The Seroprevalence and Hidden Burden of Chikungunya Endemicity and Malaria Mono- and Coinfection in Nigeria
Abstract
1. Background
2. Methods
2.1. Study Design and Site
2.2. Study Population
2.3. Screening of Study Participants
2.4. Total Number of Samples Collected
2.5. Laboratory Analysis
2.6. Statistical Tests
2.7. Ethics Statement
3. Results
3.1. The Demographic Profiles and Seroprevalence of Chikungunya and Malaria
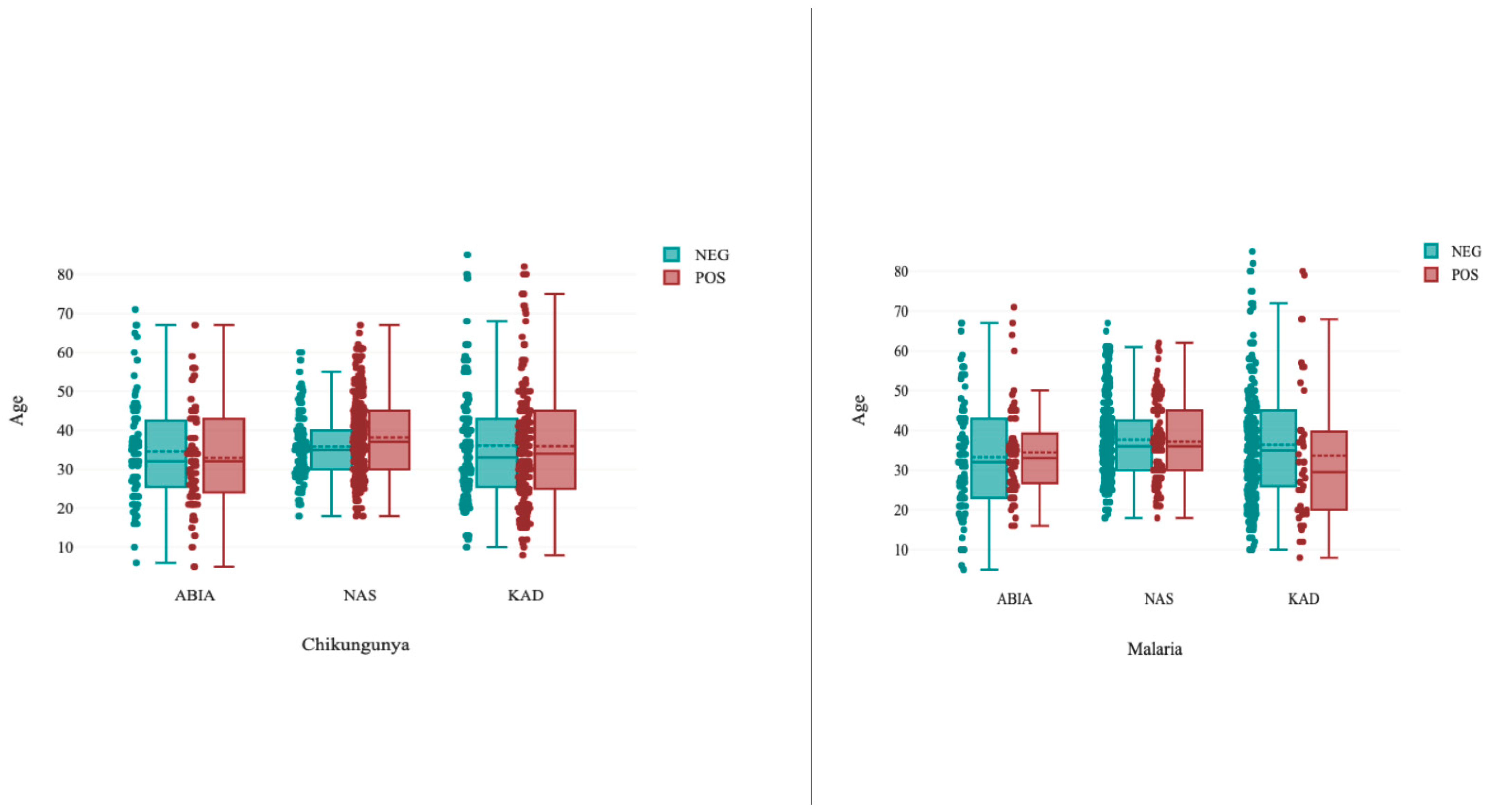
3.2. Age-Specific Seroprevalence of Chikungunya and Malaria in the Study Population
3.3. Sex-Specific Seroprevalence of Chikungunya and Malaria
3.4. Place Specific Seroprevalence of Chikungunya and Malaria
3.5. Pregnant and Nonpregnant Female Seroprevalence of Chikungunya and Malaria in the Study Population
3.6. Seroprevalence of Chikungunya–Malaria Coinfection in the Study Population
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
List of Abbreviations
| AFIs | Acute febrile illnesses |
| CHIKV | chikungunya virus |
| CI | confidence interval |
| pLDH | Parasite lactate dehydrogenase |
| HRP | Histidine-rich protein 2 |
| RDT | Rapid Diagnostic Test |
| VLP | Viral Live Particle |
References
- Abdullahi, I.N.; Akande, A.O.; Muhammed, Y.; Rogo, L.D.; Oderinde, B. Prevalence Pattern of Chikungunya Virus Infection in Nigeria: A Four Decade Systematic Review and Meta-analysis. Pathog. Glob. Health 2020, 114, 120–125. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). Dengue and Severe Dengue. Available online: https://www.who.int/newsroom/factsheets/detail/dengue-and-severe-dengue (accessed on 26 August 2020).
