Physiological Role of Orexinergic System for Health
Abstract
1. Introduction
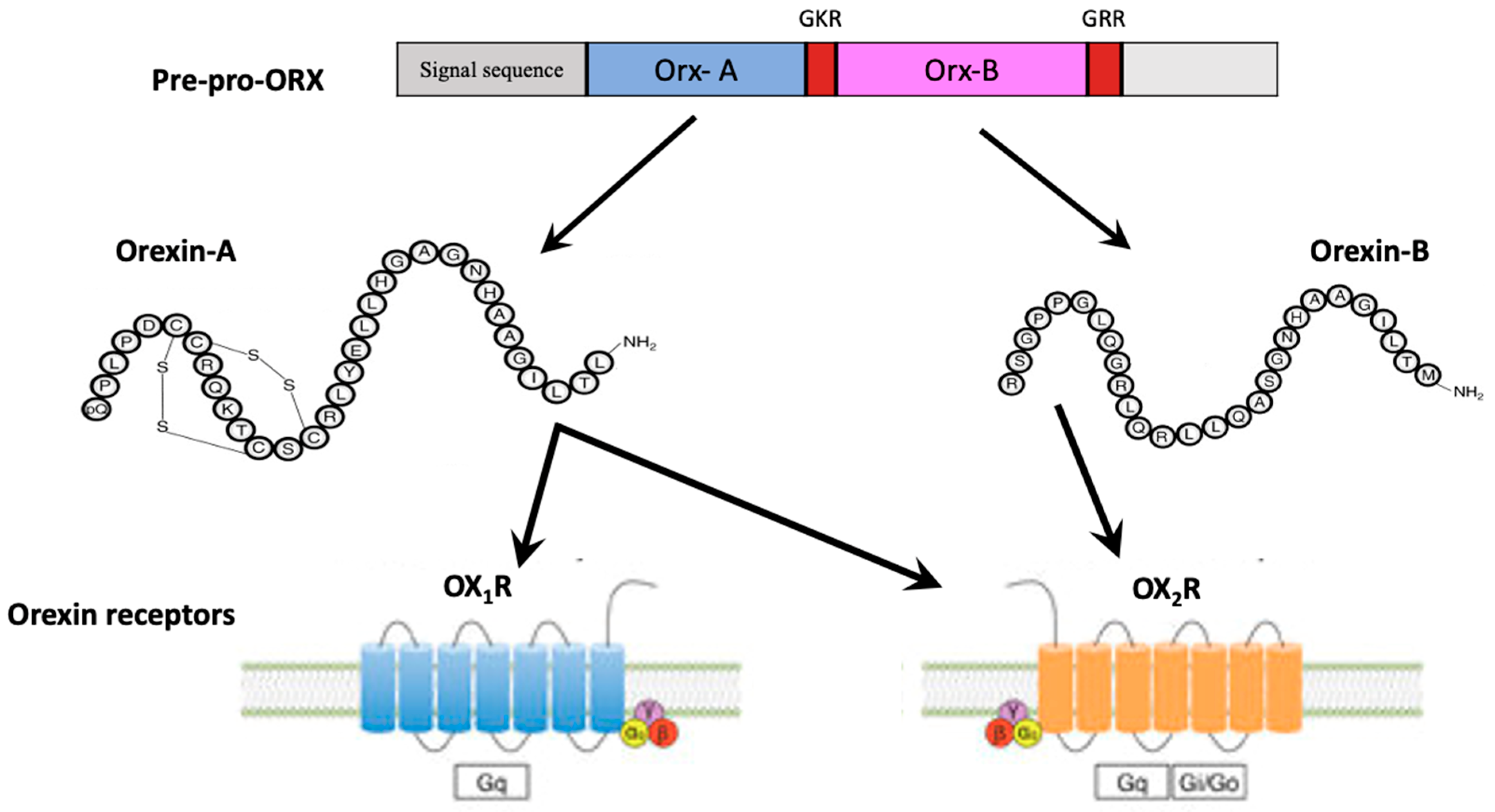
2. The Orexin/Hypocretin System
3. Sleep/Wakefulness States Regulation
3.1. Orexinergic Neurons and Basal Forebrain (BF) Projections
3.2. Orexinergic Neurons and Locus Coeruleus (LC) Projections
3.3. Orexinergic Neurons and Dorsal Raphe Nuclei (DRN) Projections
3.4. Orexinergic Neurons and Tuberomammillary Nucleus (TMN) Projections
3.5. Orexinergic Neurons and Bed Nucleus of Stria Terminalis (BNST) Projections
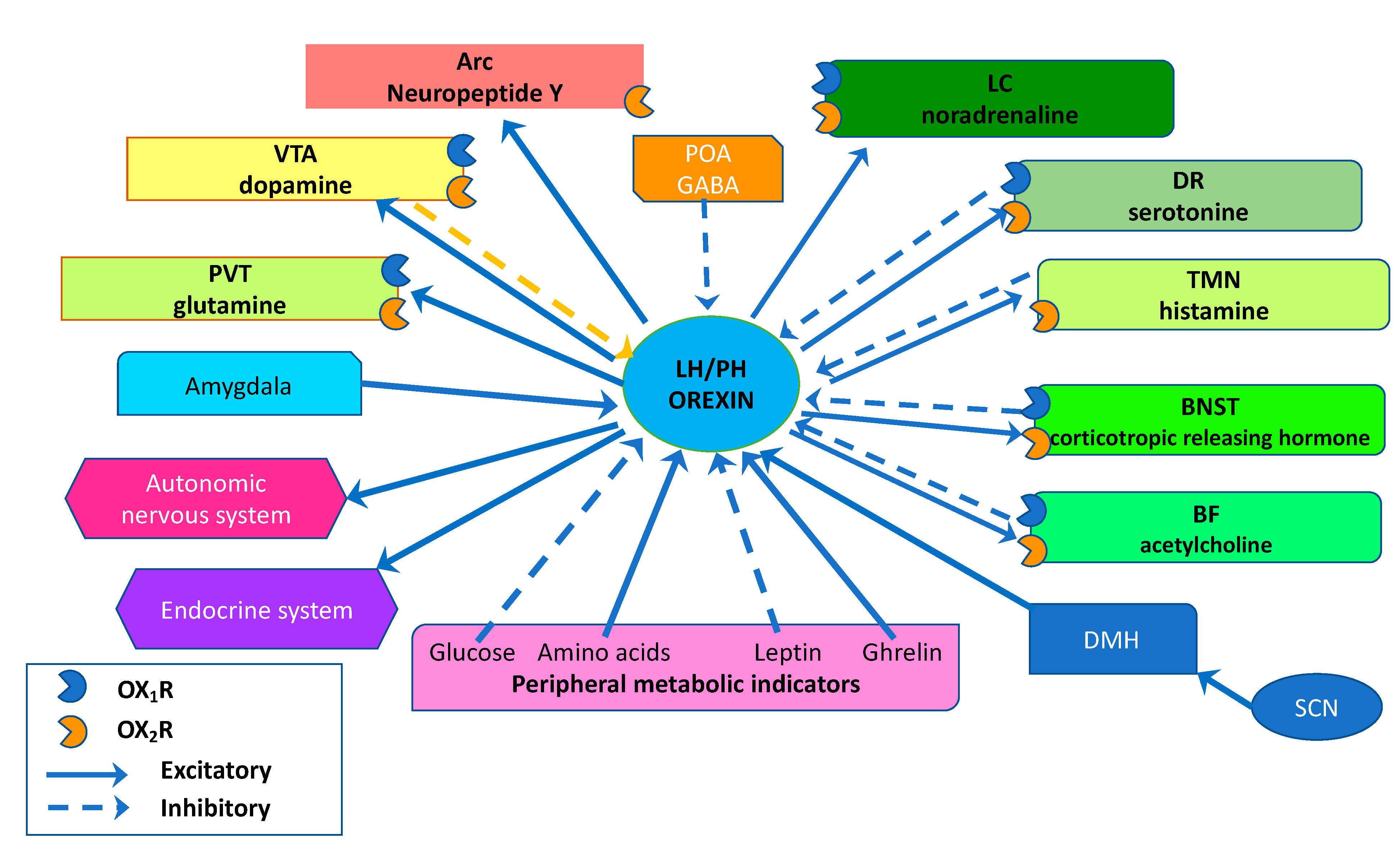
4. Feeding Behavior, Energy Homeostasis, and Obesity
4.1. Orexin and Feeding Behavior
4.2. Orexin and Energy Expenditure
4.3. Orexin and Obesity
5. Reward System and Addiction
6. Ageing and Neurogenesis
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AC | anterior commissure |
| Arc | arcuate nucleus |
| BAT | brown adipose tissue |
| BF | basal forebrain |
| BNST | bed nucleus of the stria terminalis |
| CNS | central nervous system |
| CSF | cerebrospinal fluid |
| DG | dentate gyrus |
| DMH | dorsomedial hypothalamic nucleus |
| DRN | dorsal raphe nucleus |
| GABA | gamma-aminobutyric acid |
| GPCR | G protein-coupled receptor |
| HA | high activity |
| IC | insular cortex |
| ICV | intracerebroventricular |
| LA | low activity |
| LC | locus coeruleus |
| LHA | lateral hypothalamic area |
| LS | lateral septum |
| MS | medial septum |
| NAc | nucleus accumbens |
| NEAT | non-exercise induced thermogenesis |
| NREM | non-rapid eye movement |
| NTS | nucleus of the solitary tract |
| OB | olfactory bulb |
| OP | obesity prone |
| OR | obesity resistant |
| OrxA | Orexin-A |
| OrxB | orexin-B |
| PD | Parkinson’s disease |
| POA | preoptic area |
| PPT/LDT | pedunculopontine and latero-dorsal tegmental nucleus |
| PVN | paraventricular nucleus |
| PVT | paraventricular thalamus |
| REM | rapid eye movement |
| SPA | spontaneous physical activity |
| TMN | tuberomammillary nucleus |
| VP | ventral pallidum |
| VTA | ventral tegmental area |
References
- Sakurai, T.; Amemiya, A.; Ishii, M.; Matsuzaki, I.; Chemelli, R.M.; Tanaka, H.; Williams, S.C.; Richardson, J.A.; Kozlowski, G.P.; Wilson, S.; et al. Orexins and orexin receptors: A family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell 1998, 92, 573–585. [Google Scholar] [CrossRef]
- Sakurai, T. The neural circuit of orexin (hypocretin): Maintaining sleep and wakefulness. Nat. Rev. Neurosci. 2007, 8, 171–181. [Google Scholar] [CrossRef] [PubMed]
- de Lecea, L.; Kilduff, T.S.; Peyron, C.; Gao, X.B.; Foye, P.E.; Danielson, P.E.; Fukuhara, C.; Battenberg, E.L.F.; Gautvik, V.T.; Bartlett, F.S., II; et al. The hypocretins: Hypothalamus-specific peptides with neuroexcitatory activity. Proc. Natl. Acad. Sci. USA 1998, 95, 322–327. [Google Scholar] [CrossRef] [PubMed]
- Peyron, C.; Faraco, J.; Rogers, W.; Ripley, B.; Overeem, S.; Charnay, Y.; Nevsimalova, S.; Aldrich, M.; Reynolds, D.; Albin, R.; et al. A mutation in a case of early onset narcolepsy and a generalized absence of hypocretin peptides in human narcoleptic brains. Nat. Med. 2000, 6, 991–997. [Google Scholar] [CrossRef] [PubMed]
- Chemelli, R.M.; Willie, J.T.; Sinton, C.M.; Elmquist, J.K.; Scammell, T.; Lee, C.; Richardson, J.A.; Williams, S.C.; Xiong, Y.; Kisanuki, Y.; et al. Narcolepsy in orexin knockout mice: Molecular genetics of sleep regulation. Cell 1999, 98, 437–451. [Google Scholar] [CrossRef]
- Lin, L.; Faraco, J.; Li, R.; Kadotani, H.; Rogers, W.; Lin, X.; Qiu, X.; de Jong, P.J.; Nishino, S.; Mignot, E. The sleep disorder canine narcolepsy is caused by a mutation in the hypocretin (orexin) receptor 2 gene. Cell 1999, 98, 365–376. [Google Scholar] [CrossRef]
- Thannickal, T.C.; Moore, R.Y.; Nienhuis, R.; Ramanathan, L.; Gulyani, S.; Aldrich, M.; Cornford, M.; Siegel, J.M. Reduced number of hypocretin neurons in human narcolepsy. Neuron 2000, 27, 469–474. [Google Scholar] [CrossRef]
- Messina, A.; Monda, V.; Avola, R.; Moscatelli, F.; Avalenzano, A.A.V.; Villano, I.; Ruberto, M.; Monda, E.; La Marra, M.; Tafuri, D.; et al. Role of the orexin system on arousal, attention, feeding behaviour and sleep disorders. Acta Med. 2017, 33, 635. [Google Scholar] [CrossRef]
- Soya, S.; Sakurai, T. Evolution of Orexin Neuropeptide System: Structure and Function. Front. Neurosci. 2020, 14, 691. [Google Scholar] [CrossRef]
- Mehr, J.B.; Bilotti, M.M.; James, M.H. Orexin (hypocretin) and addiction. Trends Neurosci. 2021, 44, 852–855. [Google Scholar] [CrossRef]
- Hara, J.; Beuckmann, C.T.; Nambu, T.; Willie, J.T.; Chemelli, R.M.; Sinton, C.M.; Sugiyama, F.; Yagami, K.I.; Goto, K.; Yanagisawa, M.; et al. Genetic ablation of orexin neurons in mice results in narcolepsy, hypophagia, and obesity. Neuron 2001, 30, 345–354. [Google Scholar] [CrossRef]
- Yamanaka, A.; Beuckmann, C.T.; Willie, J.T.; Hara, J.; Tsujino, N.; Mieda, M.; Tominaga, M.; Yagami, K.; Sugiyama, F.; Goto, K.; et al. Hypothalamic orexin neurons regulate arousal according to energy balance in mice. Neuron 2003, 38, 701–713. [Google Scholar] [CrossRef]
- Harris, G.C.; Wimmer, M.; Aston-Jones, G. A role for lateral hypothalamic orexin neurons in reward seeking. Nature 2005, 437, 556–559. [Google Scholar] [CrossRef]
- Narita, M.; Nagumo, Y.; Hashimoto, S.; Narita, M.; Khotib, J.; Miyatake, M.; Sakurai, T.; Yanagisawa, M.; Nakamachi, T.; Shioda, S.; et al. Direct involvement of orexinergic systems in the activation of the mesolimbic dopamine pathway and related behaviors induced by morphine. J. Neurosci. 2006, 26, 398–405. [Google Scholar] [CrossRef]
- Villano, I.; Messina, A.; Valenzano, A.; Moscatelli, F.; Esposito, T.; Monda, V.; Esposito, M.; Precenzano, F.; Carotenuto, M.; Viggiano, A.; et al. Basal Forebrain Cholinergic System and Orexin Neurons: Effects on Attention. Front. Behav. Neurosci. 2017, 11, 10. [Google Scholar] [CrossRef]
