Abstract
Metabolic syndrome (MetS) has become the most important issue in family medicine and primary care because it is a cluster of metabolic abnormalities that are a burden on health care in many countries. Highly sensitive C-reactive protein (hsCRP), which is elevated in inflammatory situations, can be produced by monocyte-derived macrophages in adipose tissue. People with MetS tend to have more adipose tissue. Therefore, we aimed to investigate the association between hsCRP and MetS among elderly individuals aged 50 years and older in northern Taiwan. This study was a cross-sectional community-based study that included 400 middle-aged and elderly Taiwanese adults, and 400 participants were eligible for analysis. We divided the participants into a MetS group and a non-MetS group. Pearson’s correlations were calculated between hsCRP and other related risk factors. Furthermore, the relationship between hsCRP and MetS was analyzed with logistic regression. People in the MetS group were more likely to have higher hsCRP levels. The Pearson’s correlation analysis showed a positive correlation with hsCRP. In the logistic regression, hsCRP was significantly associated with MetS, even with the adjustment for BMI, uric acid, age, sex, smoking status, drinking status, hypertension, diabetes mellitus, and dyslipidemia. In summary, our research indicated that hsCRP could be an independent risk factor for MetS.
1. Introduction
With wealthy lifestyles and longevity, metabolic syndrome (MetS) has become the most important issue in family medicine and primary care because it is a cluster of metabolic abnormalities, such as overweight, glucose intolerance, elevated blood pressure (BP) and dyslipidemia [1], leading to a higher risk of diabetes mellitus (DM) and cardiovascular disease [2,3]. Moreover, people with MetS tend to have more adipose tissue, resulting in the excessive release of free fatty acids, thereby reducing the peripheral insulin sensitivity and causing insulin resistance (IR) [4]. Expanded adipose tissue also leads to the overproduction of proinflammatory cytokines, including C-reactive protein (CRP), interleukin-6 (IL-6) and tumor necrosis factor alpha (TNF-α), by monocyte-derived macrophages in adipose tissue [5,6]. In addition to IR, inflammation appears to be a pathophysiologic phenomenon of MetS. Several studies have shown that high-sensitivity CRP (hsCRP) is positively associated with fasting insulin, IR and MetS [7,8,9,10]. Similar correlations have been found in different age groups (children and adults) [11,12,13]. Previous studies have also indicated that hsCRP positively correlates with many chronic diseases, such as hypertension (HTN), DM and dyslipidemia [14,15,16]. Lifestyle can also have an impact on hsCRP levels [17]. Conversely, the prevalence of chronic diseases and metabolic syndrome increases as people age [18]. Furthermore, lifestyle factors, such as smoking and drinking, can elevate the hsCRP level [17]. Elevated hsCRP has seemed to be impacted by multiple factors. However, there has been a lack of studies that have considered comprehensive parameters in the relationships between hsCRP and metabolic syndrome. In our research, we gathered many factors, from laboratory data to anthropometric parameters, to evaluate the association between hsCRP levels and metabolic syndrome among elderly individuals aged 50 years and older in northern Taiwan. The results of our research could provide a reference for primary care providers surveying metabolic syndrome.
2. Materials and Methods
2.1. Study Design and Study Population
This study was a cross-sectional community-based study designed to examine possible independent predictors of MetS. Participants were recruited from a community health promotion project of Linkou Chang Gung Memorial Hospital between 1 February 2014 and 31 August 2014. The project enrolled participants aged 50 years or older through posters or notifications from the community office. Each participant completed a questionnaire that included personal information and a medical history in a face-to-face interview. Anthropometric measurements were obtained, and blood sampling was performed by trained research assistants or nurses under the supervision of a medical doctor. The project was approved by the Institutional Review Board of Linkou Chang Gung Memorial Hospital, and all participants provided written informed consent prior to enrollment. We excluded participants based on the following exclusion criteria: (1) disability; (2) declining of participation; and (3) acute illness at enrollment or recently. Finally, a total of 400 participants were included in the analysis.
