Degradation and Detoxification of Chlorophenols with Different Structure by LAC-4 Laccase Purified from White-Rot Fungus Ganoderma lucidum
Abstract
:1. Introduction
2. Materials and Methods
2.1. Strain and Medium
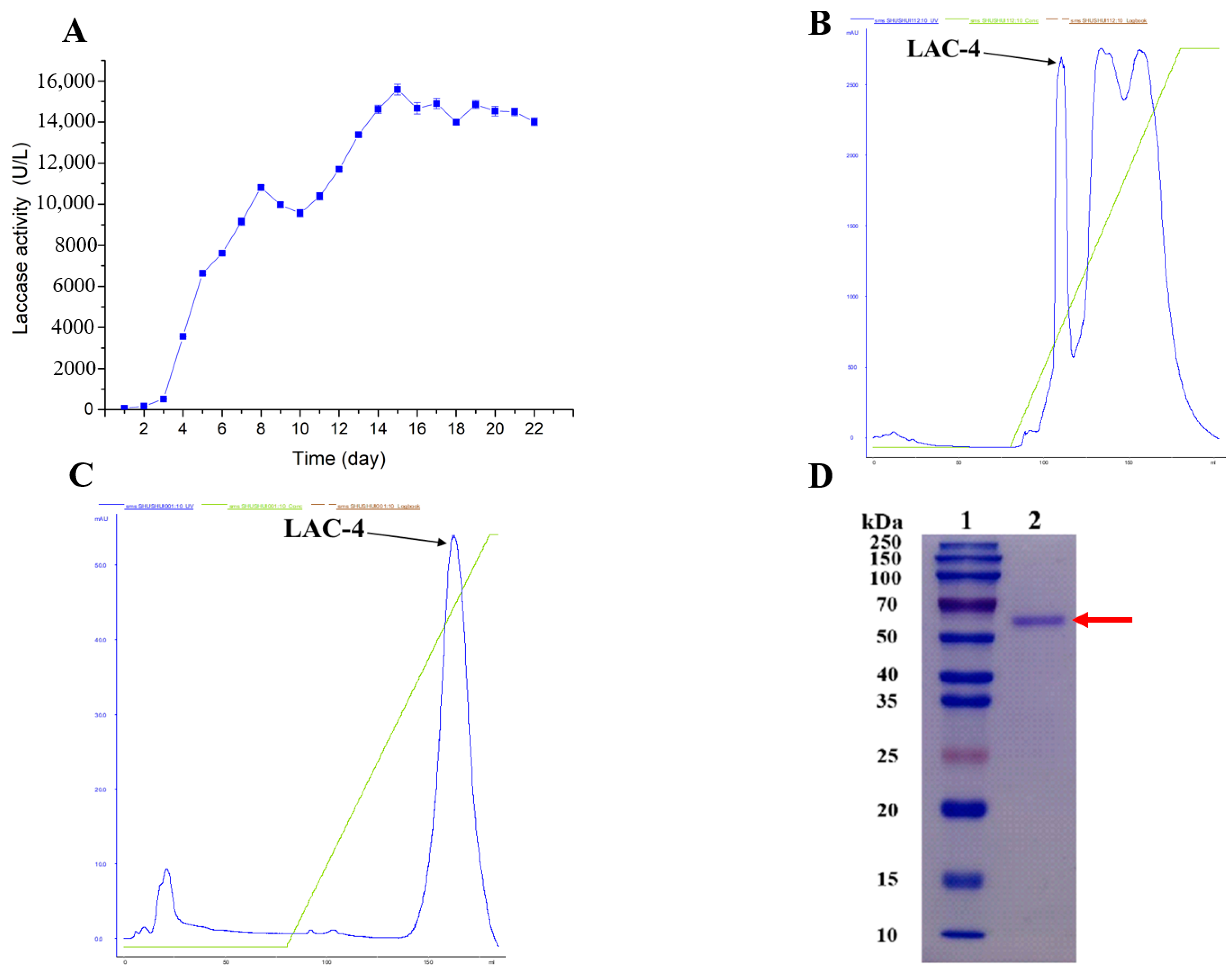
2.2. Purification of LAC-4 Laccase
2.3. Study on Enzymatic Properties of Purified LAC-4
2.3.1. Kinetic Studies on LAC-4 Laccase
2.3.2. Optimal Reaction Temperature and Stability of Laccase at Different Temperatures
2.3.3. Optimal Reaction pH and Stability of Laccase at Different pH
2.3.4. Effect of Inhibitors on the Activity of LAC-4
2.3.5. Effect of Different Metal Ions and Organic Solvents on the Activity of LAC-4
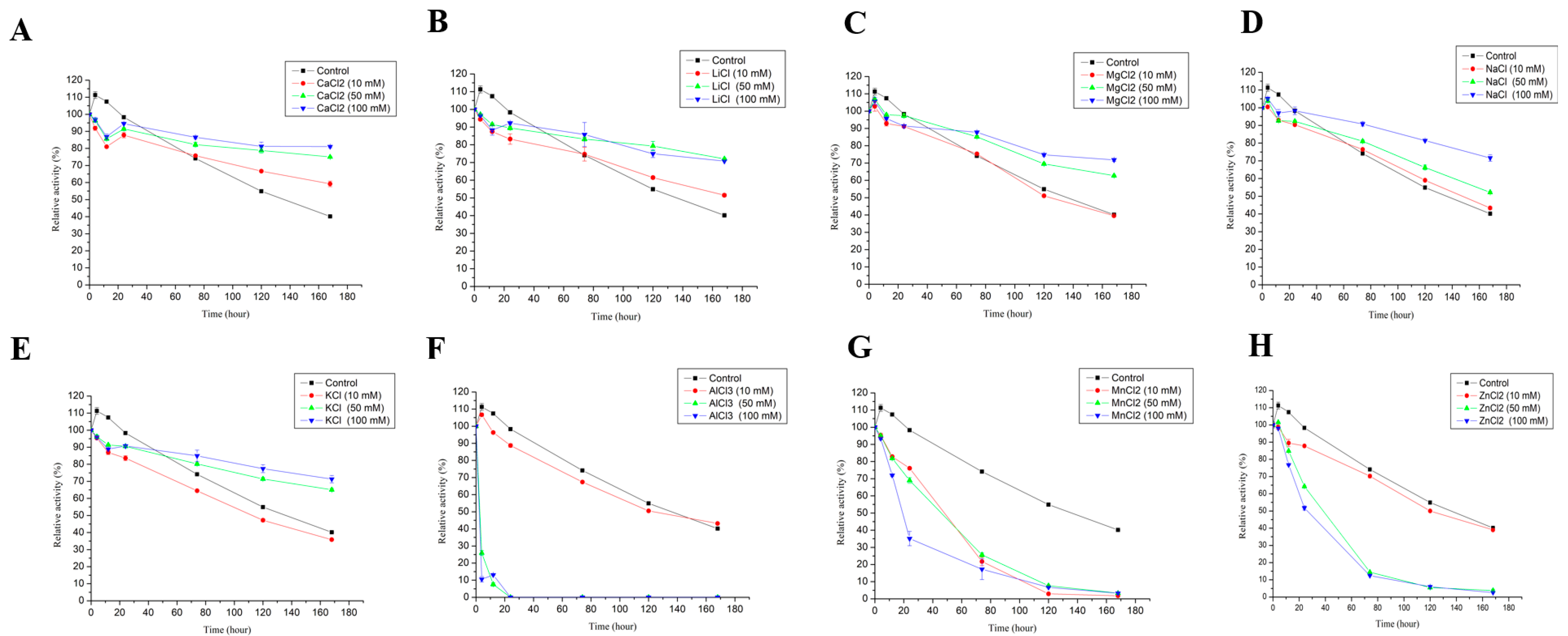
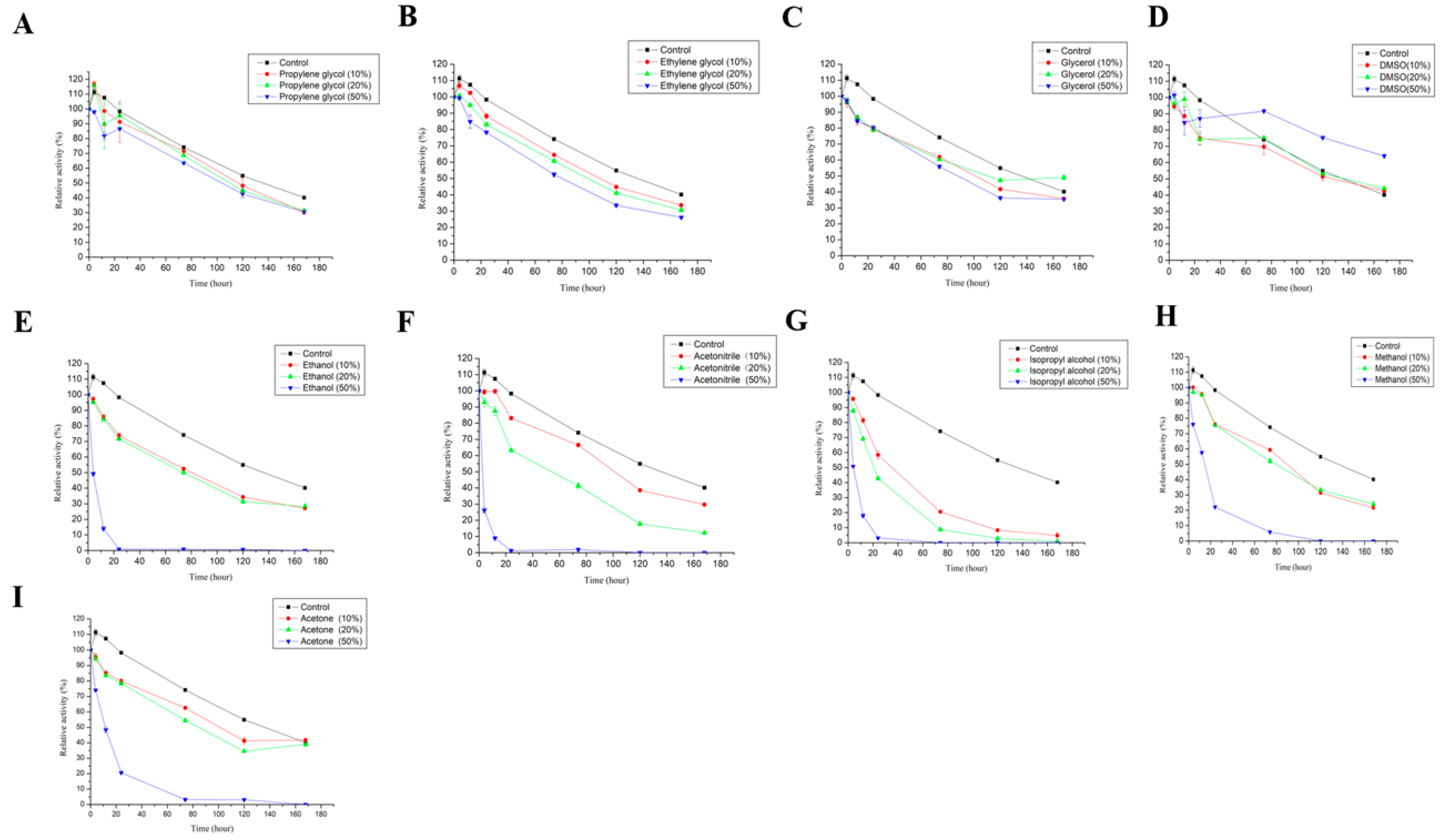
2.3.6. Effect of Different Metal Ions and Organic Solvents on the Stability of LAC-4
2.4. Degradation of Different Concentrations of Chlorophenols by LAC-4
2.5. Study on the Kinetics of Degradation of Two Chlorophenols by LAC-4
2.6. The Effects of Metal Salts and Organic Solvents on the Degradation of Chlorophenols by LAC-4
2.7. Phytotoxicity Testing of Degradation Products
2.8. Determination of Chloride Ion Concentration by Silver Nitrate Turbidimetry: Dechlorination Study
2.9. HPLC Detection of Chlorophenol
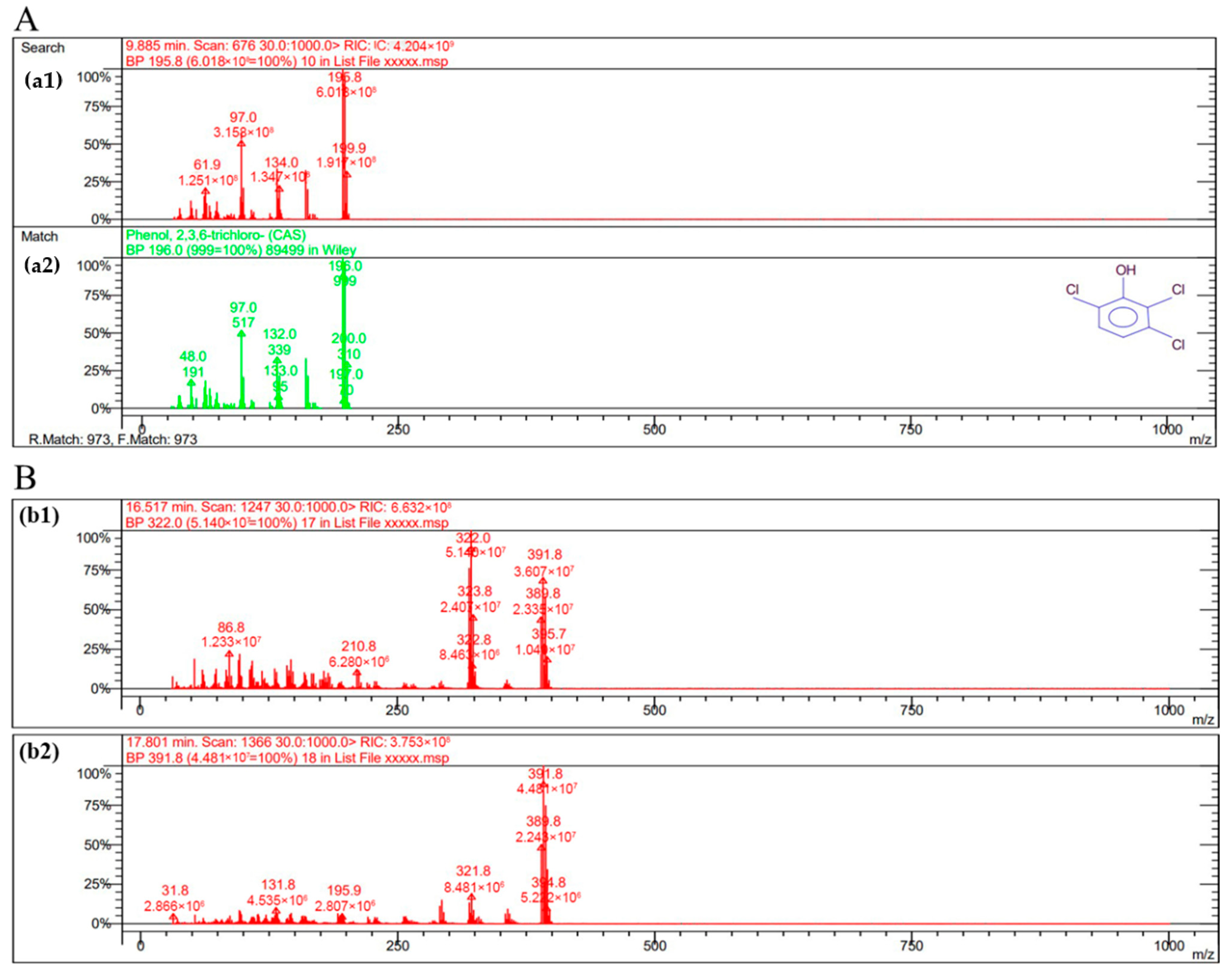
2.10. GC-MS Detection of LAC-4 Degradation of the 2,6-Dichlorophenol Intermediate
2.11. Statistical Analysis
3. Results
3.1. Purification of LAC-4 Laccase from Ganoderma lucidum
3.2. Study on the Enzymatic Properties of Purified LAC-4 Laccase
3.2.1. Kinetic Studies on the Purified LAC-4
3.2.2. Effect of Temperature on the Activity and Stability of LAC-4
3.2.3. Effect of pH on the Activity and Stability of LAC-4
3.2.4. Effect of Inhibitors on the Activity of LAC-4
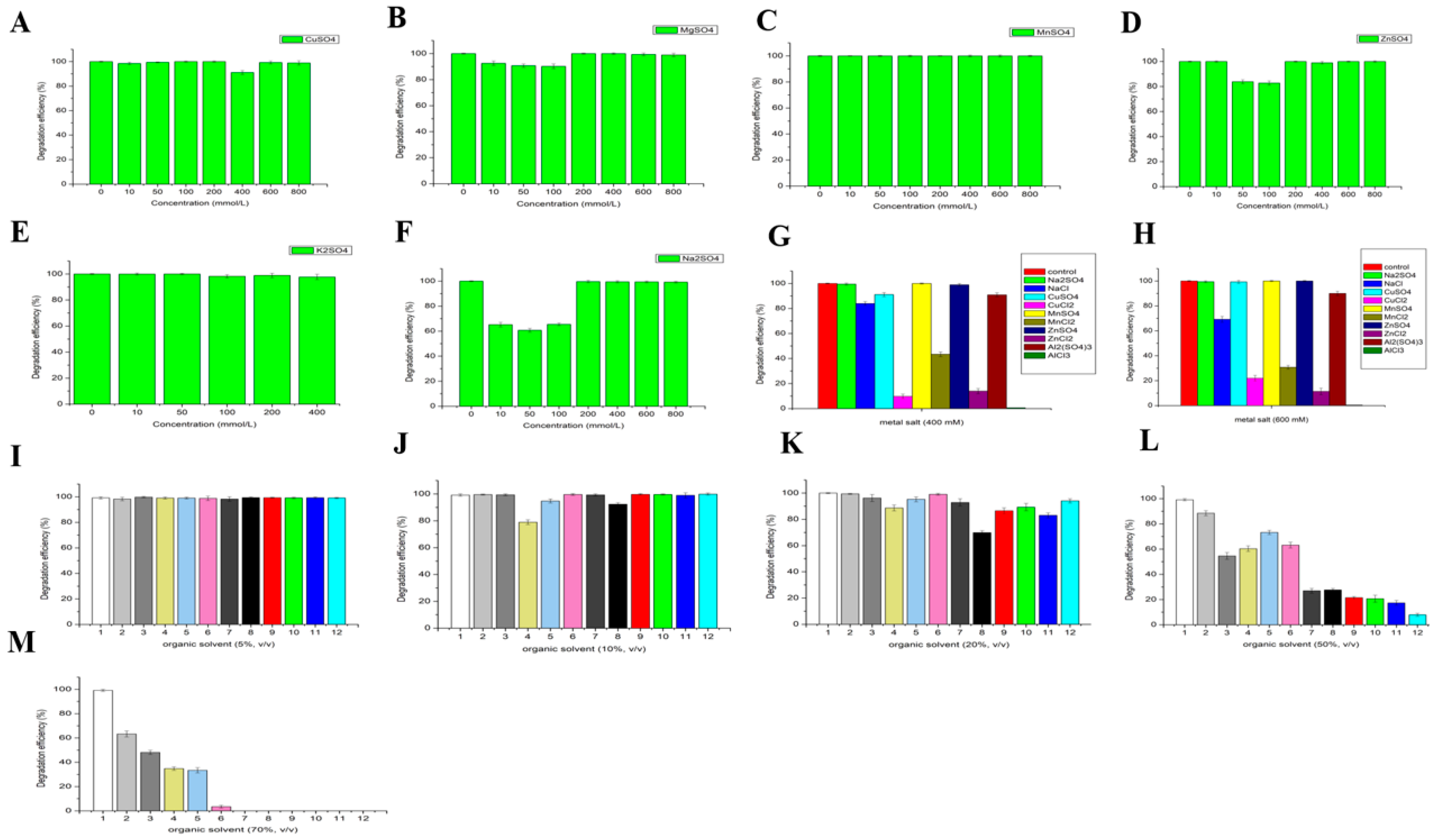
3.2.5. Effect of Metal Ions and Organic Solvents on the Activity of LAC-4
3.2.6. Effect of Metal Ions and Organic Solvents on the Stability of LAC-4
3.3. Study on the Degradation of Chlorophenols with Different Structures by LAC-4
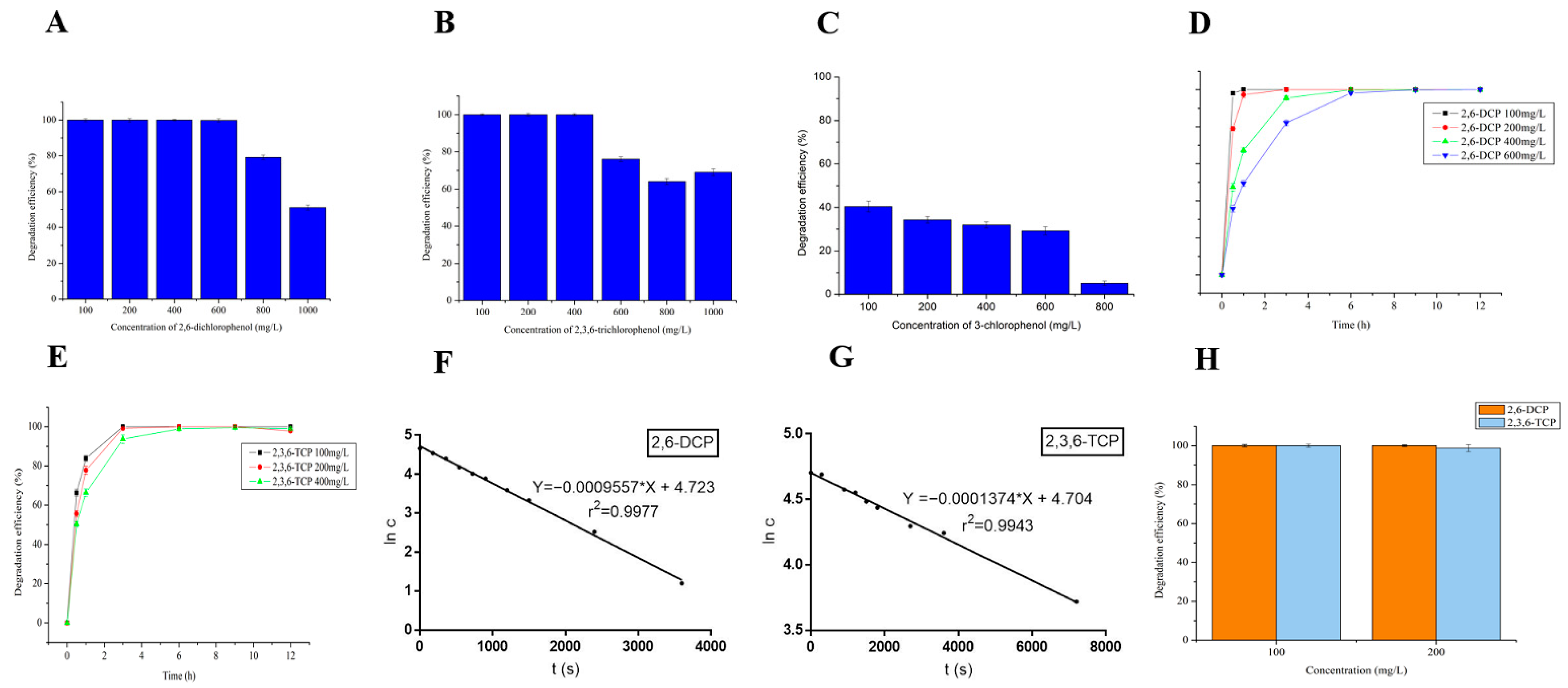
