Heavy Metals in Unprocessed or Minimally Processed Foods Consumed by Humans Worldwide: A Scoping Review
Abstract
1. Introduction
2. Methods
2.1. Search Strategy and Data Extraction
2.2. Statistical Analysis and Data Presentation
2.3. Creation of Variables for the Food Groups and Subgroups
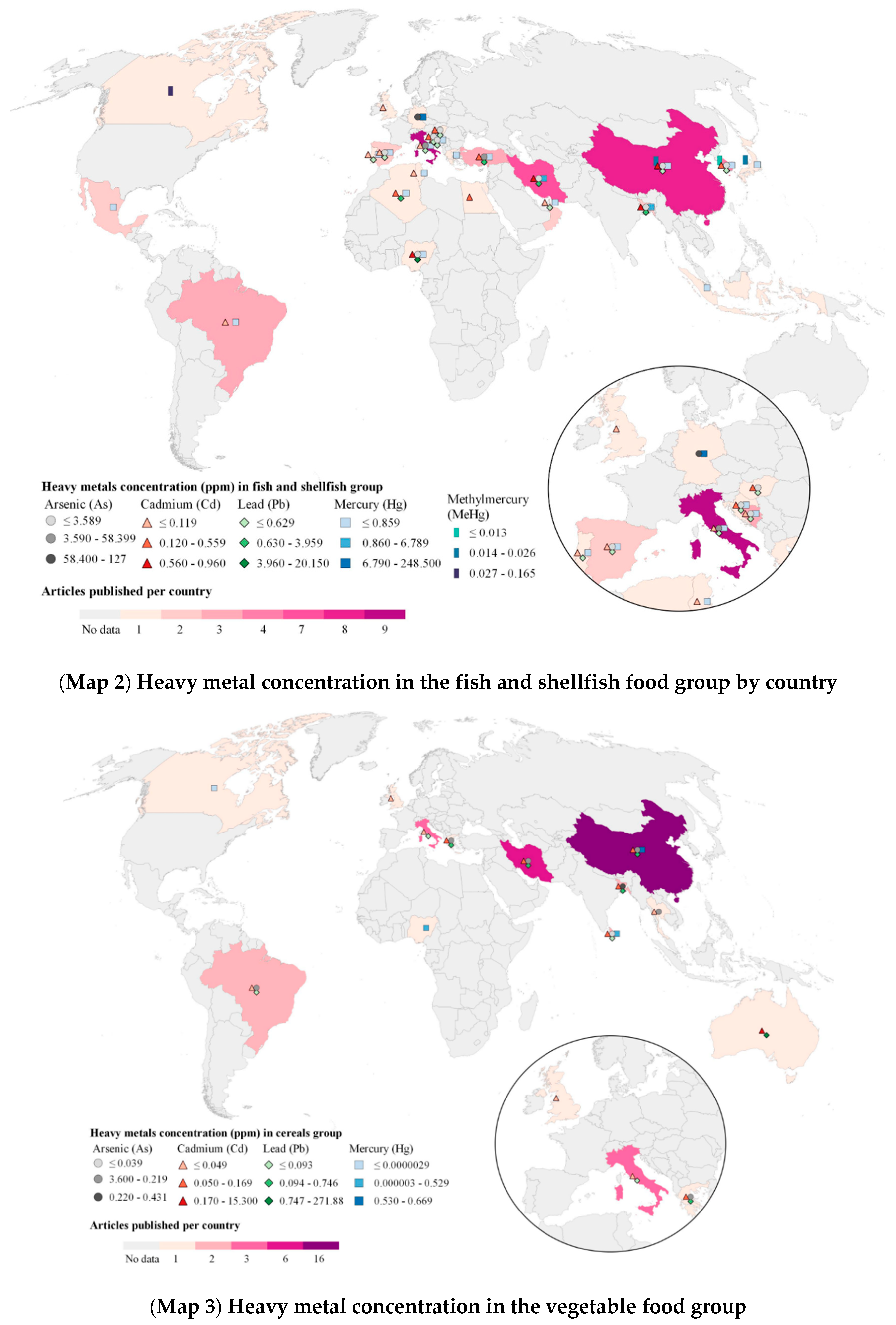
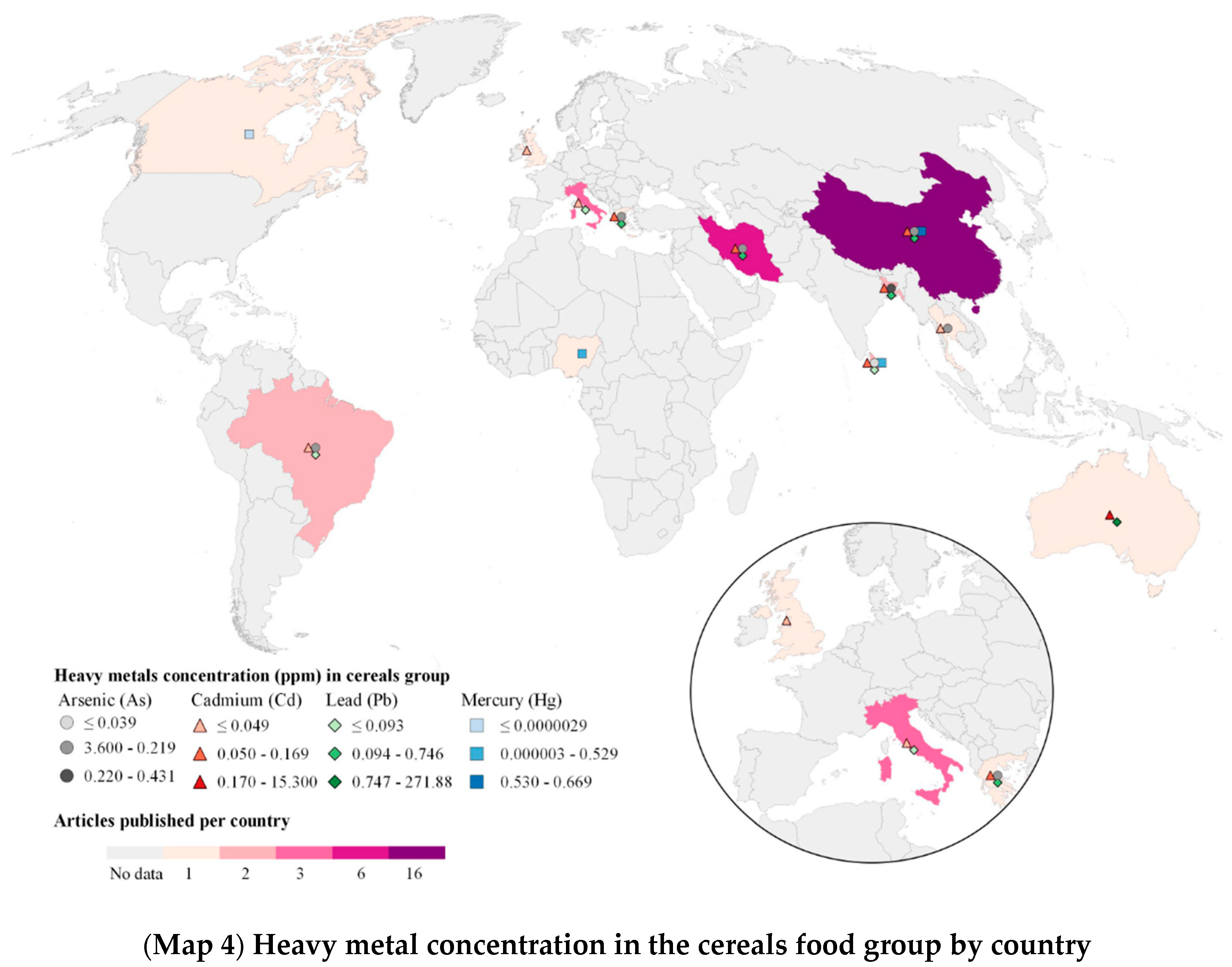
2.4. Heavy Metals (HM) in Food Geographical Analysis
2.5. Reference Values for Maximum Limits (MLs) of Heavy Metals (HM) in Foods
3. Results
3.1. Study Inclusion
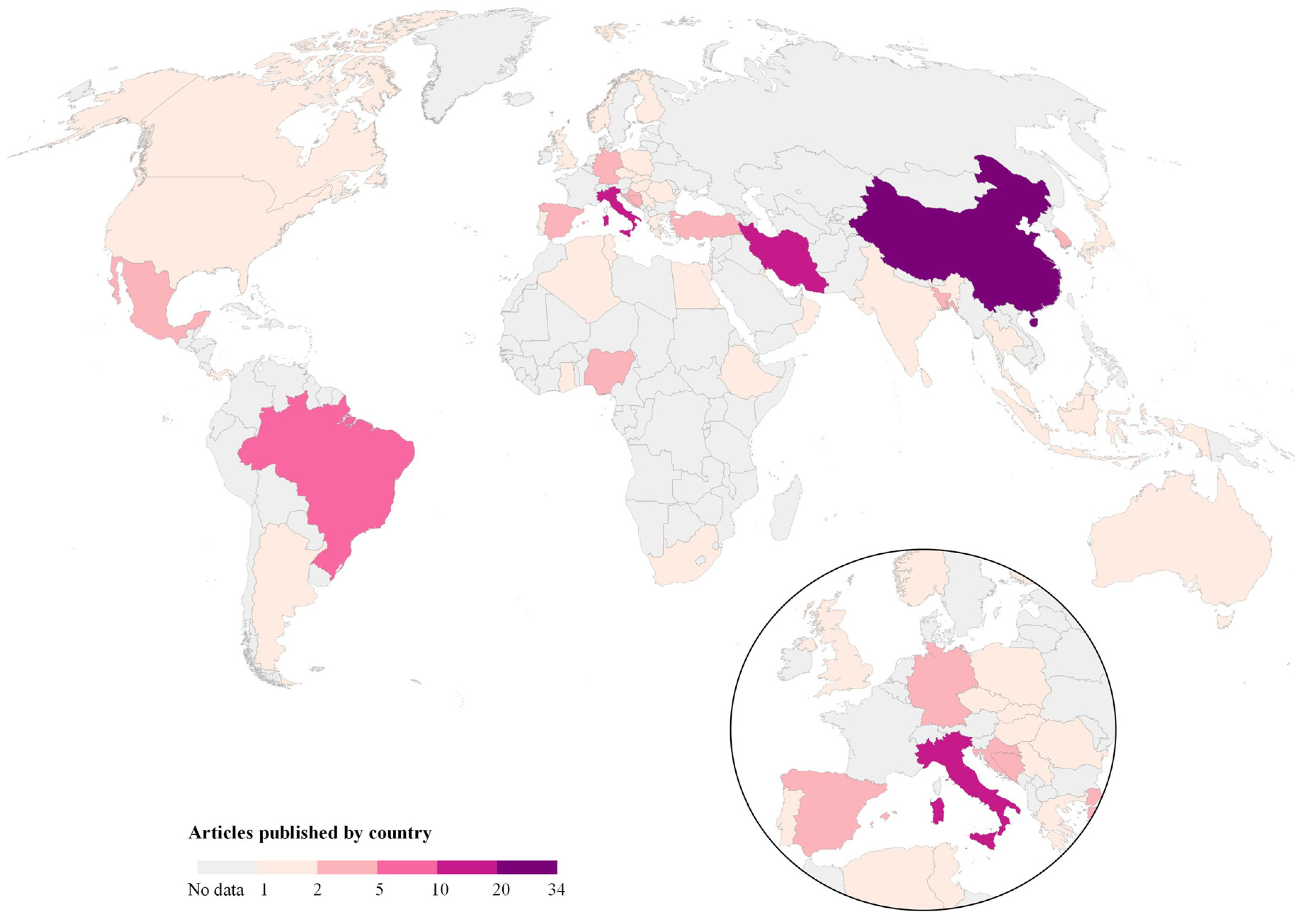
3.2. Worldwide Evidence
3.3. Principal Food Groups and Heavy Metals Identified
3.4. Food Subgroups by Food Group and Heavy Metals Identified
3.5. Heavy Metals (HM) in Food Subgroups in Comparison with the FAO/OMS Maximum Limits (Mls) for Contaminants in Food
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Balali-Mood, M.; Naseri, K.; Tahergorabi, Z.; Khazdair, M.R.; Sadeghi, M. Toxic Mechanisms of Five Heavy Metals: Mercury, Lead, Chromium, Cadmium, and Arsenic. Front. Pharmacol. 2021, 12, 643972. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, A.; Malik, R.N. Human Health Risk Assessment of Heavy Metals via Consumption of Contaminated Vegetables Collected from Different Irrigation Sources in Lahore, Pakistan. Arab. J. Chem. 2014, 7, 91–99. [Google Scholar] [CrossRef]
- Nkansah, M.A.; Agorsor, P.I.; Opoku, F. Heavy Metal Contamination and Health Risk Assessment of Mechanically Milled Delicacy Called Fufu. Int. J. Food Contam. 2021, 8, 1–7. [Google Scholar] [CrossRef]
- United States Environmental Protection Agency Metals. Available online: https://www.epa.gov/caddis-vol2/metals (accessed on 11 June 2022).