2.2. Measurements
Trained assistants or nurses collected personal information, including age, gender, smoking habit (self-reported current smoker or not), drinking status (drinking ≥ 2 days/week or not) and medical history, during a face-to-face interview. Anthropometric data, such as height, weight, waist circumference (WC) and BP, were measured. WC was measured at the level midway between the iliac crest and the lower border of the 12th rib while the subject stood with their feet 25–30 cm apart. BP was measured using an automated sphygmomanometer placed on the participant’s right arm after a 10-minute rest in a seated position, and the lowest reading was recorded. Body mass index (BMI) was calculated as the person’s weight in kilograms divided by the square of the height in meters. Participants were requested to fast for a minimum of 12 h and to avoid a high-fat diet or alcohol consumption for at least 24 h prior to blood sampling. Venous blood samples were obtained between 7 am and 11 am and stored in a refrigerator at 4 °C before analysis in the hospital laboratory. Clinical biochemical tests included hsCRP, fasting plasma glucose (FPG), total cholesterol (Total-C), triglyceride (TG), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C) and uric acid (UA). Blood samples were collected in a hospital laboratory accredited by the College of American Pathologists (CAP). Trained clinical laboratory technicians performed all assessments. All information was entered into a centralized electronic database that was under strict quality control and monitored on a regular basis.
2.3. Definition of Metabolic Syndrome and Other Diseases
MetS was defined according to the modified criteria of the National Cholesterol Education Program Adult Treatment Panel III (NCEP-ATP III) [19], but the standard of WC has been adjusted by the Minister of Health and Welfare of Taiwan. MetS is defined by the presence of three or more of the following components: (1) WC ≥ 90 cm for men and ≥80 cm for women; (2) TG ≥ 150 mg/dL; (3) HDL-C < 40 mg/dL for men and <50 mg/dL for women; (4) BP ≥ 130/85 mm Hg or current use of antihypertensive medications; and (5) FPG ≥ 100 mg/dL. The participants who met the criteria of MetS were classified in the metabolic group, whereas the participants who did not meet the criteria were classified in the non-metabolic group. Hypertension (HTN) was defined as systolic BP (SBP) ≥ 140 mm Hg, diastolic BP (DBP) ≥ 90 mm Hg, or the use of medications for HTN. Diabetes mellitus (DM) was defined as fasting plasma glucose ≥ 126 mg/dL or the use of oral hypoglycemic agents or insulin. Dyslipidemia was defined as LDL-C ≥ 130 mg/dL and HDL-C < 40 mg/dL in men, HDL-C < 50 mg/dL in women, TG ≥ 150 mg/dL, total cholesterol ≥ 200 mg/dL, or the use of lipid-lowering medications. According to the Ministry of Health and Welfare of Taiwan, obesity was defined as BMI ≥ 27 kg/m2 [20]. Based on the Centers for Disease Control and the American Heart Association, elevated hsCRP was defined as plasma hsCRP ≥ 1 mg/L for a higher risk of cardiovascular disease [21,22].
2.4. Statistical Analysis
The participants were divided into two groups according to the metabolic syndrome. In Table 1, the categorical variables are expressed as n (%) and were analyzed using the chi-square test. The normality of the continuous variables was checked by the Shapiro-Wilk normality test. Continuous variables are presented as the mean ± [SD] if the variables (age, WC, BMI, SBP, DBP, HDL-C, LDL-C, total C and uric acid) were consistent with normally distributed variables and as the median [Q1, Q3] if the variables (hsCRP, FPG and TG) deviated significantly from a normal distribution. We obtained p values from the independent sample t test for data consistent with a normal distribution and the Mann-Whitney U test for data consistent with a nonnormal distribution. Pearson’s correlation coefficient was calculated to analyze correlations between age, WC, BMI, SBP, DBP, FPG, HDL-C, TG, LDL-C, Total-C and uric acid, as shown in Table 2. In the multivariate analysis shown in Table 3, a binary logistic regression was used to evaluate the relationship between metabolic syndrome and hsCRP level with adjustment for BMI, uric acid level, age, sex, smoking status, drinking status, HTN, DM and dyslipidemia. All tests were two-sided, and a p value of less than 0.05 was considered statistically significant. Data were analyzed by SPSS Statistics software, version 22 (IBM, SPSS Armonk, IMM Co, Armonk, NY, USA).

Table 1.
General characteristics of participants between non-MetS group and MetS group.

Table 2.
The Pearson correlation between cardiometabolic risk factors and hsCRP.

Table 3.
