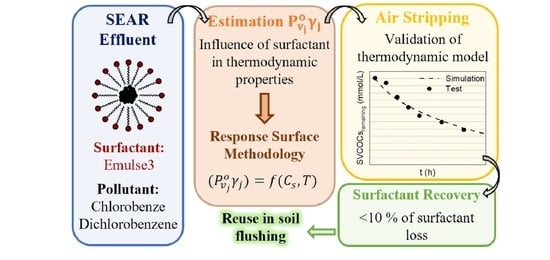
Non-Ionic Surfactant Recovery in Surfactant Enhancement Aquifer Remediation Effluent with Chlorobenzenes by Semivolatile Chlorinated Organic Compounds Volatilization
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Experimental Procedure
2.2.1. Surfactant Stability (B1)
2.2.2. B2. Estimation of (B2)
2.2.3. Volatlization Tests (B3)
2.3. SVCOCs Analysis
3. Results and Discussions
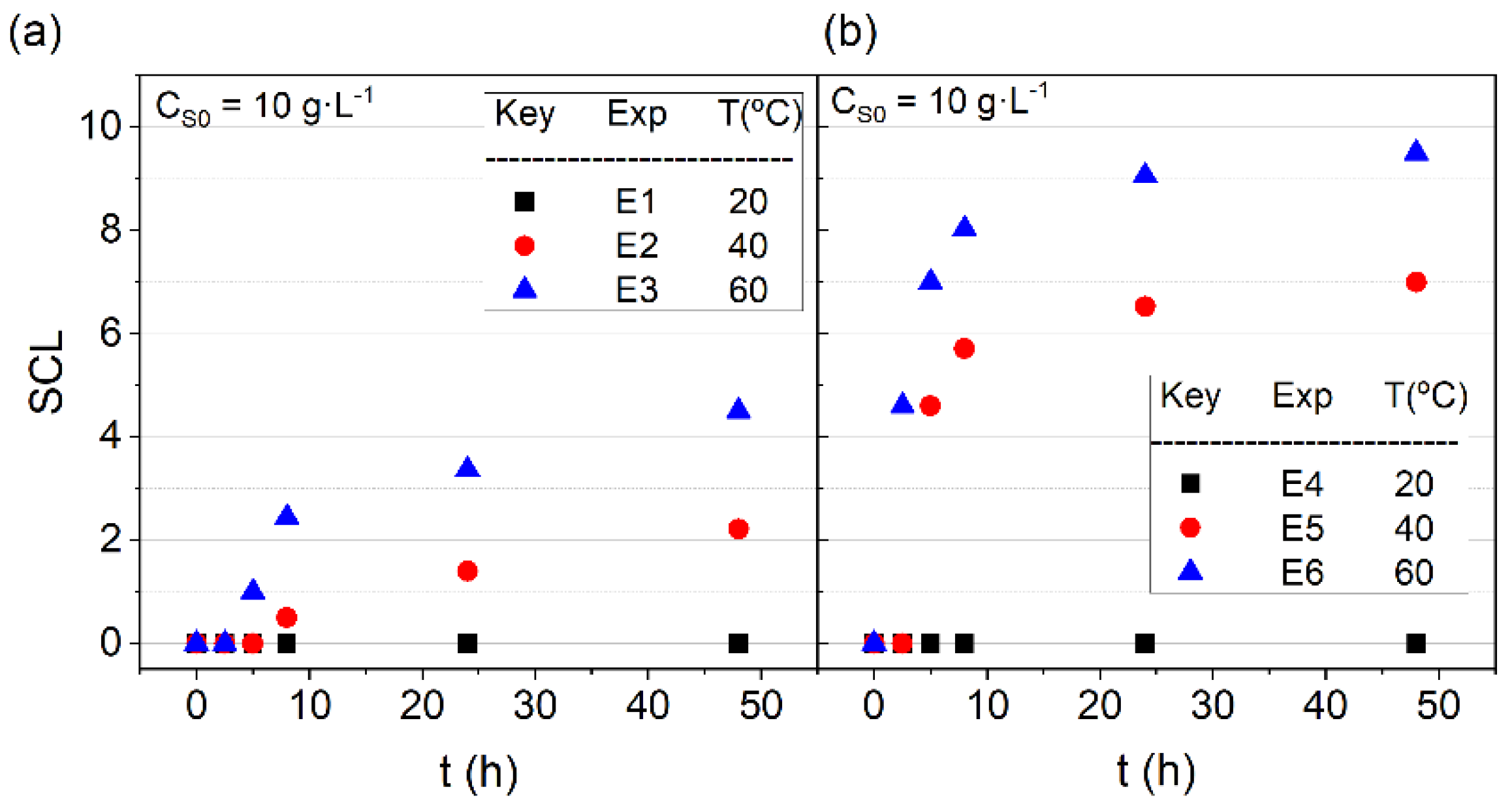
3.1. Surfactant Stability
3.2. Estimation and Correlation
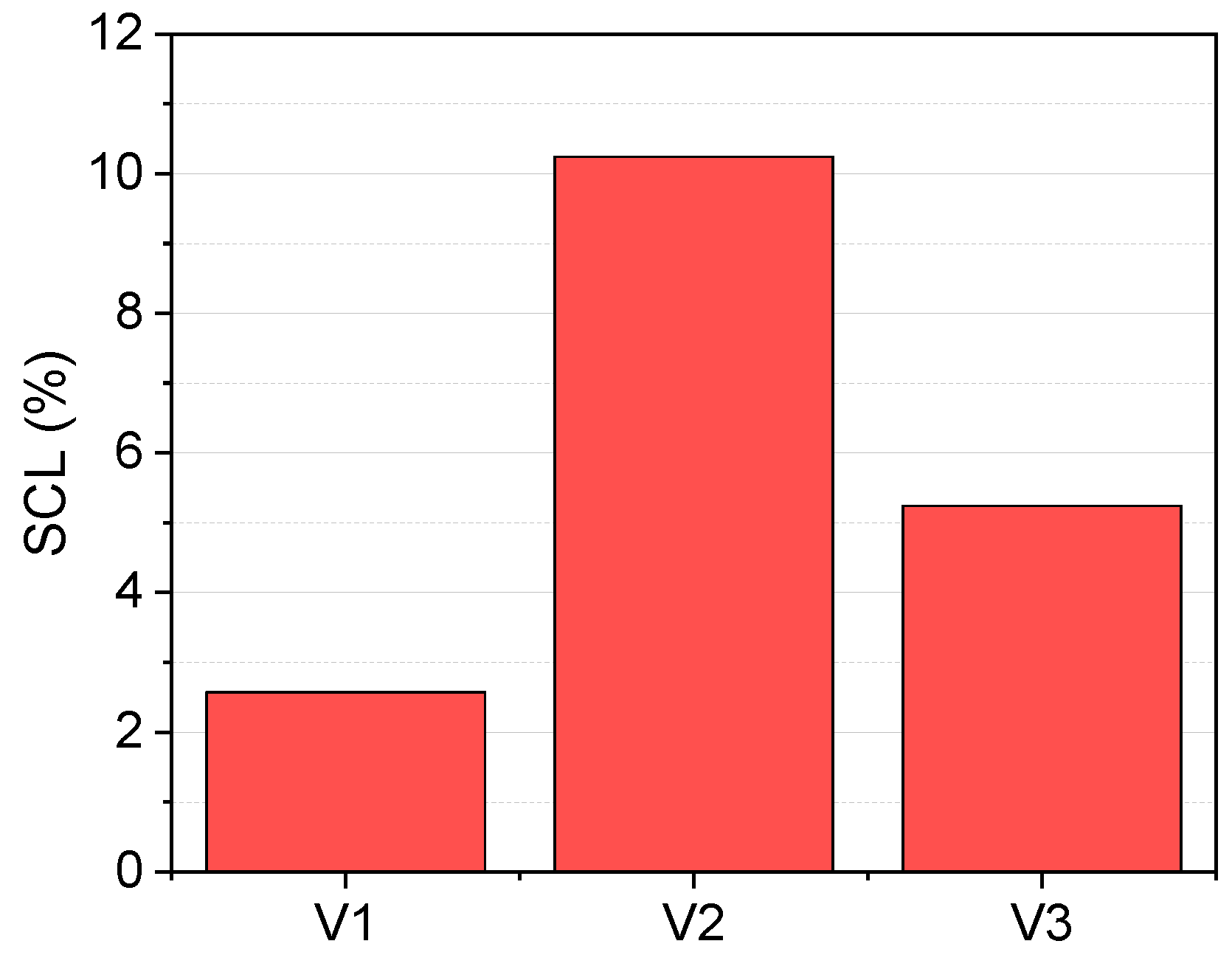
3.3. Volatilization of SVCOCs from Emulsion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Payá Perez, A.; Rodríguez Eugenio, N. Status of Local Soil Contamination in Europe; European Comission: Brussels, Belgium, 2018. [Google Scholar]
- Siegrist, R.L.; Crimi, M.; Simpkin, T.J. In Situ Chemical Oxidation for Groundwater Remediation; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2011; Volume 3. [Google Scholar]
- Huo, L.; Liu, G.; Yang, X.; Ahmad, Z.; Zhong, H. Surfactant-enhanced aquifer remediation: Mechanisms, influences, limitations and the countermeasures. Chemosphere 2020, 252, 126620. [Google Scholar] [CrossRef] [PubMed]
- Ramezanzadeh, M.; Aminnaji, M.; Rezanezhad, F.; Ghazanfari, M.H.; Babaei, M. Dissolution and remobilization of NAPL in surfactant-enhanced aquifer remediation from microscopic scale simulations. Chemosphere 2022, 289, 133177. [Google Scholar] [CrossRef] [PubMed]
- Rosen, M.J.; Kunjappu, J.T. Surfactants and Interfacial Phenomena; John Wiley & Sons: Hoboken, NJ, USA, 2012. [Google Scholar]
- Babaei, M.; Copty, N.K. Numerical modelling of the impact of surfactant partitioning on surfactant-enhanced aquifer remediation. J. Contam. Hydrol. 2019, 221, 69–81. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.W.; Wei, K.H.; Xu, S.W.; Cui, J.; Ma, J.; Xiao, X.L.; Xi, B.D.; He, X.S. Surfactant-enhanced remediation of oil-contaminated soil and groundwater: A review. Sci. Total Environ. 2021, 756, 144142. [Google Scholar] [CrossRef]
