Oral Health and Frailty in Community-Dwelling Older Adults in the Northern Netherlands: A Cross-Sectional Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Measurements
2.2.1. Frailty
2.2.2. Oral Health
2.2.3. Co-Variables
2.3. Data Analysis
3. Results
3.1. Sample Characteristics
3.2. Oral Health
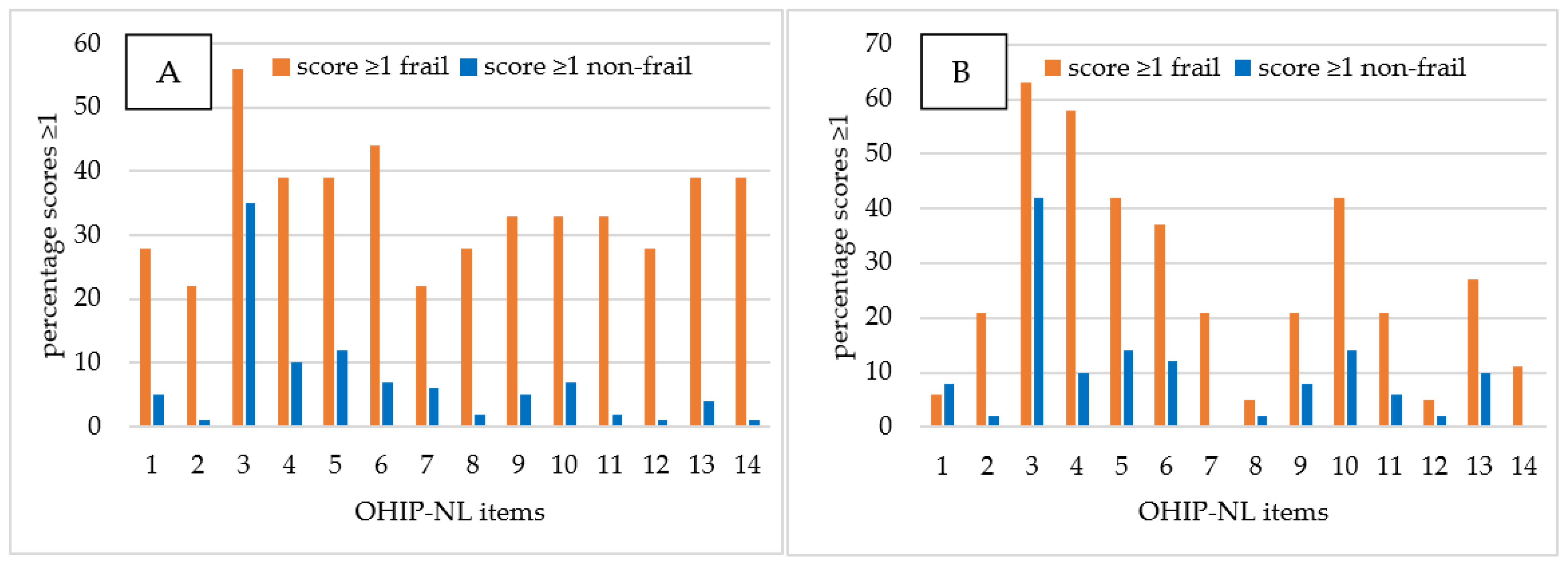
- Item 2, i.e., sense of taste worsened;
- Item 6, i.e., tense feeling;
- Item 8, i.e., interrupt meals because of problems;
- Item 9, i.e., difficult to relax;
- Item 11, i.e., irritable with other people;
- Item 12, i.e., difficulty doing your usual jobs;
- Item 13, i.e., life in general less satisfying;
- Item 14, i.e., totally unable to function.
3.3. Oral Health and Frailty
4. Discussion
4.1. Strengths and Limitations
4.2. Implications of Findings for Research and Practice
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- United Nations Population Division World Population Ageing 2017 (ST/ESA/SER.A/408); United Nations: New York, NY, USA, 2017.
