal City, WA, USA), and the accuracy and precision of heavy metals analyses were verified using the standard reference samples (GSS-1). The mean recovery (%) in different chemical phases was 80–120%, and the relative error was generally <10%.
2.5. Sequential Extraction Procedure (BCR)
Acid-soluble, reducible, oxidizable, and residual fractions were extracted sequentially using the improved BCR sequential extraction method [30]. Table 1 shows the extraction agent and target sediment fraction employed in each step: Firstly, 1.00 g of sample was weighed into a 100 mL polypropylene centrifuge tube for the acid soluble fraction (F1, 0.11 mol/L CH3COOH), reducible fraction (F2, 0.5 mol/L NH2OH-HCl, pH 1.5), oxidizable fraction (F3, 1.0 mol/L CH3COONH4, pH 2.0), and residual fraction (F4, HF–HNO3–HClO4) of sediments. At the end of each extraction, the centrifuge tube was placed in a centrifuge at 4000 rpm for 10 min. The supernatant was collected, and the residue was cleaned with ultrapure water twice, transferred into a 50 mL volumetric flask, and fixed with 3% dilute nitric acid. After passing through a 45 μm filter membrane, the centrifuge tube was loaded with 50 mL for testing.

Table 1.
Improved BCR sequential extraction procedure.
2.6. Contamination and Risk Assessment of Heavy Metals in Sediments
2.6.1. Geo-Accumulation Index (Igeo)
The geo-accumulation index (Igeo) was first suggested by Müller, a scientist from the Sediment Research Institute of Heidelberg University in Germany, in 1969 [31,32]. It shows the pollution level through the heavy metal content in sediments, indicates the natural variation characteristics of the distribution of heavy metals, and determines the effect of human activities on the environment, which is a crucial parameter. It is determined by the following Equation (1):
where Cn represents the determined concentration of heavy metal (mg/kg); k represents the correction coefficient considered due to the geological differences of rocks in various areas, which is generally 1.5; and Bn denotes the heavy metal background concentration n (mg/kg). The background concentrations of heavy metals in the soil employed in this study are as follows: Mn = 159.32, Zn = 48.25, Co = 14.60, Ni = 24.00, Cd = 0.07, Cr = 21.41, Cu = 11.31, and Pb = 20.43 [33,34] (Fe rarely contaminates the environment, and therefore, there is no background value). According to the various Igeo values, the heavy metal pollution levels can be interpreted as follows: Igeo, no pollution; 0 < Igeo ≤ 1, low pollution; 1 < Igeo ≤ 2, near moderate pollution; 2 < Igeo ≤ 3, moderate pollution; 3 < Igeo ≤ 4, near high pollution; 4 < Igeo ≤ 5, high pollution; 5 < Igeo ≤ 6, very high pollution.
2.6.2. Potential Ecological Risk Index
The potential ecological RI method is a set of approaches for evaluating heavy metal pollution and ecological damage developed by the Swedish scientist Hakanson based on sedimentology [35]. It covers several research fields combining biotoxicology, environmental chemistry, and ecology. The ecological risk of heavy metals on soil is comprehensively assessed, and the potential damage degree is quantitatively categorized (Table 2). The value of RI was computed using the following equations:
where represents the pollution coefficient of heavy metals i, represents the determined concentration of heavy metals i (mg/kg), denotes the background values of heavy metals (mg/kg), represents the sum of pollution coefficients of different heavy metals, denotes the potential ecological risk factor, represents the potential ecological RIs, denotes the toxicity coefficient of heavy metals, and i denotes the toxicity level of heavy metals and the organisms’ sensitivity to heavy metal pollution, and its values are Zn = 1, Cr = 2, Co = Ni = Cu = Pb = 5, and Cd = 30 [35]. According to the determined level of toxicity of the heavy metals, we also considered the high heavy metal content and biological activity in the study area, defining five categories of and four categories of (Table 2) [36,37]. However, other evaluation criteria have been used by Barcauskaite et al. 2020, Wu et al. 2010, Zhu et al. 2012 [38,39,40].

Table 2.
Relationship between potential ecological hazard index and potential ecological hazard level.
2.6.3. Sediment Quality Guidelines
The SQGs can be employed to evaluate the heavy metal pollution level in sediments. Theoretically, SQGs are derived from the accumulation of datasets of sediment chemistry and corresponding adverse biological impacts [41,42], whereas the empirical assessment of SQGs is based on the total amounts of heavy metals in sediments [43]. There are two concentration thresholds for SQGs; one is unlikely to generate toxic reactions, and the other is likely to generate toxic reactions, and the pollutant concentrations between the two thresholds have significant uncertainty. To resolve this situation, it is crucial to conduct a site-specific analysis by observing the health and behavior of benthic organisms at the site. One frequently employed approach is to use the threshold effect level (TEL) and possible effect level (PEL) to compare with the heavy metal concentration to assess the degree of harmful impacts of sediment-related chemical states on benthic organisms [44,45]. These two levels defined three ranges of benthic hazards: no harm (<TEL); may cause harm (>TEL and <PEL); and harm (>PEL).
Furthermore, as heavy metals always appear in complex mixtures in sediments, their ecological risk can also be further assessed by their PEL and the resulting mass fraction to yield the mean probable-effect-level quotient (mPEL-Q) [46]; the formula is as follows:
where represents the measured concentration of the heavy metal i, represents the PEL for the heavy metal i, and n is the number of heavy metal species. The mPEL-Q indices can be grouped into the following four categories: mPEL-Q ≤ 0.1, low risk; 0.1 < mPEL-Q ≤ 1, considerable risk; 1 < mPEL-Q ≤ 5, high risk; mPEL-Q > 5, very high risk.