- Tchounwou, P.B.; Yedjou, C.G.; Patlolla, A.K.; Sutton, D.J. Heavy Metal Toxicity and the Environment. Exp Suppl. 2012, 101, 133–164. [Google Scholar] [CrossRef] [PubMed]
- Witkowska, D.; Słowik, J.; Chilicka, K. Heavy Metals and Human Health: Possible Exposure Pathways and the Competition for Protein Binding Sites. Molecules 2021, 26, 6060. [Google Scholar] [CrossRef]
- Li, G.; Xiong, C.; Xu, W.; Mei, R.; Cheng, T.; Yu, X. Factors Affecting the Aluminum, Arsenic, Cadmium and Lead Concentrations in the Knee Joint Structures. Front. Public Health 2021, 9, 758074. [Google Scholar] [CrossRef]
- Igbokwe, I.O.; Igwenagu, E.; Igbokwe, N.A. Aluminium Toxicosis: A Review of Toxic Actions and Effects. Interdiscip. Toxicol. 2019, 12, 45–70. [Google Scholar] [CrossRef]
- Fu, Z.; Xi, S. The Effects of Heavy Metals on Human Metabolism. Toxicol. Mech. Methods 2020, 30, 167–176. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration. Metals and Your Food. Available online: https://www.fda.gov/food/chemicals-metals-pesticides-food/metals-and-your-food (accessed on 15 March 2022).
- Khan, A.; Khan, S.; Khan, M.A.; Qamar, Z.; Waqas, M. The Uptake and Bioaccumulation of Heavy Metals by Food Plants, Their Effects on Plants Nutrients, and Associated Health Risk: A Review. Environ. Sci. Pollut. Res. 2015, 22, 13772–13799. [Google Scholar] [CrossRef]
- Rajaram, R.; Ganeshkumar, A.; Vinothkannan, A. Health Risk Assessment and Bioaccumulation of Toxic Metals in Commercially Important Finfish and Shellfish Resources Collected from Tuticorin Coast of Gulf of Mannar, Southeastern India. Mar. Pollut. Bull. 2020, 159, 111469. [Google Scholar] [CrossRef]
- Boudebbouz, A.; Boudalia, S.; Bousbia, A.; Habila, S.; Boussadia, M.I.; Gueroui, Y. Heavy Metals Levels in Raw Cow Milk and Health Risk Assessment across the Globe: A Systematic Review. Sci. Total Environ. 2021, 751, 141830. [Google Scholar] [CrossRef] [PubMed]
- Rather, I.A.; Koh, W.Y.; Paek, W.K.; Lim, J. The Sources of Chemical Contaminants in Food and Their Health Implications. Front. Pharmacol. 2017, 8, 830. [Google Scholar] [CrossRef] [PubMed]
- Parkpian, P.; Leong, S.; Laortanakul, P.; Thunthaisong, N. Regional Monitoring of Lead and Cadmium Contamination in a Tropical Grazing Land Site, Thailand. Environ. Monit. Assess. 2003, 85, 157–173. [Google Scholar] [CrossRef] [PubMed]
- Téllez-Rojo, M.M.; Bautista-Arredondo, L.F.; Trejo-Valdivia, B.; Cantoral, A.; Estrada-Sánchez, D.; Kraiem, R.; Pantic, I.; Rosa-Parra, A.; Gómez-Acosta, L.M.; Romero-Martínez, M.; et al. Reporte Nacional de Niveles de Plomo En Sangre y Uso de Barro Vidriado En Población Infantil Vulnerable. Salud Publica Mex. 2019, 61, 787. [Google Scholar] [CrossRef]
- Weidenhamer, J.D.; Kobunski, P.A.; Kuepouo, G.; Corbin, R.W.; Gottesfeld, P. Lead Exposure from Aluminum Cookware in Cameroon. Sci. Total Environ. 2014, 496, 339–347. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, C.A.; Cannon, G.; Lawrence, M.; da Costa Louzada, M.L.; Pereira Machado, P. Ultra-Processed Foods, Diet Quality, and Health Using the NOVA Classification System; FAO: Rome, Italy, 2019. [Google Scholar]
- Ogunkunle, J.; Bello, O.S.; Ojofeitimi, O.S. Determination of Heavy Metal Contamination of Street-Vended Fruits and Vegetables in Lagos State, Nigeria. Int. Food Res. J. 2014, 5, 1725–1730. [Google Scholar]
- Food and Drug Administration. Chemical Contaminants in Food; USA. 2021. Available online: https://www.fda.gov/food/chemicals-metals-pesticides-food/chemical-contaminants-food (accessed on 14 April 2022).
- Mengistu, D.A. Public Health Implications of Heavy Metals in Foods and Drinking Water in Ethiopia (2016 to 2020): Systematic Review. BMC Public Health 2021, 21, 2114. [Google Scholar] [CrossRef]
- FAO; WHO. Codex Alimentarius. In General Standard for Contaminants and Toxins in Food and Feed; FAO: Rome, Italy, 1995; Available online: https://www.fao.org/fao-who-codexalimentarius/sh-proxy/en/?lnk=1&url=https%253A%252F%252Fworkspace.fao.org%252Fsites%252Fcodex%252FStandards%252FCXS%2B193-1995%252FCXS_193e.pdf (accessed on 10 January 2022).
- Food and Agriculture Organization of the United Nations. World Health Organization Codex Alimentarius. International Food Standards. Available online: https://www.fao.org/fao-who-codexalimentarius/about-codex/en (accessed on 20 January 2022).
- Peters, M.D.J.; Godfrey, C.; McInerney, P.; Munn, Z.; Tricco, A.C.; Khalil, H. Chapter 11: Scoping Reviews (2020 Version). In JBI Manual for Evidence Synthesis; Aromataris, E., Munn, Z., Eds.; JBI: Adelaide, SA, Australia, 2020. [Google Scholar]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef]
- Open Science Framework, Open Science Framework Registries. Available online: https://osf.io/25gps (accessed on 29 April 2021).
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]
- FAO; WHO. FAO/WHO Global Individual Food Consumption Data Tool (GIFT): Methodological Document FOOD Groups and Subgroups; FAO: Rome, Italy, 2020. [Google Scholar]
- Food and Agriculture Organization of the United Nations. AGROVOC. Available online: https://www.fao.org/agrovoc/home (accessed on 29 April 2022).
- QGIS—The Leading Open Source Desktop GIS; OSGeo: Beaverton, OR, USA. Available online: https://www.qgis.org/en/site/about/index.html (accessed on 16 March 2022).
