‘Breathing Fire’: Impact of Prolonged Bushfire Smoke Exposure in People with Severe Asthma
Abstract
:1. Introduction
2. Materials and Methods
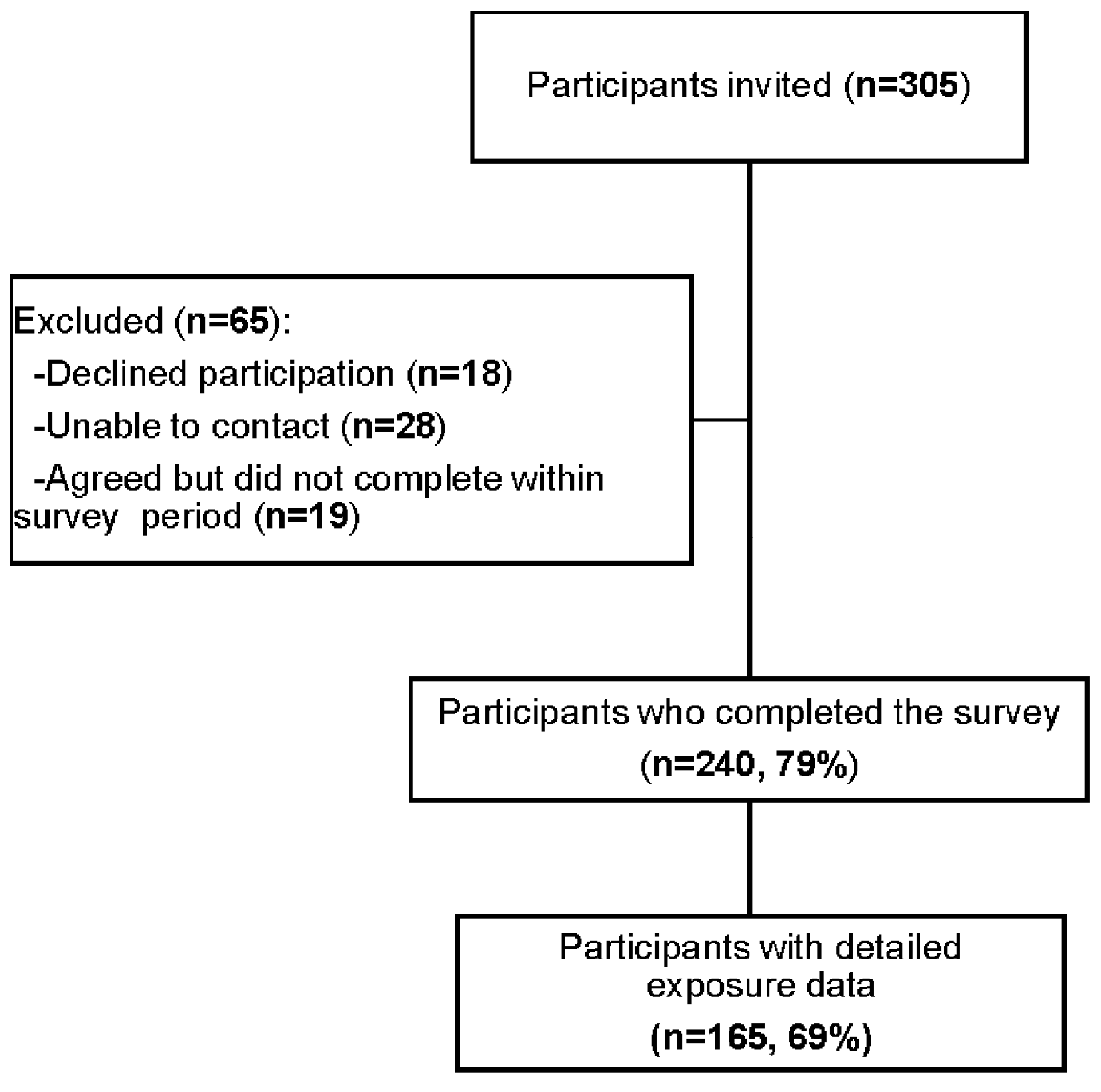
2.1. Study Design
2.2. Data Collection: Clinical Data and Health Outcomes
2.3. Data Collection: Exposure Measures
2.4. Statistical Analysis
3. Results
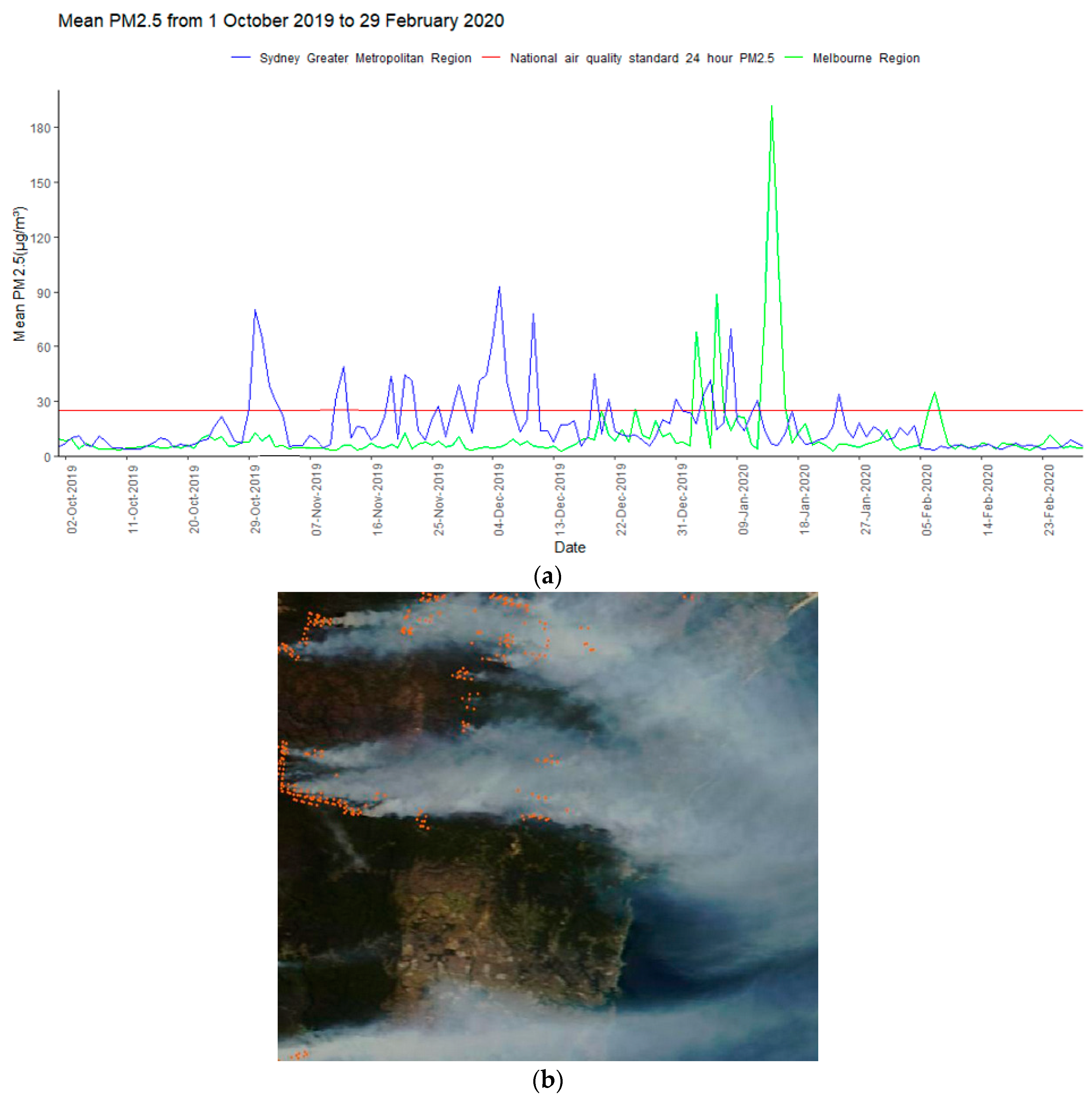
3.1. Bushfire Smoke Exposure
3.2. Demographic and Clinical Characteristics
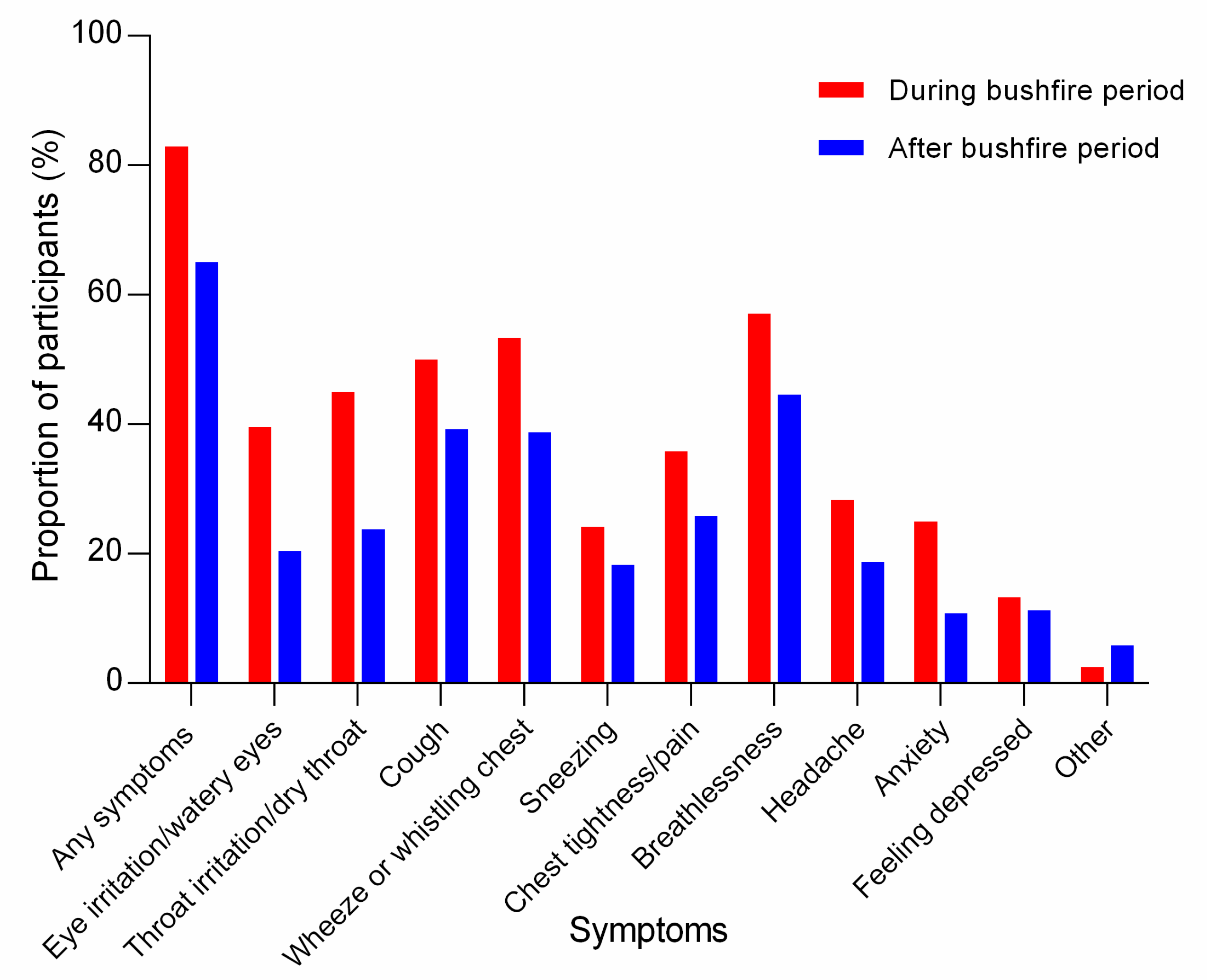
3.3. Symptoms during Bushfire Smoke Exposure
3.4. Comparison of Asthma between the 2019/2020 Bushfire Period and the 2018/2019 Bushfire Period
3.5. Comparison of Asthma before and during the 2019/2020 Bushfire Period
3.6. Persistent Symptoms Following the 2019/2020 Bushfire Period
3.7. Smoke Exposure Risk Mitigation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Flannigan, M.; Cantin, A.S.; de Groot, W.J.; Wotton, M.; Newbery, A.; Gowman, L.M. Global wildland fire season severity in the 21 st century. For. Ecol. Manag. 2013, 294, 54–61. [Google Scholar] [CrossRef]
- Wagner, R.; Jahn, M.; Schepanski, K. Wildfires as a source of airborne mineral dust—Revisiting a conceptual model using large-eddy simulation (LES). Atmos. Chem. Phys. 2018, 18, 11863–11884. [Google Scholar] [CrossRef] [Green Version]