2.6.4. Risk Assessment Code
The RAC was employed to evaluate the bioavailability and mobility of heavy metals in sediments [47], which is closely related to the concentration of heavy metals in the sequentially extracted acidic soluble. The RAC equation is as follows:
The indices can be grouped into the following five categories: RAC < 1%, no risk; 1% < RAC < 10%, low risk; 11% < RAC < 30%, considerable risk; 31% < RAC < 50%, high risk; RAC > 50%, very high risk.
3. Results and Discussion
3.1. Total Concentration and Physicochemical Properties of Heavy Metals in Sediments of the Qinjiang River
Figure 2 presents the pH values, salinity, and TOC concentration of sediments in the Qinjiang River. The pH of sediments in the Qinjiang River ranged from 6.119 to 7.147, with a mean value of 6.61 and a weak acidity generally; the variation of salinity ranged from 0.01% to 0.24%; and the variation of ω (TOC) ranged from 3.12% to 6.43%, with a mean value of 4.43%. When the point is closer to the Qinjiang River estuary, the pH tends to decrease as a whole, and salinity and TOC content tend to increase as a whole. Remarkably, pH has considerably lower values at S2, S12, S13, and S14, probably because the sampling area is close to the industrial park and the agricultural planting area. The sediment acidity was aggravated by the acidity of the water bodies caused by the sewage discharge and waste accumulation in the sampling area’s vicinity. Furthermore, the stations with significant heavy metal concentrations also have high TOC, showing that the TOC content influences the heavy metals’ enrichment.
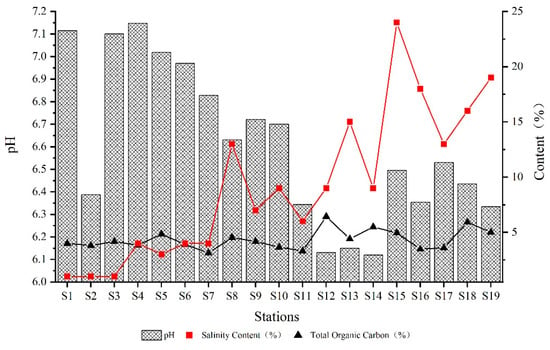
Figure 2.
Physicochemical characteristics of sediment at the different stations.
Table 3 summarizes the characteristics of heavy metal concentrations in sediments in the Qinjiang River. Average total heavy metal concentrations (mg/kg) were discovered in the decreasing order of Fe (31401.95) > Mn (500.27) > Zn (112.49) > Cu (65.45) > Cr (29.78) > Pb (22.99) > Ni (17.82) > Co (9.05) > Cd (0.02); Mn, Zn, Cr, Cu, and Pb had higher concentration values than their background values by 3.14, 2.33, 1.39, 5.79, and 1.13 times, respectively. High concentrations of Mn, Cd, and Cu were discovered in S5, which is situated in the city center and has hospitals, markets, and Nixing pottery factories nearby. High concentrations of Fe and Pb were discovered in S12, which has several agricultural planting areas and industrial parks. A high concentration of Cr is distributed in the dock area. High concentrations of Zn, Co, and Ni were distributed in the aquaculture area (S18). Heavy metals in waste and polluted soil are readily leached into the near-source water via runoff, and heavy metals migrate through rivers and accumulate in sediments [48]. Thus, these buildings may become the heavy metal pollution’s primary source of sediments and must be addressed in the subsequent analysis.

Table 3.
Values of maximum, minimum, median, average, background (in mg/kg), and coefficient of variation (CV%) for total concentration of heavy metals in the surface sediments from the Qinjiang River.
The coefficient of variation (CV) can show the uniformity and degree of variation of heavy metals in soil stations. Generally, the larger the CV, the greater the spatial dispersion that may be influenced by human activities. The CV of Fe, Co, Ni, Cd, Cr, Cu, and Pb ranges from 7.64% to 26.49%, less than 30%, showing that the distribution of these seven heavy metals in the watershed is relatively stable, and these heavy metals are primarily affected by natural factors. The coefficients of variation of Mn (53.55%) and Zn (37.02%) both exceeded 30%, suggesting that the spatial dispersion of Mn and Zn was greater, and it is speculated that Mn and Zn are primarily affected by human activities.
3.2. Geochemical Fractionations of Heavy Metals
Figure 3 shows four geochemical fractions of nine heavy metals (Fe, Mn, Zn, Co, Ni, Cd, Cr, Cu, and Pb) in surface sediments at 19 stations in the Qinjiang River extracted using the improved BCR sequential extraction method. Therefore, different proportions of geochemical fractions of heavy metals can be grouped into the following three categories: The first category is Mn, which is primarily found in the acid-soluble fraction (F1) and accounts for 38.92%, suggesting that Mn has high bioavailability and is easily released under acidic conditions; the second category is Zn, which is mainly found in the oxidizable fraction (F3) and accounts for 54.50%. Such heavy metals primarily occur in the form of iron and manganese oxides and organically bound states, which migrate with the change of redox potential, resulting in secondary pollution of water; the third category is Fe, Co, Ni, Cd, Cr, Cu, and Pb, and these seven heavy metals are primarily found in the residual fraction (F4). The average proportion of F4 is 78.13%, 57.39%, 56.60%, 55.36%, 55.31%, 73.74%, and 62.88%, respectively, and some investigations have revealed [49] that the residual fraction of heavy metals was almost unused by organisms. This can have a specified effect on organisms only by converting them into a soluble fraction by chemical reactions. Thus, these seven heavy metals are relatively stable in the Qinjiang River sediments and cannot easily pollute the ecological environment.
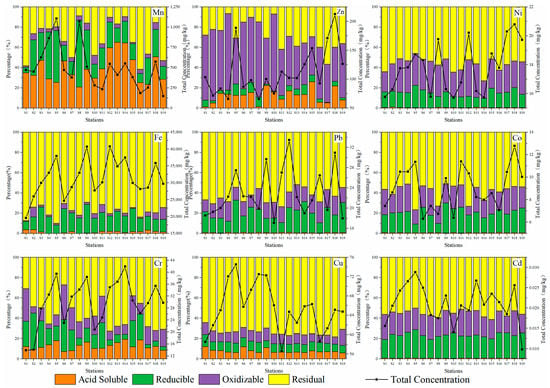
Figure 3.