- Kang-Tsung, C. Chapter 9. Data Display and Cartography. In Introduction to Geographic Information Systems; McGraw-Hill: New York, NY, USA, 2018; pp. 172–203. [Google Scholar]
- Malavolti, M.; Fairweather-tait, S.J.; Malagoli, C.; Vescovi, L. Lead Exposure in an Italian Population: Food Content, Dietary Intake and Risk Assessment. Food Res. Int. 2020, 137, 109370. [Google Scholar] [CrossRef]
- Năstăsescu, V.; Mititelu, M.; Goumenou, M.; Docea, A.O.; Renieri, E.; Udeanu, D.I.; Oprea, E.; Arsene, A.L.; Dinu-Pîrvu, C.E.; Ghica, M. Heavy Metal and Pesticide Levels in Dairy Products: Evaluation of Human Health Risk. Food Chem. Toxicol. 2020, 146, 111844. [Google Scholar] [CrossRef] [PubMed]
- Branciari, R.; Franceschini, R.; Roila, R.; Valiani, A.; Pecorelli, I.; Piersanti, A.; Haouet, N.; Framboas, M.; Ranucci, D. Nutritional Value and Contaminant Risk Assessment of Some Commercially Important Fishes and Crawfish of Lake Trasimeno, Italy. Int. J. Environ. Res. Public Health 2020, 17, 2545. [Google Scholar] [CrossRef] [PubMed]
- Adetunji, V.O.; Famakin, I.O.; Chen, J. Lead and Cadmium Levels in Cattle Muscle and Edible Tissues Collected from a Slaughter Slab in Nigeria. Food Addit. Contam. Part B 2014, 7, 79–83. [Google Scholar] [CrossRef] [PubMed]
- Castro-González, N.P.; Moreno-Rojas, R.; Calderón Sánchez, F.; Moreno-Ortega, A.; Tamariz-Flores, J.V. Metales Pesados En Leche de Vacas Alimentadas Con Alfalfa Producida En Suelos Irrigados Con Aguas Residuales En Puebla y Tlaxcala, México. Rev. Mex. Ciencias Pecu. 2018, 9, 466–485. [Google Scholar] [CrossRef]
- Moyo, B.; Matodzi, V.; Legodi, M.A.; Pakade, V.E.; Tavengwa, N.T. Determination of Cd, Mn and Ni Accumulated in Fruits, Vegetables and Soil in the Thohoyandou Town Area, South Africa. Water SA 2020, 46, 285–290. [Google Scholar] [CrossRef]
- Mehouel, F.; Bouayad, L.; Hammoudi, A.H.; Ayadi, O.; Regad, F. Evaluation of the Heavy Metals (Mercury, Lead, and Cadmium) Contamination of Sardine (Sardina pilchardus) and Swordfish (Xiphias gladius) Fished in Three Algerian Coasts. Vet. World 2019, 12, 7–11. [Google Scholar] [CrossRef]
- Ametepey, S.T.; Cobbina, S.J.; Akpabey, F.J.; Duwiejuah, A.B.; Abuntori, Z.N. Health Risk Assessment and Heavy Metal Contamination Levels in Vegetables from Tamale Metropolis, Ghana. Int. J. Food Contam. 2018, 5, 5. [Google Scholar] [CrossRef]
- Majlesi, M.; Malekzadeh, J.; Berizi, E.; Toori, M.A. Heavy Metal Content in Farmed Rainbow Trout in Relation to Aquaculture Area and Feed Pellets. Foods Raw Mater. 2019, 329–338. [Google Scholar] [CrossRef]
- Kribi-Boukhris, S.E.; Boughattas, I.; Zitouni, N.; Helaoui, S.; Sappin-Didier, V.; Coriou, C.; Bussiere, S.; Banni, M. Ecotoxicity of Trace Elements to Chicken GALLUS Gallus Domesticus Exposed to a Gradient of Polymetallic-Polluted Sites. Environ. Pollut. 2020, 265, 114831. [Google Scholar] [CrossRef]
- Jolly, Y.N.; Iqbal, S.; Rahman, M.S.; Kabir, J.; Akter, S.; Ahmad, I. Energy Dispersive X-Ray Fluorescence Detection of Heavy Metals in Bangladesh Cows’ Milk. Heliyon 2017, 3, e00403. [Google Scholar] [CrossRef]
- Alvarenga, P.; Simões, I.; Palma, P.; Amaral, O.; Matos, J.X. Field Study on the Accumulation of Trace Elements by Vegetables Produced in the Vicinity of Abandoned Pyrite Mines. Sci. Total Environ. 2014, 470–471, 1233–1242. [Google Scholar] [CrossRef] [PubMed]
- Ravanbakhsh, M.; Zare Javid, A.; Hadi, M.; Jaafarzadeh Haghighi Fard, N. Heavy Metals Risk Assessment in Fish Species (Johnius belangerii (C) and Cynoglossus arel) in Musa Estuary, Persian Gulf. Environ. Res. 2020, 188, 109560. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Khan, S.; Khan, A.; Tang, Y.; Nunes, L.M.; Yan, J.; Ye, X.; Li, G. Methylmercury Concentrations in Seafood Collected from Zhoushan Islands, Zhejiang, China, and Their Potential Health Risk for the Fishing Community. Environ. Int. 2020, 137, 105420. [Google Scholar] [CrossRef] [PubMed]
- Säumel, I.; Kotsyuk, I.; Hölscher, M.; Lenkereit, C.; Weber, F.; Kowarik, I. How Healthy Is Urban Horticulture in High Traffic Areas? Trace Metal Concentrations in Vegetable Crops from Plantings within Inner City Neighbourhoods in Berlin, Germany. Environ. Pollut. 2012, 165, 124–132. [Google Scholar] [CrossRef]
- Zheng, S.; Wang, Q.; Yuan, Y.; Sun, W. Human Health Risk Assessment of Heavy Metals in Soil and Food Crops in the Pearl River Delta Urban Agglomeration of China. Food Chem. 2020, 316, 126213. [Google Scholar] [CrossRef]
- Bortey-Sam, N.; Nakayama, S.M.M.; Ikenaka, Y.; Akoto, O.; Baidoo, E.; Yohannes, Y.B.; Mizukawa, H.; Ishizuka, M. Human Health Risks from Metals and Metalloid via Consumption of Food Animals near Gold Mines in Tarkwa, Ghana: Estimation of the Daily Intakes and Target Hazard Quotients (THQs). Ecotoxicol. Environ. Saf. 2015, 111, 160–167. [Google Scholar] [CrossRef]
- Miedico, O.; Pompa, C.; Moscatelli, S.; Chiappinelli, A.; Carosielli, L.; Chiaravalle, A.E. Lead, Cadmium and Mercury in Canned and Unprocessed Tuna: Six-Years Monitoring Survey, Comparison with Previous Studies and Recommended Tolerable Limits. J. Food Compos. Anal. 2020, 94, 103638. [Google Scholar] [CrossRef]
- Esposito, M.; Canzanella, S.; Lambiase, S.; Scaramuzzo, A.; La Nucara, R.; Bruno, T.; Picazio, G.; Colarusso, G.; Brunetti, R.; Gallo, P. Organic Pollutants (PCBs, PCDD/Fs, PAHs) and Toxic Metals in Farmed Mussels from the Gulf of Naples (Italy): Monitoring and Human Exposure. Reg. Stud. Mar. Sci. 2020, 40, 101497. [Google Scholar] [CrossRef]
- Adeogun, A.O.; Ibor, O.R.; Omiwole, R.; Chukwuka, A.V.; Adewale, A.H.; Kumuyi, O.; Arukwe, A. Sex-Differences in Physiological and Oxidative Stress Responses and Heavy Metals Burden in the Black Jaw Tilapia, Sarotherodon Melanotheron from a Tropical Freshwater Dam (Nigeria). Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2020, 229, 108676. [Google Scholar] [CrossRef]
- He, M.; Shen, H.; Li, Z.; Wang, L.; Wang, F.; Zhao, K.; Liu, X.; Wendroth, O.; Xu, J. Ten-Year Regional Monitoring of Soil-Rice Grain Contamination by Heavy Metals with Implications for Target Remediation and Food Safety. Environ. Pollut. 2019, 244, 431–439. [Google Scholar] [CrossRef]
- Mok, J.S.; Kwon, J.Y.; Son, K.T.; Choi, W.S.; Kang, S.R.; Ha, N.Y.; Jo, M.R.; Kim, J.H. Contents and Risk Assessment of Heavy Metals in Marine Invertebrates from Korean Coastal Fish Markets. J. Food Prot. 2014, 77, 1022–1030. [Google Scholar] [CrossRef] [PubMed]
- Najarnezhad, V.; Akbarabadi, M. Heavy Metals in Raw Cow and Ewe Milk from North-East Iran. Food Addit. Contam. Part B 2013, 6, 158–162. [Google Scholar] [CrossRef] [PubMed]
- Jokanović, M.R.; Tomović, V.M.; Šojić, B.V.; Škaljac, S.B.; Tasić, T.A.; Ikonić, P.M.; Kevrešan, Ž.S. Cadmium in Meat and Edible Offal of Free-Range Reared Swallow-Belly Mangulica Pigs from Vojvodina (Northern Serbia). Food Addit. Contam. Part B 2013, 6, 98–102. [Google Scholar] [CrossRef] [PubMed]
- Abd-Elghany, S.M.; Mohammed, M.A.; Abdelkhalek, A.; Saad, F.S.S.; Sallam, K.I. Health Risk Assessment of Exposure to Heavy Metals from Sheep Meat and Offal in Kuwait. J. Food Prot. 2020, 83, 503–510. [Google Scholar] [CrossRef] [PubMed]
- Luis, G.; Rubio, C.; González-Weller, D.; Gutiérrez, A.J.; Revert, C.; Hardisson, A. Evaluation of Content and Estimation of Daily Intake of Cadmium and Lead in Several Varieties of Potatoes (Solanum tuberosum L.) Cultivated in the Canary Islands (Spain). J. Food Prot. 2014, 77, 659–664. [Google Scholar] [CrossRef] [PubMed]
- Von Hoffen, L.P.; Säumel, I. Orchards for Edible Cities: Cadmium and Lead Content in Nuts, Berries, Pome and Stone Fruits Harvested within the Inner City Neighbourhoods in Berlin, Germany. Ecotoxicol. Environ. Saf. 2014, 101, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.A.; Rahman, M.M.; Reichman, S.M.; Lim, R.P.; Naidu, R. Heavy Metals in Australian Grown and Imported Rice and Vegetables on Sale in Australia: Health Hazard. Ecotoxicol. Environ. Saf. 2014, 100, 53–60. [Google Scholar] [CrossRef]
- Chang, C.Y.; Yu, H.Y.; Chen, J.J.; Li, F.B.; Zhang, H.H.; Liu, C.P. Accumulation of Heavy Metals in Leaf Vegetables from Agricultural Soils and Associated Potential Health Risks in the Pearl River Delta, South China. Environ. Monit. Assess. 2014, 186, 1547–1560. [Google Scholar] [CrossRef]
- Zhou, H.; Zeng, M.; Zhou, X.; Liao, B.-H.; Liu, J.; Lei, M.; Zhong, Q.-Y.; Zeng, H. Assessment of Heavy Metal Contamination and Bioaccumulation in Soybean Plants from Mining and Smelting Areas of Southern Hunan Province, China. Environ. Toxicol. Chem. 2013, 32, 2719–2727. [Google Scholar] [CrossRef]