Logistic regression analysis of the relationship between cardiometabolic risk factors and MetS.
3. Results
This study recruited 619 participants through posters and notifications from the community office. A total of 219 subjects with incomplete data, with disabilities, refusing participation, or with acute illnesses at enrollment or recently were excluded. The final number of participants was 400. Table 1 shows the general characteristics of the participants. There were 141 men (35.30%) and 259 women (64.70%), with a mean age of 64.47 years. The mean SBP was 129.50 mm Hg, and the mean DBP was 76.93 mm Hg. The mean WC and BMI were 85.07 cm and 24.55 kg/m2, respectively. A total of 10.80% of the participants smoked, and 19.50% drank alcohol frequently. Overall, 50.39%, 19.80% and 65.00% of the participants had HTN, DM and dyslipidemia, respectively. The results of clinical biochemical tests showed that the average levels of hsCRP, FPG, total C, TG, HDL-C, LDL-C and UA were 1.28, 91.00, 197.15, 107.00, 54.43, 118.37 and 5.75 mg/dL, respectively. According to the definition of NCEP-ATP III, we divided the participants into a non-MetS group and a MetS group. The average levels of WC, BMI, SBP, DBP, hsCRP, FPG, TG and UA in the MetS group were significantly higher than those in the non-MetS group. Additionally, the MetS group had a significantly larger proportion of HTN, DM and dyslipidemia. There were no statistically significant differences between the groups in terms of age, sex, smoking, LDL-C or total C. Participants in the non-MetS group tended to have a higher HDL-C.
Table 2 demonstrates the correlations between baseline characteristics and hsCRP using Pearson’s correlation analysis. The results show that hsCRP was only negatively associated with HDL. The correlations of hsCRP with age, WC, BMI, SBP, DBP, FPG, TG, LDL-C, total C and uric acid were not significant.
In Figure 1, the elevated hsCRP was defined as hsCRP ≥ 1 mg/L, and we observed a higher prevalence of MetS in the elevated hsCRP group. The prevalence of MetS in the normal hsCRP group and elevated hsCRP group were 21.7 and 43.2, respectively, with a p-value < 0.001. Table 3 shows the results of the binary logistic regression analysis of the relationships between cardiometabolic risk factors and MetS. Traditional cardiometabolic risk factors, such as obesity, HTN, DM, dyslipidemia, age, sex, smoking, drinking, hsCRP and uric acid, were included in the multivariate analysis. Obesity was defined as BMI ≥ 27 kg/m2, and elevated hsCRP was defined as hsCRP ≥ 1 mg/L. In the univariate logistic regression, elevated hsCRP, obesity, uric acid, drinking status, HTN, DM and dyslipidemia were significantly associated with MetS. Furthermore, the multivariate logistic regression showed that elevated hsCRP (p = 0.01, OR: 2.24, 95% CI: 1.23–4.08), uric acid (p = 0.05, OR: 1.22, 95% CI: 1.00–1.48), drinking status (p = 0.04, OR: 0.46, 95% CI: 0.22–0.98), HTN (p < 0.001, OR: 4.92, 95% CI: 2.90–8.34), DM (p < 0.001, OR: 5.88, 95% CI: 3.13–11.05) and dyslipidemia (p < 0.001, OR: 3.26, 95% CI: 1.83–5.81) remained significantly associated with MetS.
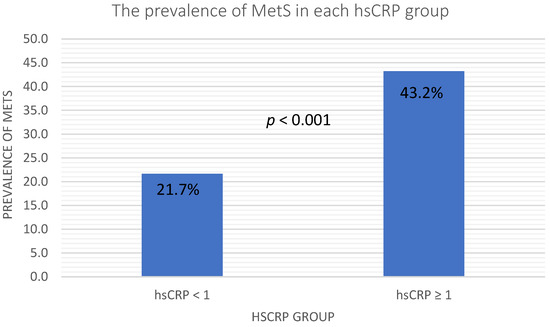
Figure 1.
Prevalence of MetS in the different hsCRP groups. Abbreviations: hsCRP, high sensitive C-reactive protein; MetS, metabolic syndrome.