Percentage distribution of heavy metals in four geochemical fractions at 19 stations.
3.3. Pollution and Risk Assessment
3.3.1. Geo-Accumulation Index (Igeo)
Figure 4 shows the Igeo values of the eight heavy metals. All heavy metals are in the decreasing order of Cu (1.94) > Mn (0.87) > Zn (0.18) > Cr (−0.17) > Pb (−0.44) > Ni (−1.02) > Co (−1.31) > Cd (−2.40). The negative Igeo values for Co, Ni, and Cd at all stations show a no pollution level; although the mean value of Cr and Pb is negative, there are still individual stations, which have low pollution. The mean value of Mn and Zn is between 0 and 1 (low pollution). However, at individual stations, Mn reaches 1 to 2 (moderate pollution) and 2 to 3 (moderate pollution). The mean Igeo value of Cu is much higher than other heavy metals, i.e., close to 2 to 3 (moderate pollution), showing that Cu is the most polluting heavy metal in the study area.
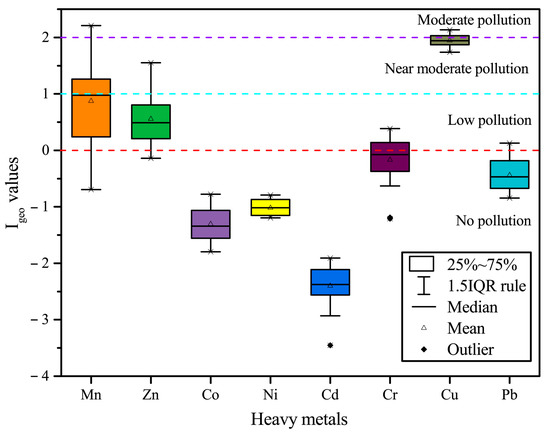
Figure 4.
Geo-accumulation index (Igeo) values of heavy metals in surface sediments from the Qinjiang River.
3.3.2. Potential Ecological Risk Index
Figure 5 shows the values of potential ecological risk factors (). The of all heavy metals were discovered in the decreasing order of Cu (28.95) > Cd (8.78) > Pb (5.63) > Ni (3.71) > Co (3.10) > Mn (3.14) > Cr (2.78) > Zn (2.33), and all heavy metals exhibited low risk. The values of Cu, Mn, Co, Ni, Cr, Cu, and Pb were consistent with the results generated by the Igeo values. However, Cd and Zn show a different result, which is probably because the primarily shows the heavy metal toxicity level and the organisms’ sensitivity to heavy metal pollution. When this approach is employed for risk assessment of heavy metals, the heavy metals’ toxicity coefficient has a significant influence on the results.
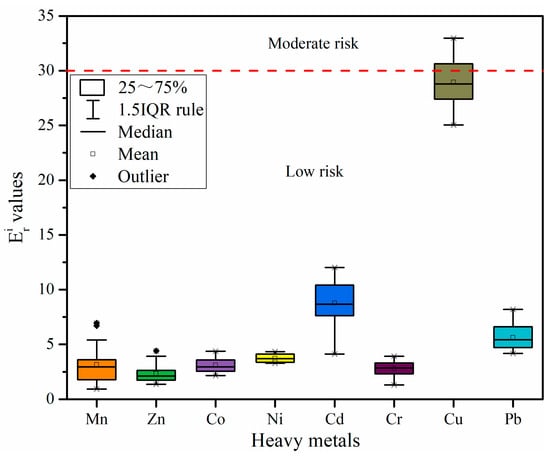
Figure 5.
Values of potential ecological risk factor () of heavy metals in the sediments of Qinjiang River.
Figure 6 shows the values of the potential ecological risk index (RI). There are six stations in the Qinjiang River with RI values greater than 60 (medium ecological risk), and the RI values of these stations were discovered in the decreasing order of S5 (67.05) > S4 (65.09) > S9 (64.47) > S8 (61.91) > S12 (61.44). Consistent with the results of the total concentration of heavy metals, higher RI values were discovered at S5 and S18, showing a higher ecological risk at these two stations. According to the field investigation, it is speculated that the pollution of S5 is due to intensive human activities and industrial pollution, including hospitals, markets, and Nixing pottery factories, whereas the pollution at S18 arises from the adjacent aquaculture area.
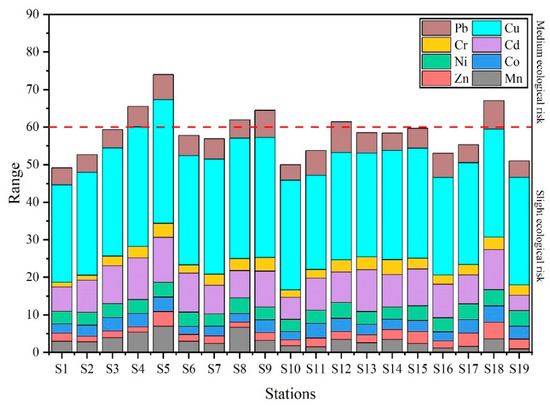
Figure 6.
Values of potential ecological risk index (RI) at different stations.