- Santos, A.; Domínguez, C.M.; Lorenzo, D.; García-Cervilla, R.; Lominchar, M.A.; Fernández, J.; Gómez, J.; Guadaño, J. Soil flushing pilot test in a landfill polluted with liquid organic wastes from lindane production. Heliyon 2019, 5, e02875. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dominguez, C.M.; Romero, A.; Santos, A. Selective removal of chlorinated organic compounds from lindane wastes by combination of nonionic surfactant soil flushing and Fenton oxidation. Chem. Eng. J. 2019, 376, 120009. [Google Scholar] [CrossRef]
- Hanafiah, S.A.; Mohamed, M.A.; Caradec, S.; Fatin-Rouge, N. Treatment of heavy petroleum hydrocarbons polluted soil leachates by ultrafiltration and oxidation for surfactant recovery. J. Environ. Chem. Eng. 2018, 6, 2568–2576. [Google Scholar] [CrossRef]
- Huang, K.; Liang, J.; Jafvert, C.T.; Li, Q.; Chen, S.; Tao, X.; Zou, M.; Dang, Z.; Lu, G. Effects of ferric ion on the photo-treatment of nonionic surfactant Brij35 washing waste containing 2,2′,4,4′-terabromodiphenyl ether. J. Hazard. Mater. 2021, 415, 125572. [Google Scholar] [CrossRef]
- Li, Y.; Hu, J.; Liu, H.; Zhou, C.; Tian, S. Electrochemically reversible foam enhanced flushing for PAHs-contaminated soil: Stability of surfactant foam, effects of soil factors, and surfactant reversible recovery. Chemosphere 2020, 260, 127645. [Google Scholar] [CrossRef]
- García-Cervilla, R.; Santos, A.; Romero, A.; Lorenzo, D. Compatibility of nonionic and anionic surfactants with persulfate activated by alkali in the abatement of chlorinated organic compounds in aqueous phase. Sci. Total Environ. 2021, 751, 141782. [Google Scholar] [CrossRef]