4. Discussion
The criteria for MetS were WC, BP, FPG, HDL-C and TG [23]. Significant differences in these criteria between the nonmetabolic group and metabolic group were observed in our study. Previous studies have reported that serum uric acid was positively related to MetS [24,25], and our results in Table 1 also corresponded with previous studies. There was a high prevalence of DM, HTN and dyslipidemia in the MetS group, as also indicated by former studies [2,3]. Furthermore, hsCRP showed a significant, positive relationship with MetS, and this finding led us to speculate about the association between hsCRP and MetS.
In MetS, WC, SBP, DBP, FPG and TG are risk factors. HDL-C acts as a protective factor against MetS. Several studies have shown significant associations of hsCRP with the components of MetS [26,27,28]. We also observed a similar trend in the correlation between hsCRP and MetS. In Table 2, Pearson’s correlation analysis indicated significant correlations between hsCRP and MetS criteria, while a negative correlation was found between hsCRP and HDL-C. Because hsCRP shared the same risk and protective factors with MetS in our study, this finding raised the question of whether hsCRP could be an independent risk factor for MetS.
Figure 1 showed that the prevalence of metabolic syndrome in the elevated hsCRP level group was significantly higher than in the low hsCRP level group. Table 3 shows the logistic regression analysis. In Model 1, the univariate logistic regression revealed positive relationships of MetS with hsCRP levels, obesity, uric acid, drinking status, HTN, DM and dyslipidemia. In the Model 2 multivariate logistic regression, only hsCRP levels, uric acid, drinking status, HTN, DM and dyslipidemia remained positively related. According to the results of our study, the hsCRP level was an independent risk factor for MetS. Obesity shares similar risk factors to metabolic syndrome [29], and we also observed a positive relationship between obesity and metabolic syndrome in the univariate logistic regression. The consumption of fructose is higher in the obese population than in the normal population. The metabolism of fructose also elevates serum uric acid [30], and obesity is also linked to metabolic syndrome [31]. We also found a significantly positive relationship between uric acid and metabolic syndrome in both the univariate and multivariate logistic regressions. Elevated plasma glucose levels, triglyceride levels and blood pressure are part of the definition of DM, dyslipidemia and hypertension, respectively [32,33,34]. The definition of metabolic syndrome also includes elevated plasma glucose levels, triglyceride levels and blood pressure [35]. It is not surprising that metabolic syndrome has significant associations with HTN, DM and dyslipidemia in both univariate and multivariate logistic regressions, and this association also corresponded to previous studies [2].
MetS has been considered a risk factor for obesity, HTN, insulin resistance, DM and dyslipidemia, and these diseases associated with MetS have become a heavy burden on health care systems in modern society. A risk factor that can predict MetS would be valuable to alleviate the burden on the health care system related to MetS. People with MetS tend to have more adipocytes, which can produce the chemokines CCL5, monocyte chemotactic protein, macrophage inflammatory protein, macrophage migration inhibition factor and macrophage colony stimulating factor [36,37,38]. These cytokines support the chemotaxis and differentiation of monocytes in adipose tissue, especially in visceral adipose tissue [38]. Eventually, monocytes become macrophages in adipose tissue and secrete proinflammatory cytokines, including IL-6, which stimulate the liver to produce acute-phase reactants, such as hsCRP [4,39].
According to previous cardiovascular disease (CVD) studies, LDL-C in atheromatous plaques can be oxidized and enzyme-modified after the binding of hsCRP. hsCRP can deposit in plaques directly, and the proinflammatory property of hsCRP contributes to the pathogenesis of CVD [40,41] because atherosclerosis is also an inflammatory disease [42]. Furthermore, IL-6, a cytokine that induces hsCRP production in the liver, also accelerates inflammation in atherosclerosis [43]. Overall, the excessive adipocytes in patients with metabolic syndrome elevate proinflammatory substances, including hsCRP and interleukins, in serum. Subsequently, these proinflammatory substances facilitate the atherogenic process from the initial chemotaxis of leukocytes in the arterial wall to the rupture of the plaque [44,45].
The proinflammatory property of hsCRP could also contribute to DM because the inflammation related to CRP alters the endothelial permeability of insulin [46]. The limitation of insulin delivery promotes IR in metabolically active tissue [47].