3.3.1. Degradation of 2,6-Dichlorophenol (2,6-DCP), 2,3,6-Trichlorophenol (2,3,6-TCP) and 3-Chlorophenol (3-CP) at Different Concentrations by LAC-4
3.3.2. Kinetics of Degradation of Chlorophenols by LAC-4
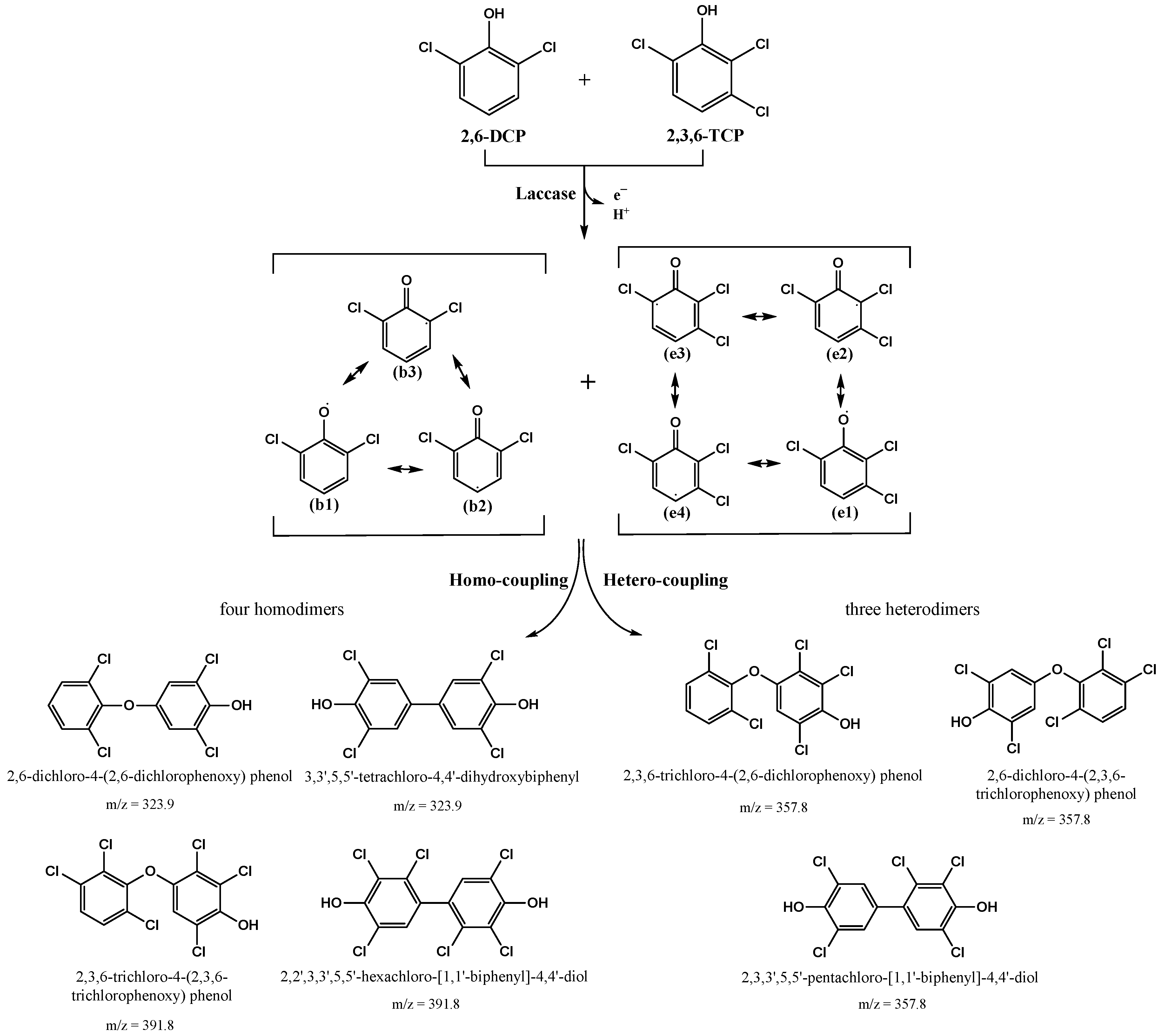
3.3.3. Degradation of Chlorophenol Mixtures by LAC-4
3.3.4. Effects of Different Concentrations of Metal Ions and Organic Solvents on the Degradation of 2,6-Dichlorophenol and 2,3,6-Trichlorophenol by LAC-4
Effects of Different Concentrations of Metal ions on the Degradation of 2,6-Dichlorophenol and 2,3,6-Trichlorophenol by LAC-4
- 2,6-DCP
- 2,3,6-TCP
Effect of Different Concentrations of Organic Solvents on the Degradation of 2,6-Dichlorophenol and 2,3,6-Trichlorophenol by LAC-4
- 2,6-DCP
- 2,3,6-TCP
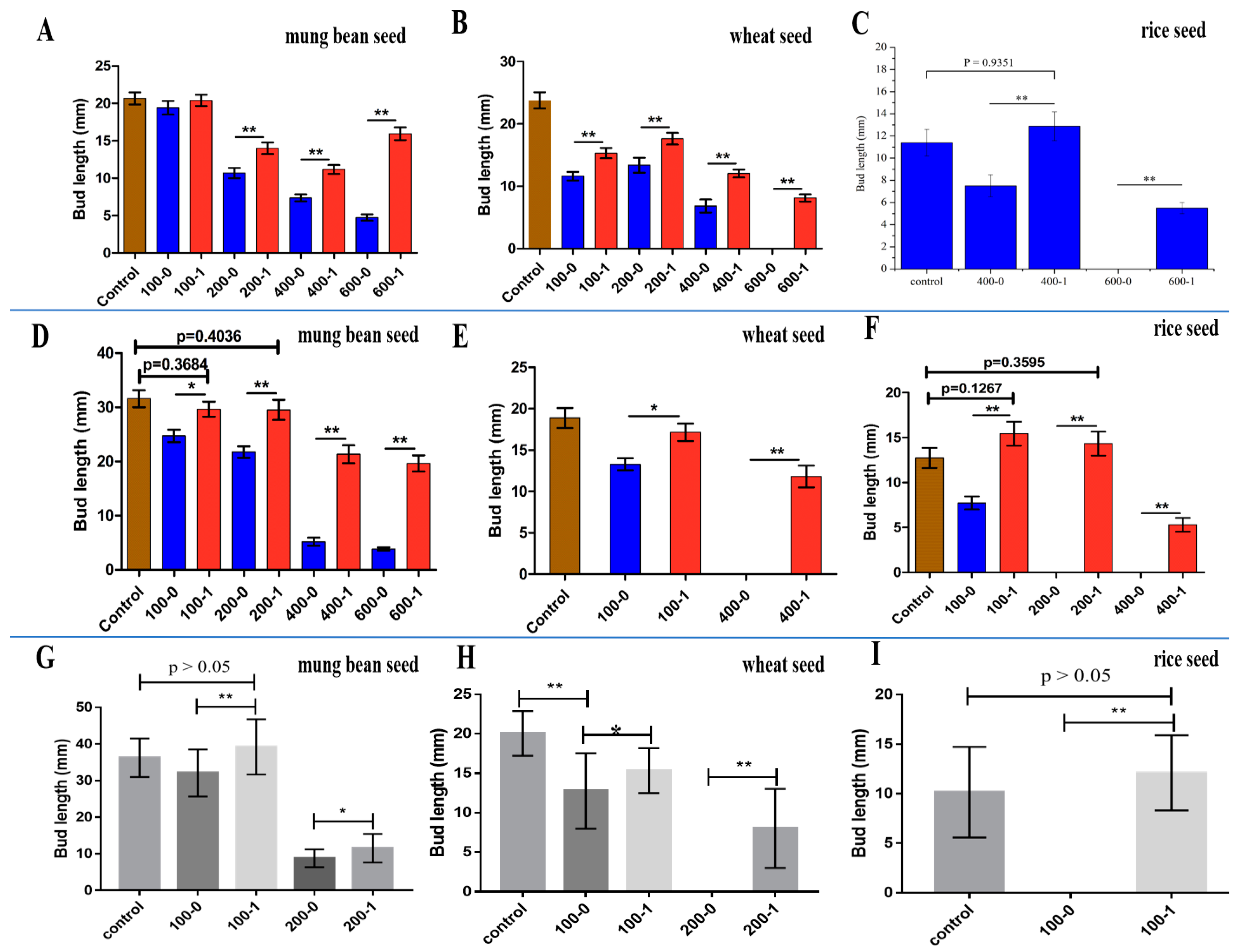
3.4. Study on the Detoxification of Chlorophenols by LAC-4
3.4.1. 2,6-DCP
Mung Bean Seed
Wheat Seed
Rice Seed
3.4.2. 2,3,6-TCP
Mung Bean Seed
Wheat Seed
Rice Seed
3.4.3. 2,6-DCP + 2,3,6-TCP
Mung Bean Seeds
Wheat Seeds
Rice Seeds
3.5. Study on the LAC-4 Degradation Mechanism of Chlorophenols
3.5.1. 2,6-DCP
Study of the Dechlorination of 2,6-Dichlorophenol and 2,3,6-Trichlorophenol by LAC-4
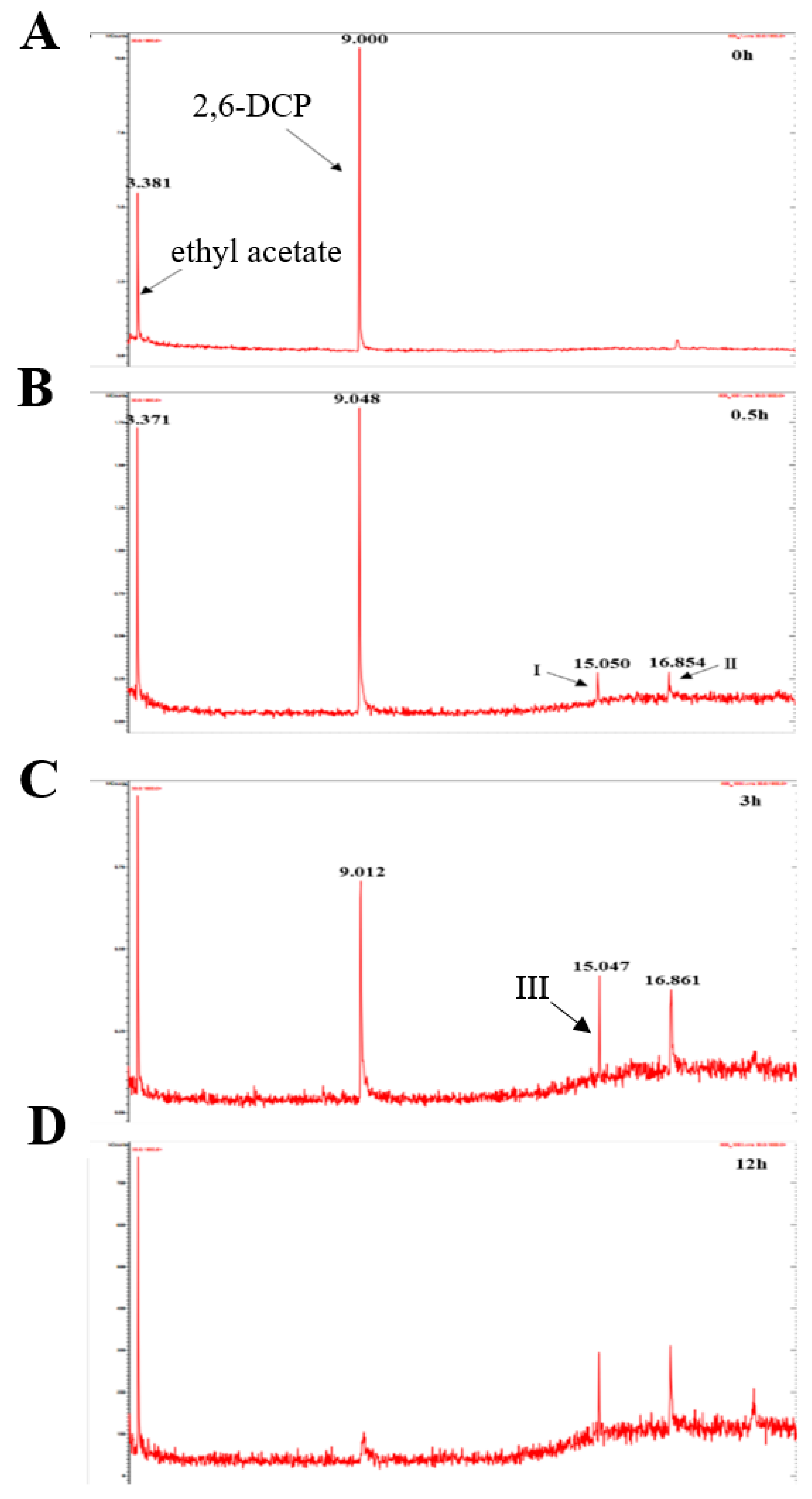
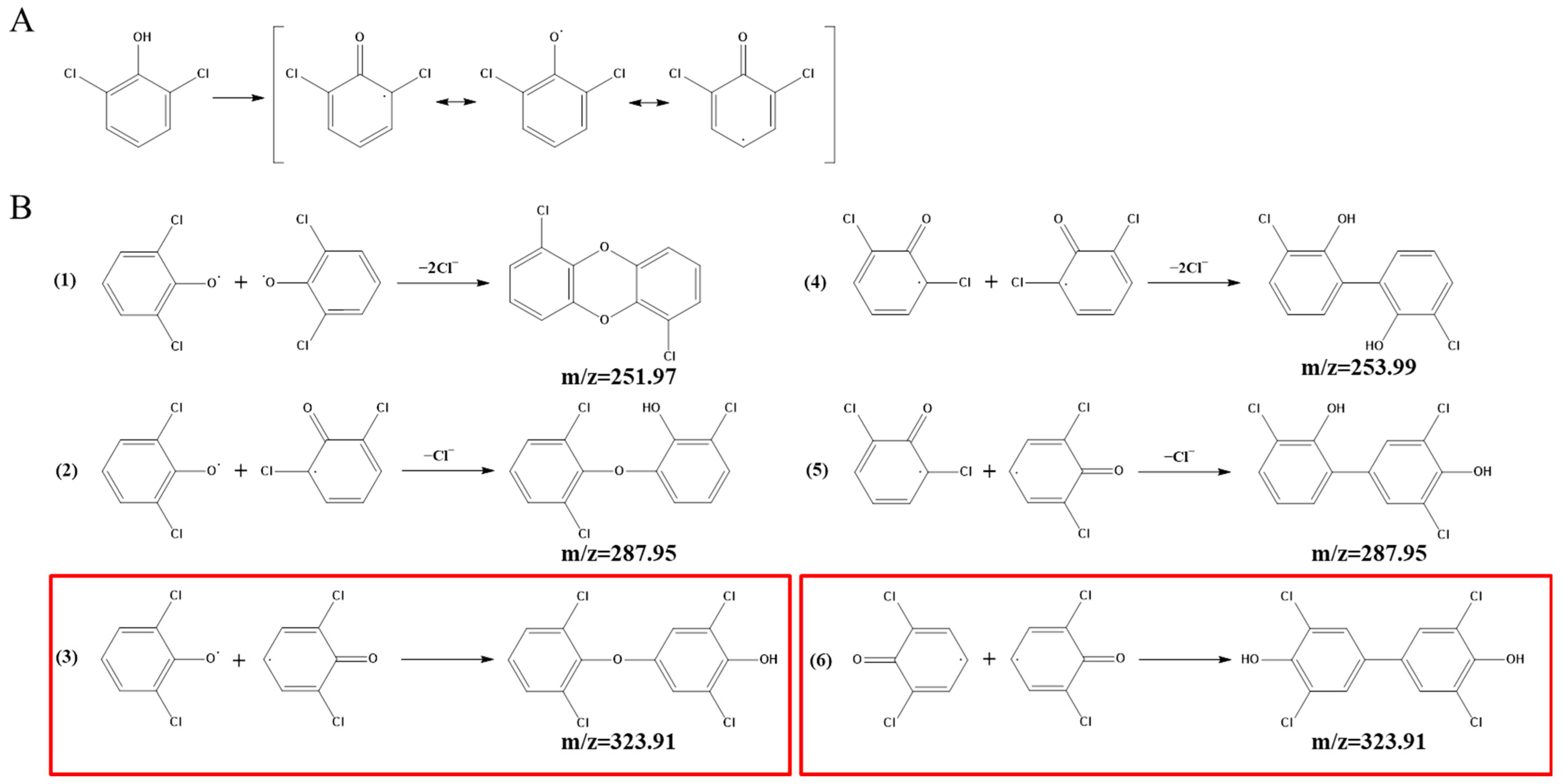
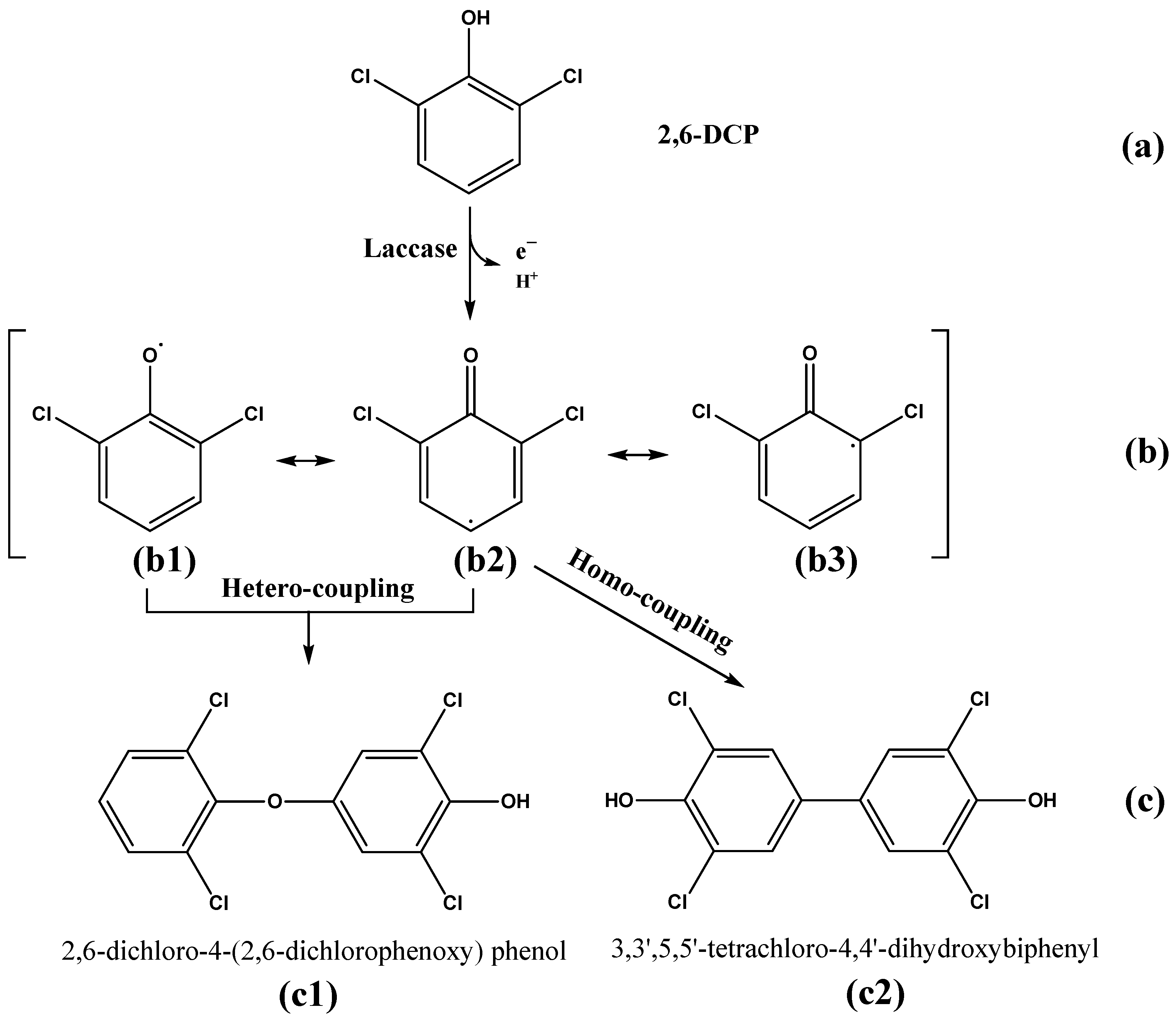
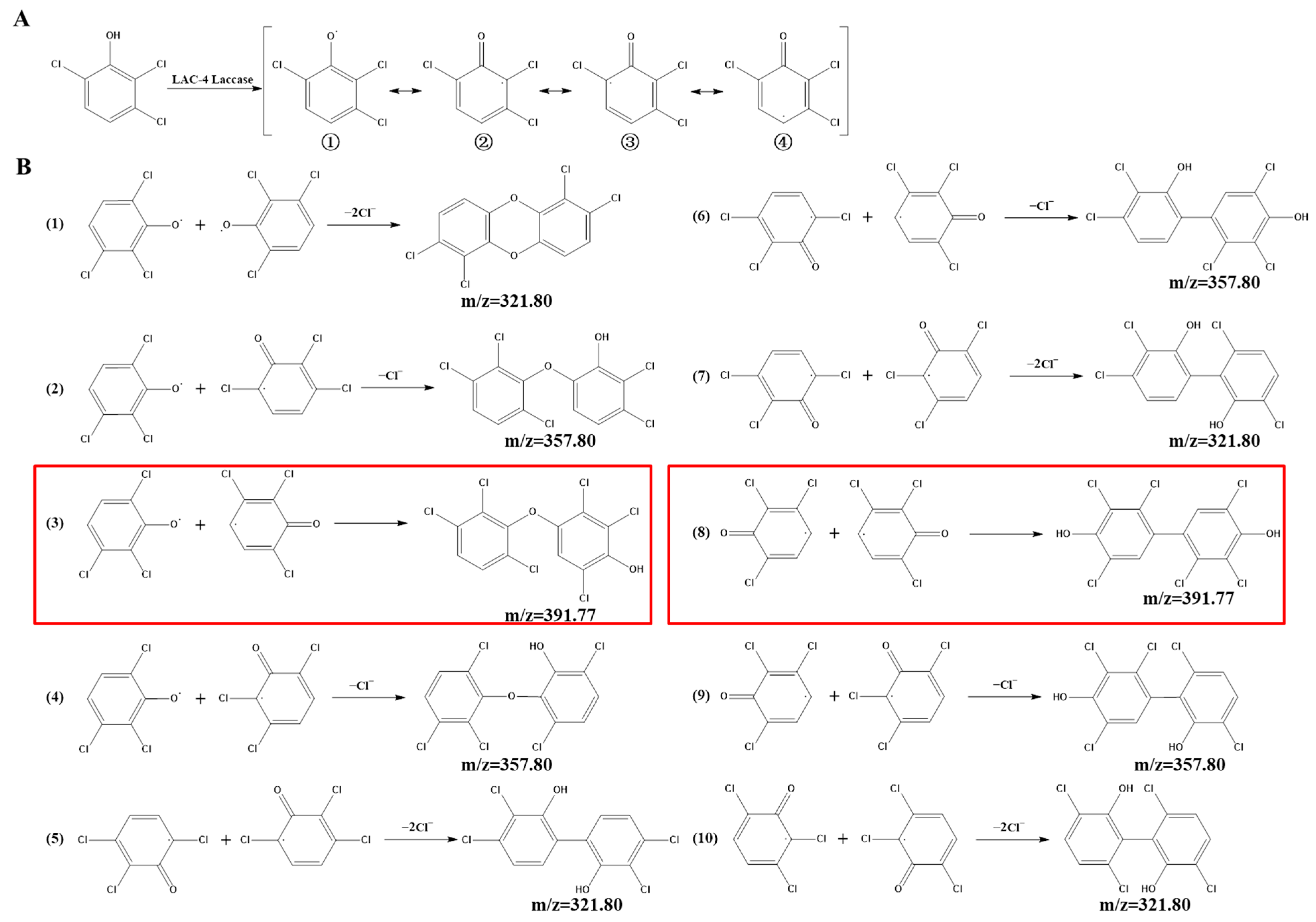
Identification of Intermediate Products of 2,6-Dichlorophenol Degraded by LAC-4 and Investigation of Degradation Pathways
3.5.2. 2,3,6-TCP
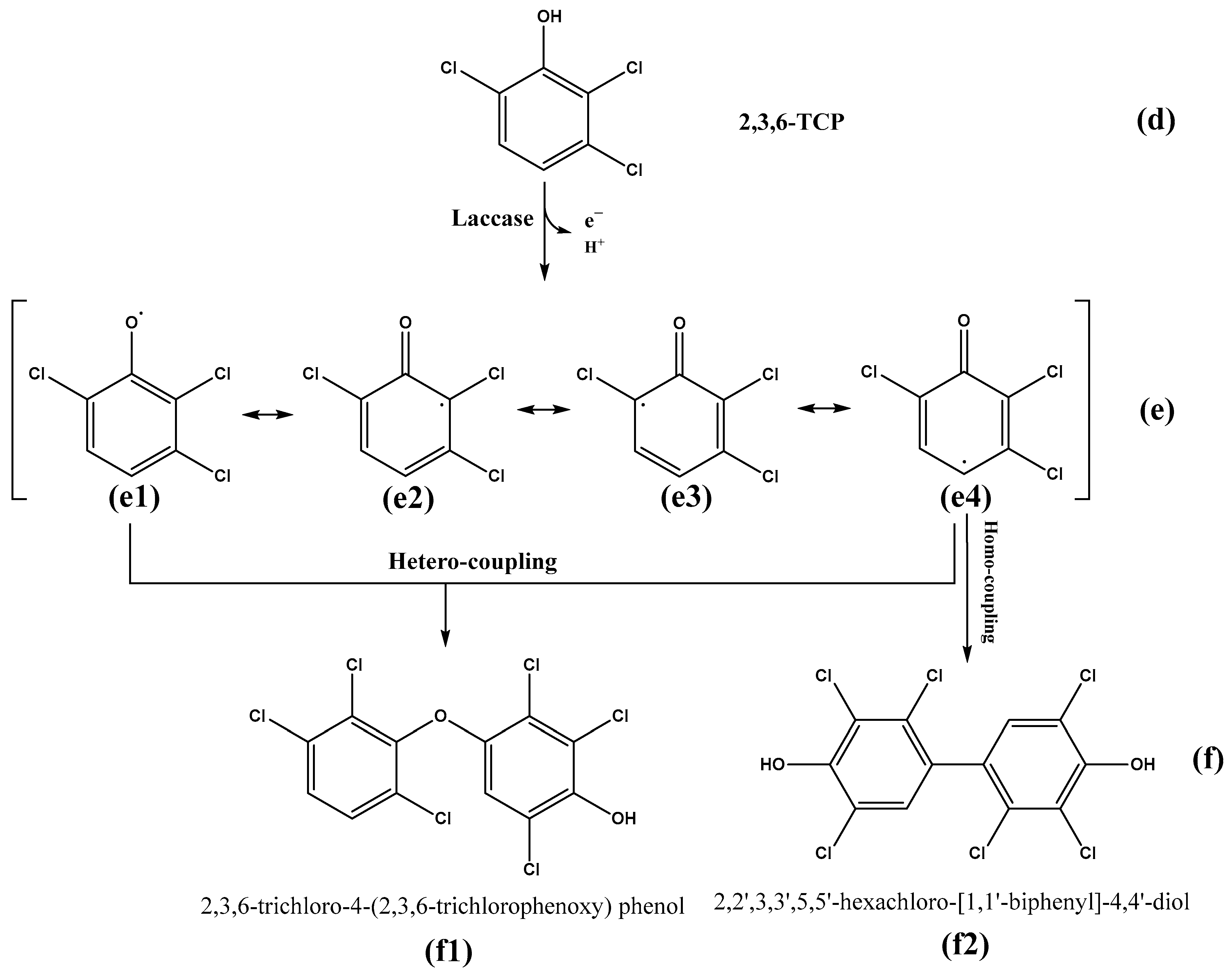
Identification of Intermediate Products of 2,3,6-Trichlorophenol Degraded by LAC-4 and Investigation of Degradation Pathways
3.5.3. 2,6-DCP + 2,3,6-TCP
4. Discussion
4.1. Enzymatic Properties of LAC-4 Laccase
4.2. Degradation Mechanism of Chlorophenols by LAC-4
4.3. Tolerance of LAC-4 for Different Metal Salts and Organic Solvents during Degradation of the Two Chlorophenols
4.4. Detoxification of Chlorophenols 2,6-DCP and 2,3,6-TCP by LAC-4