- Heidari, B.; Riyahi Bakhtiari, A.; Shirneshan, G. Concentrations of Cd, Cu, Pb and Zn in Soft Tissue of Oyster (Saccostrea cucullata) Collected from the Lengeh Port Coast, Persian Gulf, Iran: A Comparison with the Permissible Limits for Public Health. Food Chem. 2013, 141, 3014–3019. [Google Scholar] [CrossRef]
- Xia, C.; Wu, X.; Lam, J.C.W.; Xie, Z.; Lam, P.K.S. Methylmercury and Trace Elements in the Marine Fish from Coasts of East China. J. Environ. Sci. Health Part A 2013, 48, 1491–1501. [Google Scholar] [CrossRef] [PubMed]
- Olmedo, P.; Pla, A.; Hernández, A.F.; Barbier, F.; Ayouni, L.; Gil, F. Determination of Toxic Elements (Mercury, Cadmium, Lead, Tin and Arsenic) in Fish and Shellfish Samples. Risk Assessment for the Consumers. Environ. Int. 2013, 59, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Al-Mughairi, S.; Yesudhason, P.; Al-Busaidi, M.; Al-Waili, A.; Al-Rahbi, W.A.K.; Al-Mazrooei, N.; Al-Habsi, S.H. Concentration and Exposure Assessment of Mercury in Commercial Fish and Other Seafood Marketed in Oman. J. Food Sci. 2013, 78, T1082–T1090. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Zhou, P.; Zhan, J.; Gao, Y.; Dou, C.; Sun, Q. Assessment of Trace Metal Bioavailability in Garden Soils and Health Risks via Consumption of Vegetables in the Vicinity of Tongling Mining Area, China. Ecotoxicol. Environ. Saf. 2013, 90, 103–111. [Google Scholar] [CrossRef]
- Shahriar, S.; Rahman, M.M.; Naidu, R. Geographical Variation of Cadmium in Commercial Rice Brands in Bangladesh: Human Health Risk Assessment. Sci. Total Environ. 2020, 716, 137049. [Google Scholar] [CrossRef]
- Batista, B.L.; Grotto, D.; Carneiro, M.F.H.; Barbosa, F. Evaluation of the Concentration of Nonessential and Essential Elements in Chicken, Pork, and Beef Samples Produced in Brazil. J. Toxicol. Environ. Health Part A 2012, 75, 1269–1279. [Google Scholar] [CrossRef]
- Salazar, M.J.; Rodriguez, J.H.; Nieto, G.L.; Pignata, M.L. Effects of Heavy Metal Concentrations (Cd, Zn and Pb) in Agricultural Soils near Different Emission Sources on Quality, Accumulation and Food Safety in Soybean [Glycine max (L.) Merrill]. J. Hazard. Mater. 2012, 233–234, 244–253. [Google Scholar] [CrossRef]
- Li, Q.; Chen, Y.; Fu, H.; Cui, Z.; Shi, L.; Wang, L.; Liu, Z. Health Risk of Heavy Metals in Food Crops Grown on Reclaimed Tidal Flat Soil in the Pearl River Estuary, China. J. Hazard. Mater. 2012, 227–228, 148–154. [Google Scholar] [CrossRef]
- Cheraghi, M.; Lorestani, B.; Merrikhpour, H.; Rouniasi, N. Heavy Metal Risk Assessment for Potatoes Grown in Overused Phosphate-Fertilized Soils. Environ. Monit. Assess. 2013, 185, 1825–1831. [Google Scholar] [CrossRef]
- Shao, D.D.; Wu, S.C.; Liang, P.; Kang, Y.; Fu, W.J.; Zhao, K.L.; Cao, Z.H.; Wong, M.H. A Human Health Risk Assessment of Mercury Species in Soil and Food around Compact Fluorescent Lamp Factories in Zhejiang Province, PR China. J. Hazard. Mater. 2012, 221–222, 28–34. [Google Scholar] [CrossRef]
- Lourenço, H.M.; Afonso, C.; Anacleto, P.; Martins, M.F.; Nunes, M.L.; Lino, A.R. Elemental Composition of Four Farmed Fish Produced in Portugal. Int. J. Food Sci. Nutr. 2012, 63, 853–859. [Google Scholar] [CrossRef] [PubMed]
- Al-Rmalli, S.W.; Jenkins, R.O.; Haris, P.I. Dietary Intake of Cadmium from Bangladeshi Foods. J. Food Sci. 2012, 77, T26–T33. [Google Scholar] [CrossRef] [PubMed]
- Al-Busaidi, M.; Yesudhason, P.; Al-Mughairi, S.; Al-Rahbi, W.A.K.; Al-Harthy, K.S.; Al-Mazrooei, N.A.; Al-Habsi, S.H. Toxic Metals in Commercial Marine Fish in Oman with Reference to National and International Standards. Chemosphere 2011, 85, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Xu, Y.; Liu, S.; He, J.; Long, F. Concentration and Potential Health Risk of Heavy Metals in Market Vegetables in Chongqing, China. Ecotoxicol. Environ. Saf. 2011, 74, 1664–1669. [Google Scholar] [CrossRef]
- Lei, L.; Liang, D.; Yu, D.; Chen, Y.; Song, W.; Li, J. Human Health Risk Assessment of Heavy Metals in the Irrigated Area of Jinghui, Shaanxi, China, in Terms of Wheat Flour Consumption. Environ. Monit. Assess. 2015, 187, 647. [Google Scholar] [CrossRef]
- Li, L.; Feng, H.; Wei, J. Toxic Element (As and Hg) Content and Health Risk Assessment of Commercially Available Rice for Residents in Beijing Based on Their Dietary Consumption. Environ. Sci. Pollut. Res. 2020, 27, 13205–13214. [Google Scholar] [CrossRef]
- Burioli, E.A.V.; Squadrone, S.; Stella, C.; Foglini, C.; Abete, M.C.; Prearo, M. Trace Element Occurrence in the Pacific Oyster Crassostrea Gigas from Coastal Marine Ecosystems in Italy. Chemosphere 2017, 187, 248–260. [Google Scholar] [CrossRef]
- Chijioke, N.O.; Uddin Khandaker, M.; Tikpangi, K.M.; Bradley, D.A. Metal Uptake in Chicken Giblets and Human Health Implications. J. Food Compos. Anal. 2020, 85, 103332. [Google Scholar] [CrossRef]
- Damerau, A.; Venäläinen, E.R.; Peltonen, K. Heavy Metals in Meat of Finnish City Rabbits. Food Addit. Contam. Part B 2012, 5, 246–250. [Google Scholar] [CrossRef]
- Hassan, A.A.; Brustad, M.; Sandanger, T.M. Concentrations and Geographical Variations of Selected Toxic Elements in Meat from Semi-Domesticated Reindeer (Rangifer tarandus Tarandus L.) in Mid- and Northern Norway: Evaluation of Risk Assessment. Int. J. Environ. Res. Public Health 2012, 9, 1699–1714. [Google Scholar] [CrossRef]
- Zupka, S.; Vollmannová, A.; Harangozo, Ľ.; Slávik, M.; Medvecký, M. Risk of Contamination of Wild Berries from Upper Orava Region by Cadmium. Potravin. Slovak J. Food Sci. 2016, 10, 126–131. [Google Scholar] [CrossRef][Green Version]
- Filippini, T.; Tancredi, S.; Malagoli, C.; Cilloni, S.; Malavolti, M.; Violi, F.; Vescovi, L.; Bargellini, A.; Vinceti, M. Aluminum and Tin: Food Contamination and Dietary Intake in an Italian Population. J. Trace Elem. Med. Biol. 2019, 52, 293–301. [Google Scholar] [CrossRef]
- Akele, M.L.; Abebe, D.Z.; Alemu, A.K.; Assefa, A.G.; Madhusudhan, A.; de Oliveira, R.R. Analysis of Trace Metal Concentrations in Raw Cow’s Milk from Three Dairy Farms in North Gondar, Ethiopia: Chemometric Approach. Environ. Monit. Assess. 2017, 189, 499. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Han, J.; Abeysinghe, K.S.; Atapattu, A.J.; De Silva, P.M.C.S.; Xu, Z.; Long, S.; Qiu, G. Dietary Exposure Assessment of Total Mercury and Methylmercury in Commercial Rice in Sri Lanka. Chemosphere 2020, 239, 124749. [Google Scholar] [CrossRef] [PubMed]
- Brizio, P.; Benedetto, A.; Squadrone, S.; Curcio, A.; Pellegrino, M.; Ferrero, M.; Abete, M.C. Heavy Metals and Essential Elements in Italian Cereals. Food Addit. Contam. Part B 2016, 9, 261–267. [Google Scholar] [CrossRef]
- Zhao, H.; Yan, H.; Zhang, L.; Sun, G.; Li, P.; Feng, X. Mercury Contents in Rice and Potential Health Risks across China. Environ. Int. 2019, 126, 406–412. [Google Scholar] [CrossRef]
- Heshmati, A.; Mehri, F.; Karami-Momtaz, J.; Khaneghah, A.M. Concentration and Risk Assessment of Potentially Toxic Elements, Lead and Cadmium, in Vegetables and Cereals Consumed in Western Iran. J. Food Prot. 2020, 83, 101–107. [Google Scholar] [CrossRef]
- Potí, P.; Pajor, F.; Bodnár, Á.; Bárdos, L. Accumulation of Some Heavy Metals (Pb, Cd and Cr) in Milk of Grazing Sheep in North-East Hungary. J. Microbiol. Biotechnol. Food Sci. 2012, 2, 389–394. [Google Scholar]
- Ihedioha, J.N.; Ekere, N.R.; Okoye, C.O.B. Cadmium in Locally Grown Rice (Oryza Sativa) in Nigeria. Food Addit. Contam. Part B 2013, 6, 275–278. [Google Scholar] [CrossRef]
- Malvandi, H.; Alahabadi, A. Evaluation of Potential Human Health Risk Due to the Exposure to Mercury via Fish Consumption of Alosa Spp. from the Southern Caspian Sea. Mar. Pollut. Bull. 2019, 143, 66–71. [Google Scholar] [CrossRef]
- Gong, Y.; Chai, M.; Ding, H.; Shi, C.; Wang, Y.; Li, R. Bioaccumulation and Human Health Risk of Shellfish Contamination to Heavy Metals and As in Most Rapid Urbanized Shenzhen, China. Environ. Sci. Pollut. Res. 2020, 27, 2096–2106. [Google Scholar] [CrossRef] [PubMed]
- Djermanovic, M.; Baralic, I.; Pejic, S. Total Mercury Levels in Commercial Fish in Market of the Republic of Srpska, Bosnia and Herzegovina. Biol. Trace Elem. Res. 2020, 194, 545–551. [Google Scholar] [CrossRef] [PubMed]