These studies explained the possible role of hsCRP in the connection between MetS, CVD and IR. Indeed, our study revealed a significant relationship between MetS, CVD and IR. An intensive relationship between hsCRP and MetS was observed even after adjusting for other risk factors. The results of our study not only implied that hsCRP could be an imperative connection of MetS with CVD and IR, but it also indicated that hsCRP could be an independent risk factor for MetS.
Our study had several strengths. First, the study had a sufficient sample size, relevant confounders and appropriate statistics. Second, we confirmed that hsCRP could be an independent risk factor for metabolic syndrome after we considered many related confounders in middle-aged and elderly populations. The results could provide a reference for primary care providers screening for metabolic syndrome. However, there remain some limitations in our study. Regarding the drinking status, although we recorded the frequency of drinking, we did not have the number of types of alcoholic beverages. Since hsCRP levels correlate with alcohol consumption [48], the amount of alcohol intake should be considered in future studies. That all participants came from northern Taiwan is another problem. Selection bias should be considered, so the findings might not represent the entire middle-aged and elderly population.
5. Conclusions
In this study, hsCRP was an independent risk factor for MetS in middle-aged and elderly people in northern Taiwan. Thus, our findings could provide valuable information for primary care physicians to alert subjects in this age group regarding the increased risk of MetS.
Author Contributions
Project administration, J.-Y.C.; Supervision, Y.-L.S. and J.-Y.C.; Writing–original draft, Y.-L.S., J.-Y.C. and Y.L.; Writing–review & editing, Y.-L.S., J.-Y.C. All authors have read and agreed to the published version of the manuscript.
Funding
Linkou Chang Gung Memorial Hospital (CORPG3C0171~3C0172, CZRPG3C0053, CORPG3G0021, CORPG3G0022).
Institutional Review Board Statement
The studies involving human participants were reviewed and approved by Chang Gung Medical Foundation Institutional Review Board (102-2304B).
Informed Consent Statement
We explained the informed consent to the patients/participants, and they provided their written informed consent before they participated in this study.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the corresponding author, without undue reservation.
Conflicts of Interest
The authors declare no conflict of interest.
References
- aHeianza, Y.; Kato, K.; Kodama, S.; Ohara, N.; Suzuki, A.; Tanaka, S.; Hanyu, O.; Sato, K.; Sone, H. Risk of the development of Type 2 diabetes in relation to overall obesity, abdominal obesity and the clustering of metabolic abnormalities in Japanese individuals: Does metabolically healthy overweight really exist? The Niigata Wellness Study. Diabet. Med. 2015, 32, 665–672. [Google Scholar] [CrossRef] [PubMed]
- Grundy, S.M. Metabolic syndrome: Connecting and reconciling cardiovascular and diabetes worlds. J. Am. Coll. Cardiol. 2006, 47, 1093–1100. [Google Scholar] [CrossRef] [PubMed]
- Sutherland, J.P.; McKinley, B.; Eckel, R.H. The metabolic syndrome and inflammation. Metab. Syndr. Relat. Disord. 2004, 2, 82–104. [Google Scholar] [CrossRef] [PubMed]
- Russo, L.; Lumeng, C.N. Properties and functions of adipose tissue macrophages in obesity. Immunology 2018, 155, 407–417. [Google Scholar] [CrossRef] [PubMed]
- Matulewicz, N.; Karczewska-Kupczewska, M. Insulin resistance and chronic inflammation. Postepy Hig. Med. Dosw. Online 2016, 70, 1245–1258. [Google Scholar] [CrossRef]
- Amit, K.S.; Harsh, V.S.; Arun, R.; Sanjeev, K.S. C-reactive protein, inflammation and coronary heart disease. Egypt. Heart J. 2015, 67, 89–97. [Google Scholar] [CrossRef]