- Xu, R.; Yu, P.; Abramson, M.J.; Johnston, F.H.; Samet, J.M.; Bell, M.L.; Haines, A.; Ebi, K.L.; Li, S.; Guo, Y. Wildfires, Global Climate Change, and Human Health. N. Engl. J. Med. 2020, 383, 2173–2181. [Google Scholar] [CrossRef] [PubMed]
- Urbanski, S.P.; Hao, W.M.; Baker, S. Chemical Composition of Wildland Fire Emissions; Wildland fires and air pollution; Elsevier: Amsterdam, The Netherlands, 2009; Volume 8, pp. 79–107. [Google Scholar]
- Xing, Y.-F.; Xu, Y.-H.; Shi, M.-H.; Lian, Y.-X. The impact of PM2.5 on the human respiratory system. J. Thorac. Dis. 2016, 8, E69–E74. [Google Scholar]
- Feng, S.; Gao, D.; Liao, F.; Zhou, F.; Wang, X. The health effects of ambient PM2.5 and potential mechanisms. Ecotoxicol. Environ. Saf. 2016, 128, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Walter, C.M.; Schneider-Futschik, E.K.; Knibbs, L.D.; Irving, L.B. Health impacts of bushfire smoke exposure in Australia. Respirology 2020, 25, 495–501. [Google Scholar] [CrossRef] [PubMed]
- Johnston, F.H.; Purdie, S.; Jalaludin, B.; Martin, K.L.; Henderson, S.B.; Morgan, G.G. Air pollution events from forest fires and emergency department attendances in Sydney, Australia 1996–2007: A case-crossover analysis. Environ. Health 2014, 13, 105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martin, K.L.; Hanigan, I.C.; Morgan, G.G.; Henderson, S.B.; Johnston, F.H. Air pollution from bushfires and their association with hospital admissions in Sydney, Newcastle and Wollongong, Australia 1994-2007. Aust. N. Z. J. Public Health 2013, 37, 238–243. [Google Scholar] [CrossRef]