- Aendo, P.; Thongyuan, S.; Songserm, T.; Tulayakul, P. Carcinogenic and Non-Carcinogenic Risk Assessment of Heavy Metals Contamination in Duck Eggs and Meat as a Warning Scenario in Thailand. Sci. Total Environ. 2019, 689, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Custódio, F.B.; Andrade, A.M.G.F.; Guidi, L.R.; Leal, C.A.G.; Gloria, M.B.A. Total Mercury in Commercial Fishes and Estimation of Brazilian Dietary Exposure to Methylmercury. J. Trace Elem. Med. Biol. 2020, 62, 126641. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Wang, D.; Wang, M.; Zhou, F.; Huang, J.; Xue, M.; Dinh, Q.T.; Liang, D. Heavy Metals and Associated Health Risk of Wheat Grain in a Traditional Cultivation Area of Baoji, Shaanxi, China. Environ. Monit. Assess. 2019, 191, 428. [Google Scholar] [CrossRef]
- Hwang, D.-W.; Shim, K.; Lee, C. Il Concentrations and Risk Assessment of Heavy Metals in Tissues of Walleye Pollock (Gadus chalcogrammus) Captured from the Northeastern Coast of Korea. J. Food Prot. 2019, 82, 903–909. [Google Scholar] [CrossRef]
- Annibaldi, A.; Truzzi, C.; Carnevali, O.; Pignalosa, P.; Api, M.; Scarponi, G.; Illuminati, S. Determination of Hg in Farmed and Wild Atlantic Bluefin Tuna (Thunnus thynnus L.) Muscle. Molecules 2019, 24, 1273. [Google Scholar] [CrossRef]
- Darwish, W.S.; Chiba, H.; Elhelaly, A.E.; Hui, S.-P. Estimation of Cadmium Content in Egyptian Foodstuffs: Health Risk Assessment, Biological Responses of Human HepG2 Cells to Food-Relevant Concentrations of Cadmium, and Protection Trials Using Rosmarinic and Ascorbic Acids. Environ. Sci. Pollut. Res. 2019, 26, 15443–15457. [Google Scholar] [CrossRef]
- Kim, S.W.; Han, S.J.; Kim, Y.; Jun, J.W.; Giri, S.S.; Chi, C.; Yun, S.; Kim, H.J.; Kim, S.G.; Kang, J.W.; et al. Heavy Metal Accumulation in and Food Safety of Shark Meat from Jeju Island, Republic of Korea. PLoS ONE 2019, 14, e0212410. [Google Scholar] [CrossRef]
- Kato, L.S.; De Nadai Fernandes, E.A.; Raab, A.; Bacchi, M.A.; Feldmann, J. Arsenic and Cadmium Contents in Brazilian Rice from Different Origins Can Vary More than Two Orders of Magnitude. Food Chem. 2019, 286, 644–650. [Google Scholar] [CrossRef]
- Lin, H.; Santa-Rios, A.; Barst, B.D.; Basu, N.; Bayen, S. Occurrence and Bioaccessibility of Mercury in Commercial Rice Samples in Montreal (Canada). Food Chem. Toxicol. 2019, 126, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Zeinali, T.; Salmani, F.; Naseri, K. Dietary Intake of Cadmium, Chromium, Copper, Nickel, and Lead through the Consumption of Meat, Liver, and Kidney and Assessment of Human Health Risk in Birjand, Southeast of Iran. Biol. Trace Elem. Res. 2019, 191, 338–347. [Google Scholar] [CrossRef]
- Rahman, M.; Islam, M.A. Concentrations and Health Risk Assessment of Trace Elements in Cereals, Fruits, and Vegetables of Bangladesh. Biol. Trace Elem. Res. 2019, 191, 243–253. [Google Scholar] [CrossRef]
- Paz, S.; Rubio, C.; Frías, I.; Gutiérrez, Á.J.; González-Weller, D.; Martín, V.; Revert, C.; Hardisson, A. Toxic Metals (Al, Cd, Pb and Hg) in the Most Consumed Edible Seaweeds in Europe. Chemosphere 2019, 218, 879–884. [Google Scholar] [CrossRef] [PubMed]
- Di Bella, G.; Bua, G.D.; Fede, M.R.; Mottese, A.F.; Potortì, A.G.; Cicero, N.; Benameur, Q.; Dugo, G.; Lo Turco, V. Potentially Toxic Elements in Xiphias gladius from Mediterranean Sea and Risks Related to Human Consumption. Mar. Pollut. Bull. 2020, 159, 111512. [Google Scholar] [CrossRef] [PubMed]
- Traina, A.; Bono, G.; Bonsignore, M.; Falco, F.; Giuga, M.; Quinci, E.M.; Vitale, S.; Sprovieri, M. Heavy Metals Concentrations in Some Commercially Key Species from Sicilian Coasts (Mediterranean Sea): Potential Human Health Risk Estimation. Ecotoxicol. Environ. Saf. 2019, 168, 466–478. [Google Scholar] [CrossRef] [PubMed]
- Zariff, R.Z.; Pumpa, L.; Simon, D.L.; Lewis, C. Locally Produced Chicken Eggs—a Source of Dietary Lead for an Australian Community Living with an Active Lead Smelter? J. Expo. Sci. Environ. Epidemiol. 2019, 29, 688–696. [Google Scholar] [CrossRef]
- Rahmdel, S.; Rezaei, M.; Ekhlasi, J.; Zarei, S.H.; Akhlaghi, M.; Abdollahzadeh, S.M.; Sefidkar, R.; Mazloomi, S.M. Heavy Metals (Pb, Cd, Cu, Zn, Ni, Co) in Leafy Vegetables Collected from Production Sites: Their Potential Health Risk to the General Population in Shiraz, Iran. Environ. Monit. Assess. 2018, 190, 650. [Google Scholar] [CrossRef]
- Zamora-Arellano, N.; Betancourt-Lozano, M.; Ilizaliturri-Hernández, C.; García-Hernández, J.; Jara-Marini, M.; Chávez-Sánchez, C.; Ruelas-Inzunza, J.R. Mercury Levels and Risk Implications Through Fish Consumption on the Sinaloa Coasts (Gulf of California, Northwest Mexico). Risk Anal. 2018, 38, 2646–2658. [Google Scholar] [CrossRef]
- Fakhri, Y.; Mousavi Khaneghah, A.; Conti, G.O.; Ferrante, M.; Khezri, A.; Darvishi, A.; Ahmadi, M.; Hasanzadeh, V.; Rahimizadeh, A.; Keramati, H.; et al. Probabilistic Risk Assessment (Monte Carlo Simulation Method) of Pb and Cd in the Onion Bulb (Allium cepa) and Soil of Iran. Environ. Sci. Pollut. Res. 2018, 25, 30894–30906. [Google Scholar] [CrossRef]
- Costa, R.G.; da Silva Araújo, C.F.; Ferreol Bah, A.H.; Junior, E.A.G.; de Melo Rodrigues, Y.J.; Menezes-Filho, J.A. Lead in Mangrove Root Crab (Goniopsis cruentata) and Risk Assessment Due to Exposure for Estuarine Villagers. Food Addit. Contam. Part B 2018, 11, 293–301. [Google Scholar] [CrossRef] [PubMed]
- Lehel, J.; Bartha, A.; Dankó, D.; Lányi, K.; Laczay, P. Heavy Metals in Seafood Purchased from a Fishery Market in Hungary. Food Addit. Contam. Part B 2018, 11, 302–308. [Google Scholar] [CrossRef] [PubMed]
- Huo, J.; Huang, Z.; Li, R.; Song, Y.; Lan, Z.; Ma, S.; Wu, Y.; Chen, J.; Zhang, L. Dietary Cadmium Exposure Assessment in Rural Areas of Southwest China. PLoS ONE 2018, 13, e0201454. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Yang, X.; Wang, P.; Wang, Z.; Li, M.; Zhao, F.-J. Dietary Cadmium Intake from Rice and Vegetables and Potential Health Risk: A Case Study in Xiangtan, Southern China. Sci. Total Environ. 2018, 639, 271–277. [Google Scholar] [CrossRef]
- Bi, C.; Zhou, Y.; Chen, Z.; Jia, J.; Bao, X. Heavy Metals and Lead Isotopes in Soils, Road Dust and Leafy Vegetables and Health Risks via Vegetable Consumption in the Industrial Areas of Shanghai, China. Sci. Total Environ. 2018, 619–620, 1349–1357. [Google Scholar] [CrossRef]
- Djedjibegovic, J.; Marjanovic, A.; Tahirovic, D.; Caklovica, K.; Turalic, A.; Lugusic, A.; Omeragic, E.; Sober, M.; Caklovica, F. Heavy Metals in Commercial Fish and Seafood Products and Risk Assessment in Adult Population in Bosnia and Herzegovina. Sci. Rep. 2020, 10, 13238. [Google Scholar] [CrossRef] [PubMed]
- Djahed, B.; Taghavi, M.; Farzadkia, M.; Norzaee, S.; Miri, M. Stochastic Exposure and Health Risk Assessment of Rice Contamination to the Heavy Metals in the Market of Iranshahr, Iran. Food Chem. Toxicol. 2018, 115, 405–412. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Tang, Z.; Wang, P.; Zhao, F.-J. Geographical Variations of Cadmium and Arsenic Concentrations and Arsenic Speciation in Chinese Rice. Environ. Pollut. 2018, 238, 482–490. [Google Scholar] [CrossRef]
- Mallory, M.L.; O’Driscoll, N.J.; Klapstein, S.; Varela, J.L.; Ceapa, C.; Stokesbury, M.J. Methylmercury in Tissues of Atlantic Sturgeon (Acipenser oxyrhynchus) from the Saint John River, New Brunswick, Canada. Mar. Pollut. Bull. 2018, 126, 250–254. [Google Scholar] [CrossRef]
- Ding, Z.; Li, Y.; Sun, Q.; Zhang, H. Trace Elements in Soils and Selected Agricultural Plants in the Tongling Mining Area of China. Int. J. Environ. Res. Public Health 2018, 15, 202. [Google Scholar] [CrossRef]
- Giri, S.; Singh, A.K. Heavy Metals in Eggs and Chicken and the Associated Human Health Risk Assessment in the Mining Areas of Singhbhum Copper Belt, India. Arch. Environ. Occup. Health 2019, 74, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Hoang, V.A.T.; Sakamoto, M.; Yamamoto, M. Mercury and Selenium Levels, and Their Molar Ratios in Several Species of Commercial Shrimp in Japan Regarding the Health Risk of Methylmercury Exposure. J. Toxicol. Sci. 2017, 42, 509–517. [Google Scholar] [CrossRef] [PubMed]