- Ahmed, N.; Mandel, R.; Fain, M.J. Frailty: An Emerging Geriatric Syndrome. Am. J. Med. 2007, 11, 748–753. [Google Scholar] [CrossRef]
- Ye, L.; Elstgeest, L.E.M.; Zhang, X.; Alhambra-Borrás, T.; Tan, S.S.; Raat, H. Factors associated with physical, psychological and social frailty among community-dwelling older persons in Europe: A cross-sectional study of Urban Health Centres Europe (UHCE). BMC Geriatr. 2021, 21, 1–422. [Google Scholar] [CrossRef]
- Jansen-Kosterink, S.; Van Velsen, L.; Frazer, S.; Dekker-Van Weering, M.; O’Caoimh, R.; Vollenbroek-Hutten, M. Identification of community-dwelling older adults at risk of frailty using the PERSSILAA screening pathway: A methodological guide and results of a large-scale deployment in the Netherlands. BMC Public Health 2019, 19, 504. [Google Scholar] [CrossRef]
- van Campen, C. Frail Older Persons in The Netherlands; The Netherlands Institute for Social Research: The Hague, The Netherlands, 2012; p. 218. ISBN 9789037705539. [Google Scholar]
- Gobbens, R.J.; Luijkx, K.G.; Wijnen-Sponselee, M.T.; Schols, J.M. In Search of an integral conceptual definition of frailty: Opinions of experts. J. Am. Med. Dir. Assoc. 2010, 11, 338–343. [Google Scholar] [CrossRef]
- Kojima, G.; Iliffe, S.; Jivraj, S.; Walters, K. Association between frailty and quality of life among community-dwelling older people: A systematic review and meta-analysis. J. Epidemiol. Community Health 2016, 70, 716–721. [Google Scholar] [CrossRef]
- Vermeiren, S.; Vella-Azzopardi, R.; Beckwée, D.; Habbig, A.K.; Scafoglieri, A.; Jansen, B.; Bautmans, I.; Gerontopole Brussels Study Group. Frailty and the Prediction of Negative Health Outcomes: A Meta-Analysis. J. Am. Med. Dir. Assoc. 2016, 17, 1163.e1–1163.e17. [Google Scholar] [CrossRef]
- Puts, M.T.E.; Toubasi, S.; Andrew, M.K.; Ashe, M.C.; Ploeg, J.; Atkinson, E.; Ayala, A.P.; Roy, A.; Rodríguez Monforte, M.; Bergman, H.; et al. Interventions to prevent or reduce the level of frailty in community-dwelling older adults: A scoping review of the literature and international policies. Age Ageing 2017, 46, 383–392. [Google Scholar] [CrossRef] [Green Version]
- Gobbens, R.J.J.; van der Ploeg, T. The Development of Multidimensional Frailty Over Seven Years A longitudinal study among Dutch community-dwelling older people using the Tilburg Frailty Indicator. Arch. Gerontol. Geriatr. 2021, 95, 104393. [Google Scholar] [CrossRef]
- Gussekloo, J. Preventie bij ouderen: Focus op zelfredzaamheid. Huisarts Wet. 2009, 52, 426–427. [Google Scholar] [CrossRef]
- Association, J.D. The Current Evidence of Dental Care and Oral Health for Achieving Healthy Longevity in an Aging Society 2015; Japan Dental Association: Tokyo, Japan, 2015; Available online: https://www.jda.or.jp/en/pdf/world_concgress_2015_evidence_en_summary.pdf (accessed on 24 February 2020).
- Statistics Netherlands (CBS) Meerderheid Volwassenen Tevreden Met Mondgezondheid. Available online: https://www.cbs.nl/nl-nl/nieuws/2020/10/meerderheid-volwassenen-tevreden-met-mondgezondheid (accessed on 18 December 2020).