- Nattie, E.; Li, A. Respiration and autonomic regulation and orexin. Prog. Brain Res. 2012, 198, 25–46. [Google Scholar] [CrossRef]
- Nambu, T.; Sakurai, T.; Mizukami, K.; Hosoya, Y.; Yanagisawa, M.; Goto, K. Distribution of orexin neurons in the adult rat brain. Brain Res. 1999, 827, 243–260. [Google Scholar] [CrossRef]
- Azeez, I.A.; Igado, O.O.; Olopade, J.O. An overview of the orexinergic system in different animal species. Metab. Brain Dis. 2021, 36, 1419–1444. [Google Scholar] [CrossRef]
- Kukkonen, J.P. Physiology of the orexinergic/hypocretinergic system: A revisit in 2012. Am. J. Physiol. Cell Physiol. 2013, 304, C2–C32. [Google Scholar] [CrossRef]
- Xu, T.R.; Yang, Y.; Ward, R.; Gao, L.; Liu, Y. Orexin receptors: Multi-functional therapeutic targets for sleeping disorders, eating disorders, drug addiction, cancers and other physiological disorders. Cell. Signal. 2013, 25, 2413–2423. [Google Scholar] [CrossRef]
- Kukkonen, J.P.; Turunen, P.M. Cellular signaling mechanisms of hypocretin/orexin. The Orexin System. Basic Sci. Role Sleep Pathol. 2021, 45, 91–102. [Google Scholar] [CrossRef]
- Kukkonen, J.P.; Leonard, C.S. Orexin/hypocretin receptor signalling cascades. Br. J. Pharmacol. 2014, 171, 294–313. [Google Scholar] [CrossRef]
- Mieda, M.; Hasegawa, E.; Kisanuki, Y.Y.; Sinton, C.M.; Yanagisawa, M.; Sakurai, T. Differential roles of orexin receptor-1 and -2 in the regulation of non-REM and REM sleep. J. Neurosci. 2011, 31, 6518–6526. [Google Scholar] [CrossRef]
- Boss, C.; Roch, C. Recent trends in orexin research—2010 to 2015. Bioorg. Med. Chem. Lett. 2015, 25, 2875–2887. [Google Scholar] [CrossRef]
- Li, S.B.; de Lecea, L. The hypocretin (orexin) system: From a neural circuitry perspective. Neuropharmacology 2020, 167, 107993. [Google Scholar] [CrossRef]
- Sagi, D.; de Lecea, L.; Appelbaum, L. Heterogeneity of Hypocretin/Orexin Neurons. Front. Neurol. Neurosci. 2021, 45, 61–74. [Google Scholar] [CrossRef]
- Moore, R.Y.; Abrahamson, E.A.; Van Den Pol, A. The hypocretin neuron system: An arousal system in the human brain. Arch. Ital. Biol. 2001, 139, 195–205. [Google Scholar] [CrossRef]
- Steininger, T.L.; Kilduff, T.S. Anatomy of the hypocretin system. In Hypocretins: Integrators of Physiological Functions; Springer: Boston, MA, USA, 2005. [Google Scholar] [CrossRef]
- Henny, P.; Brischoux, F.; Mainville, L.; Stroh, T.; Jones, B.E. Immunohistochemical evidence for synaptic release of glutamate from orexin terminals in the locus coeruleus. Neuroscience 2010, 169, 1150–1157. [Google Scholar] [CrossRef]
- Sears, R.M.; Fink, A.E.; Wigestrand, M.B.; Farb, C.R.; de Lecea, L.; Ledoux, J.E. Orexin/hypocretin system modulates amygdala-dependent threat learning through the locus coeruleus. Proc. Natl. Acad. Sci. USA 2013, 110, 20260–20265. [Google Scholar] [CrossRef]
- González, J.A.; Jensen, L.T.; Fugger, L.; Burdakov, D. Convergent inputs from electrically and topographically distinct orexin cells to locus coeruleus and ventral tegmental area. Eur. J. Neurosci. 2012, 35, 1426–1432. [Google Scholar] [CrossRef] [PubMed]
- Davoudi, M.; Azizi, H.; Mirnajafi-Zadeh, J.; Semnanian, S. Decrease of inhibitory synaptic currents of locus coeruleus neurons via orexin type 1 receptors in the context of naloxone-induced morphine withdrawal. J. Physiol. Sci. 2019, 69, 281–293. [Google Scholar] [CrossRef] [PubMed]
- Morales, M.; Margolis, E.B. Ventral tegmental area: Cellular heterogeneity, connectivity and behaviour. Nat. Rev. Neurosci. 2017, 18, 73–85. [Google Scholar] [CrossRef] [PubMed]
- Fadel, J.; Deutch, A.Y. Anatomical substrates of orexin-dopamine interactions: Lateral hypothalamic projections to the ventral tegmental area. Neuroscience 2002, 111, 379–387. [Google Scholar] [CrossRef]
- Chowdhury, S.; Matsubara, T.; Miyazaki, T.; Ono, D.; Fukatsu, N.; Abe, M.l.; Sakimura, K.; Sudo, Y.; Yamanaka, A. GABA neurons in the ventral tegmental area regulate non-rapid eye movement sleep in mice. Elife 2019, 4, e44928. [Google Scholar] [CrossRef]
- Arrigoni, E.; Mochizuki, T.; Scammell, T.E. Activation of the basal forebrain by the orexin/hypocretin neurones. Acta Physiol. 2010, 198, 223–235. [Google Scholar] [CrossRef]
- Chieffi, S.; Castaldi, C.; Di Maio, G.; La Marra, M.; Messina, A.; Monda, V.; Villano, I. Attentional bias in the radial and vertical dimensions of space. Comptes Rendus Biol. 2019, 342, 97–100. [Google Scholar] [CrossRef]
- Fadel, J.; Burk, J.A. Orexin/hypocretin modulation of the basal forebrain cholinergic system: Role in attention. Brain Res. 2010, 1314, 112–123. [Google Scholar] [CrossRef]
- Fadel, J.; Frederick-Duus, D. Orexin/hypocretin modulation of the basal forebrain cholinergic system: Insights from in vivo microdialysis studies. Pharmacol. Biochem. Behav. 2008, 90, 156–162. [Google Scholar] [CrossRef]
- Agostinelli, L.J.; Ferrari, L.L.; Mahoney, C.E.; Mochizuki, T.; Lowell, B.B.; Arrigoni, E.; Scammell, T.E. Descending projections from the basal forebrain to the orexin neurons in mice. J. Comp. Neurol. 2017, 525, 1668–1684. [Google Scholar] [CrossRef]
- Chowdhury, S.; Yamanaka, A. Optogenetic activation of serotonergic terminals facilitates GABAergic inhibitory input to orexin/hypocretin neurons. Sci. Rep. 2016, 6, 36039. [Google Scholar] [CrossRef]
- Yamanaka, A.; Muraki, Y.; Tsujino, N.; Goto, K.; Sakurai, T. Regulation of orexin neurons by the monoaminergic and cholinergic systems. Biochem. Biophys. Res. Commun. 2003, 303, 120–129. [Google Scholar] [CrossRef]
- Li, Y.; Zhong, W.; Wang, D.; Feng, Q.; Liu, Z.; Zhou, J.; Jia, C.; Hu, F.; Zeng, J.; Guo, Q.; et al. Serotonin neurons in the dorsal raphe nucleus encode reward signals. Nat. Commun. 2016, 7, 10503. [Google Scholar] [CrossRef]