- Varol, M.; Kaya, G.K.; Alp, A. Heavy Metal and Arsenic Concentrations in Rainbow Trout (Oncorhynchus mykiss) Farmed in a Dam Reservoir on the Firat (Euphrates) River: Risk-Based Consumption Advisories. Sci. Total Environ. 2017, 599–600, 1288–1296. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Zhang, X.; Tang, Z.; Yang, Y.; Nie, Z.; Huang, Q. Concentrations and Human Health Implications of Heavy Metals in Market Foods from a Chinese Coal-Mining City. Environ. Toxicol. Pharmacol. 2017, 50, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Stančić, Z.; Vujević, D.; Gomaz, A.; Bogdan, S.; Vincek, D. Detection of Heavy Metals in Common Vegetables at Varaždin City Market, Croatia. Arch. Ind. Hyg. Toxicol. 2016, 67, 340–350. [Google Scholar] [CrossRef]
- Roya, A.Q.; Ali, M.S. Heavy Metals in Rice Samples on the Torbat-Heidarieh Market, Iran. Food Addit. Contam. Part B 2017, 10, 59–63. [Google Scholar] [CrossRef]
- Di Bella, C.; Traina, A.; Giosuè, C.; Carpintieri, D.; Lo Dico, G.M.; Bellante, A.; Del Core, M.; Falco, F.; Gherardi, S.; Uccello, M.M.; et al. Heavy Metals and PAHs in Meat, Milk, and Seafood from Augusta Area (Southern Italy): Contamination Levels, Dietary Intake, and Human Exposure Assessment. Front. Public Health 2020, 8, 273. [Google Scholar] [CrossRef]
- García, M.Á.; Núñez, R.; Alonso, J.; Melgar, M.J. Total Mercury in Fresh and Processed Tuna Marketed in Galicia (NW Spain) in Relation to Dietary Exposure. Environ. Sci. Pollut. Res. 2016, 23, 24960–24969. [Google Scholar] [CrossRef]
- Xie, L.H.; Tang, S.Q.; Wei, X.J.; Shao, G.N.; Jiao, G.A.; Sheng, Z.H.; Luo, J.; Hu, P.S. The Cadmium and Lead Content of the Grain Produced by Leading Chinese Rice Cultivars. Food Chem. 2017, 217, 217–224. [Google Scholar] [CrossRef]
- Adel, M.; Oliveri Conti, G.; Dadar, M.; Mahjoub, M.; Copat, C.; Ferrante, M. Heavy Metal Concentrations in Edible Muscle of Whitecheek Shark, Carcharhinus dussumieri (Elasmobranchii, chondrichthyes) from the Persian Gulf: A Food Safety Issue. Food Chem. Toxicol. 2016, 97, 135–140. [Google Scholar] [CrossRef]
- Ross, D.A.; Guzmán, H.M.; Van Hinsberg, V.J.; Potvin, C. Metal Contents of Marine Turtle Eggs (Chelonia Mydas; Lepidochelys Olivacea) from the Tropical Eastern Pacific and the Implications for Human Health. J. Environ. Sci. Health Part B 2016, 51, 675–687. [Google Scholar] [CrossRef] [PubMed]
- López-Alonso, M.; Miranda, M.; Benedito, J.L.; Pereira, V.; García-Vaquero, M. Essential and Toxic Trace Element Concentrations in Different Commercial Veal Cuts in Spain. Meat Sci. 2016, 121, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Avci, H.; Deveci, T. Assessment of Trace Element Concentrations in Soil and Plants from Cropland Irrigated with Wastewater. Ecotoxicol. Environ. Saf. 2013, 98, 283–291. [Google Scholar] [CrossRef]
- Islam, M.S.; Ahmed, M.K.; Habibullah-Al-Mamun, M.; Masunaga, S. Assessment of Trace Metals in Foodstuffs Grown around the Vicinity of Industries in Bangladesh. J. Food Compos. Anal. 2015, 42, 8–15. [Google Scholar] [CrossRef]
- Djedjibegovic, J.; Larssen, T.; Skrbo, A.; Marjanović, A.; Sober, M. Contents of Cadmium, Copper, Mercury and Lead in Fish from the Neretva River (Bosnia and Herzegovina) Determined by Inductively Coupled Plasma Mass Spectrometry (ICP-MS). Food Chem. 2012, 131, 469–476. [Google Scholar] [CrossRef]
- Derakhshan, Z.; Mahvi, A.H.; Faramarzian, M.; Dehghani, M.; Salari, M.; Fakhri, Y.; Afsharnia, M.; Hosseini, M.S.; Marzban, A.; Taghavi, M. Data on Heavy Metal Concentration in Common Carp Fish Consumed in Shiraz, Iran. Data Br. 2018, 21, 1890–1894. [Google Scholar] [CrossRef]
- Wu, X.-Y.; Yang, Y.-F. Heavy Metal (Pb, Co, Cd, Cr, Cu, Fe, Mn and Zn) Concentrations in Harvest-Size White Shrimp Litopenaeus Vannamei Tissues from Aquaculture and Wild Source. J. Food Compos. Anal. 2011, 24, 62–65. [Google Scholar] [CrossRef]
- Liu, L.; Han, J.; Xu, X.; Xu, Z.; Abeysinghe, K.S.; Atapattu, A.J.; De Silva, P.M.C.S.; Lu, Q.; Qiu, G. Dietary Exposure Assessment of Cadmium, Arsenic, and Lead in Market Rice from Sri Lanka. Environ. Sci. Pollut. Res. 2020, 27, 42704–42712. [Google Scholar] [CrossRef]
- Peng, M.; Zhao, C.; Ma, H.; Yang, Z.; Yang, K.; Liu, F.; Li, K.; Yang, Z.; Tang, S.; Guo, F.; et al. Heavy Metal and Pb Isotopic Compositions of Soil and Maize from a Major Agricultural Area in Northeast China: Contamination Assessment and Source Apportionment. J. Geochemical Explor. 2020, 208, 106403. [Google Scholar] [CrossRef]
- Keshavarzi, B.; Hassanaghaei, M.; Moore, F.; Rastegari Mehr, M.; Soltanian, S.; Lahijanzadeh, A.R.; Sorooshian, A. Heavy Metal Contamination and Health Risk Assessment in Three Commercial Fish Species in the Persian Gulf. Mar. Pollut. Bull. 2018, 129, 245–252. [Google Scholar] [CrossRef]
- Rabiul Islam, G.M.; Habib, M.R.; Waid, J.L.; Rahman, M.S.; Kabir, J.; Akter, S.; Jolly, Y.N. Heavy Metal Contamination of Freshwater Prawn (Macrobrachium rosenbergii) and Prawn Feed in Bangladesh: A Market-Based Study to Highlight Probable Health Risks. Chemosphere 2017, 170, 282–289. [Google Scholar] [CrossRef] [PubMed]
- Antoniadis, V.; Golia, E.E.; Liu, Y.-T.; Wang, S.-L.; Shaheen, S.M.; Rinklebe, J. Soil and Maize Contamination by Trace Elements and Associated Health Risk Assessment in the Industrial Area of Volos, Greece. Environ. Int. 2019, 124, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Zhang, C.; Zhang, X.; Wang, G.; Li, L.; Geng, H.; Liu, Y.; Nie, C. Survey of Aflatoxin B1 and Heavy Metal Contamination in Peanut and Peanut Soil in China during 2017–2018. Food Control 2020, 118, 107372. [Google Scholar] [CrossRef]
- Naghipour, D.; Chenari, M.A.; Taheri, N.; Naghipour, F.; Mehrabian, F.; Attarchi, M.S.; Jaafari, J.; Roubakhsh, E. The Concentration Data of Heavy Metals in Vegetables of Guilan Province, Iran. Data Br. 2018, 21, 1704–1708. [Google Scholar] [CrossRef] [PubMed]
- De Souza Araújo, D.F.; da Silva, A.M.R.B.; de Andrade Lima, L.L.; da Silva Vasconcelos, M.A.; Andrade, S.A.C.; Asfora Sarubbo, L. The Concentration of Minerals and Physicochemical Contaminants in Conventional and Organic Vegetables. Food Control 2014, 44, 242–248. [Google Scholar] [CrossRef]
- Anandkumar, A.; Li, J.; Prabakaran, K.; Xi Jia, Z.; Leng, Z.; Nagarajan, R.; Du, D. Accumulation of Toxic Elements in an Invasive Crayfish Species (Procambarus clarkii) and Its Health Risk Assessment to Humans. J. Food Compos. Anal. 2020, 88, 103449. [Google Scholar] [CrossRef]
- Krejčová, A.; Návesník, J.; Jičínská, J.; Černohorský, T. An Elemental Analysis of Conventionally, Organically and Self-Grown Carrots. Food Chem. 2016, 192, 242–249. [Google Scholar] [CrossRef]
- Nikolic, N.; Borisev, M.; Pajevic, S.; Arsenov, D.; Zupunski, M. Comparative Assessment of Mineral Elements and Heavy Metals Accumulation in Vegetable Species. Food Feed Res. 2014, 41, 115–123. [Google Scholar] [CrossRef]
- Kukusamude, C.; Sricharoen, P.; Limchoowong, N.; Kongsri, S. Heavy Metals and Probabilistic Risk Assessment via Rice Consumption in Thailand. Food Chem. 2021, 334, 127402. [Google Scholar] [CrossRef]
- Bat, L.; Öztekin, A.; Arici, E.; Şahin, F. Health Risk Assessment: Heavy Metals in Fish from the Southern Black Sea. Foods Raw Mater. 2020, 8, 115–124. [Google Scholar] [CrossRef]
- Taghipour, H.; Mosaferi, M. Heavy Metals in the Vegetables Collected from Production Sites. Health Promot Perspect 2013, 3, 185–193. [Google Scholar] [PubMed]
- Guerra, F.; Trevizam, A.R.; Muraoka, T.; Marcante, N.C.; Canniatti-Brazaca, S.G. Heavy Metals in Vegetables and Potential Risk for Human Health. Sci. Agric. 2012, 69, 54–60. [Google Scholar] [CrossRef]
- Melai, V.; Giovannini, A.; Chiumiento, F.; Bellocci, M.; Migliorati, G. Occurrence of Metals in Vegetables and Fruits from Areas near Landfill in Southern Italy and Implications for Human Exposure. Int. J. Food Contam. 2018, 5, 8. [Google Scholar] [CrossRef]
- Shaheen, N.; Irfan, N.M.; Khan, I.N.; Islam, S.; Islam, M.S.; Ahmed, M.K. Presence of Heavy Metals in Fruits and Vegetables: Health Risk Implications in Bangladesh. Chemosphere 2016, 152, 431–438. [Google Scholar] [CrossRef]
- Zhou, H.; Yang, W.-T.; Zhou, X.; Liu, L.; Gu, J.-F.; Wang, W.-L.; Zou, J.-L.; Tian, T.; Peng, P.-Q.; Liao, B.-H. Accumulation of Heavy Metals in Vegetable Species Planted in Contaminated Soils and the Health Risk Assessment. Int. J. Environ. Res. Public Health 2016, 13, 289. [Google Scholar] [CrossRef] [PubMed]
- Bilandžić, N.; Sedak, M.; Čalopek, B.; Luburić, Đ.B.; Solomun Kolanović, B.; Varenina, I.; Đokić, M.; Kmetič, I.; Murati, T. Lead Concentrations in Raw Cow and Goat Milk Collected in Rural Areas of Croatia from 2010 to 2014. Bull. Environ. Contam. Toxicol. 2016, 96, 645–649. [Google Scholar] [CrossRef]
