A Pilot Study on the Association of Lead, 8-Hydroxyguanine, and Malondialdehyde Levels in Opium Addicts’ Blood Serum with Illicit Drug Use and Non-Addict Persons
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Sample Preparation and Analysis
2.3. Quality Assurance and Quality Control (QA/QC)
2.4. Statistical Analysis
3. Results and Discussion
4. Study Limitations and Strengths
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Heilig, M.; MacKillop, J.; Martinez, D.; Rehm, J.; Leggio, L.; Vanderschuren, L.J. Addiction as a brain disease revised: Why it still matters, and the need for consilience. Neuropsychopharmacology 2021, 46, 1715–1723. [Google Scholar] [CrossRef]
- Koob, G.F. Drug addiction: Hyperkatifeia/negative reinforcement as a framework for medications development. Pharmacol. Rev. 2021, 73, 163–201. [Google Scholar] [CrossRef]
- Lee, M.Y.; Lee, B.H.; Kim, H.Y.; Yang, C.H. Bidirectional role of acupuncture in the treatment of drug addiction. Neurosci. Biobehav. Rev. 2021, 126, 382–397. [Google Scholar] [CrossRef]
- Takahashi, T.T.; Ornello, R.; Quatrosi, G.; Torrente, A.; Albanese, M.; Vigneri, S.; Guglielmetti, M.; Maria De Marco, C.; Dutordoir, C.; Colangeli, E.; et al. Medication overuse and drug addiction: A narrative review from addiction perspective. J. Headache Pain 2021, 22, 1–11. [Google Scholar]
- Loganathan, K.; Ho, E.T.W. Value, drug addiction and the brain. Addict. Behav. 2021, 116, 106816. [Google Scholar] [CrossRef]
- Calpe-López, C.; Martínez-Caballero, M.A.; García-Pardo, M.P.; Aguilar, M.A. Resilience to the effects of social stress on vulnerability to developing drug addiction. World J. Psychiatry 2022, 12, 24. [Google Scholar] [CrossRef]
- Antonini, G.; Palmieri, G.; Millefiorini, E.; Spagnoli, L.; Millefiorini, M. Lead poisoning during heroin addiction. Ital. J. Neurol. Sci. 1989, 10, 105–108. [Google Scholar] [CrossRef] [PubMed]
- Soltaninejad, K.; Shadnia, S. Lead poisoning in opium abuser in Iran: A systematic review. Int. J. Prev. Med. 2018, 9, 3. [Google Scholar]
- Soltaninejad, K.; Flückiger, A.; Shadnia, S. Opium addiction and lead poisoning. J. Subst. Use 2011, 16, 208–212. [Google Scholar] [CrossRef]