- Muraki, Y.; Yamanaka, A.; Tsujino, N.; Kilduff, T.S.; Goto, K.; Sakurai, T. Serotonergic regulation of the orexin/hypocretin neurons through the 5-HT1A receptor. J. Neurosci. 2004, 24, 7159–7166. [Google Scholar] [CrossRef]
- Matzeu, A.; Martin-Fardon, R. Drug seeking and relapse: New evidence of a role for orexin and dynorphin Co-transmission in the paraventricular nucleus of the thalamus. Front. Neurol. 2018, 9, 720. [Google Scholar] [CrossRef]
- Kodani, S.; Soya, S.; Sakurai, T. Excitation of GABAergic neurons in the bed nucleus of the stria terminalis triggers immediate transition from non-rapid eye movement sleep to wakefulness in mice. J. Neurosci. 2017, 37, 7164–7176. [Google Scholar] [CrossRef]
- Takahashi, K.; Lin, J.S.; Sakai, K. Neuronal activity of histaminergic tuberomammillary neurons during wake-sleep states in the mouse. J. Neurosci. 2006, 26, 10292–10298. [Google Scholar] [CrossRef]
- Thakkar, M.M. Histamine in the regulation of wakefulness. Sleep Med. Rev. 2011, 15, 65–74. [Google Scholar] [CrossRef]
- Eriksson, K.S.; Sergeeva, O.; Brown, R.E.; Haas, H.L. Orexin/hypocretin excites the histaminergic neurons of the tuberomammillary nucleus. J. Neurosci. 2001, 21, 9273–9279. [Google Scholar] [CrossRef]
- Satoh, N.; Ogawa, Y.; Katsuura, G.; Hayase, M.; Tsuji, T.; Imagawa, K.; Yoshimasa, Y.; Nishi, S.; Hosoda, K.; Nakao, K. The arcuate nucleus as a primary site of satiety effect of leptin in rats. Neurosci. Lett. 1997, 224, 149–152. [Google Scholar] [CrossRef]
- Muroya, S.; Funahashi, H.; Yamanaka, A.; Kohno, D.; Uramura, K.; Nambu, T.; Shibahara, M.; Kuramochi, M.; Takigawa, M.; Yanagisawa, M.; et al. Orexins (hypocretins) directly interact with neuropeptide Y, POMC and glucose-responsive neurons to regulate Ca2+ signaling in a reciprocal manner to leptin: Orexigenic neuronal pathways in the mediobasal hypothalamus. Eur. J. Neurosci. 2004, 19, 1524–1534. [Google Scholar] [CrossRef]
- Fu, L.Y.; Acuna-Goycolea, C.; van den Pol, A.N. Neuropeptide Y inhibits hypocretin/orexin neurons by multiple presynaptic and postsynaptic mechanisms: Tonic depression of the hypothalamic arousal system. J. Neurosci. 2004, 24, 8741–8751. [Google Scholar] [CrossRef][Green Version]
- Weinhold, S.L.; Seeck-Hirschner, M.; Nowak, A.; Hallschmid, M.; Göder, R.; Baier, P.C. The effect of intranasal orexin-A (hypocretin-1) on sleep, wakefulness and attention in narcolepsy with cataplexy. Behav. Brain Res. 2014, 262, 8–13. [Google Scholar] [CrossRef]
- Chieffi, S.; Carotenuto, M.; Monda, V.; Valenzano, A.; Villano, I.; Precenzano, F.; Tafuri, D.; Salerno, M.; Filippi, N.; Nuccio, F.; et al. Orexin System: The Key for a Healthy Life. Front. Physiol. 2017, 8, 357. [Google Scholar] [CrossRef]
- Precenzano, F.; Ruberto, M.; Parisi, L.; Salerno, M.; Maltese, A.; Verde, D.; Tripi, G.; Romano, P.; Di Folco, A.; Di Filippo, T.; et al. Sleep habits in children affected by autism spectrum disorders: A preliminary case-control study. Acta Med. Mediterr. 2017, 33, 405. [Google Scholar] [CrossRef]
- Sakurai, T. The role of orexin in motivated behaviours. Nat. Rev. Neurosci. 2014, 15, 719–731. [Google Scholar] [CrossRef]
- Yoshida, K.; McCormack, S.; España, R.A.; Crocker, A.; Scammell, T.E. Afferents to the orexin neurons of the rat brain. J. Comp. Neurol. 2006, 494, 845–861. [Google Scholar] [CrossRef]
- Kiyashchenko, L.I.; Mileykovskiy, B.Y.; Maidment, N.; Lam, H.A.; Wu, M.-F.; John, J.; Peever, J.; Siegel, J.M. Release of hypocretin (orexin) during waking and sleep states. J. Neurosci. 2002, 22, 5282–5286. [Google Scholar] [CrossRef]
- Lee, M.G.; Hassani, O.K.; Jones, B.E. Discharge of identified orexin/ hypocretin neurons across the sleep-waking cycle. J. Neurosci. 2005, 25, 6716–6720. [Google Scholar] [CrossRef]
- Goforth, P.B.; Myers, M.G. Roles for Orexin/Hypocretin in the Control of Energy Balance and Metabolism; Springer: Cham, Switzerland, 2016; pp. 137–156. [Google Scholar]
- Belle, M.D.C.; Hughes, A.T.L.; Bechtold, D.A.; Cunningham, P.; Pierucci, M.; Burdakov, D.; Piggins, H.D. Acute suppressive and long-term phase modulation actions of orexin on the mammalian circadian clock. J. Neurosci. 2014, 34, 3607–3621. [Google Scholar] [CrossRef]
- Northeast, R.C.; Vyazovskiy, V.V.; Bechtold, D.A. Eat, sleep, repeat: The role of the circadian system in balancing sleep-wake control with metabolic need. Curr. Physiol. 2020, 15, 183–191. [Google Scholar] [CrossRef]
- Mistlberger, R.E.; Antle, M.C. Entrainment of circadian clocks in mammals by arousal and food. Essays Biochem. 2011, 49, 119–136. [Google Scholar] [PubMed]
- Mistlberger, R.E. Neurobiology of food anticipatory circadian rhythms. Physiol. Behav. 2011, 104, 535–545. [Google Scholar] [CrossRef] [PubMed]
- Marchant, E.G.; Mistlberger, R.E. Anticipation and entrainment to feeding time in intact and SCN-ablated C57BL/6j mice. Brain Res. 1997, 765, 273–282. [Google Scholar] [CrossRef]
- Delezie, J.; Dumont, S.; Sandu, C.; Reibel, S.; Pevet, P.; Challet, E. Rev-erba in the brain is essential for circadian food entrainment. Sci. Rep. 2016, 6, 29386. [Google Scholar] [CrossRef]
- Chavan, R.; Feillet, C.; Costa, S.S.F.; Delorme, J.E.; Okabe, T.; Ripperger, J.A.; Albrecht, U. Liver-derived ketone bodies are necessary for food anticipation. Nat. Commun. 2016, 7, 10580. [Google Scholar] [CrossRef]
- Mistlberger, R.E. Food as circadian time cue for appetitive behavior. F1000Research 2020, 9, 61. [Google Scholar] [CrossRef]
- Saper, C.B.; Scammell, T.E.; Lu, J. Hypothalamic regulation of sleep and circadian rhythms. Nature 2005, 437, 1257–1263. [Google Scholar] [CrossRef]
- Dong, H.I.; Fukuda, S.; Murata, E.; Zhu, Z.H.; Higuchi, T. Orexins increase cortical acetylcholine release and electroencephalographic activation through orexin-1 receptor in the rat basal forebrain during isoflurane anesthesia. Anesthesiology 2006, 104, 1023–1032. [Google Scholar] [CrossRef]
