Serum Trimethylamine N-Oxide Levels Correlate with Metabolic Syndrome in Coronary Artery Disease Patients
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Anthropometric Analysis
2.3. Biochemical Investigations
2.4. Metabolic Syndrome and Its Components
2.5. High-Performance Liquid Chromatography-Mass Spectrometry
2.6. Statistical Analysis
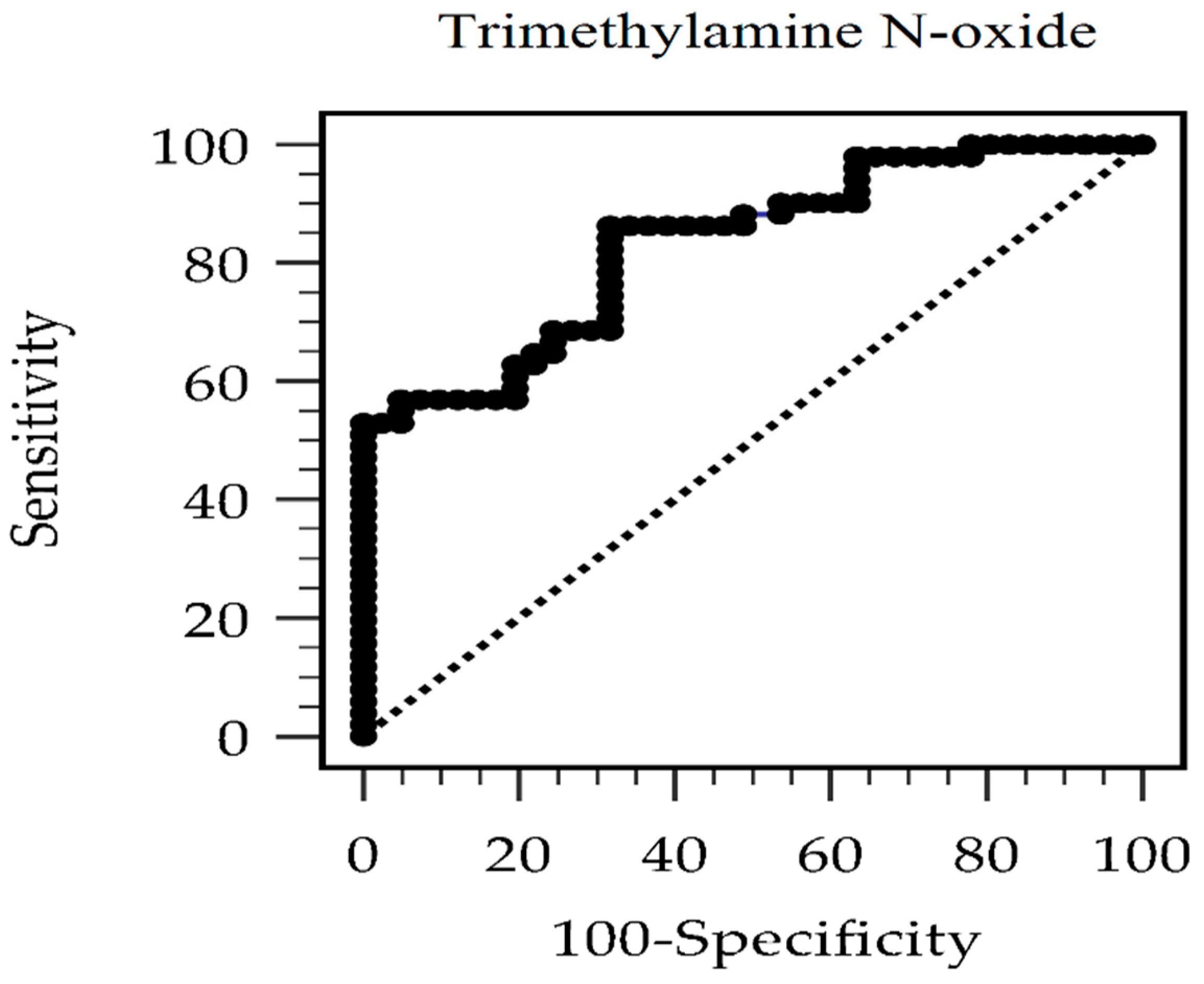
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Grundy, S.M. Metabolic syndrome update. Trends Cardiovasc. Med. 2016, 26, 364–373. [Google Scholar] [CrossRef] [PubMed]
- Mottillo, S.; Filion, K.B.; Genest, J.; Joseph, L.; Pilote, L.; Poirier, P.; Rinfret, S.; Schiffrin, E.L.; Eisenberg, M.J. The metabolic syndrome and cardiovascular risk a systematic review and meta-analysis. J. Am. Coll. Cardiol. 2010, 56, 1113–1132. [Google Scholar] [CrossRef] [Green Version]
- Janeiro, M.H.; Ramírez, M.J.; Milagro, F.I.; Martínez, J.A.; Solas, M. Implication of trimethylamine n-oxide (TMAO) in disease: Potential biomarker or new therapeutic target. Nutrients 2018, 10, 1398. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhuang, R.; Ge, X.; Han, L.; Yu, P.; Gong, X.; Meng, Q.; Zhang, Y.; Fan, H.; Zheng, L.; Liu, Z.; et al. Gut microbe–generated metabolite trimethylamine N-oxide and the risk of diabetes: A systematic review and dose-response meta-analysis. Obes. Rev. 2019, 20, 883–894. [Google Scholar] [CrossRef] [PubMed]
- Senthong, V.; Wang, Z.; Li, X.S.; Fan, Y.; Wu, Y.; Tang, W.H.; Hazen, S.L. Intestinal microbiota-generated metabolite trimethylamine-N-oxide and 5-year mortality risk in stable coronary artery disease: The contributory role of intestinal microbiota in a COURAGE-like patient cohort. J. Am. Heart Assoc. 2016, 5, e002816. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, K.; Yuan, Y.; Yu, H.; Dai, X.; Wang, S.; Sun, Z.; Wang, F.; Fei, H.; Lin, Q.; Jiang, H.; et al. The gut microbial metabolite trimethylamine N-oxide aggravates GVHD by inducing M1 macrophage polarization in mice. Blood 2020, 136, 501–515. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.; Huang, Y.; Yang, Z.; Zhu, L.; Yu, B. Reduction of TMAO level enhances the stability of carotid atherosclerotic plaque through promoting macrophage M2 polarization and efferocytosis. Biosci. Rep. 2021, 41, BSR20204250. [Google Scholar] [CrossRef]
- Shan, Z.; Sun, T.; Huang, H.; Chen, S.; Chen, L.; Luo, C.; Yang, W.; Yang, X.; Yao, P.; Cheng, J.; et al. Association between microbiota-dependent metabolite trimethylamine-N-oxide and type 2 diabetes. Am. J. Clin. Nutr. 2017, 106, 888–894. [Google Scholar] [CrossRef] [Green Version]