- Nasab, H.; Rajabi, S.; Eghbalian, M.; Malakootian, M.; Hashemi, M.; Mahmoudi-Moghaddam, H. Association of As, Pb, Cr, and Zn urinary heavy metals levels with predictive indicators of cardiovascular disease and obesity in children and adolescents. Chemosphere 2022, 294, 133664. [Google Scholar] [CrossRef]
- Kroese, L.J.; Scheffer, P.G. 8-hydroxy-2′-deoxyguanosine and cardiovascular disease: A systematic review. Curr. Atheroscler. Rep. 2014, 16, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, A.J.; Clement, H.W.; Gebhardt, S.; Hemmeter, U.M.; Schulz, E.; Krieg, J.C.; Kircher, T.; Heiser, P. Impact of psychostimulants and atomoxetine on the expression of 8-hydroxyguanine glycosylase 1 in human cells. J. Neural Transm. 2010, 117, 793–797. [Google Scholar] [CrossRef] [PubMed]
- Rybus-Kalinowska, B.; Zwirska-Korczala, K.; Kalinowski, M.; Kukla, M.; Birkner, E.; Jochem, J. Activity of antioxidative enzymes and concentration of malondialdehyde as oxidative status markers in women with newly diagnosed Graves-Basedow disease and after thiamazole therapy leading to euthyroidism. Polskie Archiwum Medycyny Wewnetrznej 2008, 118, 420–425. [Google Scholar] [CrossRef][Green Version]
- Rybus-Kalinowska, B.; Żwirska-Korczala, K.; Kalinowski, M.; Kukla, M.; Birkner, E.; Jochem, J. Activity of antioxidative enzymes and concentration of malondialdehyde as oxidative status markers in women with non-autoimmunological subclinical hyperthyroidism. Endokrynologia Polska 2009, 60, 199–202. [Google Scholar]
- Luan, X.; Chen, H.; Qiu, H.; Shen, H.; Zhao, K.; Ren, W.; Gu, Y.; Su, H.; Zhang, J.; Lv, D.; et al. Association between serum malondialdehyde levels and depression during early methamphetamine withdrawal. Neurosci. Lett. 2018, 687, 22–25. [Google Scholar] [CrossRef]
- Polachova, A.; Gramblicka, T.; Bechynska, K.; Parizek, O.; Parizkova, D.; Dvorakova, D.; Honkova, K.; Rossnerova, A.; Rossner, P.; Sram, R.J.; et al. Biomonitoring of 89 POPs in blood serum samples of Czech city policemen. Environ. Pollut. 2021, 291, 118140. [Google Scholar] [CrossRef]
- Nasab, H.; Mirzaee, M.; Hashemi, M.; Rajabi, S. Measurement of Urinary Triclocarban and 2, 4-Dichlorophenol Concentration and Their Relationship with Obesity and Predictors of Cardiovascular Diseases among Children and Adolescents in Kerman, Iran. J. Environ. Public Health 2022, 2022, 2939022. [Google Scholar] [CrossRef]