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Giardina, P.; Faraco, V.; Pezzella, C.; Piscitelli, A.; Vanhulle, S.; Sannia, G. Laccases: A never-ending story. Cell. Mol. Life Sci. 2010, 67, 369–385. [Google Scholar] [CrossRef]
- Baldrian, P. Fungal laccases—Occurrence and properties. FEMS Microbiol. Rev. 2006, 30, 215–242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Janusz, G.; Pawlik, A.; Świderska-Burek, U.; Polak, J.; Sulej, J.; Jarosz-Wilkołazka, A.; Paszczyński, A. Laccase Properties, Physio-logical Functions, and Evolution. Int. J. Mol. Sci. 2020, 21, 966. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, D.; Gupta, N. Microbial Laccase: A robust enzyme and its industrial applications. Biologia 2020, 75, 1183–1193. [Google Scholar] [CrossRef]
- Debnath, R.; Saha, T. An insight into the production strategies and applications of the ligninolytic enzyme laccase from bacteria and fungi. Biocatal. Agric. Biotechnol. 2020, 26, 101645. [Google Scholar] [CrossRef]
- Singh, G.; Arya, S.K. Utility of laccase in pulp and paper industry: A progressive step towards the green technology. Int. J. Biol. Macromol. 2019, 134, 1070–1084. [Google Scholar] [CrossRef] [PubMed]
- Bilal, M.; Rasheed, T.; Nabeel, F.; Iqbal, H.M.; Zhao, Y. Hazardous contaminants in the environment and their laccase-assisted degradation—A review. J. Environ. Manag. 2019, 234, 253–264. [Google Scholar] [CrossRef]
- Bilal, M.; Iqbal, H.M.N.; Barceló, D. Persistence of pesticides-based contaminants in the environment and their effective degradation using laccase-assisted biocatalytic systems. Sci. Total Environ. 2019, 695, 133896. [Google Scholar] [CrossRef] [PubMed]
- Iark, D.; Buzzo, A.J.D.R.; Garcia, J.A.A.; Côrrea, V.G.; Helm, C.V.; Corrêa, R.C.G.; Peralta, R.A.; Moreira, R.D.F.P.M.; Bracht, A.; Peralta, R.M. Enzymatic degradation and detoxification of azo dye Congo red by a new laccase from Oudemansiella canarii. Bioresour. Technol. 2019, 289, 121655. [Google Scholar] [CrossRef] [PubMed]
- Olaniran, A.O.; Igbinosa, E. Chlorophenols and other related derivatives of environmental concern: Properties, distribution and microbial degradation processes. Chemosphere 2011, 83, 1297–1306. [Google Scholar] [CrossRef] [PubMed]