3.3.3. Sediment Quality Guidelines (SQGs)
Considering the limitations of the Igeo and RI approaches, another approach was used according to SQGs, based on the heavy metals’ total concentration. In this study, a set of SQGs for the TEL and PEL was employed to determine the ecotoxicological implications of seven heavy metals (Zn, Ni, Cd, Cr, Cu, and Pb) in the Qinjiang River. Table 4 shows the results. The mean concentrations of Cd and Cr at all stations are less than TEL, suggesting that neither Cd nor Cr will harm benthic organisms; 19%, 21%, and 85% of Zn, Ni, and Pb samples, respectively, are lower than TEL, respectively, and 81%, 79%, and 15% are between TEL and PEL, showing that Zn and Ni may occasionally have harmful impacts on benthic organisms at some stations. Mn was lower than the PEL at most stations (94%); however, it was the only heavy metal in the study that exceeded the PEL at one station (S5). Thus, Mn contamination at station S5 must be addressed. The mean concentrations of Cu at all stations were between TEL and PEL, indicating that Cu may be harmful to benthic organisms.

Table 4.
Percentage of heavy metals in each category associated with biological risks.
Additionally, the mPEL-Q values of each station were computed to further investigate its risk level, and Figure 7 shows the results. The mPEL-Q varied within the range 0.16–0.26, between 0.1 and 1, indicating considerable risk. The mPEL-Q’s relatively higher value appeared at S5 and S18, which is consistent with the results of the potential ecological risk assessment of RI. Thus, it can be determined that the high-value areas of heavy metals in the sampling area are situated at stations S5 and S18.
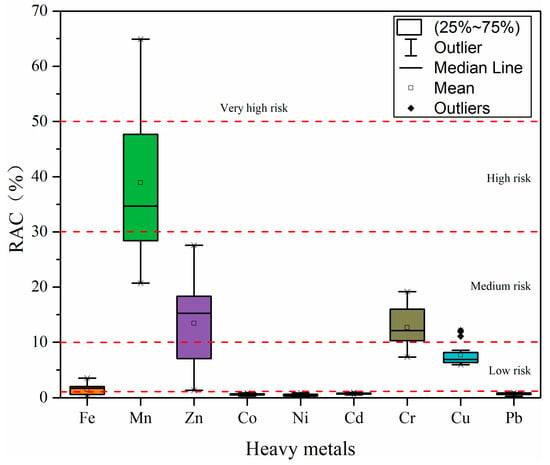
Figure 7.
Risk assessment code (RAC) values of heavy metals in surface sediments from the Qinjiang River.
3.3.4. Risk Assessment Code
In the study by Singh et al. [51], it was revealed that among numerous heavy metal geochemical fractions, changes in acid-soluble fractions caused by human activities influence the bioavailability or mobility of heavy metals in sediment. Thus, the higher the percentage of acid-soluble fraction in geochemical fractions, the higher the migration capacity of heavy metals in sediments, the higher the bioavailability, and the higher the level of potential ecological risk. Figure 7 shows the RAC evaluation results of heavy metals in sediments in the Qinjiang River. The mean RAC value of each heavy metal decreased in the order of Mn (38.92%) > Zn (13.43%) > Cr (12.68%) > Cu (7.70%) > Fe (1.48%) > Cd (0.75%) > Pb (0.69%) > Co (0.62%) > Ni (0.49%). According to RAC, Mn appeared to pose a high risk, Zn and Cr were grouped as medium risk, Fe and Cu were grouped as low risk, and Co, Ni, Cd, and Pb were grouped as no risk. However, it should be noted that the results of Cu are different from those of Igeo and RI, probably because the RAC approach is based on the heavy metals’ geochemical fractions and their proportion to characterize the risk level and does not consider the total concentration of heavy metals, and the bioavailability expressed in the geochemical fractions is unequal to biological toxicity considered by Igeo and RI. In this study, the total concentration of Cu is higher than the background values; however, Cu is primarily present in the residual fractions, which are more stable and less likely to be released in the sediment and cause harm to the ecological environment.
3.4. Source Analysis
Based on the risk assessment of different heavy metals in Qinjiang River sediments, personal correlation analysis, principal component analysis, and cluster analysis were conducted on nine heavy metals in the study area using SPSS25.0 to further investigate the possible sources of heavy metals in Qinjiang River sediments.
Principal Component Analysis
Based on personal correlation analysis, the sources of heavy metals were further examined using principal component analysis (PCA) of nine heavy metals. The matrix Kaiser–Meyer–Olkin test value was 0.598, indicating that the data were suitable for PCA (Figure 8). It was observed that the eigenvalues of principal components 1 and 2 are 3.948 and 1.784, respectively, and the variance contribution rates are 43.869 and 19.819, which can better demonstrate the data situation. In the first component (PC1), Fe, Cr, Pb, and Cd demonstrated highly positive loadings, indicating that Fe, Cr, Pb, and Cd may have the same source; in second component (PC2), Cu and Mn have highly positive loadings, suggesting that Cu and Mn may have the same source, and Zn, Co, and Ni have comparable positive loadings, indicating that Zn, Co, and Ni enrichment may be related to Mn.
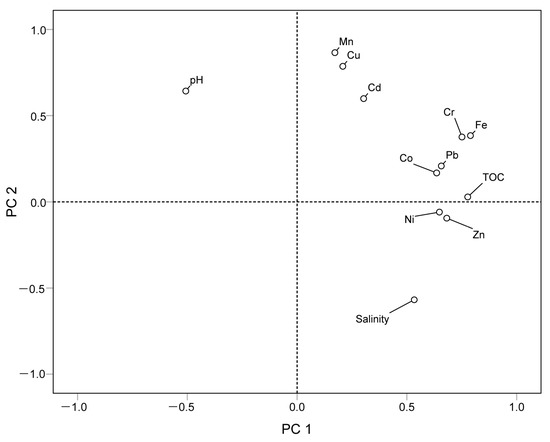
Figure 8.
PCA loadings of components 1 and 2 for the nine heavy metals in surface sediments from the Qinjiang River.