- Borchers Arriagada, N.; Horsley, J.A.; Palmer, A.J.; Morgan, G.G.; Tham, R.; Johnston, F.H. Association between fire smoke fine particulate matter and asthma-related outcomes: Systematic review and meta-analysis. Environ. Res. 2019, 179 Pt A, 108777. [Google Scholar] [CrossRef]
- Aguilera, R.; Corringham, T.; Gershunov, A.; Benmarhnia, T. Wildfire smoke impacts respiratory health more than fine particles from other sources: Observational evidence from Southern California. Nat. Commun. 2021, 12, 1493. [Google Scholar] [CrossRef]
- DeFlorio-Barker, S.; Crooks, J.; Reyes, J.; Rappold, A.G. Cardiopulmonary Effects of Fine Particulate Matter Exposure among Older Adults, during Wildfire and Non-Wildfire Periods, in the United States 2008–2010. Environ. Health Perspect. 2019, 127, 37006. [Google Scholar] [CrossRef] [PubMed]
- Verma, V.; Polidori, A.; Schauer, J.J.; Shafer, M.M.; Cassee, F.R.; Sioutas, C. Physicochemical and toxicological profiles of particulate matter in Los Angeles during the October 2007 southern California wildfires. Environ. Sci. Technol. 2009, 43, 954–960. [Google Scholar] [CrossRef]