- Pesko, K.; Westbrook, C.J.; Mores, C.N.; Lounibos, L.P.; Reiskind, M.H. Effects of infectious virus dose and blood meal delivery method on susceptibility of Aedes aegypti and Aedes albopic- tus to chikungunya virus. J. Entomol. 2019, 46, 395–399. [Google Scholar]
- Baba, M.; Logue, C.H.; Oderinde, B.; Abdulmaleek, H.; Williams, J.; Lewis, J.; Laws, T.R.; Hewson, R.; Marcello, A.; Agaro, P.D. Evidence of arbovirus coinfection in suspected febrile malaria and typhoid patients in Nigeria. J. Infect. Dev. Ctries. 2013, 7, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Ayorinde, A.F.; Oyeyiga, A.M.; Nosegbe, N.O.; Folarin, O.A. A survey of malaria and some arboviral infections among sus- pected febrile patients visiting a health centre in Simawa, Ogun State, Nigeria. J. Infect. Public Health 2016, 9, 52–59. [Google Scholar] [CrossRef][Green Version]
- Mala, W.; Wilairatana, P.; Kotepui, K.U.; Kotepui, M. Prevalence of Malaria and Chikungunya Co-Infection in Febrile Patients: A Systematic Review and Meta-Analysis. Trop. Med. Infect. Dis. 2021, 6, 119. [Google Scholar] [CrossRef]
- Adusei, J.A.; Narkwa, P.W.; Owusu, M.; Domfeh, S.A.; Alhassan, M.; Appau, E.; Salam, A.; Mutocheluh, M. Evidence of chikungunya virus infections among febrile patients at three secondary health facilities in the Ashanti and the Bono Regions of Ghana. PLoS Negl. Trop. Dis. 2021, 15, e0009735. [Google Scholar] [CrossRef]
- Olajiga, O.M.; Adesoye, O.E.; Emilolorun, A.P.; Adeyemi, A.J.; Adeyefa, E.O.; Aderibigbe, I.A.; Adejumo, S.A.; Adebimpe, W.O.; Opaleye, O.O.; Sule, W.F.; et al. Chikungunya Virus Seroprevalence and Associated Factors among Hospital Attendees in Two States of Southwest Nigeria: A Preliminary Assessment. Immunol. Investig. 2017, 46, 552–565. [Google Scholar] [CrossRef]
- Igbasi, U.; Oyibo, W. Seroprevalence of immunoglobulin G and E among outpatients with malaria in Ikorodu Lga, Lagos, Nigeria. Microbes Infect. Chemother. 2022, 2, e1376. [Google Scholar] [CrossRef]
- Gaviria, R.R.; Santhekadur, P. A case of coinfection with malaria and chikungunya in a returning traveler from Nigeria. J. Vector Borne Dis. 2021, 58, 178–180. [Google Scholar] [CrossRef]
- Mohamed, N.; Magzoub, M.; Mohamed, R.E.H.; Aleanizy, F.S.; Alqahtani, F.Y.; Nour, B.Y.M.; Alkarsany, M.M. Prevalence and identification of arthropod-transmitted viruses in Kassala State, Eastern Sudan. Libyan J. Med. 2019, 14, 1564511. [Google Scholar] [CrossRef]
- Chattopadhyay, S.; Mukherjee, R.; Nandi, A.; Bhattacharya, N. Chikungunya virus infection in West Bengal, India. Indian J. Med. Microbiol. 2016, 34, 213–215. [Google Scholar] [CrossRef] [PubMed]
- Emeribe, A.U.; Abdullahi, I.N.; Isong, I.K.; Nwofe, J.O.; Shuaib, B.I.; Gwarzo, A.M.; Usman, Y.; Sadi, M.; Umeozuru, C.M.; Dangana, A.; et al. Dengue Virus is Hyperendemic in Nigeria from 2009 to 2020: A Contemporary Systematic Review. Infect. Chemother. 2021, 53, 284–299. [Google Scholar] [CrossRef] [PubMed]