- Drabsch, T.; Holzapfel, C.; Stecher, L.; Petzold, J.; Skurk, T.; Hauner, H. Associations Between C-Reactive Protein, Insulin Sensitivity, and Resting Metabolic Rate in Adults: A Mediator Analysis. Front. Endocrinol. 2018, 9, 556. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Chen, R.; Wang, H.; Liang, F. Mechanisms Linking Inflammation to Insulin Resistance. Int. J. Endocrinol. 2015, 2015, 508409. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Tian, M.; Zhou, Y. The relationship between insulin resistance, adiponectin and C-reactive protein and vascular endothelial injury in diabetic patients with coronary heart disease. Exp. Ther. Med. 2018, 16, 2022–2026. [Google Scholar] [CrossRef] [PubMed]
- Yousuf, O.; Mohanty, B.D.; Martin, S.S.; Joshi, P.H.; Blaha, M.J.; Nasir, K.; Blumenthal, R.S.; Budoff, M.J. High-sensitivity C-reactive protein and cardiovascular disease: A resolute belief or an elusive link? J. Am. Coll. Cardiol. 2013, 62, 397–408. [Google Scholar] [CrossRef] [PubMed]
- Kyithar, M.P.; Bonner, C.; Bacon, S.; Kilbride, S.M.; Schmid, J.; Graf, R.; Prehn, J.H.; Byrne, M.M. Effects of hepatocyte nuclear factor-1A and -4A on pancreatic stone protein/regenerating protein and C-reactive protein gene expression: Implications for maturity-onset diabetes of the young. J. Transl. Med. 2013, 11, 156. [Google Scholar] [CrossRef]
- Suhett, L.G.; Hermsdorff, H.H.M.; Rocha, N.P.; Silva, M.A.; Filgueiras, M.S.; Milagres, L.C.; Peluzio, M.D.C.G.; de Novaes, J.F. Increased C-Reactive Protein in Brazilian Children: Association with Cardiometabolic Risk and Metabolic Syndrome Components (PASE Study). Cardiol. Res. Pract. 2019, 2019, 3904568. [Google Scholar] [CrossRef]
- Olza, J.; Aguilera, C.M.; Gil-Campos, M.; Leis, R.; Bueno, G.; Valle, M.; Cañete, R.; Tojo, R.; Moreno, L.A.; Gil, Á. A Continuous Metabolic Syndrome Score Is Associated with Specific Biomarkers of Inflammation and CVD Risk in Prepubertal Children. Ann. Nutr. Metab. 2015, 66, 72–79. [Google Scholar] [CrossRef]
- Lakoski, S.G.; Cushman, M.; Siscovick, D.S.; Blumenthal, R.S.; Palmas, W.; Burke, G.; Herrington, D.M. The relationship between inflammation, obesity and risk for hypertension in the Multi-Ethnic Study of Atherosclerosis (MESA). J. Hum. Hypertens. 2011, 25, 73–79. [Google Scholar] [CrossRef]
- Noordam, R.; Oudt, C.H.; Bos, M.M.; Smit, R.A.J.; van Heemst, D. High-sensitivity C-reactive protein, low-grade systemic inflammation and type 2 diabetes mellitus: A two-sample Mendelian randomization study. Nutr. Metab. Cardiovasc. Dis. 2018, 28, 795–802. [Google Scholar] [CrossRef]
- Koenig, W. Low-Grade Inflammation Modifies Cardiovascular Risk Even at Very Low LDL-C Levels: Are We Aiming for a Dual Target Concept? Circulation 2018, 138, 150–153. [Google Scholar] [CrossRef]
- Blaum, C.; Brunner, F.J.; Kröger, F.; Braetz, J.; Lorenz, T.; Goßling, A.; Ojeda, F.; Koester, L.; Karakas, M.; Zeller, T.; et al. Modifiable lifestyle risk factors and C-reactive protein in patients with coronary artery disease: Implications for an anti-inflammatory treatment target population. Eur. J. Prev. Cardiol. 2021, 28, 152–158. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Chen, G.; Zhao, R.; Huang, D.; Tao, L. Temporal trends in the prevalence of metabolic syndrome among middle-aged and elderly adults from 2011 to 2015 in China: The China health and retirement longitudinal study (CHARLS). BMC Public Health 2021, 21, 1045. [Google Scholar] [CrossRef]
- Kassi, E.; Pervanidou, P.; Kaltsas, G.; Chrousos, G. Metabolic syndrome: Definitions and controversies. BMC Med. 2011, 9, 48. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, T.H.; Lee, J.J.; Yu, E.W.; Hu, H.Y.; Lin, S.Y.; Ho, C.Y. Association between obesity and education level among the elderly in Taipei, Taiwan between 2013 and 2015: A cross-sectional study. Sci. Rep. 2020, 10, 20285. [Google Scholar] [CrossRef] [PubMed]
- Sabatine, M.S.; Morrow, D.A.; Jablonski, K.A.; Rice, M.M.; Warnica, J.W.; Domanski, M.J.; Hsia, J.; Gersh, B.J.; Rifai, N.; Ridker, P.M.; et al. Prognostic significance of the Centers for Disease Control/American Heart Association high-sensitivity C-reactive protein cut points for cardiovascular and other outcomes in patients with stable coronary artery disease. Circulation 2007, 115, 1528–1536. [Google Scholar] [CrossRef] [PubMed]
- Nehring, S.M.; Goyal, A.; Bansal, P.; Patel, B.C. C Reactive Protein. Available online: https://www.ncbi.nlm.nih.gov/books/NBK441843 (accessed on 18 July 2022).