- Igbinosa, E.O.; Odjadjare, E.E.; Chigor, V.N.; Igbinosa, I.H.; Emoghene, A.O.; Ekhaise, F.O.; Igiehon, N.O.; Idemudia, O.G. Toxicological profile of chlorophenols and their derivatives in the environment: The public health perspective. Sci. World J. 2013, 2013, 460215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Czaplicka, M. Sources and transformations of chlorophenols in the natural environment. Sci. Total Environ. 2004, 322, 21–39. [Google Scholar] [CrossRef] [PubMed]
- Ge, T.; Han, J.; Qi, Y.; Gu, X.; Ma, L.; Zhang, C.; Naeem, S.; Huang, D. The toxic effects of chlorophenols and associated mechanisms in fish. Aquat. Toxicol. 2017, 184, 78–93. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, D.; Shoji, R. Toxicological effects of chlorophenols to green algae observed at various pH and concentration of humic acid. J. Hazard. Mater. 2020, 400, 123079. [Google Scholar] [CrossRef] [PubMed]
- Zeljezic, D.; Garaj-Vrhovac, V. Chromosomal aberration and single cell gel electrophoresis (Comet) assay in the longitudinal risk assessment of occupational exposure to pesticides. Mutagenesis 2001, 16, 359–363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hattula, M.-L.; Knuutinen, J. Mutagenesis of mammalian cells in culture by chlorophenols, chlorocatechols and chloroguaiacols. Chemosphere 1985, 14, 1617–1625. [Google Scholar] [CrossRef]
- Cooper, G.S.; Jones, S. Pentachlorophenol and Cancer Risk: Focusing the Lens on Specific Chlorophenols and Contaminants. Environ. Health Perspect. 2008, 116, 1001–1008. [Google Scholar] [CrossRef] [Green Version]
- Chhabra, R.S.; Maronpot, R.M.; Bucher, J.R.; Haseman, J.K.; Toft, J.D.; Hejtmancik, M.R. Toxicology and carcinogenesis studies of pentachlorophenol in rats. Toxicol. Sci. 1999, 48, 14–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Itoh, K.; Fujita, M.; Kumano, K.; Suyama, K.; Yamamoto, H. Phenolic acids affect transformations of chlorophenols by a Coriolus versicolor laccase. Soil Biol. Biochem. 2000, 32, 85–91. [Google Scholar] [CrossRef]