- Boer, J.C.L.D.; Van Der Sanden, W.J.M.; Bruers, J.J.M. Developments in oral health care in the Netherlands between 1995 and 2018. BMC Oral Health 2020, 20, 192. [Google Scholar] [CrossRef]
- Dörfer, C.; Benz, C.; Aida, J.; Campard, G. The relationship of oral health with general health and NCDs: A brief review. Int. Dent. J. 2017, 67, 14–18. [Google Scholar] [CrossRef] [Green Version]
- Hajishengallis, G.; Chavakis, T. Local and systemic mechanisms linking periodontal disease and inflammatory comorbidities. Nat. Rev. Immunol. 2021, 21, 426–440. [Google Scholar] [CrossRef]
- Butera, A.; Gallo, S.; Pascadopoli, M.; Maiorani, C.; Milone, A.; Alovisi, M.; Scribante, A. Paraprobiotics in Non-Surgical Periodontal Therapy: Clinical and Microbiological Aspects in a 6-Month Follow-Up Domiciliary Protocol for Oral Hygiene. Microorganisms 2022, 10, 337. [Google Scholar] [CrossRef]
- Kassebaum, N.J.; Smith, A.G.C.; Bernabé, E.; Fleming, T.D.; Reynolds, A.E.; Vos, T.; Murray, C.J.L.; Marcenes, W. Global, Regional, and National Prevalence, Incidence, and Disability-Adjusted Life Years for Oral Conditions for 195 Countries, 1990–2015: A Systematic Analysis for the Global Burden of Diseases, Injuries, and Risk Factors. J. Dent. Res. 2017, 96, 380–387. [Google Scholar] [CrossRef]
- Dibello, V.; Zupo, R.; Sardone, R.; Lozupone, M.; Castellana, F.; Dibello, A.; Daniele, A.; De Pergola, G.; Bortone, I.; Lampignano, L.; et al. Oral frailty and its determinants in older age: A systematic review. Lancet Healthy Longev. 2021, 2, e507. [Google Scholar] [CrossRef]
- Everaars, B.; Jerković-Ćosić, K.; Bleijenberg, N.; De Wit, N.J.; Van Der Heijden, G.J.M. G Exploring Associations Between Oral Health and Frailty in Community-Dwelling Older People. J. Frailty Aging 2021, 10, 56–62. [Google Scholar] [CrossRef]
- Peters, L.L.; Boter, H.; Burgerhof, J.G.; Slaets, J.P.; Buskens, E. Construct validity of the Groningen Frailty Indicator established in a large sample of home-dwelling elderly persons: Evidence of stability across age and gender. Exp. Gerontol. 2015, 69, 129–141. [Google Scholar] [CrossRef]
- Hoeksema, A.R.; Spoorenberg, S.L.W.; Peters, L.L.; Meijer, H.J.A.; Raghoebar, G.M.; Vissink, A.; Wynia, K.; Visser, A. Elderly with remaining teeth report less frailty and better quality of life than edentulous elderly: A cross-sectional study. Oral Dis. 2017, 23, 526–536. [Google Scholar] [CrossRef]
- Peters, L.L.; Boter, H.; Buskens, E.; Slaets, J.P. Measurement Properties of the Groningen Frailty Indicator in Home-Dwelling and Institutionalized Elderly People. J. Am. Med. Dir. Assoc. 2012, 13, 546–551. [Google Scholar] [CrossRef] [Green Version]
- Steverink, N. Measuring frailty: Developing and testing the GFI (Groningen Frailty Indicator). Gerontologist 2001, 41, 236–237. [Google Scholar]
- Van Der Meulen, M.J.; John, M.T.; Naeije, M.; Lobbezoo, F. The Dutch version of the Oral Health Impact Profile (OHIP-NL): Translation, reliability and construct validity. BMC Oral Health 2008, 8, 11. [Google Scholar] [CrossRef] [Green Version]