- Barrea, L.; Annunziata, G.; Muscogiuri, G.; Di Somma, C.; Laudisio, D.; Maisto, M.; de Alteriis, G.; Tenore, G.C.; Colao, A.; Savastano, S.; et al. Trimethylamine-N-oxide (TMAO) as novel potential biomarker of early predictors of metabolic syndrome. Nutrients 2018, 10, 1971. [Google Scholar] [CrossRef] [Green Version]
- Lian, S.H.; Hsu, B.G.; Wang, J.H.; Chen, M.C. Positive correlation of serum angiopoietin-like protein 3 levels with metabolic syndrome in patients with coronary artery disease. Tzu Chi Med. J. 2021, 34, 75–81. [Google Scholar]
- Alberti, K.G.; Zimmet, P.Z.; Shaw, J. Metabolic syndrome: A new world-wide definition: A consensus statement from the International Diabetes Federation. Diabet. Med. 2006, 23, 469–480. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.F.; Lin, T.J.; Liu, C.H.; Chen, Y.C.; Tang, S.C.; Yang, J.H.; Meng, T.C.; Cheng, C.F. Eating right for a healthier heart: Food choice contributes to cardiometabolic benefits and reduction of carotid intima-media thickness. Nutrition 2020, 78, 110892. [Google Scholar] [CrossRef] [PubMed]
- Mazidi, M.; Rezaie, P.; Kengne, A.P.; Mobarhan, M.G.; Ferns, G.A. Gut microbiome and metabolic syndrome. Diabetes Metab. Syndr. 2016, 10 (Suppl. 1), S150–S157. [Google Scholar] [CrossRef] [PubMed]
- Nowiński, A.; Ufnal, M. Trimethylamine N-oxide: A harmful, protective or diagnostic marker in lifestyle diseases? Nutrition 2018, 46, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Dinicolantonio, J.J.; Mccarty, M.; Okeefe, J. Association of moderately elevated trimethylamine N-oxide with cardiovascular risk: Is TMAO serving as a marker for hepatic insulin resistance. Open Heart 2019, 6, e000890. [Google Scholar] [CrossRef]
- Chen, M.L.; Yi, L.; Zhang, Y.; Zhou, X.; Ran, L.; Yang, J.; Zhu, J.D.; Zhang, Q.Y.; Mi, M.T. Resveratrol attenuates trimethylamine-N-Oxide (TMAO)-induced atherosclerosis by regulating TMAO synthesis and bile acid metabolism via remodeling of the gut microbiota. mBio 2016, 7, e02210–e02215. [Google Scholar] [CrossRef] [Green Version]
- Cho, C.E.; Caudill, M.A. Trimethylamine-N-oxide: Friend, foe, or simply caught in the cross-fire? Trends Endocrinol. Metab. 2017, 28, 121–130. [Google Scholar] [CrossRef]