Table 5 shows the correlations between the nine investigated heavy metals, pH, salinity, and TOC. It was observed that there is a considerable negative correlation between pH and salinity and TOC, suggesting that salinity and TOC decrease with the increase in pH. Fe, Zn, Ni, Cr, Pb, and TOC manifested substantial positive correlations, suggesting that there is a close relationship between the TOC in the soil and total heavy metal concentrations. It was revealed in a study [52] that Fe is less influenced by human interference because of its high concentration, and therefore, it can be employed as a characterization of human and natural factors. In this study, Fe has a considerable positive correlation with Cr and Pb (p < 0.01), and therefore, both Cr and Pb are derived from natural factors. Cd has a substantial positive correlation with Fe, Cr, and Pb (p < 0.05) and indicates low risk in both the Igeo and RI, indicating that Cd also belongs to natural factors; Cu and Mn were considerably positively correlated (p < 0.01).

Table 5.
Pearson correlation matrix for heavy metals concentration in surface sediments from the Qinjiang River.
Igeo indicated that Mn and Zn were higher than soil background values and had evident characteristics of foreign pollution. PCA and correlation analysis indicated that the two groups of heavy gold denoted by Mn and Zn belong to various sources; the contents of Mn, Cu, and Cd are related to pH, while Zn, Fe, Pb, Co, Ni, and Cr are related to TOC and salinity. Combined with the analysis of heavy metal components, the composition of Mn includes the acid soluble fraction (F1) and the reducible fraction (F2), and the Zn component is the oxidizable fraction (F3). Qinzhou Port is also the largest distribution center of manganese ore in China [53]. The Upper Devonian Liujiang Formation is the primary manganese-bearing strata in the Qinzhou area. Manganese is present in the form of manganese carbonate minerals, such as rhodochrosite, calcite, manganese siderite, and manganese dolomite, while manganese in sediments or weathering crust is in the form of secondary oxides, accompanied by Cu, Cd, and other elements [54]. Zinc deposits in the Qinjiang River Basin occur in the Indo-Hercynian granitic rock mass in Pubei County, Qinzhou City (Figure 9). The zinc deposits are primarily sphalerite, which has a symbiotic relationship with Pb, generating lead–zinc deposits and forming an organic combination with OM and pyrite after weathering and leaching [55].
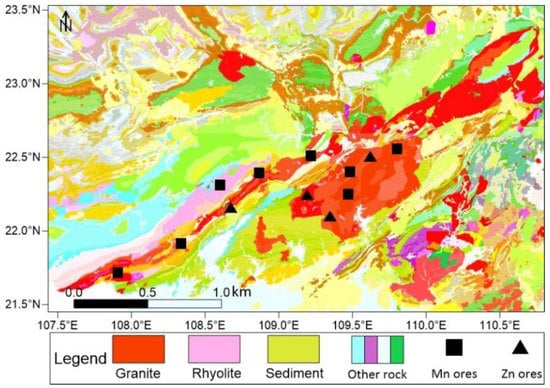
Figure 9.
Geological map (1:200,000) and mineral distribution around the Qinjiang River (Adapted with permission from Ref. [56]).
The survey of soil heavy metals in the Guangxi region showed that the spatial distribution of Cd, As, Cr, Cu, Hg, Ni, Pb, and Zn was mainly controlled by geological background. The content of heavy metals in the soils of carbonate source area is higher than that of clastic source area soils. The enrichment of Cd in surface soils is the result of secondary enrichment and weathering of parent rocks, and the enrichment of other metals is mainly the result of secondary enrichment. During weathering of the carbonate bedrock, the vast majority of the intrinsic heavy metals were leached out, and only 2% of Cd was retained in situ. Soil Fe, Al, Mn oxides, organic carbon, and clay content are closely related to the enrichment of heavy metals [57]. High concentrations of Pb, Cr, Cd, Cu, Zn, As, and Hg in surface sediments were found in Qinzhou Bay, Fangchenggang, and other coastal areas, with heavy metals mainly attributable to industrial sources, including petrochemicals, coal combustion, processing of metals and metal compounds, leather tanning, and human activities: anthropogenic sources accounted for about 70% of all pollution [34]. The sources of heavy metals in atmospheric deposition in the Beibu Gulf region reveal that Pb, Se, and S are mainly derived from coal combustion in coal-fired power plants, Mn, Cd and Hg are largely associated with the possession of Mn mines and the Mn industry, while Zn and Cu in atmospheric deposition are mainly derived from suspended soil particles [58]. Therefore, heavy metal pollution in surface sediments was primarily from manganese ore and zinc ore mining in the Qinjiang River because manganese ore is more common than zinc ore, and the manganese pollution level is higher than that of zinc [59]. Manganese carbonate and its weathered secondary manganese oxide are typically associated with a significant amount of residual Cu and Cd, and their concentrations are usually influenced by pH; that is, high pH is suitable for the deposition and enrichment of these heavy metals, but low pH may lead to acidic dissolved states being formed and migration to the estuarine shelf. Lead–zinc ore and its weathering products generate organic compounds with residual Fe, Co, Cr, and Ni, and their content is related to salinity. A possible reason is that the weathering process of lead–zinc ore causes the conversion of low sulfur into sulfate radicals and the release of associated metal elements.
4. Conclusions
The total concentrations of heavy metals were discovered in the decreasing order of Fe > Mn > Zn > Cu > Cr > Pb > Ni > Co > Cd, and they are all significantly lower than the background values, except Mn, Zn, Cr, Cu, and Pb. Cu, Mn, and Zn cause pollution and have certain ecological risks in the Qinjiang River. However, Cu poses less ecological risk than Mn and Zn. Fe, Co, Ni, Cd, Cr, and Pb are not harmful to the environment because of their low content in sediments and natural source heavy metals, but attention must be paid toward their prevention and control. The heavy metal pollution in surface sediments is primarily due to manganese and zinc ore mining in the Qinjiang River. Manganese carbonate and its weathered secondary manganese oxide are typically associated with a significant amount of residual Cu and Cd, and a higher pH is suitable for the deposition and enrichment of these heavy metals. Lead–zinc ore and its weathering products generate organic compounds with residual Fe, Co, Cr, and Ni, and their content is related to salinity.