- Ahn, C.K.; Woo, S.H.; Park, J.M. Selective adsorption of phenanthrene in nonionic-anionic surfactant mixtures using activated carbon. Chem. Eng. J. 2010, 158, 115–119. [Google Scholar] [CrossRef]
- Rosas, J.M.; Santos, A.; Romero, A. Soil-Washing Effluent Treatment by Selective Adsorption of Toxic Organic Contaminants on Activated Carbon. Water Air Soil Pollut. 2013, 224, 10. [Google Scholar] [CrossRef]
- Ahn, C.K.; Lee, M.W.; Lee, D.S.; Woo, S.H.; Park, J.M. Mathematical evaluation of activated carbon adsorption for surfactant recovery in a soil washing process. J. Hazard. Mater. 2008, 160, 13–19. [Google Scholar] [CrossRef]
- Virga, E.; Parra, M.A.; de Vos, W.M. Fouling of polyelectrolyte multilayer based nanofiltration membranes during produced water treatment: The role of surfactant size and chemistry. J. Colloid Interface Sci. 2021, 594, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Cao, W.; Zhang, L.; Miao, Y.; Qiu, L. Research progress in the enhancement technology of soil vapor extraction of volatile petroleum hydrocarbon pollutants. Environ. Sci. Processes Impacts 2021, 23, 1650–1662. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Yang, Y.; Dai, X.; Li, C.; Wang, Q.; Chen, C.; Yan, G.; Guo, S. Bioremediation Enhances the Pollutant Removal Efficiency of Soil Vapor Extraction (SVE) in Treating Petroleum Drilling Waste. Water Air Soil Pollut. 2016, 227, 465. [Google Scholar] [CrossRef]
- Lee, J.H.; Woo, H.J.; Jeong, K.S. Removal of non-aqueous phase liquids (NAPLs) from TPH-saturated sandy aquifer sediments using in situ air sparging combined with soil vapor extraction. J. Environ. Sci. Health A Toxic Hazard. Subst. Environ. Eng. 2018, 53, 1253–1266. [Google Scholar] [CrossRef]
- Huan, J.C.; Shang, C. Air Stripping. In Advanced Physicochemical Treatment Processes. Handbook of Environmental Engineering; Wang, L.K., Hung, Y.T., Shammas, N.K., Eds.; Humana Press: Totowa, NJ, USA, 2006; Volume 4. [Google Scholar] [CrossRef]