- Nasab, H.; Rajabi, S.; Mirzaee, M.; Hashemi, M. Association of urinary triclosan, methyl triclosan, triclocarban, and 2,4-dichlorophenol levels with anthropometric and demographic parameters in children and adolescents in 2020 (case study: Kerman, Iran). Environ. Sci. Pollut. Res. 2022, 29, 30754–30763. [Google Scholar] [CrossRef]
- Nasab, H.; Mirzaee, M.; Ebrahimpour, K.; Hashemi, M. Association of urinary triclosan and methyl-triclosan levels with predictive indicators of cardiovascular disease and obesity in children and adolescents in 2020 (case study: Kerman, Iran). Environ. Health Eng. Manag. J. 2021, 8, 187–195. [Google Scholar] [CrossRef]
- Asiamah, N.; Mensah, H.K.; Oteng-Abayie, E.F. General, target, and accessible population: Demystifying the concepts for effective sampling. Qual. Rep. 2017, 22, 1607. [Google Scholar] [CrossRef]
- Suljević, D.; Handžić, N.; Fočak, M.; Lasić, I.; Sipović, F.; Sulejmanović, J.; Begić, S.; Alijagic, A. Lead exposure influences serum biomarkers, hepatocyte survival, bone marrow hematopoiesis, and the reproductive cycle in Japanese quails. Biol. Trace Elem. Res. 2021, 199, 1574–1583. [Google Scholar] [CrossRef]
- Malavika, L.; Mitra, P.; Goyal, T.; Sharma, S.; Purohit, P.; Sharma, P. Association of blood lead level with neurobehavior and neurotransmitter expressions in Indian children. Toxicol. Rep. 2021, 8, 971–976. [Google Scholar]
- Park, E.; Kim, J.; Kim, B.; Park, E.Y. Association between environmental exposure to cadmium and risk of suspected non-alcoholic fatty liver disease. Chemosphere 2021, 266, 128947. [Google Scholar] [CrossRef] [PubMed]
- Masoudi, M.; Zali, M.R.; Ehsani, M.; Mohammadalizadeh, A.H.; Aiassofi, K.; Aghazadeh, R.; Shavakhi, A.; Soumi, M.; Antikchi, M.H.; Yazdani, S. Abdominal pain due to lead-contaminated opium: A new source of inorganic lead poisoning in Iran. Arch. Iran. Med. 2006, 9, 72–75. [Google Scholar]
- Busse, F.P.; Fiedler, G.M.; Leichtle, A.; Hentschel, H.; Stumvoll, M. Lead poisoning due to adulterated marijuana in Leipzig. Deutsches Ärzteblatt Int. 2008, 105, 757. [Google Scholar] [CrossRef]