- Fagbami, A.H.; Onoja, A.B. Dengue haemorrhagic fever: An emerging disease in Nigeria, West Africa. J. Infect. Public Health 2018, 11, 757–762. [Google Scholar] [CrossRef] [PubMed]
- Metz, S.W.; Gardner, J.; Geertsema, C.; Le, T.T.; Goh, L.; Vlak, J.M.; Suhrbier, A.; Pijlman, G.P. Effective Chikungunya Virus-like Particle Vaccine Produced in Insect Cells. PLoS Negl. Trop. Dis. 2013, 7, e2124. [Google Scholar] [CrossRef]
- Ingoba, L.L.; Adedoja, A.; Peko, S.M.; Vairo, F.; Haider, N.; Kock, R.; Ippolito, G.; Zumla, A.; Nguimbi, E.; Pallerla, S.R.; et al. Diagnosis of Chikungunya Virus in Febrile Patients from a Malaria Holoendemic Area. Int. J. Infect. Dis. 2021, 109, 247–252. [Google Scholar] [CrossRef]
- Charrel, R.N.; De Lamballerie, X.; Raoult, D. Chikungunya outbreaks–the globalization of vector-borne diseases. N. Engl. J. Med. 2007, 356, 769–771. [Google Scholar] [CrossRef]
- Fritel, X.; Rollot, O.; Gérardin, P.; Gaüzère, B.-A.; Bideault, J.; Lagarde, L.; Dhuime, B.; Orvain, E.; Cuillier, F.; Ramful, D.; et al. Chikungunya virus infection during pregnancy, Réunion, France, 2006. Emerg. Infect. Dis. 2010, 16, 418–425. [Google Scholar] [CrossRef]
- Omatola, C.A.; Onoja, B.A.; Fassan, P.K.; Osaruyi, S.A.; Iyeh, M.; Samuel, M.A.; Haruna, P.U. Seroprevalence of chikungunya virus infection in five hospitals within Anyigba, Kogi State of Nigeria. Braz. J. Infect. Dis. 2020, 24, 1–6. [Google Scholar] [CrossRef]
- Ndosi, R.; Kwigizile, E.; Ibrahim, U.; Dossajee, U.; Rwiza, J.; Kabanyana, C.; Ndaro, A.; Chilongola, J. Risk factors for concurrent malaria and arbovirus infections in Handeni, Northeastern Tanzania. Int. J. Trop. Dis. Health 2016, 20, 1–7. [Google Scholar] [CrossRef]
- Thiberville, S.-D.; Boisson, V.; Gaudart, J.; Simon, F.; Flahault, A.; de Lamballerie, X. Chikungunya fever: A clinical and virological investigation of outpatients on Reunion Island, South-West Indian Ocean. PLoS Negl. Trop. Dis. 2013, 7, 2004. [Google Scholar] [CrossRef]
- Yactayo, S.; Staples, J.E.; Millot, V.; Cibrelus, L.; Ramon-Pardo, P. Epidemiology of Chikungunya in Americas. J. Infect. Dis. 2016, 214, S441–S445. [Google Scholar] [CrossRef] [PubMed]
- Ferede, G.; Tiruneh, M.; Abate, E.; Wondimeneh, Y.; Gadisa, E.; Howe, R.; Aseffa, A.; Tessema, B. Evidence of chikungunya virus infection among febrile patients in northwest Ethiopia. Int. J. Infect. Dis. 2021, 104, 183–188. [Google Scholar] [CrossRef] [PubMed]
- Salam, N.; Mustafa, S.; Hafiz, A.; Chaudhary, A.A.; Deeba, F.; Parveen, S. Global prevalence and distribution of coinfection of malaria, dengue and chikungunya: A systematic review. BMC Public Health 2018, 18, 710. [Google Scholar] [CrossRef] [PubMed]
- Robinson, M.L.; Kadam, D.; Khadse, S.; Balasubramanian, U.; Raichur, P.; Valvi, C.; Marbaniang, I.; Kanade, S.; Sachs, J.; Basavaraj, A.; et al. Vector-borne disease is a common cause of hospitalized febrile illness in India. Am. J. Trop. Med. Hyg. 2018, 98, 1526–1533. [Google Scholar] [CrossRef] [PubMed]