- Pitt, R.; Shirley, C.; Field, R. Groundwater contamination potential from stormwater infiltration practices. Urban Water 1999, 1, 217–236. [Google Scholar] [CrossRef]
- Braeckevelt, M.; Mirschel, G.; Wiessner, A.; Rueckert, M.; Reiche, N.; Vogt, C.; Schultz, A.; Paschke, H.; Kuschk, P.; Kaestner, M. Treatment of chlorobenzene-contaminated groundwater in a pilot-scale constructed wetland. Ecol. Eng. 2008, 33, 45–53. [Google Scholar] [CrossRef]
- Lin, X.; Xu, C.; Zhou, Y.; Liu, S.; Liu, W. A new perspective on volatile halogenated hydrocarbons in Chinese agricultural soils. Sci. Total Environ. 2020, 703, 134646. [Google Scholar] [CrossRef]
- Santos, A.; Fernandez, J.; Guadano, J.; Lorenzo, D.; Romero, A. Chlorinated organic compounds in liquid wastes (DNAPL) from lindane production dumped in landfills in Sabinanigo (Spain). Environ. Pollut. 2018, 242, 1616–1624. [Google Scholar] [CrossRef] [PubMed]
- García-Cervilla, R.; Romero, A.; Santos, A.; Lorenzo, D. Surfactant-enhanced solubilization of chlorinated organic compounds contained in dnapl from lindane waste: Effect of surfactant type and ph. Int. J. Environ. Res. Public Health 2020, 17, 4494. [Google Scholar] [CrossRef] [PubMed]


| Set B1 | |||||
|---|---|---|---|---|---|
| Exp | T (°C) | CSVCOCs () | |||
| E1 | 20 | 10 | 0 | ||
| E2 | 40 | 10 | 0 | ||
| E3 | 60 | 10 | 0 | ||
| E4 | 20 | 10 | 94.11 | ||
| E5 | 40 | 10 | 94.11 | ||
| E6 | 60 | 10 | 94.11 | ||
| Set B2 | |||||
| Exp | T (°C) | CVCOCs () | |||
| P1 | 30, 40, 60 | 1.5 | 3.1, 6.3 | ||
| P2 | 30, 40, 60 | 3.5 | 7.5, 19.6 | ||
| P3 | 30, 40, 60 | 7.0 | 7.8, 23.5, 39.2 | ||
| P4 | 30, 40, 60 | 15.0 | 15.6, 31.3, 62.8 | ||
| Set B3 | |||||
| Exp | T (°C) | V emulsion (L) | |||
| V1 | 40 | 3.5 | 23.5 | 1.8 | 0.3 |
| V2 | 60 | 3.5 | 23.5 | 1.8 | 0.3 |
| V3 | 40 | 3.5 | 23.5 | 3.6 | 0.3 |
| a | b | c | d | e | f | F-Value | p-Value | ||