- Canyelles, M.; Tondo, M.; Cedó, L.; Farràs, M.; Escolà-Gil, J.; Blanco-Vaca, F. Trimethylamine N-Oxide: A link among diet, gut microbiota, gene regulation of liver and intestine cholesterol homeostasis and HDL function. Int. J. Mol. Sci. 2018, 19, 3228. [Google Scholar] [CrossRef] [Green Version]
- Koeth, R.A.; Wang, Z.; Levison, B.S.; Buffa, J.A.; Org, E.; Sheehy, B.T.; Britt, E.B.; Fu, X.; Wu, Y.; Li, L.; et al. Intestinal microbiota metabolism of L-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat. Med. 2013, 19, 576–585. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Klipfell, E.; Bennett, B.J.; Koeth, R.; Levison, B.S.; Dugar, B.; Feldstein, A.E.; Britt, E.B.; Fu, X.; Chung, Y.M.; et al. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature 2011, 472, 57–63. [Google Scholar] [CrossRef] [Green Version]
- Ufnal, M.; Jazwiec, R.; Dadlez, M.; Drapala, A.; Sikora, M.; Skrzypecki, J. Trimethylamine-N-oxide: A carnitine-derived metabolite that prolongs the hypertensive effect of angiotensin II in rats. Can. J. Cardiol. 2014, 30, 1700–1705. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Randrianarisoa, E.; Lehn-Stefan, A.; Wang, X.; Hoene, M.; Peter, A.; Heinzmann, S.S.; Zhao, X.; Königsrainer, I.; Königsrainer, A.; Balletshofer, B.; et al. Relationship of serum trimethylamine N-oxide (TMAO) levels with early atherosclerosis in humans. Sci. Rep. 2016, 6, 26745. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schugar, R.C.; Shih, D.M.; Warrier, M.; Helsley, R.N.; Burrows, A.; Ferguson, D.; Brown, A.L.; Gromovsky, A.D.; Heine, M.; Chatterjee, A.; et al. The TMAO-producing enzyme flavin-containing monooxygenase 3 regulates obesity and the beiging of white adipose tissue. Cell Rep. 2017, 20, 279. [Google Scholar] [CrossRef] [Green Version]
- Al-Waiz, M.; Mitchell, S.C.; Idle, J.R.; Smith, R.L. The metabolism of14C-labelled trimethylamine and its N-oxide in man. Xenobiotica 1987, 17, 551–558. [Google Scholar] [CrossRef] [PubMed]
- Teft, W.A.; Morse, B.L.; Leake, B.F.; Wilson, A.; Mansell, S.E.; Hegele, R.A.; Ho, R.H.; Kim, R.B. Identification and characterization of trimethylamine-N-oxide uptake and efflux transporters. Mol. Pharm. 2017, 14, 310–318. [Google Scholar] [CrossRef] [PubMed]
- Missailidis, C.; Hällqvist, J.; Qureshi, A.R.; Barany, P.; Heimbürger, O.; Lindholm, B.; Stenvinkel, P.; Bergman, P. Serum trimethylamine-N-oxide is strongly related to renal function and predicts outcome in chronic kidney disease. PLoS ONE 2016, 11, e0141738. [Google Scholar] [CrossRef] [Green Version]