- Vossoughinia, H.; Pourakbar, A.; Esfandiari, S.; Sharifianrazavi, M. Severe abdominal pain caused by lead toxicity without response to oral chelators: A case report. Middle East, J. Dig. Dis. 2016, 8, 67. [Google Scholar] [CrossRef][Green Version]
- Beigmohammadi, M.T.; Aghdashi, M.; Najafi, A.; Mojtahedzadeh, M.; Karvandian, K. Quadriplegia due to lead-contaminated opium. MEJ ANESTH 2008, 19, 1411–1416. [Google Scholar]
- Tabrizi, R.; Sarihi, S.; Moazzen, F.; Hosseini-Bensenjan, M.; Malekpour, F.; Asadikaram, G.; Momeni-Moghaddam, M.A.; Akbari, H. A systematic review and meta-analysis on blood lead level in opium addicts: An emerging health threat. Biol. Trace Elem. Res. 2021, 199, 3634–3641. [Google Scholar] [CrossRef]
- Khatibi-Moghadam, H.; Khadem-Rezaiyan, M.; Afshari, R. Comparison of serum and urine lead levels in opium addicts with healthy control group. Hum. Exp. Toxicol. 2016, 35, 861–865. [Google Scholar] [CrossRef]
- Ahmadinejad, M.; Ahmadipour, M.; Divsalar, K. Blood lead level in opiate addicts hospitalized in the intensive care unit of a trauma referral center in Kerman, Iran. Addict. Health 2019, 11, 11. [Google Scholar]
- Shafikhani, A.A.; Kazemifar, A.M. Comparison of blood lead levels between oral and inhalation opium addicts and its relationship with hematological parameters. Indian J. Forensic Med. Toxicol. 2019, 13, 326–331. [Google Scholar] [CrossRef]
- Shojaeepour, S.; Fazeli, M.; Mandegary, A.; Sayed-Mirzaei, S.M.; Ahmadi, N.; Saeedi, A.; Oghabian, Z. Evaluation of oxidative stress in combination therapy with d-penicillamine and n-acetylcysteine (NAC) in lead poisoning in opium addicts. Asia Pac. J. Med. Toxicol. 2017, 6, 123–128. [Google Scholar]
- Shahramian, I.; Noori, N.M.; Afshari, M.; Delaramnasab, M.; Bazi, A.; Abdollahi, M. The clinical relevance of elevated blood lead levels in opium addicts with severe abdominal pain. Arch. Psychiatry Res. Int. J. Psychiatry Relat. Sci. 2019, 55, 165–172. [Google Scholar] [CrossRef]
- Farzaneh, E.; Habibzadeh, A.; Mehrpour, O. Lead toxicity among oral opium addicts with abdominal pain: A case series of 17 cases. Prof. RK Sharma 2017, 11, 222. [Google Scholar] [CrossRef]