- Koistinen, S.; Olai, L.; Ståhlnacke, K.; Fält, A.; Ehrenberg, A. Oral health-related quality of life and associated factors among older people in short-term care. Int. J. Dent. Hyg. 2020, 18, 163. [Google Scholar] [CrossRef]
- Feng, Z.; Lugtenberg, M.; Franse, C.; Fang, X.; Hu, S.; Jin, C.; Raat, H. Risk factors and protective factors associated with incident or increase of frailty among community-dwelling older adults: A systematic review of longitudinal studies. PLoS ONE 2017, 12, e0178383. [Google Scholar] [CrossRef] [Green Version]
- Bunt, S.; O’Caoimh, R.; Krijnen, W.P.; Molloy, D.W.; Goodijk, G.P.; van der Schans, C.P.; Hobbelen, H.J.S.M. Validation of the dutch version of the quick mild cognitive impairment screen (qmci-d). BMC Geriatr. 2015, 15, 115. [Google Scholar] [CrossRef] [Green Version]
- Schroevers, M.J.; Sanderman, R.; Van Sonderen, E.; Ranchor, A.V. The evaluation of the Center for Epidemiologic Studies Depression (CES-D) scale: Depressed and Positive Affect in cancer patients and healthy reference subjects. Qual. Life Res. 2000, 9, 1015–1029. [Google Scholar] [CrossRef]
- Charlson, M.; Szatrowski, T.P.; Peterson, J.; Gold, J. Validation of a combined comorbidity index. J. Clin. Epidemiol. 1994, 47, 1245–1251. [Google Scholar] [CrossRef]
- Breheny, P.; Huang, J. Coordinate descent algorithms for nonconvex penalized regression, with applications to biological feature selection. Ann. Appl. Stat. 2011, 5, 232–253. [Google Scholar] [CrossRef] [Green Version]
- Fan, J.; Peng, H. Nonconcave penalized likelihood with a diverging number of parameters. Ann. Stat. 2004, 32, 928–961. [Google Scholar] [CrossRef] [Green Version]
- Hoeksema, A.R.; Peters, L.L.; Raghoebar, G.M.; Meijer, H.J.A.; Vissink, A.; Visser, A. Health and quality of life differ between community living older people with and without remaining teeth who recently received formal home care: A cross sectional study. Clin. Oral Investig. 2018, 22, 2615–2622. [Google Scholar] [CrossRef] [Green Version]
- Bakker, M.H.; Vissink, A.; Raghoebar, G.M.; Peters, L.L.; Visser, A. General health, healthcare costs and dental care use of elderly with a natural dentition, implant-retained overdenture or conventional denture: An 8-year cohort of Dutch elderly (aged 75 and over). BMC Geriatr. 2021, 21, 1–477. [Google Scholar] [CrossRef]
- Slade, G.D.; Sanders, A.E. The Paradox of Better Subjective Oral Health in Older Age. J. Dent. Res. 2011, 90, 1279–1285. [Google Scholar] [CrossRef]
- Takehara, S.; Wright, F.A.C.; Naganathan, V.; Hirani, V.; Blyth, F.M.; Couteur, D.G.L.; Waite, L.M.; Seibel, M.J.; Handelsman, D.J.; Cumming, R.G. A Cross-Sectional Study of Perceived Dental Treatment Needs and Oral Health Status in Community-Dwelling Older Australian Men: The Concord Health and Ageing in Men Project. Int. Dent. J. 2021, 71, 224–232. [Google Scholar] [CrossRef]
- Walls, A.W.G.; Steele, J.G. The relationship between oral health and nutrition in older people. Mech. Ageing Dev. 2004, 125, 853–857. [Google Scholar] [CrossRef]