- Farhangi, M.A.; Vajdi, M. Novel findings of the association between gut microbiota-derived metabolite trimethylamine N-oxide and inflammation: Results from a systematic review and dose-response meta-analysis. Crit. Rev. Food Sci. Nutr. 2020, 60, 2801–2823. [Google Scholar] [CrossRef]
- Rahman, M.M.; Islam, F.; -Or-Rashid, M.H.; Mamun, A.A.; Rahaman, M.S.; Islam, M.M.; Meem, A.F.K.; Sutradhar, P.R.; Mitra, S.; Mimi, A.A.; et al. The gut microbiota (microbiome) in cardiovascular disease and its therapeutic regulation. Front. Cell Infect. Microbiol. 2022, 12, 903570. [Google Scholar] [CrossRef]
- Agnoletti, D.; Piani, F.; Cicero, A.F.G.; Borghi, C. The gut microbiota and vascular aging: A state-of-the-art and systematic review of the literature. J. Clin. Med. 2022, 11, 3557. [Google Scholar] [CrossRef]
- Rutkowski, K.; Rahman, Y.; Halter, M. Development and feasibility of the use of an assessment tool measuring treatment efficacy in patients with trimethylaminuria: A mixed methods study. J. Inherit. Metab. Dis. 2019, 42, 362–370. [Google Scholar] [CrossRef] [Green Version]
- Alibrandi, S.; Nicita, F.; Donato, L.; Scimone, C.; Rinaldi, C.; D’Angelo, R.; Sidoti, A. Adaptive modelling of mutated FMO3 enzyme could unveil unexplored scenarios linking variant haplotypes to TMAU phenotypes. Molecules 2021, 26, 7045. [Google Scholar] [CrossRef] [PubMed]
- Scicchitano, P.; Cameli, M.; Maiello, M.; Modesti, P.A.; Muiesan, M.L.; Novo, S. Nutraceuticals and dyslipidaemia: Beyond the common therapeutics. J. Funct. Foods 2014, 6, 11–32. [Google Scholar] [CrossRef]
- Jia, X.; Osborn, L.J.; Wang, Z. Simultaneous measurement of urinary trimethylamine (TMA) and trimethylamine N-oxide (TMAO) by liquid chromatography-mass spectrometry. Molecules 2020, 25, 1862. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sapa, H.; Gutiérrez, O.M.; Shlipak, M.G.; Katz, R.; Ix, J.H.; Sarnak, M.J.; Cushman, M.; Rhee, E.P.; Kimmel, P.L.; Vasan, R.S.; et al. Association of uremic solutes with cardiovascular death in diabetic kidney disease. Am. J. Kidney Dis. 2022; in press. [Google Scholar]

| Variables | All Patients (n = 92) | No Metabolic Syndrome Group (n = 41) | Metabolic Syndrome Group (n = 51) | p Value |
|---|---|---|---|---|
| Age (years) | 65.44 ± 9.37 | 66.29 ± 8.77 | 64.75 ± 9.86 | 0.435 |
| Height (cm) | 161.30 ± 7.88 | 161.54 ± 6.26 | 161.12 ± 9.03 | 0.802 |
| Body weight (kg) | 68.59 ± 12.19 | 64.59 ± 9.51 | 71.82 ± 13.20 | 0.004 * |
| Waist circumference (cm) | 92.52 ± 10.20 | 86.98 ± 7.99 | 96.98 ± 9.63 | <0.001 * |