- Kotepui, M.; Kotepui, K.U. Prevalence and laboratory analysis of malaria and dengue coinfection: A systematic review and meta-analysis. BMC Public Health 2019, 19, 1148. [Google Scholar] [CrossRef]
- Kotepui, M.; Kotepui, K.U.; Milanez, G.D.J.; Masangkay, F.R. Prevalence of and risk factors for severe malaria caused by Plasmodium and dengue virus coinfection: A systematic review and meta-analysis. Infect. Dis. Poverty 2020, 9, 134. [Google Scholar] [CrossRef]
- Kotepui, M.; Masangkay, F.R.; Kotepui, K.U.; Milanez, G.D.J. Preliminary review on the prevalence, proportion, geographical distribution, and characteristics of naturally acquired Plasmodium cynomolgi infection in mosquitoes, macaques, and humans: A systematic review and meta-analysis. BMC Infect. Dis. 2021, 21, 259. [Google Scholar] [CrossRef]
- Doucoure, S.; Thiaw, O.; Wotodjo, A.N.; Bouganali, C.; Diagne, N.; Parola, P.; Sokhna, C. Anopheles arabiensis and Anopheles funestus biting patterns in Dielmo, an area of low-level exposure to malaria vectors. Malar. J. 2020, 19, 230. [Google Scholar] [CrossRef]
- Nasir, I.A.; Agbede, O.; Dangana, A.; Baba, M.; Haruna, A.S. Dengue virus nonstructural Protein-1 expression and associated risk factors among febrile Patients attending University of Abuja Teaching Hospital, Nigeria. Virus Res. 2017, 230, 7–12. [Google Scholar] [CrossRef]
- Available online: https://www.mikrogen.de/produkte/produktuebersicht/testsystem/tropical-fever-igg.html (accessed on 22 August 2021).
- WHO Tool Kit. Available online: https://apps.who.int/iris/bitstream/handle/10665/277257/WHO-CDS-NTD-VEM-2018.05-eng.pdf?sequence=1&isAllowed=y (accessed on 22 August 2021).

| Chikungunya | Malaria | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Negative | Positive | Total Examined (n) | 95% CI | Negative | Positive | Total Examined (n) | 95% CI | |||||
| Regions | ||||||||||||
| South (Abia) | 79 (52.0%) | 73 (48.0%) | 152 (100%) | 0.46–0.50 | p = 0.03 | R = 0.16 | 84 (55.3%) | 68 (44.7%) | 152 (100%) | 0.33–0.57 | p = 0.01 | R = 0.25 |
| North (Kaduna) | 99 (33%) | 201 (67.0%) | 300 (100%) | 0.65–0.69 | 287 (68.5%) | 132 (31.5%) | 419 (100%) | 0.24–0.39 | ||||
| Central (Nasarawa) | 128 (30.5%) | 291 (69.5%) | 419 (100%) | 0.68–0.70 | 258 (86.0%) | 42 (14.0%) | 300 (100%) | 0.4–0.24 | ||||
| Total (N) | 306 (35.1%) | 565 (64.9%) | 87 (100%) | 0.63–0.67 | 629 (72.3%) | 242 (27.7%) | 871 (100%) | 0.25–0.31 | ||||
| Sex | ||||||||||||
| Male | 90 (35.7%) | 162 (64.3%) | 252 (100%) | 0.61–0.67 | p = 0.82 | R = 0.01 | 195 (77.4%) | 57 (22.6%) | 252 (100%) | 0.19–0.25 | p = 0.03 | R = 0.01 |
| Female | 216 (34.9%) | 403 (65.1%) | 619 (100%) | 0.62–0.68 | 434 (70.1%) | 185 (29.9%) | 619 (100%) | 0.27–0.33 | ||||
| Total (N) | 306 (35.1%) | 565 (64.9%) | 871 (100%) | 0.62–0.68 | 629 (72.3%) | 242 (27.7%) | 871 (100%) | 0.25–0.31 | ||||
| Domicile | ||||||||||||