- Lorenzo-López, L.; Maseda, A.; De Labra, C.; Regueiro-Folgueira, L.; Rodríguez-Villamil, J.L.; Millán-Calenti, J.C. Nutritional determinants of frailty in older adults: A systematic review. BMC Geriatr. 2017, 17, 108. [Google Scholar] [CrossRef] [Green Version]
- Gale, C.R.; Westbury, L.; Cooper, C. Social isolation and loneliness as risk factors for the progression of frailty: The English Longitudinal Study of Ageing. Age Ageing 2018, 47, 392–397. [Google Scholar] [CrossRef] [Green Version]
- Kandelman, D.; Petersen, P.E.; Ueda, H. Oral health, general health, and quality of life in older people. Spec. Care Dent. 2008, 28, 224–236. [Google Scholar] [CrossRef]
- Kroese, J.M.; Volgenant, C.M.C.; van Schaardenburg, D.; van Boheemen, L.; van Selms, M.K.A.; Visscher, C.M.; Crielaard, W.; Loos, B.G.; Lobbezoo, F. Oral health-related quality of life in patients with early rheumatoid arthritis is associated with periodontal inflammation and painful temporomandibular disorders: A cross-sectional study. Clin. Oral Investig. 2022, 26, 555–563. [Google Scholar] [CrossRef]
- Cademartori, M.G.; Gastal, M.T.; Nascimento, G.G.; Demarco, F.F.; Corrêa, M.B. Is depression associated with oral health outcomes in adults and elders? A systematic review and meta-analysis. Clin. Oral Investig. 2018, 22, 2685–2702. [Google Scholar] [CrossRef]
- Avlund, K.; Holm-Pedersen, P.; Schroll, M. Functional ability and oral health among older people: A longitudinal study from age 75 to 80. J. Am. Geriatr. Soc. 2001, 49, 954–962. [Google Scholar] [CrossRef]
- Tanaka, T.; Takahashi, K.; Hirano, H.; Kikutani, T.; Watanabe, Y.; Ohara, Y.; Furuya, H.; Tetsuo, T.; Akishita, M.; Iijima, K. Oral frailty as a risk factor for physical frailty and mortality in community-dwelling elderly. J. Gerontol. Ser. A 2018, 73, 1661–1667. [Google Scholar] [CrossRef]
- Parisius, K.G.; Wartewig, E.; Schoonmade, L.J.; Aarab, G.; Gobbens, R.; Lobbezoo, F. Oral frailty dissected and conceptualized: A scoping review. Arch. Gerontol. Geriatr. 2022, 100, 104653. [Google Scholar] [CrossRef]
- Menegaz, A.M.; Silva, A.E.R.; Cascaes, A.M. Intervenções educativas em serviços de saúde e saúde bucal: Revisão sistemática. Rev. De Saúde Pública 2018, 52, 52. [Google Scholar] [CrossRef] [Green Version]
- Jepsen, S.; Blanco, J.; Buchalla, W.; Carvalho, J.C.; Dietrich, T.; Dörfer, C.; Eaton, K.A.; Figuero, E.; Frencken, J.E.; Graziani, F.; et al. Prevention and control of dental caries and periodontal diseases at individual and population level: Consensus report of group 3 of joint EFP/ORCA workshop on the boundaries between caries and periodontal diseases. J. Clin. Periodontol. 2017, 44, S85–S93. [Google Scholar] [CrossRef]
| Characteristics | Total | Frail | Non-Frail | |
|---|---|---|---|---|
| n = 170 (100) | n = 37 (22) | n = 133 (78) | ||
| Frailty (GFI; median (IQR)) | 1 (0–2) | 5 (4–6) | 1 (0–2) | |
| Oral health (OHIP-NL14; median (IQR)) | 1 (0–2) | 3 (0–12) | 2 (0–2) | |
| Age (in years; median (IQR)) | 64 (59–69) | 64 (59–69) | 66 (62–71) | |
| Gender (%) | Male | 75 (44) | 14 (38) | 61 (46) |