| Body mass index (kg/m2) | 26.26 ± 3.59 | 24.73 ± 3.13 | 27.50 ± 3.48 | <0.001 * |
| Systolic blood pressure (mmHg) | 130.27 ± 16.47 | 124.44 ± 13.64 | 134.96 ± 17.16 | 0.002 * |
| Diastolic blood pressure (mmHg) | 72.25 ± 10.29 | 70.73 ± 8.33 | 73.47 ± 11.56 | 0.206 |
| Total cholesterol (mg/dL) | 167.36 ± 37.17 | 164.85 ± 33.71 | 169.37 ± 39.94 | 0.565 |
| Triglycerides (mg/dL) | 120.00 (91.25–183.00) | 104.00 (86.50–127.50) | 151.00 (101.00–238.00) | <0.001 * |
| HDL-C (mg/dL) | 45.41 ± 12.15 | 49.27 ± 13.49 | 42.31 ± 10.05 | 0.006 * |
| LDL-C (mg/dL) | 96.23 ± 27.47 | 95.56 ± 27.1 | 96.76 ± 28.00 | 0.836 |
| Fasting glucose (mg/dL) | 113.00 (98.25–157.00) | 100.00 (92.00–146.50) | 125.00 (105.00–157.00) | 0.003 * |
| Blood urea nitrogen (mg/dL) | 16.00 (13.00–20.00) | 15.00 (12.00–17.50) | 19.00 (13.00–22.00) | 0.003 * |
| Creatinine (mg/dL) | 1.10 (0.90–1.30) | 1.00 (0.90–1.20) | 1.20 (0.90–1.50) | 0.004 * |
| eGFR (mL/min) | 67.02 ± 19.42 | 75.03 ± 13.44 | 60.57 ± 21.14 | <0.001 * |
| C-reactive protein (mg/dL) | 0.19 (0.14–0.26) | 0.18 (0.14–0.22) | 0.22 (0.15–0.30) | 0.048 * |
| Insulin (uIU/mL) | 12.57 (9.34–17.13) | 11.18 (7.09–15.69) | 14.64 (9.89–19.60) | 0.041 * |
| HOMA-IR | 3.97 (2.81–5.39) | 3.56 (2.17–4.72) | 4.23 (3.30–6.22) | 0.004 * |
| TMAO (μg/L) | 119.58 (98.00–176.72) | 99.96 (88.95–128.41) | 153.67 (109.41–219.80) | <0.001 * |
| Female (n, %) | 21 (22.8) | 6 (14.6) | 15 (29.4) | 0.093 |
| Hypertension (n, %) | 72 (78.3) | 24 (58.5) | 48 (94.1) | <0.001 * |
| Diabetes (n, %) | 41 (44.6) | 10 (24.4) | 31 (60.8) | <0.001 * |
| ACE inhibitor use (n, %) | 22 (23.9) | 6 (14.6) | 16 (31.4) | 0.061 |
| ARB use (n, %) | 34 (37.0) | 12 (29.3) | 22 (43.1) | 0.171 |
| β-blocker use (n, %) | 52 (56.5) | 21 (51.2) | 31 (60.8) | 0.358 |
| CCB use (n, %) | 34 (37.0) | 11 (26.8) | 23 (45.1) | 0.071 |
| Statin use (n, %) | 64 (69.6) | 25 (61.0) | 39 (76.5) | 0.108 |
| Fibrate use (n, %) | 15 (16.3) | 4 (9.8) | 11 (21.6) | 0.127 |
| TMAO (μg/L) | Unadjusted | Model 1 | Model 2 | |||
|---|---|---|---|---|---|---|
| OR (95% CI) | p Value | OR (95% CI) | p Value | OR (95% CI) | p Value | |
| Per 1 μg/L TMAO increase | 1.034 (1.017–1.052) | <0.001 * | 1.033 (1.009–1.058) | 0.007 * | 1.036 (1.005–1.067) | 0.023 * |
| Variables | Log-Transformed TMAO (μg/L) | ||||
|---|---|---|---|---|---|
| Simple Regression | Multivariate Regression | ||||
| r | p Value | Beta | Adjusted R2 Change | p Value | |
| Female | 0.162 | 0.122 | - | - | - |