- Thakkar, M.M.; Ramesh, V.; Strecker, R.E.; McCarley, R.W. Microdialysis perfusion of orexin-A in the basal forebrain increases wakefulness in freely behaving rats. Arch. Ital. Biol. 2001, 139, 313–328. [Google Scholar]
- Aston-Jones, G.; Bloom, F.E. Activity of norepinephrine-containing locus coeruleus neurons in behaving rats anticipates fluctuations in the sleep-waking cycle. J. Neurosci. 1981, 1, 876–886. [Google Scholar] [CrossRef]
- Eschenko, O.; Magri, C.; Panzeri, S.; Sara, S.J. Noradrenergic neurons of the locus coeruleus are phase locked to cortical up-down states during sleep. Cereb. Cortex 2012, 22, 426–435. [Google Scholar] [CrossRef]
- Takahashi, K.; Kayama, Y.; Lin, J.S.; Sakai, K. Locus coeruleus neuronal activity during the sleep-waking cycle in mice. Neuroscience 2010, 169, 1115–1126. [Google Scholar] [CrossRef]
- Carter, M.E.; Brill, J.; Bonnavion, P.; Huguenard, J.R.; Huerta, R.; de Lecea, L. Mechanism for Hypocretin-mediated sleep-to-wake transitions. Proc. Natl. Acad. Sci. USA 2012, 109, E2635–E2644. [Google Scholar] [CrossRef]
- Bourgin, P.; Huitron-Resendiz, S.; Spier, A.D.; Fabre, V.; Morte, B.; Criado, J.R.; Sutcliffe, J.G.; Henriksen, S.J.; de Lecea, L. Hypocretin-1 modulates rapid eye movement sleep through activation of locus coeruleus neurons. J. Neurosci. 2000, 20, 7760–7765. [Google Scholar] [CrossRef]
- Del Cid-Pellitero, E.; Garzon, M. Hypocretin1/OrexinA-Containing axons innervate locus coeruleus neurons that project to the rat medial prefrontal cortex. Implication in the sleep-wakefulness cycle and cortical activation. Synapse 2011, 65, 843–857. [Google Scholar] [CrossRef]
- Wang, Q.P.; Guan, J.L.; Matsuoka, T.; Hirayana, Y.; Shioda, S. Electron microscopic examination of the orexin immunoreactivity in the dorsal raphe nucleus. Peptides 2003, 24, 925–930. [Google Scholar] [CrossRef]
- Wang, Q.P.; Koyama, Y.; Guan, J.L.; Takahashi, K.; Kayama, Y.; Shioda, S. The orexinergic synaptic innervation of serotonin- and orexin 1-receptor-containing neurons in the dorsal raphe nucleus. Regul. Pept. 2005, 126, 35–42. [Google Scholar] [CrossRef]
- Bayer, L.; Eggermann, E.; Serafin, M.; Saint-Mleux, B.; Machard, D.; Jones, B.; Muhlethaler, M. Orexins (hypocretins) directly excite tuberomammillary neurons. Eur. J. Neurosci. 2001, 14, 1571–1575. [Google Scholar] [CrossRef]
- Eriksson, K.S.; Sergeeva, O.A.; Selbach, O.; Haas, H.L. Orexin (hypocretin)/dynorphin neurons control GABAergic inputs to tuberomammillary neurons. Eur. J. Neurosci. 2004, 19, 1278–1284. [Google Scholar] [CrossRef]
- Schöne, C.; Apergis-Schoute, J.; Sakurai, T.; Adamantidis, A.; Burdakov, D. Coreleased orexin and glutamate evoke nonredundant spike outputs and computations in histamine neurons. Cell Rep. 2014, 7, 697–704. [Google Scholar] [CrossRef]
- Lebow, M.A.; Chen, A. Overshadowed by the amygdala: The bed nucleus of the stria terminalis emerges as key to psychiatric disorders. Mol. Psychiatry 2016, 21, 450–463. [Google Scholar] [CrossRef]
- Valassi, E.; Scacchi, M.; Cavagnini, F. Neuroendocrine control of food intake. Nutr. Metab. Cardiovasc. Dis. 2008, 18, 158–168. [Google Scholar] [CrossRef]
- Konturek, S.J.; Konturek, J.W.; Pawlik, T.; Brzozowski, T. Brain-gut axis and its role in the control of food intake. J. Physiol. Pharmacol. 2004, 55 Pt 2, 137–154. [Google Scholar]
- Parker, J.A.; Bloom, S.R. Hypothalamic neuropeptides and the regulation of appetite. Neuropharmacology 2012, 63, 18–30. [Google Scholar] [CrossRef]
- Lee, J.; Raycraft, L.; Johnson, A.W. The dynamic regulation of appetitive behavior through lateral hypothalamic orexin and melanin concentrating hormone expressing cells. Physiol. Behav. 2021, 229, 113234. [Google Scholar] [CrossRef]
- Mieda, M.; Yanagisawa, M. Sleep, feeding, and neuropeptides: Roles of orexins and orexin receptors. Curr. Opin. Neurobiol. 2002, 12, 339–345. [Google Scholar] [CrossRef]
- Arora, S.; Anubhuti. Role of neuropeptides in appetite regulation and obesity—A review. Neuropeptides 2006, 40, 375–401. [Google Scholar] [CrossRef]
- Fukushima, A.; Hagiwara, H.; Fujioka, H.; Kimura, F.; Akema, T.; Funabashi, T. Sex differences in feeding behavior in rats: The relationship with neuronal activation in the hypothalamus. Front. Neurosci. 2015, 9, 88. [Google Scholar] [CrossRef]
- Nakamachi, T.; Matsuda, K.; Maruyama, K.; Miura, T.; Uchiyama, M.; Funahashi, H.; Sakurai, T.; Shioda, S. Regulation by orexin of feeding behaviour and locomotor activity in the goldfish. J. Neuroendocr. 2006, 18, 290–297. [Google Scholar] [CrossRef]
- Mistlberger, R.E.; Antle, M.C.; Kilduff, T.S.; Jones, M. Food- and light- entrained circadian rhythms in rats with hypocretin-2-saporin ablations of the lateral hypothalamus. Brain Res. 2003, 980, 161–168. [Google Scholar] [CrossRef]
- Choi, D.L.; Davis, J.F.; Magrisso, I.J.; Fitzgerald, M.E.; Lipton, J.W.; Benoit, S.C. Orexin signaling in the paraventricular thalamic nucleus modulates mesolimbic dopamine and hedonic feeding in the rat. Neuroscience 2012, 210, 243–248. [Google Scholar] [CrossRef] [PubMed]
- Crespo, I.; Gomez de Heras, R.; Rodriguez de Fonseca, F.; Navarro, M. Pretreatment with subeffective doses of Rimonabant attenuates orexigenic actions of orexin A-hypocretin 1. Neuropharmacology 2008, 54, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Ford, G.K.; Al-Barazanji, K.A.; Wilson, S.; Jones, D.N.; Harbuz, M.S.; Jessop, D.S. Orexin expression and function: Glucocorticoid manipulation, stress, and feeding studies. Endocrinology 2005, 146, 37243731. [Google Scholar] [CrossRef] [PubMed]
- Haynes, A.C.; Jackson, B.; Overend, P.; Buckingham, R.E.; Wilson, S.; Tadayyon, M.; Arch, J.R.S. Effects of single and chronic intracerebroventricular administration of the orexins on feeding in the rat. Peptides 1999, 20, 1099–1105. [Google Scholar] [CrossRef]