- Pan, X.-D.; Wu, P.-G.; Jiang, X.-G. Levels and Potential Health Risk of Heavy Metals in Marketed Vegetables in Zhejiang, China. Sci. Rep. 2016, 6, 20317. [Google Scholar] [CrossRef]
- Ariano, A.; Lo Voi, A.; D’Ambola, M.; Marrone, R.; Cacace, D.; Severino, L. Levels of Cadmium in White and Brown Meat of Warty Crab (Eriphia verrucosa). J. Food Prot. 2015, 78, 2253–2256. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.-G.; Kim, M.; Shin, J.Y.; Son, S.-W. Cadmium and Lead in Animal Tissue (Muscle, Liver and Kidney), Cow Milk and Dairy Products in Korea. Food Addit. Contam. Part B 2016, 9, 33–37. [Google Scholar] [CrossRef]
- Milatou, N.; Dassenakis, M.; Megalofonou, P. Mercury Concentrations in Reared Atlantic Bluefin Tuna and Risk Assessment for the Consumers: To Eat or Not to Eat? Food Chem. 2020, 331, 127267. [Google Scholar] [CrossRef]
- Arroyo-Abad, U.; Pfeifer, M.; Mothes, S.; Stärk, H.-J.; Piechotta, C.; Mattusch, J.; Reemtsma, T. Determination of Moderately Polar Arsenolipids and Mercury Speciation in Freshwater Fish of the River Elbe (Saxony, Germany). Environ. Pollut. 2016, 208, 458–466. [Google Scholar] [CrossRef] [PubMed]
- Pirsaheb, M.; Fattahi, N.; Sharafi, K.; Khamotian, R.; Atafar, Z. Essential and Toxic Heavy Metals in Cereals and Agricultural Products Marketed in Kermanshah, Iran, and Human Health Risk Assessment. Food Addit. Contam. Part B 2016, 9, 15–20. [Google Scholar] [CrossRef]
- Rahayu, R.N.; Irawan, B.; Soegianto, A. Concentration of Mercury in Cockles (Anadara granosa and A. antiquata) Harvested from Estuaries of Western Lombok, Indonesia, and Potential Risks to Human Health. Bull. Environ. Contam. Toxicol. 2016, 96, 20–24. [Google Scholar] [CrossRef] [PubMed]
- Molognoni, L.; Vitali, L.; Ploêncio, L.A.; Santos, J.N.; Daguer, H. Determining the Arsenic, Cadmium, Lead, Copper and Chromium Contents by Atomic Absorption Spectrometry in Pangasius Fillets from Vietnam. J. Sci. Food Agric. 2016, 96, 3109–3113. [Google Scholar] [CrossRef] [PubMed]
- Musilova, J.; Bystricka, J.; Lachman, J.; Harangozo, L.; Trebichalsky, P.; Volnova, B. Potatoes—A Crop Resistant against Input of Heavy Metals from the Metallicaly Contaminated Soil. Int. J. Phytoremediation 2016, 18, 547–552. [Google Scholar] [CrossRef]
- Esposito, M.; Picazio, G.; Serpe, P.; Lambiase, S.; Cerino, P. Content of Cadmium and Lead in Vegetables and Fruits Grown in the Campania Region of Italy. J. Food Prot. 2015, 78, 1760–1765. [Google Scholar] [CrossRef]
- Tang, W.; Cheng, J.; Zhao, W.; Wang, W. Mercury Levels and Estimated Total Daily Intakes for Children and Adults from an Electronic Waste Recycling Area in Taizhou, China: Key Role of Rice and Fish Consumption. J. Environ. Sci. 2015, 34, 107–115. [Google Scholar] [CrossRef]
- Bilandžić, N.; Sedak, M.; Čalopek, B.; Džafić, N.; Ostojić, D.M.; Potočnjak, D. Metal Content in Four Shellfish Species from the Istrian Coast of Croatia. Bull. Environ. Contam. Toxicol. 2015, 95, 611–617. [Google Scholar] [CrossRef]
- Norton, G.J.; Deacon, C.M.; Mestrot, A.; Feldmann, J.; Jenkins, P.; Baskaran, C.; Meharg, A.A. Cadmium and Lead in Vegetable and Fruit Produce Selected from Specific Regional Areas of the UK. Sci. Total Environ. 2015, 533, 520–527. [Google Scholar] [CrossRef]
- Bian, B.; Wu, H.s.; Lv, L.; Fan, Y.; Lu, H. Health Risk Assessment of Metals in Food Crops and Related Soils Amended with Biogas Slurry in Taihu Basin: Perspective from Field Experiment. Environ. Sci. Pollut. Res. 2015, 22, 14358–14366. [Google Scholar] [CrossRef]
- Kosker, A.R. Metal and Fatty Acid Levels of Some Commercially Important Marine Species from the Northeastern Mediterranean: Benefits and Health Risk Estimation. Environ. Monit. Assess. 2020, 192, 358. [Google Scholar] [CrossRef] [PubMed]
- Ke, S.; Cheng, X.-Y.; Zhang, N.; Hu, H.-G.; Yan, Q.; Hou, L.-L.; Sun, X.; Chen, Z.-N. Cadmium Contamination of Rice from Various Polluted Areas of China and Its Potential Risks to Human Health. Environ. Monit. Assess. 2015, 187, 408. [Google Scholar] [CrossRef] [PubMed]
- Dziubanek, G.; Piekut, A.; Rusin, M.; Baranowska, R.; Hajok, I. Contamination of Food Crops Grown on Soils with Elevated Heavy Metals Content. Ecotoxicol. Environ. Saf. 2015, 118, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Zhao, K.; Fu, W.; Ye, Z.; Zhang, C. Contamination and Spatial Variation of Heavy Metals in the Soil-Rice System in Nanxun County, Southeastern China. Int. J. Environ. Res. Public Health 2015, 12, 1577–1594. [Google Scholar] [CrossRef] [PubMed]
- Naseri, M.; Vazirzadeh, A.; Kazemi, R.; Zaheri, F. Concentration of Some Heavy Metals in Rice Types Available in Shiraz Market and Human Health Risk Assessment. Food Chem. 2015, 175, 243–248. [Google Scholar] [CrossRef]
- Mok, J.S.; Kwon, J.Y.; Son, K.T.; Choi, W.S.; Shim, K.B.; Lee, T.S.; Kim, J.H. Distribution of Heavy Metals in Muscles and Internal Organs of Korean Cephalopods and Crustaceans: Risk Assessment for Human Health. J. Food Prot. 2014, 77, 2168–2175. [Google Scholar] [CrossRef] [PubMed]
- Rjeibi, M.; Metian, M.; Hajji, T.; Guyot, T.; Ben Chaouacha-Chekir, R.; Bustamante, P. Seasonal Survey of Contaminants (Cd and Hg) and Micronutrients (Cu and Zn) in Edible Tissues of Cephalopods from Tunisia: Assessment of Risk and Nutritional Benefits. J. Food Sci. 2015, 80, T199–T206. [Google Scholar] [CrossRef]
- Lin, K.; Lu, S.; Wang, J.; Yang, Y. The Arsenic Contamination of Rice in Guangdong Province, the Most Economically Dynamic Provinces of China: Arsenic Speciation and Its Potential Health Risk. Environ. Geochem. Health 2015, 37, 353–361. [Google Scholar] [CrossRef]
- Basu, N.; Tutino, R.; Zhang, Z.; Cantonwine, D.E.; Goodrich, J.M.; Somers, E.C.; Rodriguez, L.; Schnaas, L.; Solano, M.; Mercado, A.; et al. Mercury Levels in Pregnant Women, Children, and Seafood from Mexico City. Environ. Res. 2014, 135, 63–69. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, J.; Zhu, L.; Yang, G.; Li, D. Transfer of Cadmium from Soil to Vegetable in the Pearl River Delta Area, South China. PLoS ONE 2014, 9, e108572. [Google Scholar] [CrossRef]
- Kohrman, H.; Chamberlain, C.P. Heavy Metals in Produce from Urban Farms in the San Francisco Bay Area. Food Addit. Contam. Part B 2014, 7, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zhou, K.; Qin, W.; Tian, C.; Qi, M.; Yan, X.; Han, W. A Review on Heavy Metals Contamination in Soil: Effects, Sources, and Remediation Techniques. Soil Sediment. Contam. An. Int. J. 2019, 28, 380–394. [Google Scholar] [CrossRef]
- Laine, J.E.; Bailey, K.A.; Rubio-Andrade, M.; Olshan, A.F.; Smeester, L.; Drobná, Z.; Herring, A.H.; Stýblo, M.; García-Vargas, G.G.; Fry, R.C. Maternal Arsenic Exposure, Arsenic Methylation Efficiency, and Birth Outcomes in the Biomarkers of Exposure to ARsenic (BEAR) Pregnancy Cohort in Mexico. Environ. Health Perspect. 2015, 123, 186–192. [Google Scholar] [CrossRef]
- Hu, Y.; Wang, D.; Wei, L.; Song, B. Heavy Metal Contamination of Urban Topsoils in a Typical Region of Loess Plateau, China. J. Soils Sediments 2014, 14, 928–935. [Google Scholar] [CrossRef]
- Chen, H.; Teng, Y.; Lu, S.; Wang, Y.; Wang, J. Contamination Features and Health Risk of Soil Heavy Metals in China. Sci. Total Environ. 2015, 512–513, 143–153. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.; Karim, M.; Zheng, X.; Li, X. Heavy Metal and Metalloid Pollution of Soil, Water and Foods in Bangladesh: A Critical Review. Int. J. Environ. Res. Public Health 2018, 15, 2825. [Google Scholar] [CrossRef] [PubMed]
- Rai, P.K.; Lee, S.S.; Zhang, M.; Tsang, Y.F.; Kim, K.H. Heavy Metals in Food Crops: Health Risks, Fate, Mechanisms, and Management. Environ. Int. 2019, 125, 365–385. [Google Scholar] [CrossRef]
- Nkwunonwo, U.C.; Odika, P.O.; Onyia, N.I. A Review of the Health Implications of Heavy Metals in Food Chain in Nigeria. Sci. World J. 2020, 2020, 6594109. [Google Scholar] [CrossRef]
- Onakpa, M.M.; Njan, A.A.; Kalu, O.C. A Review of Heavy Metal Contamination of Food Crops in Nigeria. Ann. Glob. Health 2018, 84, 488–494. [Google Scholar] [CrossRef]
- Sharafi, K.; Yunesian, M.; Nodehi, R.N.; Mahvi, A.H.; Pirsaheb, M. A Systematic Literature Review for Some Toxic Metals in Widely Consumed Rice Types (Domestic and Imported) in Iran: Human Health Risk Assessment, Uncertainty and Sensitivity Analysis. Ecotoxicol. Environ. Saf. 2019, 176, 64–75. [Google Scholar] [CrossRef]

| Database | Search | Results |
|---|---|---|