|---|---|---|---|---|---|---|---|---|---|
| CB | 2.86 | −0.27 | 0.06 | 8.73 × 10−3 | −1.32 × 10−4 | −6.74 × 10−5 | 0.99 | 414 | 1.86 × 10−22 |
| 1,4-DCB | 0.29 | −0.30 | 0.09 | 1.21 × 10−2 | −2.85 × 10−4 | −8.10 × 10−4 | 0.99 | 473 | 3.73 × 10−23 |
| 1,2-DCB | 0.31 | −0.30 | 0.08 | 1.19 × 10−2 | −2.34 × 10−4 | −8.13 × 10−4 | 0.99 | 666 | 6.52 × 10−25 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sáez, P.; Santos, A.; García-Cervilla, R.; Romero, A.; Lorenzo, D. Non-Ionic Surfactant Recovery in Surfactant Enhancement Aquifer Remediation Effluent with Chlorobenzenes by Semivolatile Chlorinated Organic Compounds Volatilization. Int. J. Environ. Res. Public Health 2022, 19, 7547. https://doi.org/10.3390/ijerph19127547
Sáez P, Santos A, García-Cervilla R, Romero A, Lorenzo D. Non-Ionic Surfactant Recovery in Surfactant Enhancement Aquifer Remediation Effluent with Chlorobenzenes by Semivolatile Chlorinated Organic Compounds Volatilization. International Journal of Environmental Research and Public Health. 2022; 19(12):7547. https://doi.org/10.3390/ijerph19127547
Chicago/Turabian StyleSáez, Patricia, Aurora Santos, Raúl García-Cervilla, Arturo Romero, and David Lorenzo. 2022. "Non-Ionic Surfactant Recovery in Surfactant Enhancement Aquifer Remediation Effluent with Chlorobenzenes by Semivolatile Chlorinated Organic Compounds Volatilization" International Journal of Environmental Research and Public Health 19, no. 12: 7547. https://doi.org/10.3390/ijerph19127547
APA StyleSáez, P., Santos, A., García-Cervilla, R., Romero, A., & Lorenzo, D. (2022). Non-Ionic Surfactant Recovery in Surfactant Enhancement Aquifer Remediation Effluent with Chlorobenzenes by Semivolatile Chlorinated Organic Compounds Volatilization. International Journal of Environmental Research and Public Health, 19(12), 7547. https://doi.org/10.3390/ijerph19127547