- Forootanfar, H.; Movahednia, M.M.; Yaghmaei, S.; Tabatabaei-Sameni, M.; Rastegar, H.; Sadighi, A.; Faramarzi, M.A. Removal of chlorophenolic derivatives by soil isolated ascomycete of Paraconiothyrium variabile and studying the role of its extracellular laccase. J. Hazard. Mater. 2012, 209, 199–203. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Liu, X.; Xu, Z.; Chen, H.; Yang, Y. Degradation of chlorophenols catalyzed by laccase. Int. Biodeterior. Biodegrad. 2008, 61, 351–356. [Google Scholar] [CrossRef]
- Bao, W.; Peng, R.; Zhang, Z.; Tian, Y.; Zhao, W.; Xue, Y.; Gao, J.; Yao, Q. Expression, characterization and 2,4,6-trichlorophenol degradation of laccase from Monilinia fructigena. Mol. Biol. Rep. 2012, 39, 3871–3877. [Google Scholar] [CrossRef]
- Sajben-Nagy, E.; Manczinger, L.; Škrbić, B.; Živančev, J.; Antić, I.; Krisch, J.; Vágvölgyi, C. Characterization of an extracellular laccase of Leptosphaerulina chartarum. World J. Microbiol. Biotechnol. 2014, 30, 2449–2458. [Google Scholar] [CrossRef] [PubMed]
- Qiu, X.; Wang, Y.; Xue, Y.; Li, W.; Hu, Y. Laccase immobilized on magnetic nanoparticles modified by amino-functionalized ionic liquid via dialdehyde starch for phenolic compounds biodegradation. Chem. Eng. J. 2020, 391, 123564. [Google Scholar] [CrossRef]
- Xu, R.; Cui, J.Y.; Tang, R.Z.; Li, F.T.; Zhang, B.R. Removal of 2,4,6-trichlorophenol by laccase immobilized on nano-copper incorporated electrospun fibrous membrane-high efficiency, stability and reusability. Chem. Eng. J. 2017, 326, 647–655. [Google Scholar] [CrossRef]
- Rubilar, O.; Diez, M.C.; Gianfreda, L. Transformation of Chlorinated Phenolic Compounds by White Rot Fungi. Crit. Rev. Environ. Sci. Technol. 2008, 38, 227–268. [Google Scholar] [CrossRef]
- Gaitan, I.J.; Medina, S.C.; González, J.C.; Rodríguez, A.; Espejo, Á.J.; Osma, J.F.; Sarria, V.; Alméciga-Díaz, C.J.; Sánchez, O.F. Evaluation of toxicity and degradation of a chlorophenol mixture by the laccase produced by Trametes pubescens. Bioresour. Technol. 2011, 102, 3632–3635. [Google Scholar] [CrossRef]