- Haynes, A.C.; Jackson, B.; Chapman, H.; Tadayyon, M.; Johns, A.; Porter, R.A.; Arch, J.R.S. A selective orexin-1 receptor antagonist reduces food consumption in male and female rats. Regul. Pept. 2000, 96, 45–51. [Google Scholar] [CrossRef]
- Karasawa, H.; Yakabi, S.; Wang, L.X.; Tache, Y. Orexin-1 receptor mediates the increased food and water intake induced by intracerebroventricular injection of the stable somatostatin pan-agonist, ODT8-SST in rats. Neurosci. Lett. 2014, 576, 88–92. [Google Scholar] [CrossRef]
- Li, A.J.; Wang, Q.; Davis, H.; Wang, R.; Ritter, S. Orexin-A enhances feeding in male rats by activating hindbrain catecholamine neurons. Am. J. Physiol. Regul. 2015, 309, R358–R367. [Google Scholar] [CrossRef]
- Lubkin, M.; Stricker-Krongrad, A. Independent feeding and metabolic actions of orexins in mice. Biochem. Biophys. Res. Commun. 1998, 253, 241–245. [Google Scholar] [CrossRef]
- Dhuria, S.V.; Fine, J.M.; Bingham, D.; Svitak, A.L.; Burns, R.B.; Baillargeon, A.M.; Panter, S.S.; Kazi, A.N.; Frey, W.H.; Hanson, L.R. Food consumption and activity levels increase in rats following intranasal Hypocretin-1. Neurosci. Lett. 2016, 627, 155–159. [Google Scholar] [CrossRef]
- Muthmainah, M.; Gogos, A.; Sumithran, P.; Brown, R.M. Orexins (hypocretins): The intersection between homeostatic and hedonic feeding. J. Neurochem. 2021, 157, 1473–1494. [Google Scholar] [CrossRef]
- McElroy, S.L.; Guerdjikova, A.I.; Mori, N.; Romo-Nava, F. Progress in developing pharmacologic agents to treat bulimia nervosa. CNS Drugs 2019, 33, 31–46. [Google Scholar] [CrossRef]
- Chieffi, S.; Iavarone, A.; La Marra, M.; Messina, G.; Villano, I.; Ranucci, S.; Monda, M. Memory for proprioceptive targets in bulimia nervosa. J. Psychiatry 2015, 18, 2. [Google Scholar] [CrossRef]
- Clegg, D.J.; Air, E.L.; Woods, S.C.; Seeley, R.J. Eating elicited by Orexin-A, but not melanin-concentrating hormone, is opioid mediated. Endocrinology 2002, 143, 2995–3000. [Google Scholar] [CrossRef]
- Guerdjikova, A.I.; Mori, N.; Casuto, L.S.; McElroy, S.L. Binge eating disorder. Psychiatr. Clin. N. Am. 2017, 40, 255–266. [Google Scholar] [CrossRef]
- La Marra, M.; Caviglia, G.; Perrella, R. Using smartphones when eating increases caloric intake in young people: An overview of the literature. Front. Psychol. 2020, 11, 587886. [Google Scholar] [CrossRef]
- La Marra, M.; Villano, I.; Ilardi, C.R.; Carosella, M.; Staiano, M.; Iavarone, A.; Chieffi, S.; Messina, G.; Polito, R.; Porro, C.; et al. Executive Functions in Overweight and Obese Treatment-Seeking Patients: Cross-Sectional Data and Longitudinal Perspectives. Brain Sci. 2022, 12, 777. [Google Scholar] [CrossRef]
- Villano, I.; Ilardi, C.R.; Arena, S.; Scuotto, C.; Gleijeses, M.G.; Messina, G.; Messina, A.; Monda, V.; Monda, M.; Iavarone, A.; et al. Obese Subjects without Eating Disorders Experience Binge Episodes Also Independently of Emotional Eating and Personality Traits among University Students of Southern Italy. Brain Sci. 2021, 11, 1145. [Google Scholar] [CrossRef]
- Morganstern, I.; Chang, G.Q.; Karatayev, O.; Leibowitz, S.F. Increased orexin and melanin-concentrating hormone expression in the perifornical lateral hypothalamus of rats prone to overconsuming a fat-rich diet. Pharmacol. Biochem. Behav. 2010, 96, 413–422. [Google Scholar] [CrossRef]
- Mitchell, C.S.; Fisher, S.D.; Yeoh, J.W.; Pearl, A.J.; Burton, N.J.; Bains, J.S.; McNally, G.P.; Andrews, Z.A.; Graham, B.A.; Dayas, C.V. A ventral striatal-orexin/ hypocretin circuit modulates approach but not consumption of food. bioRxiv. 2020. [Google Scholar] [CrossRef]
- Mieda, M.; Sakurai, T. Overview of orexin/hypocretin system. Prog Brain Res. 2012, 198, 5–14. [Google Scholar] [CrossRef]
- Burdakov, D.; Gerasimenko, O.; Verkhratsky, A. Physiological changes in glucose differentially modulate the excitability of hypothalamic melanin-concentrating hormone and orexin neurons in situ. J. Neurosci. 2005, 25, 2429–2433. [Google Scholar] [CrossRef]
- Akimoto-Takano, S.; Sakurai, C.; Kanai, S.; Hosoya, H.; Ohta, M.; Miyasaka, K. Differences in the appetite-stimulating effect of orexin, neuropeptide Y and ghrelin among young, adult and old rats. Neuroendocrinology 2005, 82, 256–263. [Google Scholar] [CrossRef]
- Elias, C.F.; Saper, C.B.; Maratos-Flier, E.; Tritos, N.A.; Lee, C.; Kelly, J.; Tatro, J.B.; Hoffman, G.E.; Ollmann, M.M.; Barsh, G.S.; et al. Chemically defined projections linking the mediobasal hypothalamus and the lateral hypothalamic area. J. Comp. Neurol. 1998, 402, 442–459. [Google Scholar] [CrossRef]
- Miura, T.; Maruyama, K.; Shimakura, S.; Kaiya, H.; Uchiyama, M.; Kangawa, K.; Shioda, S.; Matsuda, K. Regulation of food intake in the goldfish by interaction between ghrelin and orexin. Peptides 2007, 28, 1207–1213. [Google Scholar] [CrossRef]
- Karnani, M.M.; Apergis-Schoute, J.; Adamantidis, A.; Jensen, L.T.; de Lecea, L.; Fugger, L.; Burdakov, D. Activation of central orexin/hypocretin neurons by dietary amino acids. Neuron 2011, 72, 616–629. [Google Scholar] [CrossRef]
- Mavanji, V.; Pomonis, B.; Kotz, C.M. Orexin, serotonin, and energy balance. Wires Mech. Dis. 2021, 14, e1536. [Google Scholar] [CrossRef]
- Kotz, C.; Nixon, J.; Butterick, T.; Perez-Leighton, C.; Teske, J.; Billington, C. Brain orexin promotes obesity resistance. Ann. N. Y. Acad. Sci. 2012, 1264, 72–86. [Google Scholar] [CrossRef]
- Kayaba, Y.; Nakamura, A.; Kasuya, Y.; Ohuchi, T.; Yanagisawa, M.; Komuro, I.; Fukuda, Y.; Kuwaki, T. Attenuated defense response and low basal blood pressure in orexin knockout mice. Am. J. Physiol. Regul. 2003, 285, R581–R593. [Google Scholar] [CrossRef]
- Zhang, W.; Sakurai, T.; Fukuda, Y.; Kuwaki, T. Orexin neuron-mediated skeletal muscle vasodilation and shift of baroreflex during defense response in mice. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2006, 290, R1654–R1663. [Google Scholar] [CrossRef]