Author Contributions
Conceptualization: B.C. Data curation: J.D. Investigation: H.S. and S.Z. Methodology: T.W. Writing—original draft: S.Z. Writing—review and editing: F.W. All authors have read and agreed to the published version of the manuscript.
Funding
This study was financially supported by the Guangxi Natural Science Foundation, China (2020GXNSFBA297128); the Special Talent Project of Guangxi Science and Technology Base, China (Guike AD20238041); the National Natural Science Foundation of China (grant no. 41872145), the High level innovation teams and excellent scholars program in Guangxi Universities (Guijiao Ren (2016)42); the Guangxi Natural Science Foundation, China (2019GXNSFAA245016); and the Innovative Training Program for Guangxi Province College Students (202011607292 and 202111607020).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data are contained within the article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- El Zokm, G.M.; Okbah, M.A.; Younis, A.M. Assessment of heavy metals pollution using AVS-SEM and fractionation techniques in Edku Lagoon sediments, Mediterranean Sea, Egypt. J. Environ. Sci. Health, Part A Toxic/Hazard. Subst. Environ. Eng. 2015, 50, 571–584. [Google Scholar]
- Okbah, M.A.; Shata, M.A.; Shridah, M.A. Geochemical forms of trace metals in mangrove sediments-Red Sea (Egypt). Chem. Ecol. 2005, 21, 23–36. [Google Scholar] [CrossRef]
- Wojtkowska, M.; Bogacki, J. Assessment of Trace Metals Contamination, Species Distribution and Mobility in River Sediments Using EDTA Extraction. Int. J. Environ. Res. Public Health 2022, 19, 6978. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.S.; Saha, N.; Molla, A.H. Potential ecological risk assessment of heavy metal contamination in sediment and water body around Dhaka export processing zone, Bangladesh. Environ. Earth Sci. 2014, 71, 2293–2308. [Google Scholar] [CrossRef]
- Zhang, M.Y.; Cui, L.J.; Sheng, L.X.; Wang, Y.F. Distribution and enrichment of heavy metals among sediments, water body and plants in Hengshuihu Wetland of Northern China. Ecol. Eng. 2009, 35, 563–569. [Google Scholar] [CrossRef]
- He, N.; Liu, L.; Wei, R.; Sun, K. Heavy Metal Pollution and Potential Ecological Risk Assessment in a Typical Mariculture Area in Western Guangdong. Int. J. Environ. Res. Public Health 2021, 18, 11245. [Google Scholar] [CrossRef]
- Abrahim, G.M.S.; Parker, R.J. Assessment of heavy metal enrichment factors and the degree of contamination in marine sediments from Tamaki Estuary, Auckland, New Zealand. Environ. Monit. Assess. 2008, 136, 227–238. [Google Scholar] [CrossRef]
- Gao, X.L.; Chen, C.T.A. Heavy metal pollution status in surface sediments of the coastal Bohai Bay. Water Res. 2012, 46, 1901–1911. [Google Scholar] [CrossRef]
- Islam, M.S.; Ahmed, M.K.; Raknuzzaman, M.; Habibullah-Al-Mamun, M.; Islam, M.K. Heavy metal pollution in surface water and sediment: A preliminary assessment of an urban river in a developing country. Ecol. Indic. 2015, 48, 282–291. [Google Scholar] [CrossRef]
- Ciszewski, D.; Grygar, T.M. A Review of Flood-Related Storage and Remobilization of Heavy Metal Pollutants in River Systems. Water Air Soil Pollut. 2016, 227, 239. [Google Scholar] [CrossRef] [Green Version]
- Shotbolt, L.A.; Thomas, A.D.; Hutchinson, S.M. The use of reservoir sediments as environmental archives of catchment inputs and atmospheric pollution. Prog. Phys. Geog. 2005, 29, 337–361. [Google Scholar] [CrossRef]
- Chapman, P.M.; Wang, F.; Caeiro, S.S. Assessing and managing sediment contamination in transitional waters. Environ. Int. 2013, 55, 71–91. [Google Scholar] [CrossRef]
- Gu, Y.-G.; Lin, Q.; Jiang, S.-J.; Wang, Z.-H. Metal pollution status in Zhelin Bay surface sediments inferred from a sequential extraction technique, South China Sea. Mar. Pollut. Bull. 2014, 81, 256–261. [Google Scholar] [CrossRef]
- Nielsen, L.P.; Risgaard-Petersen, N.; Fossing, H.; Christensen, P.B.; Sayama, M. Electric currents couple spatially separated biogeochemical processes in marine sediment. Nature 2010, 463, 1071–1074. [Google Scholar] [CrossRef]
- Luo, P.P.; Xu, C.Y.; Kang, S.X.; Huo, A.D.; Lyu, J.; Zhou, M.M.; Nover, D. Heavy metals in water and surface sediments of the Fenghe River Basin, China: Assessment and source analysis. Water Sci. Technol. 2021, 84, 3072–3090. [Google Scholar] [CrossRef]
- Zhou, Q.Q.; Yang, N.; Li, Y.Z.; Ren, B.; Ding, X.H.; Bian, H.L.; Yao, X. Total concentrations and sources of heavy metal pollution in global river and lake water bodies from 1972 to 2017. Glob. Ecol. Conserv. 2020, 22, e00925. [Google Scholar] [CrossRef]
- Morillo, J.; Usero, J.; Gracia, I. Heavy metal fractionation in sediments from the Tinto River (Spain). Int. J. Environ. Anal. Chem. 2002, 82, 245–257. [Google Scholar] [CrossRef]