| Female | 95 (56) | 23 (62) | 72 (54) | |
| Education status (%) | Low | 14 (8.2) | 2 (5) | 12 (9) |
| Middle | 49 (29) | 7 (19) | 42 (32) | |
| High | 107 (63) | 28 (76) | 79 (59) | |
| Smoking status (%) | Non-smoker | 69 (41) | 12 (32) | 57 (43) |
| Current smoker | 8 (5) | 1 (3) | 7 (5) | |
| Ex-smoker | 93 (55) | 24 (65) | 69 (52) | |
| Body mass index (kg/m2; median (IQR)) | 25 (23–28) | 25 (23–27) | 25 (23–28) | |
| Cognitive function (Qmci-D; mean (SD)) | 70 (10) | 69 (10) | 71 (10) | |
| Depressive symptoms (CES-D; median (IQR)) | 6 (2–11) | 14 (9–20) | 4 (1–8) | |
| Comorbidity (%) | No | 155 (91) | 32 (87) | 123 (93) |
| Yes | 15 (9) | 5 (14) | 10 (8) | |
| OHIP-NL14 Score | ||||||
|---|---|---|---|---|---|---|
| Item | Question | Never n (%) | Rarely n (%) | Occasionally n (%) | Rather Often n (%) | Very Often n (%) |
| 1 | Have you had trouble pronouncing certain words because of problems with your teeth, mouth or dentures? | 151 (89) | 11 (6) | 5 (3) | 2 (1) | 1 (1) |
| 2 | Have you felt that your sense of taste has worsened because of problems with your teeth, mouth or dentures? | 160 (94) | 8 (5) | 2 (1) | 0 (0) | 0 (0) |
| 3 | Have you had pain aching in your mouth? | 99 (58) | 52 (31) | 15 (9) | 4 (2) | 0 (0) |
| 4 | Have you found it uncomfortable to eat any foods because of problems with your teeth, mouth or dentures? | 139 (82) | 24 (14) | 4 (2) | 3 (2) | 0 (0) |
| 5 | Have you been self-conscious because of your teeth, mouth or dentures? | 138 (81) | 18 (11) | 12 (7) | 2 (1) | 0 (0) |
| 6 | Have you felt tense because of your teeth, mouth or dentures? | 143 (84) | 19 (11) | 8 (5) | 0 (0) | 0 (0) |
| 7 | Has your diet been unsatisfactory because of your teeth, mouth or dentures? | 157 (92) | 11 (6) | 1 (1) | 1 (1) | 0 (0) |
| 8 | Have you had to interrupt meals because of problems with your teeth, mouth or dentures? | 161 (95) | 7 (4) | 2 (1) | 0 (0) | 0 (0) |
| 9 | Have you found it difficult to relax because of problems with your teeth, mouth or dentures? | 152 (89) | 15 (9) | 2 (1) | 1 (1) | 0 (0) |
| 10 | Have you been a bit embarrassed because of problems with your teeth, mouth or dentures? | 143 (84) | 18 (10) | 8 (5) | 1 (1) | 0 (0) |
| 11 | Have you been a bit irritable with other people because of problems with your teeth, mouth or dentures? | 155 (91) | 12 (7) | 3 (2) | 0 (0) | 0 (0) |
| 12 | Have you had difficulty doing your usual jobs because of problems with your teeth, mouth or dentures? | 162 (95) | 7 (4) | 0 (0) | 1 (1) | 0 (0) |
| 13 | Have you felt that life in general was less satisfying because of problems with your teeth, mouth or dentures? | 150 (88) | 13 (8) | 5 (3) | 2 (1) | 0 (0) |
| 14 | Have you been totally unable to function because of your problems with your teeth, mouth or dentures? | 160 (94) | 9 (5) | 1 (1) | 0 (0) | 0 (0) |
| Frail | Non-Frail | Frail vs. Non-Frail | |
|---|---|---|---|
| OHIP-NL14 Item | n (%) | n (%) | p-Value |
| 1 | 11 (30) | 8 (6) | 0.000 * |
| 2 | 8 (22) | 2 (2) | 0.000 * |
| 3 | 22 (60) | 49 (37) | 0.015 |
| 4 | 18 (49) | 13 (10) | 0.000 * |