- Charames, G.S.; Bapat, B. Genomic instability and cancer. Curr. Mol. Med. 2003, 3, 589–596. [Google Scholar] [CrossRef]
- Lowe, F.J.; Luettich, K.; Gregg, E.O. Lung cancer biomarkers for the assessment of modified risk tobacco products: An oxidative stress perspective. Biomarkers 2013, 18, 183–195. [Google Scholar] [CrossRef]
- Valavanidis, A.; Vlachogianni, T.; Fiotakis, K. Tobacco smoke: Involvement of reactive oxygen species and stable free radicals in mechanisms of oxidative damage, carcinogenesis and synergistic effects with other respirable particles. Int. J. Environ. Res. Public Health 2009, 6, 445–462. [Google Scholar] [CrossRef] [PubMed]
- Valko, M.; Izakovic, M.; Mazur, M.; Rhodes, C.J.; Telser, J. Role of oxygen radicals in DNA damage and cancer incidence. Mol. Cell. Biochem. 2004, 266, 37–56. [Google Scholar] [CrossRef]
- Voulgaridou, G.-P.; Anestopoulos, I.; Franco, R.; Panayiotidis, M.I.; Pappa, A. DNA damage induced by endogenous aldehydes: Current state of knowledge. Mutat. Res. Fundam. Mol. Mech. Mutagenesis 2011, 711, 13–27. [Google Scholar] [CrossRef]
- Marnett, L.J. Oxy radicals, lipid peroxidation and DNA damage. Toxicology 2002, 181, 219–222. [Google Scholar] [CrossRef]
- Tudek, B.; Swoboda, M.; Kowalczyk, P.; Oliński, R. Modulation of oxidative DNA damage repair by the diet, inflammation and neoplastic transformation. J. Physiol. Pharmacol. Off. J. Pol. Physiol. Soc. 2006, 57, 33–49. [Google Scholar]
- Knaapen, A.M.; Güngör, N.; Schins, R.P.; Borm, P.J.; Van Schooten, F.J. Neutrophils and respiratory tract DNA damage and mutagenesis: A review. Mutagenesis 2006, 21, 225–236. [Google Scholar] [CrossRef] [PubMed]
- Faux, S.P.; Tai, T.; Thorne, D.; Xu, Y.; Breheny, D.; Gaca, M. The role of oxidative stress in the biological responses of lung epithelial cells to cigarette smoke. Biomarkers 2009, 14, 90–96. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.J.; Perfetti, T.A.; King, J.A. Perspectives on pulmonary inflammation and lung cancer risk in cigarette smokers. Inhal. Toxicol. 2006, 18, 667–677. [Google Scholar] [CrossRef] [PubMed]
- Lodovici, M.; Caldini, S.; Luceri, C.; Bambi, F.; Boddi, V.; Dolara, P. Active and passive smoking and lifestyle determinants of 8-oxo-7, 8-dihydro-2′-deoxyguanosine levels in human leukocyte DNA. Cancer Epidemiol. Prev. Biomark. 2005, 14, 2975–2977. [Google Scholar] [CrossRef]