- Grey, R.; Höfer, C.; Schlosser, D. Degradation of 2-chlorophenol and formation of 2-chloro-1,4-benzoquinone by mycelia and cell-free crude culture liquids of Trametes versicolor in relation to extracellular laccase activity. J. Basic Microbiol. 1998, 38, 371–382. [Google Scholar] [CrossRef]
- Kadhim, H.; Graham, C.; Barratt, P.; Evans, C.; Rastall, R. Removal of phenolic compounds in water using Coriolus versicolor grown on wheat bran. Enzym. Microb. Technol. 1999, 24, 303–307. [Google Scholar] [CrossRef]
- Dec, J.; Bollag, J.-M. Effect of Various Factors on Dehalogenation of Chlorinated Phenols and Anilines during Oxidative Coupling. Environ. Sci. Technol. 1995, 29, 657–663. [Google Scholar] [CrossRef] [PubMed]
- Qin, W.J.; Yu, H.S.; Fu, S.Y. Degradation of the organic contamination by laccase. China Pulp Pap. 2001, 20, 58–62. [Google Scholar]
- Leontievsky, A.; Myasoedova, N.; Baskunov, B. Transformation of 2,4,6-trichlorophenol by free and immobilized fungal laccase. Appl. Microbiol. Biotechnol. 2001, 57, 85–91. [Google Scholar]
- Yin, L.; Shen, Z.; Niu, J. Degradation of Pentachlorophenol and 2,4-Dichloronhenol by Sequential Visible-Light Driven Photocatalysis and Laccase Catalysis. Environ. Sci. Technol. 2010, 44, 9117–9122. [Google Scholar]
- Roy-Arcand, L.; Archibald, F.S. Direct dechiorination of chiorophenolic compounds by laccases from Trametes (Coriolus) versicolor. Enzym. Microb. Technol. 1991, 13, 194–203. [Google Scholar] [CrossRef]
- Leitner, C.; Hess, J.; Galhaup, C.; Ludwig, R.; Nidetzky, B.; Kulbe, K.D.; Haltrich, D. Purification and Characterization of a Laccase from the White-Rot Fungus Trametes multicolor. Appl. Biochem. Biotechnol. 2002, 98, 497–507. [Google Scholar] [CrossRef]
- Xu, F. Effects of Redox Potential and Hydroxide Inhibition on the pH Activity Profile of Fungal Laccases. J. Biol. Chem. 1997, 272, 924–928. [Google Scholar] [CrossRef] [Green Version]