- Huang, P.L. A comprehensive definition for metabolic syndrome. Dis. Model. Mech. 2009, 2, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Ali, N.; Miah, R.; Hasan, M.; Barman, Z.; Mou, A.D.; Hafsa, J.M.; Trisha, A.D.; Hasan, A.; Islam, F. Association between serum uric acid and metabolic syndrome: A cross-sectional study in Bangladeshi adults. Sci. Rep. 2020, 10, 7841. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.W.; Chen, K.H.; Tseng, C.K.; Chang, W.C.; Wu, Y.W.; Hwang, J.J. The dose-response effects of uric acid on the prevalence of metabolic syndrome and electrocardiographic left ventricular hypertrophy in healthy individuals. Nutr. Metab. Cardiovasc. Dis. 2019, 29, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Sigdel, M.; Kumar, A.; Gyawali, P.; Shrestha, R.; Tuladhar, E.T.; Jha, B. Association of high sensitivity C-reactive protein with the components of metabolic syndrome in diabetic and non-diabetic individuals. J. Clin. Diagn. Res. 2014, 8, CC11–CC13. [Google Scholar] [CrossRef]
- Pravenec, M.; Kajiya, T.; Zídek, V.; Landa, V.; Mlejnek, P.; Simáková, M.; Silhavý, J.; Malínská, H.; Oliyarnyk, O.; Kazdová, L.; et al. Effects of human C-reactive protein on pathogenesis of features of the metabolic syndrome. Hypertension 2011, 57, 731–737. [Google Scholar] [CrossRef]
- Tamakoshi, K.; Yatsuya, H.; Kondo, T.; Hori, Y.; Ishikawa, M.; Zhang, H.; Murata, C.; Otsuka, R.; Zhu, S.; Toyoshima, H. The metabolic syndrome is associated with elevated circulating C-reactive protein in healthy reference range, a systemic low-grade inflammatory state. Int. J. Obes. Relat. Metab. Disord. 2003, 27, 443–449. [Google Scholar] [CrossRef] [PubMed]
- Engin, A. The Definition and Prevalence of Obesity and Metabolic Syndrome. In Obesity and Lipotoxicity; Springer: Cham, Switzerland, 2017; Volume 960, pp. 1–17. [Google Scholar] [CrossRef]
- Caliceti, C.; Calabria, D.; Roda, A.; Cicero, A.F.G. Fructose Intake, Serum Uric Acid, and Cardiometabolic Disorders: A Critical Review. Nutrients 2017, 9, 395. [Google Scholar] [CrossRef]
- Han, T.S.; Lean, M.E. A clinical perspective of obesity, metabolic syndrome and cardiovascular disease. JRSM Cardiovasc. Dis. 2016, 5. [Google Scholar] [CrossRef]
- Inzucchi, S.E. Clinical practice. Diagnosis of diabetes. N. Engl. J. Med. 2012, 367, 542–550. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.Y.; Duan, X.Y.; Li, L.; Dai, F.; Li, Y.Y.; Li, X.J.; Fan, J.G. Dyslipidemia in Shanghai, China. Prev. Med. 2010, 51, 412–415. [Google Scholar] [CrossRef] [PubMed]
- Parati, G.; Stergiou, G.; O’Brien, E.; Asmar, R.; Beilin, L.; Bilo, G.; Clement, D.; de la Sierra, A.; de Leeuw, P.; Dolan, E.; et al. European Society of Hypertension Working Group on Blood Pressure Monitoring and Cardiovascular Variability. European Society of Hypertension practice Guidelines for Ambulatory Blood Pressure Monitoring. J. Hypertens. 2014, 32, 1359–1366. [Google Scholar] [CrossRef] [PubMed]