- Zhang, W.F.; Liu, X.P.; Cheng, H.F.; Zeng, E.Y.; Hu, Y.A. Heavy metal pollution in sediments of a typical mariculture zone in South China. Mar. Pollut. Bull. 2012, 64, 712–720. [Google Scholar] [CrossRef]
- Krupadam, R.J.; Smita, P.; Wate, S.R. Geochemical fractionation of heavy metals in sediments of the Tapi estuary. Geochem. J. 2006, 40, 513–522. [Google Scholar] [CrossRef] [Green Version]
- Marcovecchio, J.; Ferrer, L. Distribution and geochemical partitioning of heavy metals in sediments of the Bahia Blanca Estuary, Argentina. J. Coastal Res. 2005, 21, 826–834. [Google Scholar] [CrossRef]
- Jingwen, M.; Yanbo, C.; Maohong, C.; Pirajno, F. Major types and time–space distribution of Mesozoic ore deposits in South China and their geodynamic settings. Min. Dep. 2013, 48, 267–294. [Google Scholar] [CrossRef]
- Hu, R.-Z.; Chen, W.T.; Xu, D.-R.; Zhou, M.-F. Reviews and new metallogenic models of mineral deposits in South China: An introduction. J. Asian Earth Sci. 2017, 137, 1–8. [Google Scholar] [CrossRef]
- Xia, P.; Meng, X.; Yin, P.; Cao, Z.; Wang, X. Eighty-year sedimentary record of heavy metal inputs in the intertidal sediments from the Nanliu River estuary, Beibu Gulf of South China Sea. Environ. Pollut. 2011, 159, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Li, Y.; Wang, Y.; Zhang, Y.; Zhang, D.; Zhang, R.; Li, J.; Zhang, G. Spatial distribution and ecological risk of polychlorinated biphenyls in sediments from Qinzhou Bay, Beibu Gulf of South China. Mar. Pollut. Bull. 2014, 80, 338–343. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Q.; Zhang, R.; Wang, Y.; Pan, X.; Tang, J.; Zhang, G. Occurrence and distribution of antibiotics in the Beibu Gulf, China: Impacts of river discharge and aquaculture activities. Mar. Environ. Res. 2012, 78, 26–33. [Google Scholar] [CrossRef]
- Pacifico, R.; Adamo, P.; Cremisini, C.; Spaziani, F.; Ferrara, L. A geochemical analytical approach for the evaluation of heavy metal distribution in lagoon sediments. J. Soil Sci. 2007, 7, 313–325. [Google Scholar] [CrossRef]
- Lu, R. Analyse Methods of Soil and Agrochemistry; Soil Science Society of China, Chinese Agricultural Science and Technology Press: Beijing, China, 1999. (In Chinese)
- Ball, D. Carbon analysis in a mud sample based on loss on ignition. J. Soil Sci. 1964, 15, 84. [Google Scholar] [CrossRef]
- Peng, Y.; Wu, J. A method for the accurate determination of 14 metal elements in soils/sediments by ICP-MS. Environ. Chem. 2017, 36, 175–182. [Google Scholar]
- Rauret, G.; Lopez-Sanchez, J.; Sahuquillo, A.; Rubio, R.; Davidson, C.; Ure, A.; Quevauviller, P. Improvement of the BCR three step sequential extraction procedure prior to the certification of new sediment and soil reference materials. J. Environ. Monit. 1999, 1, 57–61. [Google Scholar] [CrossRef]
- Basir Kimijima, S.; Sakakibara, M.; Pateda, S.M.; Sera, K. Contamination Level in Geo-Accumulation Index of River Sediments at Artisanal and Small-Scale Gold Mining Area in Gorontalo Province, Indonesia. Int J Environ Res Public Health 2022, 19, 6094. [Google Scholar] [CrossRef]
- Müller, G. Die Schwermetallbelstung der sedimente des Neckars und seiner Nebenflusse: Eine Bestandsaufnahme. Chem. Ztg. 1981, 105, 157–164. [Google Scholar]
- Jiang, R.; Huang, S.; Wang, W.; Liu, Y.; Pan, Z.; Sun, X.; Lin, C. Heavy metal pollution and ecological risk assessment in the Maowei sea mangrove, China. Mar. Pollut. Bull. 2020, 161, 111816. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Lan, W.; Feng, Q.; Zhu, X.; Li, T.; Zhang, R.; Song, H.; Zhu, Y.; Zhao, B. Pollution and ecological risk assessment, and source identification of heavy metals in sediment from the Beibu Gulf, South China Sea. Mar. Pollut. Bull. 2021, 168, 112403. [Google Scholar] [CrossRef] [PubMed]
- Hakanson, L. An ecological risk index for aquatic pollution control. A sedimentological approach. Water Res. 1980, 14, 975–1001. [Google Scholar] [CrossRef]
- Agyeman, P.C.; John, K.; Kebonye, N.M.; Ofori, S.; Borůvka, L.; Vašát, R.; Kočárek, M. Ecological risk source distribution, uncertainty analysis, and application of geographically weighted regression cokriging for prediction of potentially toxic elements in agricultural soils. Process Saf. Environ. Prot. 2022, 164, 729–746. [Google Scholar] [CrossRef]
- Ferreira, S.L.C.; da Silva, J.B.; dos Santos, I.F.; de Oliveira, O.M.C.; Cerda, V.; Queiroz, A.F.S. Use of pollution indices and ecological risk in the assessment of contamination from chemical elements in soils and sediments–Practical aspects. Trends Environ. Anal. Chem. 2022, 35, e00169. [Google Scholar] [CrossRef]
- Barcauskaite, K.; Zydelis, R.; Mazeika, R. Screening of chemical composition and risk index of different origin composts produced in Lithuania. Environ. Sci. Pollut. Res. Int. 2020, 27, 24480–24494. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.-G.; Xu, Y.-N.; Zhang, J.-H.; Hu, S.-H. Evaluation of ecological risk and primary empirical research on heavy metals in polluted soil over Xiaoqinling gold mining region, Shaanxi, China. Trans. Nonferrous Met. Soc. China 2010, 20, 688–694. [Google Scholar] [CrossRef]