| PubMed #5 | (((#1) AND (#2)) NOT (#3)) NOT (#4) | 2324 |
| #4 | (Review) OR (Review Literature) | 1,184,623 |
| #3 | (Water) | 406,953 |
| #2 | ((Food contamination) OR (Food Contaminations)) OR (Contamination, Food) | 34,956 |
| #1 | ((Metals, Heavy) OR (Heavy Metals) OR (Heavy Metal)) | 198,701 |
| Filters applied in every search: Books and Documents, Case Reports, Clinical Study, Clinical Trial, Journal Article, Observational Study, Randomized Controlled Trial, English, Spanish, MEDLINE, from 2011–2020 | ||
| ScienceDirect | ||
| #1 | (((Metals, Heavy) OR (Heavy Metals)) OR (Heavy Metal)) AND ((Food contamination) OR (Food Contaminations) OR (Contamination, Food)) NOT (Water) NOT ((Review) OR (Review Literature))) | 266 |
| Filters applied: Year: 2011–2020 Title, abstract, keywords | ||
| FAO|AGRIS | ||
| #2 | (((Metals, Heavy) OR (Heavy Metals)) OR (Heavy Metal)) AND ((Food contamination) OR (Food Contaminations) OR (Contamination, Food)) NOT (Water) NOT ((Review) OR (Review Literature))) | 6 |
| #1 | (((Metals, Heavy) OR (Heavy Metals)) OR (Heavy Metal)) AND ((Food contamination) OR (Food Contaminations) OR (Contamination, Food)) NOT (Water) NOT ((Review) OR (Review Literature))) | 137 |
| Filters applied search #2: language:(Spanish), publication Date: [2011 TO 2020] | ||
| Filters applied search #1: language:(English), publication Date: [2011 TO 2020] | ||
| Number of Articles by Heavy Metals Studied | ||||||||
|---|---|---|---|---|---|---|---|---|
| Food Group Studied * | Number of Articles Reviewed by Food Group | Food Subgroup Studied * | Number of Articles that Report the Food Subgroup | As | Pb | Cd | Hg/MeHg | Al |
| Cereals | 37 | Maize | 6 | 5 | 6 | 6 | 2 | 1 |
| Millet | 1 | 0 | 0 | 1 | 0 | 0 | ||
| Rice | 33 | 12 | 16 | 25 | 8 | 3 | ||
| Wheat | 7 | 2 | 6 | 7 | 1 | 1 | ||
| Others (barley, oat) | 1 | 0 | 1 | 1 | 0 | 1 | ||
| Eggs | 8 | Chicken egg | 6 | 2 | 3 | 3 | 2 | 1 |
| Duck egg | 1 | 0 | 1 | 1 | 1 | 0 | ||
| Fish egg | 1 | 0 | 0 | 1 | 0 | 0 | ||
| Turtle egg | 1 | 1 | 0 | 1 | 0 | 0 | ||
| Fish and shellfish | 58 | Cephalopods | 8 | 3 | 5 | 5 | 9 | 0 |
| Crustacean | 17 | 7 | 10 | 11 | 14 | 1 | ||
| Fish | 42 | 15 | 22 | 26 | 39 | 2 | ||
| Molluses | 14 | 7 | 10 | 10 | 14 | 1 | ||
| Offal fish | 4 | 3 | 2 | 2 | 4 | 0 | ||
| Fruits | 13 | Citrus fruits | 3 | 0 | 2 | 3 | 0 | 0 |
| Pome fruits | 5 | 1 | 4 | 5 | 0 | 0 | ||
| Soft fruits | 7 | 1 | 6 | 7 | 1 | 1 | ||
| Stone fruits | 3 | 1 | 3 | 3 | 0 | 0 | ||
| Tropical fruits | 7 | 3 | 6 | 7 | 0 | 0 | ||
| Watermelons | 3 | 1 | 3 | 3 | 0 | 0 | ||
| Meat | 20 | Offal poultry | 6 | 2 | 4 | 5 | 4 | 1 |
| Offal red meat | 8 | 3 | 5 | 7 | 4 | 0 | ||
| Poultry meat | 10 | 5 | 7 | 8 | 5 | 1 | ||
| Red meat | 16 | 8 | 11 | 15 | 8 | 0 | ||
| Milk | 12 | Cow milk | 11 | 4 | 9 | 9 | 5 | 0 |
| Ewe milk | 2 | 0 | 2 | 2 | 1 | 0 | ||
| Goat milk | 1 | 0 | 1 | 0 | 0 | 0 | ||
| Pulses, seed and nuts | 16 | Nuts and seeds | 4 | 0 | 3 | 4 | 0 | 0 |
| Pulses | 11 | 7 | 10 | 11 | 3 | 0 | ||
| Soybeans | 4 | 2 | 4 | 4 | 1 | 0 | ||
| Roots and tubers | 25 | Beetroot | 4 | 0 | 3 | 4 | 0 | 0 |
| Carrot | 12 | 5 | 8 | 12 | 1 | 1 | ||
| Potato | 21 | 6 | 17 | 20 | 2 | 1 | ||
| Radish | 5 | 2 | 3 | 5 | 1 | 0 | ||
| Others | 6 | 1 | 5 | 6 | 0 | 0 | ||
| Vegetables | 39 | Brasica vegetables | 21 | 6 | 16 | 19 | 5 | 1 |
| Bulb vegetables | 19 | 4 | 13 | 19 | 2 | 0 | ||
| Fruiting vegetables | 29 | 10 | 25 | 26 | 4 | 3 | ||
| Leafy vegetables | 28 | 9 | 24 | 27 | 7 | 2 | ||
| Legume vegetables | 10 | 2 | 9 | 10 | 0 | 0 | ||
| Stalk and sterm vegetables | 9 | 4 | 7 | 9 | 2 | 0 | ||
| Total of times HM reported | 144 | 292 | 350 | 150 | 22 | |||
| Classification FAO/WHO Global Individual Food Consumption Data Tool (GIFT) a | FAO/OMS Maximum Limits (MLs) b | Number of Articles per Country that Exceed Maximum Limits (MLs) in our Study c | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Food Group | Subgroup-Short Name | As | Pb | Cd | MeHg | As | Pb | Cd | MeHg |
| Cereals | Rice | 0.2 | 0.4 | Thailand: 1 Bangladesh: 1 Iran: 2 China: 2 | China: 3 Iran: 1 Australia: 1 | ||||
| Maize | 0.2 | 0.1 | Bangladesh: 1 Greece: 1 Iran: 1 China: 1 | Bangladesh: 2 Iran: 1 China: 2 | |||||
| Wheat | 0.2 | 0.2 | Iran: 1 China: 1 | Iran: 1 China: 1 UK: 1 | |||||
| Roots and tubers | Potato | 0.1 | 0.1 | Bangladesh: 1 Brazil: 1 China: 2 Croatia: 1 Germany: 1 Iran: 1 Poland: 1 Serbia: 1 Slovakia: 1 | Brazil: 1 China: 2 Croatia: 1 Iran: 1 Poland: 1 | ||||
| Other starchy roots and tubers: | 0.1 | 0.1 | |||||||
| Beetroot | Brazil: 1 Serbia: 1 | ||||||||
| Carrot | Bangladesh: 1 Brazil: 1 China: 1 Croatia: 1 Germany: 1 Poland: 1 Serbia: 1 | Croatia: 1 Germany: 1 Poland: 1 Serbia: 1 | |||||||
| Radish | China: 1 Iran: 1 | Iran: 1 | |||||||
| Pulses, seeds and nuts | Pulses | 0.1 | 0.1 | Bangladesh: 2 Brazil: 1 Croatia: 1 Iran: 1 | Bangladesh: 1 Brazil: 1 China: 1 Iran: 1 | ||||
| Milk | Milk | 0.02 | Bangladesh: 2 Ethiopia: 1 Hungary: 1 Mexico: 1 South Korea: 1 | ||||||
| Eggs | Eggs | 0.1 | Australia: 1 Bangladesh: 1 India: 1 Thailand: 1 | ||||||
| Fish and shellfish | Freshwater, diadromous and marine fish | 0.3 | Argelia: 1 Bangladesh: 1 Bosnia and Herzegovina: 1 China: 1 Iran: 1 Italy: 1 Nigeria: 1 Turkey: 1 | ||||||
| Mollusks | 2.0 | Iran: 1 | |||||||
| Cephalopods | 2.0 | ||||||||
| Tuna | 1.2 | Italia: 1 | |||||||
| Shark | 1.6 | Mexico: 1 | |||||||
| Meat | Red meat | 0.1 | Bangladesh: 1 China: 1 Kuwait: 1 Nigeria: 1 South Korea: 1 | ||||||
| Poultry | 0.1 | Bangladesh: 1 India: 1 South Korea: 1 Thailand: 1 | |||||||
| Offal red meat | 0.2 | Kuwait: 1 Nigeria: 1 South Korea: 1 | |||||||
| Offal poultry | 0.1 | Ghana: 1 Malaysia: 1 Tunisia: 1 South Korea: 1 | |||||||
| Vegetables | Leafy vegetables | 0.3 | 0.2 | Bangladesh: 1 Brazil: 2 China: 4 Croatia: 1 Germany: 1 Iran: 2 Portugal: 1 Serbia: 1 Spain: 1 | Bangladesh: 1 China: 4 Croatia: 1 Germany: 1 Iran: 2 Serbia: 1 South Africa: 1 Spain: 1 | ||||
| Stalk and steem vegetables | 0.1 | China: 2 | |||||||
| Brassica vegetables | 0.1 | 0.05 | Bangladesh: 1 Brazil: 1 China: 6 Croatia: 1 Germany: 1 Serbia: 1 | Brazil: 1 China: 6 Croatia: 1 Germany: 1 Serbia: 1 South Africa: 1 | |||||
| Bulb vegetables | 0.1 | 0.05 | Bangladesh: 1 Brazil: 1 China: 3 Croatia: 1 Iran: 2 | Bnagladesh: 1 Brazil: 1 China: 3 Croatia: 1 Ghana: 1 Iran: 3 South Africa: 1 | |||||
| Fruiting vegetables | 0.05 | 0.05 | Bangladesh: 2 Brazil: 2 China: 4 Germany: 1 Serbia: 1 Turkey: 1 | Bangladesh: 3 Brazil: 2 China: 5 Germany: 1 Ghana: 1 Iran: 1 Serbia: 1 South Africa: 1 Turkey: 1 | |||||
| Legume vegetables | 0.1 | 0.1 | Bangladesh: 1 Brazil: 1 China: 2 Germany: 1 Iran: 1 | Bangladesh: 1 China: 1 | |||||
| Fruits | Tropical fruits | 0.1 | Bangladesh: 3 Brazil: 1 China: 1 | ||||||
| Stone fruits | 0.1 | Bangladesh: 1 Germany: 1 | |||||||
| Pome fruits | 0.1 | Bangladesh: 1 Brazil: 1 Germany: 1 | |||||||
| Soft fruits (berries and other small fruits) | 0.1 | Brazil: 1 Germany: 1 | |||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Collado-López, S.; Betanzos-Robledo, L.; Téllez-Rojo, M.M.; Lamadrid-Figueroa, H.; Reyes, M.; Ríos, C.; Cantoral, A. Heavy Metals in Unprocessed or Minimally Processed Foods Consumed by Humans Worldwide: A Scoping Review. Int. J. Environ. Res. Public Health 2022, 19, 8651. https://doi.org/10.3390/ijerph19148651
Collado-López S, Betanzos-Robledo L, Téllez-Rojo MM, Lamadrid-Figueroa H, Reyes M, Ríos C, Cantoral A. Heavy Metals in Unprocessed or Minimally Processed Foods Consumed by Humans Worldwide: A Scoping Review. International Journal of Environmental Research and Public Health. 2022; 19(14):8651. https://doi.org/10.3390/ijerph19148651
Chicago/Turabian StyleCollado-López, Sonia, Larissa Betanzos-Robledo, Martha María Téllez-Rojo, Héctor Lamadrid-Figueroa, Moisés Reyes, Camilo Ríos, and Alejandra Cantoral. 2022. "Heavy Metals in Unprocessed or Minimally Processed Foods Consumed by Humans Worldwide: A Scoping Review" International Journal of Environmental Research and Public Health 19, no. 14: 8651. https://doi.org/10.3390/ijerph19148651
APA StyleCollado-López, S., Betanzos-Robledo, L., Téllez-Rojo, M. M., Lamadrid-Figueroa, H., Reyes, M., Ríos, C., & Cantoral, A. (2022). Heavy Metals in Unprocessed or Minimally Processed Foods Consumed by Humans Worldwide: A Scoping Review. International Journal of Environmental Research and Public Health, 19(14), 8651. https://doi.org/10.3390/ijerph19148651