- Yao, Q.H.; Mei, S.R.; Weng, Q.F.; Zhang, P.D.; Yang, Q.; Wu, C.Y.; Xu, G.W. Determination of urinary oxidative DNA damage marker 8-hydroxy-2′-deoxyguanosine and the association with cigarette smoking. Talanta 2004, 63, 617–623. [Google Scholar] [CrossRef]
- Nia, A.B.; Van Schooten, F.J.; Schilderman, P.A.E.L.; De Kok, T.M.C.M.; Haenen, G.R.; Van Herwijnen, M.H.M.; Van Agen, E.; Pachen, D.M.F.A.; Kleinjans, J.C.S. A multi-biomarker approach to study the effects of smoking on oxidative DNA damage and repair and antioxidative defense mechanisms. Carcinogenesis 2001, 22, 395–401. [Google Scholar] [CrossRef][Green Version]
- van Zeeland, A.A.; de Groot, A.J.; Hall, J.; Donato, F. 8-Hydroxydeoxyguanosine in DNA from leukocytes of healthy adults: Relationship with cigarette smoking, environmental tobacco smoke, alcohol and coffee consumption. Mutat. Res./Genet. Toxicol. Environ. Mutagenesis 1999, 439, 249–257. [Google Scholar] [CrossRef]
- Shadnia, S.; Gorgzadeh, N.; Soltaninejad, K.; Abdollahi, M.; Motevalian, S. Status of Total Antioxidant Capacity and Malondialdehyde Level in Methamphetamine Addicts: A Cross Sectional Study. Int. J. Med. Toxicol. Forensic Med. 2017, 7, 19–25. [Google Scholar]
- Karajibani, M.; Montazerifar, F.; Feizabad, A.K. Study of oxidants and antioxidants in addicts. Int. J. High Risk Behav. Addict. 2017, 6, e35057. [Google Scholar] [CrossRef]
- Macotpet, A.; Suksawat, F.; Sukon, P.; Pimpakdee, K.; Pattarapanwichien, E.; Tangrassameeprasert, R.; Boonsiri, P. Oxidative stress in cancer-bearing dogs assessed by measuring serum malondialdehyde. BMC Vet. Res. 2013, 9, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Karatas, F.; Karatepe, M.; Baysar, A. Determination of free malondialdehyde in human serum by high-performance liquid chromatography. Anal. Biochem. 2002, 311, 76–79. [Google Scholar] [CrossRef]
- Rahman, T.; Hosen, I.; Islam, M.T.; Shekhar, H.U. Oxidative stress and human health. Adv. Biosci. Biotechnol. 2012, 3, 23. [Google Scholar] [CrossRef]