- Parikh, R.M.; Mohan, V. Changing definitions of metabolic syndrome. Indian J. Endocrinol. Metab. 2012, 16, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Chawla, A.; Nguyen, K.D.; Goh, Y.P. Macrophage-mediated inflammation in metabolic disease. Nat. Rev. Immunol. 2011, 11, 738–749. [Google Scholar] [CrossRef]
- Olefsky, J.M.; Glass, C.K. Macrophages, inflammation, and insulin resistance. Annu. Rev. Physiol. 2010, 72, 219–246. [Google Scholar] [CrossRef]
- Alexopoulos, N.; Katritsis, D.; Raggi, P. Visceral adipose tissue as a source of inflammation and promoter of atherosclerosis. Atherosclerosis 2014, 233, 104–112. [Google Scholar] [CrossRef]
- Thomas, D.; Apovian, C. Macrophage functions in lean and obese adipose tissue. Metabolism 2017, 72, 120–143. [Google Scholar] [CrossRef]
- Denegri, A.; Boriani, G. High Sensitivity C-reactive Protein (hsCRP) and its Implications in Cardiovascular Outcomes. Curr. Pharm. Des. 2021, 27, 263–275. [Google Scholar] [CrossRef]
- Singh, U.; Dasu, M.R.; Yancey, P.G.; Afify, A.; Devaraj, S.; Jialal, I. Human C-reactive protein promotes oxidized low density lipoprotein uptake and matrix metalloproteinase-9 release in Wistar rats. J. Lipid Res. 2008, 49, 1015–1023. [Google Scholar] [CrossRef]
- Tuttolomondo, A.; Di Raimondo, D.; Pecoraro, R.; Arnao, V.; Pinto, A.; Licata, G. Atherosclerosis as an inflammatory disease. Curr. Pharm. Des. 2012, 18, 4266–4288. [Google Scholar] [CrossRef] [PubMed]
- Tyrrell, D.J.; Goldstein, D.R. Ageing and atherosclerosis: Vascular intrinsic and extrinsic factors and potential role of IL-6. Nat. Rev. Cardiol. 2021, 18, 58–68. [Google Scholar] [CrossRef]
- Soeki, T.; Sata, M. Inflammatory Biomarkers and Atherosclerosis. Int. Heart J. 2016, 57, 134–139. [Google Scholar] [CrossRef] [PubMed]
- Rogoveanu, O.C.; Mogoşanu, G.D.; Bejenaru, C.; Bejenaru, L.E.; Croitoru, O.; Neamţu, J.; Pietrzkowski, Z.; Reyes-Izquierdo, T.; Biţă, A.; Scorei, I.D.; et al. Effects of Calcium Fructoborate on Levels of C-Reactive Protein, Total Cholesterol, Low-Density Lipoprotein, Triglycerides, IL-1β, IL-6, and MCP-1: A Double-blind, Placebo-controlled Clinical Study. Biol. Trace Elem. Res. 2015, 163, 124–131. [Google Scholar] [CrossRef][Green Version]
- Jeong, H.; Baek, S.Y.; Kim, S.W.; Park, E.J.; Lee, J.; Kim, H.; Jeon, C.H. C reactive protein level as a marker for dyslipidaemia, diabetes and metabolic syndrome: Results from the Korea National Health and Nutrition Examination Survey. BMJ Open 2019, 9, e029861. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Nie, Y.; Xiong, H.; Liu, S.; Li, G.; Huang, A.; Guo, L.; Wang, S.; Xue, Y.; Wu, B.; et al. P2X7 Receptor Expression in Peripheral Blood Monocytes Is Correlated with Plasma C-Reactive Protein and Cytokine Levels in Patients with Type 2 Diabetes Mellitus: A Preliminary Report. Inflammation 2015, 38, 2076–2081. [Google Scholar] [CrossRef]
- Park, J.Y.; Kim, M.J.; Kim, J.H. Influence of Alcohol Consumption on the Serum hs-CRP Level and Prevalence of Metabolic Syndrome-Based on the 2015 Korean National Health and Nutrition Examination Survey. J. Korean Diet. Assoc. 2019, 25, 83–104. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).