- Kang, C.; Ren, D.; Zhang, S.; Zhang, X.; He, X.; Deng, Z.; Huang, C.; Guo, H. Effect of Polyhydroxyl Compounds on the Thermal Stability and Structure of Laccase. Pol. J. Environ. Stud. 2019, 28, 3253–3259. [Google Scholar] [CrossRef]
- Jafari, M.; Mojtabavi, S.; Faramarzi, M.A.; Mehrnejad, F.; Soleimani, M.; Mirjani, R. Molecular level insight into stability, activity, and structure of Laccase in aqueous ionic liquid and organic solvents: An experimental and computational research. J. Mol. Liq. 2020, 317, 113925. [Google Scholar] [CrossRef]
- Radhakrishnan, R.; Manna, B.; Ghosh, A. Solvent induced conformational changes for the altered activity of laccase: A molecular dynamics study. J. Hazard. Mater. 2022, 423, 127123. [Google Scholar] [PubMed]
- Rosconi, F.; Fraguas, L.F.; Martínez-Drets, G.; Castro-Sowinski, S. Purification and characterization of a periplasmic laccase produced by Sinorhizobium meliloti. Enzym. Microb. Technol. 2005, 36, 800–807. [Google Scholar] [CrossRef]
- Li, C.; Xu, D.; Zhao, M.; Sun, L.; Wang, Y. Production optimization, purification, and characterization of a novel acid protease from a fusant by Aspergillus oryzae and Aspergillus niger. Eur. Food Res. Technol. 2014, 238, 905–917. [Google Scholar] [CrossRef]
- D’Acunzo, F.; Barreca, A.M.; Galli, C. Determination of the activity of laccase, and mediated oxidation of a lignin model compound, in aqueous-organic mixed solvents. J. Mol. Catal. B Enzym. 2004, 31, 25–30. [Google Scholar] [CrossRef]
- Khmelnitsky, Y.L.; Belova, A.B.; Levashov, A.V.; Mozhaev, V.V. Relationship between surface hydrophilicity of a protein and its stability against denaturation by organic solvents. FEBS Lett. 1991, 284, 267–269. [Google Scholar] [CrossRef] [Green Version]





| Km (mM) | Vmax (mM/s) | Kcat (s−1) | Kcat/Km (s−1mM−1) | |
|---|---|---|---|---|
| ABTS | 0.075 | 3.988 × 10−7 | 9.42 | 126.27 |
| 2,6-DMP | 0.803 | 1.085 × 10−7 | 2.56 | 3.19 |
| Guaiacol | 0.097 | 1.906 × 10−7 | 4.5 | 46.34 |
| Km (mM) | Vmax (mM/s) | Kcat (s−1) | Kcat/Km (s−1mM−1) | |
|---|---|---|---|---|
| 2,6-DCP | 3.636 | 2.74 × 10−3 | 1.23 | 0.338 |
| 2,3,6-TCP | 4.875 | 1.50 × 10−3 | 0.67 | 0.137 |