- Schuld, A.; Hebebrand, J.; Geller, F.; Pollmächer, T. Increased body-mass index in patients with narcolepsy. Lancet 2000, 355, 1274–1275. [Google Scholar] [CrossRef]
- Funato, H.; Tsai, A.L.; Willie, J.T.; Kisanuki, Y.; Williams, S.C.; Sakurai, T.; Yanagisawa, M. Enhanced orexin receptor-2 signaling prevents diet-induced obesity and improves leptin sensitivity. Cell Met. 2009, 9, 64–76. [Google Scholar] [CrossRef] [PubMed]
- Chang, G.Q.; Karatayev, O.; Davydova, Z.; Leibowitz, S.F. Circulating triglycerides impact on orexigenic peptides and neuronal activity in hypothalamus. Endocrinology 2004, 145, 3904–3912. [Google Scholar] [CrossRef] [PubMed]
- Julian, V.; Thivel, D.; Costes, F.; Touron, J.; Boirie, Y.; Pereira, B.; Perrault, H.; Duclos, M.; Richard, R. Eccentric training improves body composition by inducing mechanical and metabolic adaptations: A promising approach for overweight and obese individuals. Front. Physiol. 2018, 9, 1013. [Google Scholar] [CrossRef] [PubMed]
- Villano, I.; La Marra, M.; Messina, A.; Di Maio, G.; Moscatelli, F.; Chieffi, S.; Monda, M.; Messina, G.; Monda, V. Effects of vegetarian and vegan nutrition on body composition in competitive futsal athletes. Prog. Nutr. 2021, 23, e2021126. [Google Scholar] [CrossRef]
- Chieffi, S.; Messina, G.; Villano, I.; Messina, A.; Valenzano, A.; Moscatelli, F.; Salerno, M.; Sullo, A.; Avola, R.; Monda, V.; et al. Neuroprotective Effects of Physical Activity: Evidence from Human and Animal Studies. Front. Neurol. 2017, 8, 188. [Google Scholar] [CrossRef]
- Di Maio, G.; Monda, V.; Messina, A.; Polito, R.; Monda, M.; Tartaglia, N.; Ambrosio, A.; Pisanelli, D.; Asmundo, A.; Di Nunno, N.; et al. Physical activity and modification of lifestyle induce benefits on the health status. Acta Med. Mediterr. 2020, 36, 1913–1919. [Google Scholar] [CrossRef]
- Crispino, M.; Trinchese, G.; Penna, E.; Cimmino, F.; Catapano, A.; Perrone-Capano, C.; Mollica, M.P. Interplay between peripheral and central inflammation in obesity-promoted disorders: The impact on synaptic mitochondrial functions. Int. J. Mol. Sci. 2020, 21, 5964. [Google Scholar] [CrossRef]
- Kotz, C.M.; Perez-Leighton, C.E.; Teske, J.A.; Billington, C.J. Spontaneous physical activity defends against obesity. Curr. Obes. Rep. 2017, 6, 362–370. [Google Scholar] [CrossRef]
- Levine, J.A. Non-exercise activity thermogenesis (NEAT). Best Pract. Res. Clin. Endocrinol. Metab. 2002, 16, 679–702. [Google Scholar] [CrossRef]
- Flegal, K.M.; Carroll, M.D.; Ogden, C.L.; Curtin, L.R. Prevalence and trends in obesity among US adults, 1999–2008. JAMA 2010, 303, 235–241. [Google Scholar] [CrossRef]
- Monda, V.; La Marra, M.; Perrella, R.; Caviglia, G.; Iavarone, A.; Chieffi, S.; Messina, G.; Carotenuto, M.; Monda, M.; Messina, A. Obesity and brain illness: From cognitive and psychological evidences to obesity paradox. Diabetes Metab. Syndr. Obes. 2017, 10, 473. [Google Scholar] [CrossRef]
- Levin, B.E.; Dunn-Meynell, A.A.; Balkan, B.; Keesey, R.E. Selective breeding for diet-induced obesity and resistance in Sprague-Dawley rats. Am. J. Physiol. 1997, 273, R725–R730. [Google Scholar] [CrossRef]
- Teske, J.A.; Levine, A.S.; Kuskowski, M.; Levine, J.A.; Kotz, C.M. Elevated hypothalamic orexin signaling, sensitivity to orexin A, and spontaneous physical activity in obesity-resistant rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2006, 291, R889–R899. [Google Scholar] [CrossRef]
- Perez-Leighton, C.E.; Boland, K.; Teske, J.A.; Billington, C.; Kotz, C.M. Behavioral responses to orexin, orexin receptor gene expression, and spontaneous physical activity contribute to individual sensitivity to obesity. Am. J. Physiol. Endocrinol. Metab. 2012, 303, E865–E874. [Google Scholar] [CrossRef]
- Moretto, T.L.; Benfato, I.D.; de Carvalho, F.P.; Barthichoto, M.; Le Sueur-Maluf, L.; de Oliveira, C.A.M. The effects of calorie-matched high-fat diet consumption on spontaneous physical activity and development of obesity. Life Sci. 2017, 179, 30–36. [Google Scholar] [CrossRef]
- Hao, Y.Y.; Yuan, H.W.; Fang, P.H.; Zhang, Y.; Liao, Y.X.; Shen, C.; Wang, D.; Zhang, T.T.; Bo, P. Plasma orexin-A level associated with physical activity in obese people. Eat. Weight Disord. 2017, 22, 69–77. [Google Scholar] [CrossRef]
- Hara, J.; Yanagisawa, M.; Sakurai, T. Difference in obesity phenotype between orexin-knockout mice and orexin neuron-deficient mice with same genetic background and environmental conditions. Neurosci. Lett. 2005, 380, 239–242. [Google Scholar] [CrossRef]
- Burdakov, D.; Jensen, L.T.; Alexopoulos, H.; Williams, R.H.; Fearon, I.M.; O’Kelly, I.; Gerasimenko, O.; Fugger, L.; Verkhratsky, A. Tandem-pore K+ channels mediate inhibition of orexin neurons by glucose. Neuron 2006, 50, 711–722. [Google Scholar] [CrossRef]
- Teske, J.A.; Mavanji, V. Energy expenditure: Role of orexin. Vitam. Horm. 2012, 89, 91–109. [Google Scholar] [CrossRef]
- James, M.H.; Campbell, E.J.; Walker, F.R.; Smith, D.W.; Richardson, H.N.; Hodgson, D.M.; Dayas, C.V. Exercise reverses the effects of early life stress on orexin cell reactivity in male but not female rats. Front. Behav. Neurosci. 2014, 8, 244. [Google Scholar] [CrossRef]
- Martin, T.; Dauvilliers, Y.; Koumar, O.C.; Bouet, V.; Freret, T.; Besnard, S.; Bessot, N. Dual orexin receptor antagonist induces changes in core body temperature in rats after exercise. Sci. Rep. 2019, 9, 18432. [Google Scholar] [CrossRef]
- Monda, V.; Villano, I.; Messina, A.; Valenzano, A.; Salerno, M.; Santo Signorelli, S.; Monda, M.; Russo, V.; Sessa, F.; Messina, G. Aerobic exercise and Orexin A: Role of sympathetic activity and redox system. J. Biol. Regul. Homeost. Agents 2019, 33, 587–592. [Google Scholar]