- Najafi, K.; Ahmadi, S.; Rahpeyma, M.; Khazaie, H.; Vaisi-Raygani, A.; Moini, A.; Kiani, A. Study of serum malondialdehyde level in opioid and methamphetamine dependent patients. Acta Med. Iran. 2017, 55, 616–620. [Google Scholar] [PubMed]
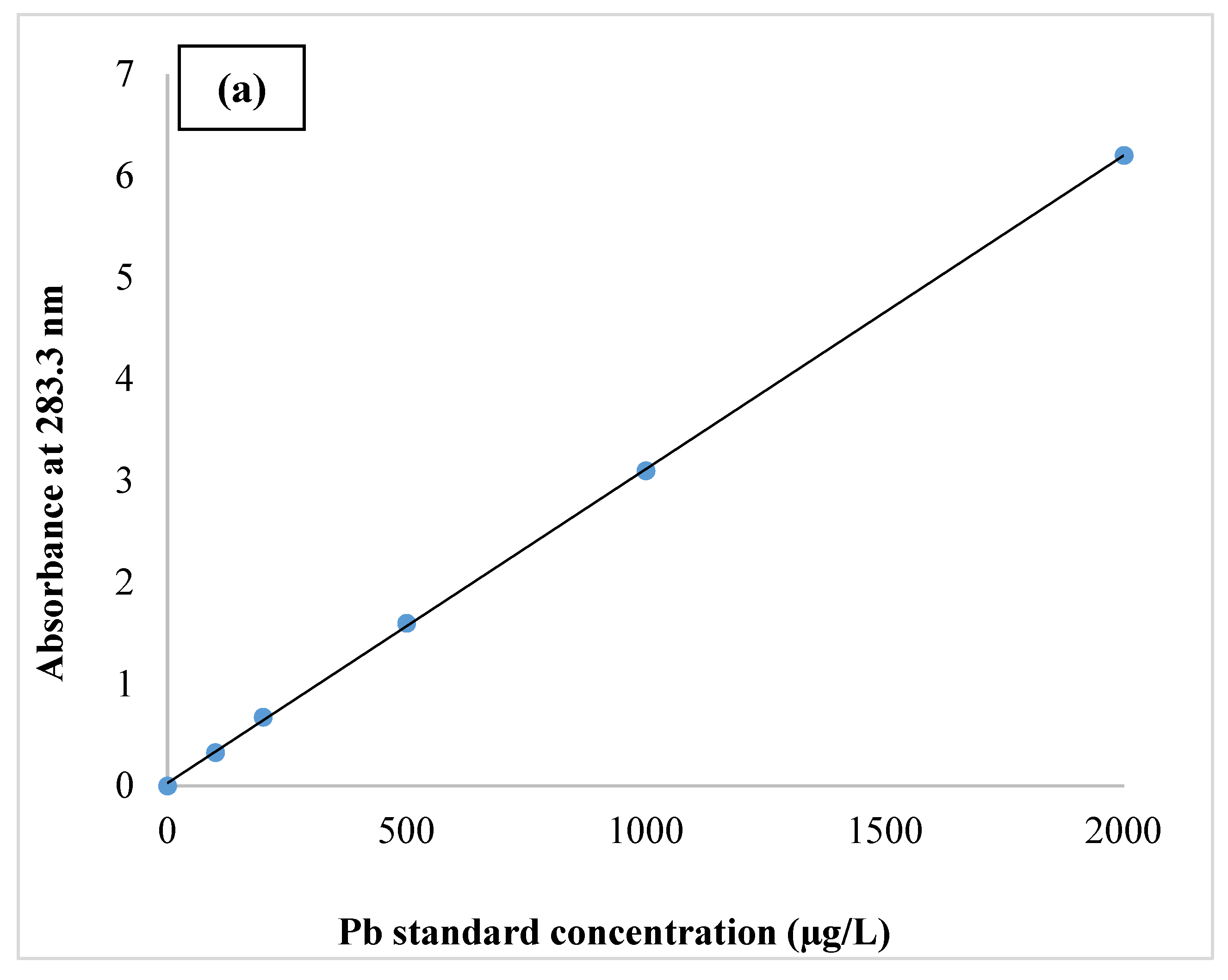
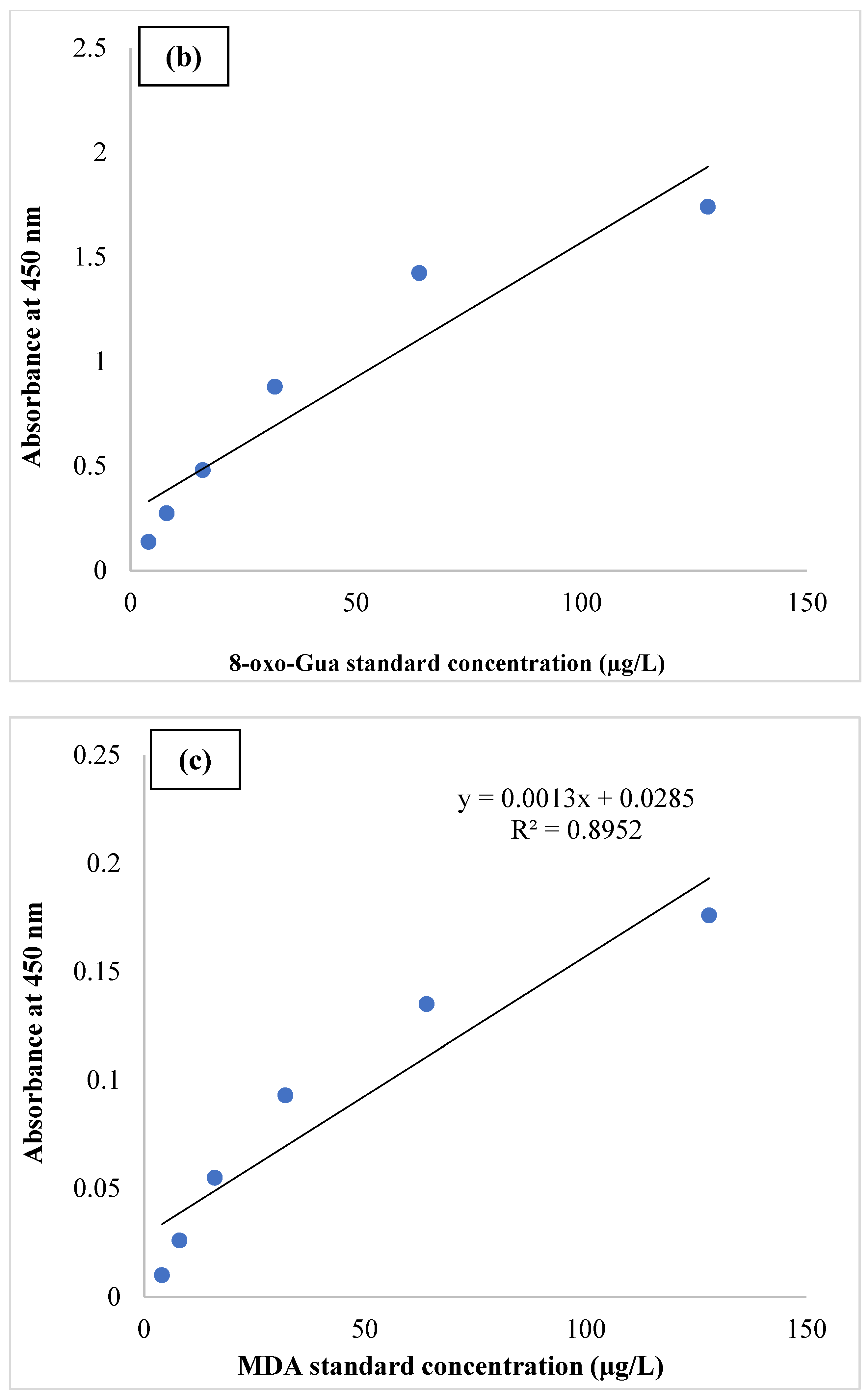
| Variable | Frequencies (%) | Std. Deviation | ||
|---|---|---|---|---|
| Addict | Non-Addict | Addict | Non-Addict | |
| Sex | ||||
| Male | 15 (60%) | 13 (52%) | 0.50 | 0.51 |
| Female | 10 (40%) | 12 (48%) | ||
| Age groups (years old) | ||||
| 20–30 | 7 (28%) | 9 (36%) | 0.73 | 0.81 |
| 30–40 | 12 (48%) | 9 (36%) | ||
| 40–50 | 6 (24%) | 7 (28%) | ||
| Education | ||||
| Illiterate | 1 (4%) | 7 (28%) | 0.59 | 0.90 |
| Non-academic (high school diploma) | 11 (44%) | 3 (4%) | ||
| Academic (e.g., Associate’s degree, BSc., MSc., and Ph.D.) | 13 (52%) | 15 (60%) | ||
| Job | ||||
| Unemployed | 3 (12%) | 8 (32%) | 0.45 | 0.69 |
| Employee | 20 (80%) | 13 (52%) | ||
| Retired | 2 (8%) | 4 (16%) | ||
| History of illicit drug use | ||||
| Never | 0 (0%) | 20 (80%) | 1.11 | 0.52 |
| 3 years | 9 (36%) | 4 (16%) | ||
| 4 years | 7 (28%) | 1 (4%) | ||
| 5 years | 5 (20%) | 0 (0%) | ||
| >5 years | 4 (16%) | 0 (0%) | ||
| Drug type | ||||
| Opium | 11 (44%) | 1 (4%) | 1.64 | 1.57 |
| Heroin | 4 (16%) | 1 (4%) | ||
| Cocaine | 0 (0%) | 0 (0%) | ||
| Marijuana | 6 (24%) | 1 (4%) | ||
| Other | 4 (16%) | 2 (8%) | ||
| Variable | Frequency (%) | Std. Deviation | ||
|---|---|---|---|---|
| Addict | Non-Addict | Addict | Non-Addict | |
| Pb (µg/dL) | ||||
| <25 | 14 (28%) | 9 (18%) | 0.94516 | 1.09240 |
| 25–35 | 7 (14%) | 8 (16%) | ||
| 35–45 | 2 (4%) | 4 (8%) | ||
| >45 | 2 (4%) | 4 (8%) | ||
| 8-oxo-Gua (ng/mL) | 1.43991 | 1.38082 | ||
| 64 | 10 (20%) | 9 (18%) | ||
| 32 | 5 (10%) | 6 (12%) | ||
| 16 | 4 (8%) | 5 (10%) | ||
| 8 | 3 (6%) | 2 (4%) | ||
| 4 | 3 (6%) | 3 (6%) | ||
| MDA (ng/mL) | ||||
| 64 | 8 (16%) | 9 (18%) | ||
| 32 | 7 (14%) | 7 (14%) | 1.25433 | 1.26754 |
| 16 | 4 (8%) | 5 (10%) | ||
| 8 | 5 (10%) | 2 (4%) | ||
| 4 | 1 (2%) | 2 (4%) | ||
| Variable Indicators | Mean Concentration (µg/dL) | Std. Deviation | p-Value * df (f) | |||
|---|---|---|---|---|---|---|
| Addict | Non-Addict | Addict | Non-Addict | |||
| Pb | Male | 22.78 | 10.11 | 13.79 | 4.19 | 0.001 48 (4.87) |