| 2,6-DCP | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Control | 100-0 | 100-1 | 200-0 | 200-1 | 400-0 | 400-1 | 600-0 | 600-1 | |
| wheat seed germination rate (%) | 95 | 95 | 95 | 80 | 95 | 60 | 100 | 0 | 80 |
| 2,6-DCP | |||||
|---|---|---|---|---|---|
| Control | 400-0 | 400-1 | 600-0 | 600-1 | |
| rice seed germination rate (%) | 95 | 70 | 85 | 0 | 50 |
| 2,3,6-TCP | |||||||
|---|---|---|---|---|---|---|---|
| Control | 100-0 | 100-1 | 200-0 | 200-1 | 400-0 | 400-1 | |
| wheat seed germination rate (%) | 90 | 35 | 95 | 15 | 95 | 0 | 25 |
| rice seed germination rate (%) | 95 | 75 | 80 | 0 | 80 | 0 | 50 |
| 2,6-DCP + 2,3,6-TCP | |||||
|---|---|---|---|---|---|
| Control | 100-0 | 100-1 | 200-0 | 200-1 | |
| mung bean seed germination rate (%) | 100 | 100 | 100 | 70 | 100 |
| wheat seed germination rate (%) | 95 | 40 | 90 | 0 | 15 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deng, W.; Zhao, W.; Yang, Y. Degradation and Detoxification of Chlorophenols with Different Structure by LAC-4 Laccase Purified from White-Rot Fungus Ganoderma lucidum. Int. J. Environ. Res. Public Health 2022, 19, 8150. https://doi.org/10.3390/ijerph19138150
Deng W, Zhao W, Yang Y. Degradation and Detoxification of Chlorophenols with Different Structure by LAC-4 Laccase Purified from White-Rot Fungus Ganoderma lucidum. International Journal of Environmental Research and Public Health. 2022; 19(13):8150. https://doi.org/10.3390/ijerph19138150
Chicago/Turabian StyleDeng, Wei, Wei Zhao, and Yang Yang. 2022. "Degradation and Detoxification of Chlorophenols with Different Structure by LAC-4 Laccase Purified from White-Rot Fungus Ganoderma lucidum" International Journal of Environmental Research and Public Health 19, no. 13: 8150. https://doi.org/10.3390/ijerph19138150
APA StyleDeng, W., Zhao, W., & Yang, Y. (2022). Degradation and Detoxification of Chlorophenols with Different Structure by LAC-4 Laccase Purified from White-Rot Fungus Ganoderma lucidum. International Journal of Environmental Research and Public Health, 19(13), 8150. https://doi.org/10.3390/ijerph19138150