| 5 | 15 (41) | 17 (13) | 0.001 * |
| 6 | 15 (41) | 12 (9) | 0.000 * |
| 7 | 8 (22) | 5 (4) | 0.001 * |
| 8 | 6 (16) | 3 (2) | 0.000 * |
| 9 | 10 (27) | 8 (6) | 0.001 * |
| 10 | 14 (38) | 13 (10) | 0.000 * |
| 11 | 10 (27) | 5 (4) | 0.000 * |
| 12 | 6 (16) | 2 (2) | 0.001 * |
| 13 | 12 (32) | 8 (6) | 0.000 * |
| 14 | 9 (24) | 1 (1) | 0.000 * |
| Univariate Analysis | Multivariate Analysis | SCAD Analysis | ||||
|---|---|---|---|---|---|---|
| RC 1 | p-Value | RC 1 | p-Value | RC 1 | ||
| Oral health (OHIP-NL14) | 0.22 | 0.000 * | 0.22 | 0.002 * | 0.12 | |
| Age (in years) | 0.06 | 0.021 * | 0.06 | 0.241 | 0.02 | |
| Female | 0.33 | 0.386 | 1.49 | 0.014 * | 1.14 | |
| Education status | High | |||||
| Low | −0.52 | 0.507 | −1.46 | 0.177 | - | |
| Middle | −0.65 | 0.159 | −1.17 | 0.074 | - | |
| Smoking status | Non-smoker | |||||
| Current smoker | −0.69 | 0.523 | −1.20 | 0.431 | - | |
| Ex-smoker | 0.53 | 0.163 | 0.31 | 0.564 | - | |
| Body mass index | 0.00 | 0.969 | 0.04 | 0.423 | - | |
| Cognitive function (Qmci-D) | −0.04 | 0.041 * | −0.04 | 0.183 | - | |
| Depressive symptoms (CES-D) | 0.20 | 0.000 * | 0.18 | 0.000 * | 0.18 | |
| Comorbidity | 0.65 | 0.262 | −0.51 | 0.651 | - | |
| Univariate Analysis | Multivariate Analysis | |||||
|---|---|---|---|---|---|---|
| OR 1 | 95% CI 2 | p-Value | OR 1 | 95% CI 2 | p-Value | |
| Oral health (OHIP-NL14) | 1.25 | 1.13–1.38 | 0.000 * | 1.21 | 1.09–1.37 | 0.000 * |
| Age (in years) | 1.06 | 1.01–1.12 | 0.021 * | 1.06 | 0.99–1.15 | 0.086 |
| Female | 1.39 | 0.66–2.94 | 0.386 | 3.26 | 1.16–10.44 | 0.032 * |
| Depressive symptoms (CES-D) | 1.22 | 1.13–1.31 | 0.000 * | 1.20 | 1.12–1.31 | 0.000 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dros, C.; Sealy, M.J.; Krijnen, W.P.; Weening-Verbree, L.F.; Hobbelen, H.; Jager-Wittenaar, H. Oral Health and Frailty in Community-Dwelling Older Adults in the Northern Netherlands: A Cross-Sectional Study. Int. J. Environ. Res. Public Health 2022, 19, 7654. https://doi.org/10.3390/ijerph19137654
Dros C, Sealy MJ, Krijnen WP, Weening-Verbree LF, Hobbelen H, Jager-Wittenaar H. Oral Health and Frailty in Community-Dwelling Older Adults in the Northern Netherlands: A Cross-Sectional Study. International Journal of Environmental Research and Public Health. 2022; 19(13):7654. https://doi.org/10.3390/ijerph19137654
Chicago/Turabian StyleDros, Coen, Martine J. Sealy, Wim P. Krijnen, Lina F. Weening-Verbree, Hans Hobbelen, and Harriët Jager-Wittenaar. 2022. "Oral Health and Frailty in Community-Dwelling Older Adults in the Northern Netherlands: A Cross-Sectional Study" International Journal of Environmental Research and Public Health 19, no. 13: 7654. https://doi.org/10.3390/ijerph19137654
APA StyleDros, C., Sealy, M. J., Krijnen, W. P., Weening-Verbree, L. F., Hobbelen, H., & Jager-Wittenaar, H. (2022). Oral Health and Frailty in Community-Dwelling Older Adults in the Northern Netherlands: A Cross-Sectional Study. International Journal of Environmental Research and Public Health, 19(13), 7654. https://doi.org/10.3390/ijerph19137654