- Zhu, H.-N.; Yuan, X.-Z.; Zeng, G.-M.; Jiang, M.; Liang, J.; Zhang, C.; Yin, J.; Huang, H.-J.; Liu, Z.-F.; Jiang, H.-W. Ecological risk assessment of heavy metals in sediments of Xiawan Port based on modified potential ecological risk index. Trans. Nonferrous Met. Soc. China 2012, 22, 1470–1477. [Google Scholar] [CrossRef]
- Long, E.R.; MacDonald, D.D.; Smith, S.L.; Calder, F.D. Incidence of adverse biological effects within ranges of chemical concentrations in marine and estuarine sediments. Environ. Manag. 1995, 19, 81–97. [Google Scholar] [CrossRef]
- Macdonald, D.D.; Carr, R.S.; Calder, F.D.; Long, E.R.; Ingersoll, C.G. Development and evaluation of sediment quality guidelines for Florida coastal waters. Ecotoxicology 1996, 5, 253–278. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, S.K.; Mondal, P.; Biswas, J.K.; Kwon, E.E.; Ok, Y.S.; Rinklebe, J. Trace elements in surface sediments of the Hooghly (Ganges) estuary: Distribution and contamination risk assessment. Environ. Geochem. Health 2017, 39, 1245–1258. [Google Scholar] [CrossRef] [PubMed]
- Long, E.R.; Field, L.J.; MacDonald, D.D. Predicting toxicity in marine sediments with numerical sediment quality guidelines. Environ. Toxicol. Chem. 1998, 17, 714–727. [Google Scholar] [CrossRef]
- MacDonald, D.D.; Ingersoll, C.G.; Berger, T. Development and evaluation of consensus-based sediment quality guidelines for freshwater ecosystems. Arch. Environ. Contam. Toxicol. 2000, 39, 20–31. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.-G. Heavy metal fractionation and ecological risk implications in the intertidal surface sediments of Zhelin Bay, South China. Mar. Pollut. Bull. 2018, 129, 905–912. [Google Scholar] [CrossRef] [PubMed]
- Perin, G.; Craboledda, L.; Lucchese, L.; Cirillo, R.; Dotta, L.; Orio, A. Heavy metal speciation in the sediments of Northern Adriatic Sea. A new approach for environmental toxicity determination. Heavy Met. Environ. 1985, 2, 454–456. [Google Scholar]
- Feng, Y.; Chenglin, L.; Bowen, W. Evaluation of heavy metal pollution in the sediment of Poyang Lake based on stochastic geo-accumulation model (SGM). Sci. Total Environ. 2019, 659, 1–6. [Google Scholar] [CrossRef]
- Teasdale, P.; Apte, S.; Ford, P.; Batley, G.; Koehnken, L. Geochemical cycling and speciation of copper in waters and sediments of Macquarie Harbour, Western Tasmania. Estuar. Coast. Shelf Sci. 2003, 57, 475–487. [Google Scholar] [CrossRef]
- Xu, Y.; Wu, Y.; Han, J.; Li, P. The current status of heavy metal in lake sediments from China: Pollution and ecological risk assessment. Ecol. Evol. 2017, 7, 5454–5466. [Google Scholar] [CrossRef]
- Singh, K.P.; Mohan, D.; Singh, V.K.; Malik, A. Studies on distribution and fractionation of heavy metals in Gomti river sediments—a tributary of the Ganges. India J. Hydrol. 2005, 312, 14–27. [Google Scholar] [CrossRef]
- Schiff, K.; Weisberg, S.B. Iron as a reference element for determining trace metal enrichment in Southern California coastal shelf sediments. Mar. Environ. Res. 1999, 48, 161–176. [Google Scholar] [CrossRef]
- Fan, D.; Yang, P. Introduction to and classification of manganese deposits of China. Ore Geol. Rev. 1999, 15, 1–13. [Google Scholar] [CrossRef]
- Lang, Y.; Li, J.; Deng, X.; Zhang, W.; Yan, D.; Chen, L. Mineralogy and geochemistry of supergene manganese ore deposits in Qinzhou-Fangcheng area, southern Guangxi, with implications for ore genesis. Min. Dep. 2007, 26, 527. [Google Scholar]
- Carter, A.; Roques, D.; Bristow, C.; Kinny, P. Understanding Mesozoic accretion in Southeast Asia: Significance of Triassic thermotectonism (Indosinian orogeny) in Vietnam. Geology 2001, 29, 211–214. [Google Scholar] [CrossRef]
- Survey, C.G. 1:200,000 Geological Map of China (Guangxi); National Geological Archive: Beijing, China, 2013.
- Yang, Q.; Yang, Z.; Filippelli, G.M.; Ji, J.; Ji, W.; Liu, X.; Wang, L.; Yu, T.; Wu, T.; Zhuo, X.; et al. Distribution and secondary enrichment of heavy metal elements in karstic soils with high geochemical background in Guangxi, China. Chem. Geol. 2021, 567, 120081. [Google Scholar] [CrossRef]
- Zhong, C.; Yang, Z.; Jiang, W.; Hu., B.; Hou, Q.; Yu, T.; Li, J. Ecological geochemical assessment and source identification of trace elements in atmospheric deposition of an emerging industrial area: Beibu Gulf economic zone. Sci. Total Environ. 2016, 573, 1519–1526. [Google Scholar] [CrossRef]
- Xia, P.; Meng, X.; Feng, A.; Yin, P.; Wang, X.; Zhang, J. Pb-210 chronology and trace metal geochemistry in the intertidal sediment of Qinjiang River estuary. J. Ocean U China 2012, 11, 165–173. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).