- Kiwaki, K.; Kotz, C.M.; Wang, C.; Lanningham-Foster, L.; Levine, J.A. Orexin A (hypocretin 1) injected into hypothalamic paraventricular nucleus and spontaneous physical activity in rats. Am. J. Physiol.-Endocrinol. Metab. 2004, 286, E551–E559. [Google Scholar] [CrossRef]
- España, R.A.; Baldo, B.A.; Kelley, A.E.; Berridge, C.W. Wake-promoting and sleep-suppressing actions of hypocretin (orexin): Basal forebrain sites of action. Neuroscience 2001, 106, 699–715. [Google Scholar] [CrossRef]
- Kotz, C.M.; Teske, J.A.; Billington, C.J. Neuroregulation of nonexercise activity thermogenesis and obesity resistance. Am. J. Physiol. Regul. 2008, 294, R699–R710. [Google Scholar] [CrossRef]
- Novak, C.M.; Kotz, C.M.; Levine, J.A. Central orexin sensitivity, physical activity, and obesity in diet-induced obese and diet-resistant rats. Am. J. Physiol. Endocrinol. Metab. 2006, 290, E396–E403. [Google Scholar] [CrossRef]
- Mavanji, V.; Perez-Leighton, C.E.; Kotz, C.M.; Billington, C.J.; Parthasarathy, S.; Sinton, C.M.; Teske, J.A. Promotion of wakefulness and energy expenditure by orexin-A in the ventrolateral preoptic area. Sleep 2015, 38, 1361–1370. [Google Scholar] [CrossRef]
- Novak, C.M.; Levine, J.A. Daily intraparaventricular orexin-A treatment induces weight loss in rats. Obesity 2009, 17, 1493–1498. [Google Scholar] [CrossRef]
- Teske, J.A.; Billington, C.J.; Kotz, C.M. Hypocretin/orexin and energy expenditure. Acta Physiol. 2010, 198, 303–312. [Google Scholar] [CrossRef] [PubMed]
- Teske, J.A.; Perez-Leighton, C.E.; Billington, C.J.; Kotz, C.M. Role of the locus coeruleus in enhanced orexin A-induced spontaneous physical activity in obesity-resistant rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2013, 305, R1337–R1345. [Google Scholar] [CrossRef] [PubMed]
- Perez-Leighton, C.E.; Butterick-Peterson, T.A.; Billington, C.J.; Kotz, C.M. Role of orexin receptors in obesity: From cellular to behavioral evidence. Int. J. Obes. 2013, 37, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Messina, A.; Monda, M.; Valenzano, A.; Messina, G.; Villano, I.; Moscatelli, F.; Cibelli, G.; Marsala, G.; Polito, R.; Ruberto, M.; et al. Functional changes induced by orexin a and adiponectin on the sympathetic/parasympathetic balance. Front. Phys. 2018, 9, 259. [Google Scholar] [CrossRef] [PubMed]
- Straat, M.E.; Schinkelshoek, M.S.; Fronczek, R.; Lammers, G.J.; Rensen, P.C.N.; Boon, M.R. Role of Brown Adipose Tissue in Adiposity Associated With Narcolepsy Type 1. Front. Endocrinol. 2020, 11, 145. [Google Scholar] [CrossRef] [PubMed]
- Messina, A.; Monda, V.; Villano, I.; Valenzano, A.A.; Salerno, M.; Tafuri, D.; Avola, R.; Chieffi, S.; Sullo, A.; Cibelli, G.; et al. Orexin system increases energy expenditure by brown adipose tissue activity. Natl. J. Physiol. Pharm. Pharmacol. 2017, 7, 658. [Google Scholar] [CrossRef]
- Morrison, S.F.; Madden, C.J.; Tupone, D. Central neural regulation of brown adipose tissue thermogenesis and energy expenditure. Cell Metab. 2014, 19, 741–756. [Google Scholar] [CrossRef]
- Burt, J.; Alberto, C.O.; Parsons, M.P.; Hirasawa, M. Local network regulation of orexin neurons in the lateral hypothalamus. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2011, 301, R572–R580. [Google Scholar] [CrossRef]
- James, M.H.; Mahler, S.V.; Moorman, D.E.; Aston-Jones, G. Decade of orexin/hypocretin and addiction: Where are we now? Curr. Top. Behav. Neurosci. 2016, 33, 247–281. [Google Scholar] [CrossRef]
- Martin-Fardon, R.; Cauvi, G.; Kerr, T.M.; Weiss, F. Differential role of hypothalamic orexin/hypocretin neurons in reward seeking motivated by cocaine versus palatable food. Addict. Biol. 2016, 23, 6–15. [Google Scholar] [CrossRef]
- Walker, L.C.; Lawrence, A.J. The Role of Orexins/Hypocretins in Alcohol Use and Abuse. Curr. Top. Behav. Neurosci. 2017, 33, 221–246. [Google Scholar] [CrossRef]
- Salerno, M.; Villano, I.; Nicolosi, D.; Longhitano, L.; Loreto, C.; Lovino, A.; Sessa, F.; Polito, A.N.; Monda, V.; Chieffi, S.; et al. Modafinil and orexin system: Interactions and medico-legal considerations. Front. Biosci. 2019, 24, 564–575. [Google Scholar] [CrossRef]
- Subramanian, S.; Ravichandran, M. Orexin receptors: Targets and applications. Fundam. Clin. Pharmacol. 2022, 36, 72–80. [Google Scholar] [CrossRef]
- James, M.H.; Stopper, C.M.; Zimmer, B.A.; Koll, N.E.; Bowrey, H.E.; Aston-Jones, G. Increased number and activity of a lateral subpopulation of hypothalamic orexin/hypocretin neurons underlies the expression of an addicted state in rats. Biol. Psychiatry 2019, 85, 925–935. [Google Scholar] [CrossRef]
- Baimel, C.; Borgland, S.L. Hypocretin modulation of drug-induced synaptic plasticity. Prog. Brain Res. 2012, 198, 123–131. [Google Scholar] [CrossRef]
- Khoo, S.Y.; Brown, R.M. Orexin/hypocretin-based pharmacotherapies for the treatment of addiction: DORA or SORA? CNS Drugs 2014, 28, 713–730. [Google Scholar] [CrossRef]
- Rasmussen, K.; White, D.A.; Acri, J.B. NIDA’s medication development priorities in response to the Opioid Crisis: Ten most wanted. Neuropsychopharmacology 2019, 44, 657–659. [Google Scholar] [CrossRef]
- Campbell, E.J.; Marchant, N.J.; Lawrence, A.J. A sleeping giant: Suvorexant for the treatment of alcohol use disorder? Brain Res. 2020, 1731, 145902. [Google Scholar] [CrossRef]
- McGregor, R.; Shan, L.; Wu, M.F.; Siegel, J.M. Diurnal fluctuation in the number of hypocretin/orexin and histamine producing: Implication for understanding and treating neuronal loss. PLoS ONE. 2017, 12, e0178573. [Google Scholar] [CrossRef]
- Ilardi, C.R.; Garofalo, E.; Chieffi, S.; Gamboz, N.; La Marra, M.; Iavarone, A. Daily exposure to digital displays may affect the clock-drawing test: From psychometrics to serendipity. Neurol. Sci. 2020, 41, 3683–3690. [Google Scholar] [CrossRef]