| Hypertension | 0.260 | 0.012 * | - | - | - |
| Diabetes | 0.075 | 0.477 | - | - | - |
| ACE inhibitor use | 0.031 | 0.768 | - | - | - |
| ARB use | 0.044 | 0.680 | - | - | - |
| β-blocker use | 0.157 | 0.134 | - | - | - |
| CCB use | 0.077 | 0.467 | - | - | - |
| Statin use | 0.102 | 0.333 | - | - | - |
| Fibrate use | 0.137 | 0.194 | - | - | - |
| Age (years) | 0.067 | 0.528 | - | - | - |
| Body weight (kg) | 0.094 | 0.372 | - | - | - |
| Waist circumference (cm) | 0.279 | 0.007 * | - | - | - |
| Body mass index (kg/m2) | 0.190 | 0.069 | - | - | - |
| Systolic blood pressure (mmHg) | 0.265 | 0.011 * | - | - | - |
| Diastolic blood pressure (mmHg) | −0.008 | 0.942 | - | - | - |
| Total cholesterol (mg/dL) | 0.041 | 0.698 | - | - | - |
| Log-Triglyceride (mg/dL) | 0.187 | 0.074 | - | - | - |
| HDL-C (mg/dL) | −0.174 | 0.096 | - | - | - |
| LDL-C (mg/dL) | 0.008 | 0.941 | - | - | - |
| Log-Glucose (mg/dL) | 0.007 | 0.951 | - | - | - |
| Log-BUN (mg/dL) | 0.246 | 0.018 * | - | - | - |
| Log-Creatinine (mg/dL) | 0.214 | 0.041 * | - | - | - |
| eGFR (mL/min) | −0.306 | 0.003 * | −0.235 | 0.042 | 0.022 * |
| Log-CRP (mg/dL) | 0.335 | 0.001 * | 0.274 | 0.103 | 0.001 * |
| Log-Insulin (uIU/mL) | 0.125 | 0.235 | - | - | - |
| Log-HOMA-IR | 0.114 | 0.279 | - | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuo, C.-H.; Liu, C.-H.; Wang, J.-H.; Hsu, B.-G. Serum Trimethylamine N-Oxide Levels Correlate with Metabolic Syndrome in Coronary Artery Disease Patients. Int. J. Environ. Res. Public Health 2022, 19, 8710. https://doi.org/10.3390/ijerph19148710
Kuo C-H, Liu C-H, Wang J-H, Hsu B-G. Serum Trimethylamine N-Oxide Levels Correlate with Metabolic Syndrome in Coronary Artery Disease Patients. International Journal of Environmental Research and Public Health. 2022; 19(14):8710. https://doi.org/10.3390/ijerph19148710
Chicago/Turabian StyleKuo, Chiu-Huang, Chin-Hung Liu, Ji-Hung Wang, and Bang-Gee Hsu. 2022. "Serum Trimethylamine N-Oxide Levels Correlate with Metabolic Syndrome in Coronary Artery Disease Patients" International Journal of Environmental Research and Public Health 19, no. 14: 8710. https://doi.org/10.3390/ijerph19148710
APA StyleKuo, C.-H., Liu, C.-H., Wang, J.-H., & Hsu, B.-G. (2022). Serum Trimethylamine N-Oxide Levels Correlate with Metabolic Syndrome in Coronary Artery Disease Patients. International Journal of Environmental Research and Public Health, 19(14), 8710. https://doi.org/10.3390/ijerph19148710