| Urban | 197 (38.9%) | 310 (61.1%) | 507 (100%) | 0.59–0.63 | p = 0.02 | R = 0.09 | 385 (75.9%) | 122 (24.1%) | 507 (100%) | 0.12–0.26 | p = 0.01 | R = 0.1 |
| Rural | 78 (30.2%) | 180 (69.8%) | 258 (100%) | 0.68–0.72 | 175 (67.8%) | 83 (32.2%) | 258 (100%) | 0.22–0.42 | ||||
| Slum | 31 (29.2%) | 75 (70.8%) | 106 (100%) | 0.69–0.73 | 69 (65.1%) | 37 (34.9%) | 106 (100%) | 0.20–0.50 | ||||
| Total (N) | 306 (35.1%) | 565 (64.9%) | 871 (100%) | 0.63–0.67 | 629 (72.3%) | 242 (27.7%) | 871 (100%) | 0.25–0.31 | ||||
| Pregnancy Status | ||||||||||||
| Pregnant | 88 (37.8%) | 145 (60.9%) | 233 (100%) | 0.60–0.64 | p = 0.32 | R = 0.05 | 154 (67.0%) | 76 (33.0%) | 230 (100%) | 0.22–0.44 | p = 0.04 | R = 0.07 |
| Non-pregnant | 218 (34.6%) | 420 (66.3%) | 638 (100%) | 0.63–0.67 | 475 (74.1.0%) | 166 (25.9%) | 641 (100%) | 0.19–0.33 | ||||
| Total (N) | 306 (35.1%) | 565 (64.9%) | 871 (100%) | 0.63–0.67 | 629 (49.0%) | 242 (27.7%) | 871 (100%) | 0.25–0.31 | ||||
| Demographic Groups | Chikungunya-Malaria Co-Infection | |||||
|---|---|---|---|---|---|---|
| Negative | Positive | Total Examined (n) | 95% CI | |||
| Regions (South, North, Central) | 20 (9.6%) | 189 (90.4%) | 209 (100%) | 0.88–0.92 | p = 0.01 | R = 0.25 |
| Age | 79 (31.6%) | 171 (68.4%) | 250 (100%) | 0.66–0.70 | ||
| Sex (Males & Females) | 52 (34.9%) | 97 (65.1%) | 149 (100%) | 0.63–0.68 | ||
| Place of domicile (Urban, Rural, Slum) | 41 (33.1%) | 83 (66.9%) | 124 (100%) | 0.68–0.72 | ||
| Pregnancy status (pregnant/nonpregnant) | 52 (37.4%) | 87 (62.6%) | 139 (100%) | 0.61–0.65 | ||
| Grand Total Examined (N) | 244 (28.0%) | 627 (71.9%) | 871 (100%) | 0.70–0.74 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Asaga Mac, P.; Airiohuodion, P.E.; Yako, A.B.; Makpo, J.K.; Kroeger, A. The Seroprevalence and Hidden Burden of Chikungunya Endemicity and Malaria Mono- and Coinfection in Nigeria. Int. J. Environ. Res. Public Health 2022, 19, 8896. https://doi.org/10.3390/ijerph19158896
Asaga Mac P, Airiohuodion PE, Yako AB, Makpo JK, Kroeger A. The Seroprevalence and Hidden Burden of Chikungunya Endemicity and Malaria Mono- and Coinfection in Nigeria. International Journal of Environmental Research and Public Health. 2022; 19(15):8896. https://doi.org/10.3390/ijerph19158896
Chicago/Turabian StyleAsaga Mac, Peter, Philomena E. Airiohuodion, Andrew B. Yako, James K. Makpo, and Axel Kroeger. 2022. "The Seroprevalence and Hidden Burden of Chikungunya Endemicity and Malaria Mono- and Coinfection in Nigeria" International Journal of Environmental Research and Public Health 19, no. 15: 8896. https://doi.org/10.3390/ijerph19158896
APA StyleAsaga Mac, P., Airiohuodion, P. E., Yako, A. B., Makpo, J. K., & Kroeger, A. (2022). The Seroprevalence and Hidden Burden of Chikungunya Endemicity and Malaria Mono- and Coinfection in Nigeria. International Journal of Environmental Research and Public Health, 19(15), 8896. https://doi.org/10.3390/ijerph19158896