| Female | 21.76 | 8.84 | 12.36 | 3.29 | 0.001 48 (4.66) | |
| 8-oxo-Gua | Male | 22.57 | 22.90 | 11.55 | 11.46 | 0.785 48 (0.88) |
| Female | 23.11 | 23.76 | 14.76 | 14.66 | 0.647 48 (0.94) | |
| MDA | Male | 25.64 | 25.55 | 12.77 | 12.75 | 0.995 48 (0.97) |
| Female | 24.84 | 23.98 | 13.34 | 13.67 | 0.867 48 (0.57) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hesaruiyeh, F.A.; Rajabi, S.; Motamed-Jahromi, M.; Sarhadi, M.; Bell, M.L.; Khaksefidi, R.; Sarhadi, S.; Mohammadi, L.; Dua, K.; Mohammadpour, A.; et al. A Pilot Study on the Association of Lead, 8-Hydroxyguanine, and Malondialdehyde Levels in Opium Addicts’ Blood Serum with Illicit Drug Use and Non-Addict Persons. Int. J. Environ. Res. Public Health 2022, 19, 9110. https://doi.org/10.3390/ijerph19159110
Hesaruiyeh FA, Rajabi S, Motamed-Jahromi M, Sarhadi M, Bell ML, Khaksefidi R, Sarhadi S, Mohammadi L, Dua K, Mohammadpour A, et al. A Pilot Study on the Association of Lead, 8-Hydroxyguanine, and Malondialdehyde Levels in Opium Addicts’ Blood Serum with Illicit Drug Use and Non-Addict Persons. International Journal of Environmental Research and Public Health. 2022; 19(15):9110. https://doi.org/10.3390/ijerph19159110
Chicago/Turabian StyleHesaruiyeh, Farzaneh Allahdinian, Saeed Rajabi, Mohadeseh Motamed-Jahromi, Mohammad Sarhadi, Michelle L. Bell, Razieh Khaksefidi, Somayeh Sarhadi, Leili Mohammadi, Kamal Dua, Amin Mohammadpour, and et al. 2022. "A Pilot Study on the Association of Lead, 8-Hydroxyguanine, and Malondialdehyde Levels in Opium Addicts’ Blood Serum with Illicit Drug Use and Non-Addict Persons" International Journal of Environmental Research and Public Health 19, no. 15: 9110. https://doi.org/10.3390/ijerph19159110
APA StyleHesaruiyeh, F. A., Rajabi, S., Motamed-Jahromi, M., Sarhadi, M., Bell, M. L., Khaksefidi, R., Sarhadi, S., Mohammadi, L., Dua, K., Mohammadpour, A., & Martelletti, P. (2022). A Pilot Study on the Association of Lead, 8-Hydroxyguanine, and Malondialdehyde Levels in Opium Addicts’ Blood Serum with Illicit Drug Use and Non-Addict Persons. International Journal of Environmental Research and Public Health, 19(15), 9110. https://doi.org/10.3390/ijerph19159110