- Chieffi, S.; Messina, G.; La Marra, M.; Iavarone, A.; Viggiano, A.; De Luca, V.; Monda, M. Distractor interference in visual motor tasks. Hor. Neurosci. Res. 2014, 13, 151–160. [Google Scholar]
- Wang, Q.; Cao, F.; Wu, Y. Orexinergic System in Neurodegenerative Diseases. Front. Aging Neurosci. 2021, 13, 713201. [Google Scholar] [CrossRef]
- Fronczek, R.; van Geest, S.; Frölich, M.; Overeem, S.; Roelandse, F.W.C.; Lammers, G.J.; Swaab, D.F. Hypocretin (orexin) loss in Alzheimer’s disease. Neurobiol. Aging. 2012, 33, 1642–1650. [Google Scholar] [CrossRef]
- Fronczek, R.; Overeem, S.; Lee, S.Y.Y.; Hegeman, I.M.; van Pelt, J.; van Duinen, S.G.; Lammers, G.J.; Swaab, D.F. Hypocretin (orexin) loss in Parkinson’s disease. Brain 2007, 130, 1577–1585. [Google Scholar] [CrossRef]
- Stanojlovic, M.; Pallais, J.P.; Kotz, C.M. Inhibition of Orexin/Hypocretin Neurons Ameliorates Elevated Physical Activity and Energy Expenditure in the A53T Mouse Model of Parkinson’s Disease. Int. J. Mol. Sci. 2021, 22, 795. [Google Scholar] [CrossRef]
- Chieffi, S.; Villano, I.; Messina, A.; Monda, V.; La Marra, M.; Messina, G.; Monda, M. Involvement of orexin in sleep disorders and neurodegenerative diseases. Curr. Top. Pept. 2015, 16, 49–54. [Google Scholar]
- Porkka-Heiskanen, T.; Alanko, L.; Kalinchuk, A.; Heiskanen, S.; Stenberg, D. The effect of age on prepro-orexin gene expression and contents of orexin A and B in the rat brain. Neurobiol. Aging 2004, 25, 231–238. [Google Scholar] [CrossRef]
- Zhang, J.H.; Sampogna, S.; Morales, F.R.; Chase, M.H. Age-related changes in hypocretin (orexin) immunoreactivity in the cat brainstem. Brain Res. 2002, 930, 206–211. [Google Scholar] [CrossRef]
- Zhao, X.; Zhang, R.X.; Tang, S.; Ren, Y.M.; Yang, W.; Liu, X.; Tang, J. Orexin-A-induced ERK1/2 activation reverses impaired spatial learning and memory in pentylenetetrazol-kindled rats via OX1R-mediated hippocampal neurogenesis. Peptides 2014, 54, 140–147. [Google Scholar] [CrossRef]
- Yang, L.; Zou, B.; Xiong, X.; Pascual, C.; Xie, J.; Malik, A.; Xie, J.; Sakurai, T.; Xie, X.S. Hypocretin/orexin neurons contribute to hippocampus-dependent social memory and synaptic plasticity in mice. J. Neurosci. 2013, 33, 5275–5284. [Google Scholar] [CrossRef]
- Chieffi, S.; Messina, G.; Villano, I.; Messina, A.; Esposito, M.; Monda, V.; Valenzano, A.; Moscatelli, F.; Esposito, T.; Carotenuto, M.; et al. Exercise Influence on Hippocampal Function: Possible Involvement of Orexin-A. Front. Physiol. 2017, 8, 85. [Google Scholar] [CrossRef]
- La Torre, M.E.; Villano, I.; Monda, M.; Messina, A.; Cibelli, G.; Valenzano, A.; Pisanelli, D.; Panaro, M.A.; Tartaglia, N.; Ambrosi, A.; et al. Role of vitamin E and the orexin system in neuroprotection. Brain Sci. 2021, 11, 1098. [Google Scholar] [CrossRef]
- Precenzano, F.; Ruberto, M.; Parisi, L.; Salerno, M.; Maltese, A.; Vagliano, C.; Messina, G.; Di Folco, A.; Di Filippo, T.; Roccella, M. Executive functioning in preschool children affected by autism spectrum disorder: A pilot study. Acta Med. 2017, 33, 35. [Google Scholar] [CrossRef]
- Chieffi, S.; Messina, A.; Villano, I.; Valenzano, A.A.; Nigro, E.; La Marra, M.; Messina, G. The use of velocity information in movement reproduction. Front. Psychol. 2017, 8, 983. [Google Scholar] [CrossRef] [PubMed]
| OX1R Expression Site | OX2R Expression Site |
|---|---|
| Nucleus of the Solitary Tract (NTS) | Nucleus of the Solitary Tract (NTS) |
| Pedunculopontine/Latero-Dorsal Tegmental Nucleus (PPT/LDT) | Arcuate Nucleus (ARC) |
| Locus Coeruleus (LC) | Pedunculopontine/Latero-Dorsal Tegmental Nucleus (PPT/LDT) |
| Ventral Tegmental Area (VTA) | Locus Coeruleus (LC) |
| Dorsal Raphe Nucleus (DRN) | Ventral Tegmental Area (VTA) |
| Anterior Hypothalamus | Dorsal Raphe Nucleus (DRN) |
| Bed Nucleus of the Stria Terminalis (BNST) | Paraventricular Thalamus (PVT) |
| Basal Forebrain (BF) | Paraventricular Nucleus (PVN) |
| Paraventricular Thalamus (PVT) | Preoptic Area (POA) |
| Paraventricular Nucleus (PVN) | Lateral Hypothalamus (LH) |
| Preoptic Area (POA) | Basal Forebrain (BF) |
| Hippocampus (CA1 And CA2) | Bed Nucleus of the Stria Terminalis (BNST) |
| Dentate Gyrus (DG) | Dorsomedial Hypothalamic Nucleus (DMH) |
| Amygdala | Tuberomammillary Nucleus (TMN) |
| Ventral Pallidum (VP) | Hippocampus (CA3) |
| Olfactory Bulb (OB) | Dentate Gyrus (DG) |
| Prefrontal and Infralimbic Cortex (IL) | Amygdala |
| Insular Cortex (IC) | Nucleus Accumbens (NAC) |
| Lateral Septum (LS) | |
| Medial Septum (MS) | |
| Anterior Commissure (AC) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Villano, I.; La Marra, M.; Di Maio, G.; Monda, V.; Chieffi, S.; Guatteo, E.; Messina, G.; Moscatelli, F.; Monda, M.; Messina, A. Physiological Role of Orexinergic System for Health. Int. J. Environ. Res. Public Health 2022, 19, 8353. https://doi.org/10.3390/ijerph19148353
Villano I, La Marra M, Di Maio G, Monda V, Chieffi S, Guatteo E, Messina G, Moscatelli F, Monda M, Messina A. Physiological Role of Orexinergic System for Health. International Journal of Environmental Research and Public Health. 2022; 19(14):8353. https://doi.org/10.3390/ijerph19148353
Chicago/Turabian StyleVillano, Ines, Marco La Marra, Girolamo Di Maio, Vincenzo Monda, Sergio Chieffi, Ezia Guatteo, Giovanni Messina, Fiorenzo Moscatelli, Marcellino Monda, and Antonietta Messina. 2022. "Physiological Role of Orexinergic System for Health" International Journal of Environmental Research and Public Health 19, no. 14: 8353. https://doi.org/10.3390/ijerph19148353
APA StyleVillano, I., La Marra, M., Di Maio, G., Monda, V., Chieffi, S., Guatteo, E., Messina, G., Moscatelli, F., Monda, M., & Messina, A. (2022). Physiological Role of Orexinergic System for Health. International Journal of Environmental Research and Public Health, 19(14), 8353. https://doi.org/10.3390/ijerph19148353