- Dong, T.T.T.; Hinwood, A.L.; Callan, A.C.; Zosky, G.; Stock, W.D. In vitro assessment of the toxicity of bushfire emissions: A review. Sci. Total Environ. 2017, 603, 268–278. [Google Scholar] [CrossRef] [PubMed]
- Borchers Arriagada, N.; Palmer, A.J.; Bowman, D.M.; Morgan, G.G.; Jalaludin, B.B.; Johnston, F.H. Unprecedented smoke-related health burden associated with the 2019-20 bushfires in eastern Australia. Med. J. Aust. 2020, 213, 282–283. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.D.; Azzi, M.; White, S.; Salter, D.; Trieu, T.; Morgan, G.; Rahman, M.; Watt, S.; Riley, M.; Chang, L.T.; et al. The Summer 2019–2020 Wildfires in East Coast Australia and Their Impacts on Air Quality and Health in New South Wales, Australia. Int. J. Environ. Res. Public Health 2021, 18, 3538. [Google Scholar] [CrossRef] [PubMed]
- Johnston, F.H.; Borchers-Arriagada, N.; Morgan, G.G.; Jalaludin, B.; Palmer, A.J.; Williamson, G.J.; Bowman, D.M.J.S. Unprecedented health costs of smoke-related PM2.5 from the 2019–20 Australian megafires. Nat. Sustain. 2021, 4, 42–47. [Google Scholar] [CrossRef]
- MacIntyre, C.R.; Nguyen, P.Y.; Trent, M.; Seale, H.; Chughtai, A.A.; Shah, S.; Marks, G.B. Adverse Health Effects in People with and without Preexisting Respiratory Conditions during Bushfire Smoke Exposure in the 2019/2020 Australian Summer. Am. J. Respir. Crit. Care Med. 2021, 204, 368–371. [Google Scholar] [CrossRef]
- Bui, D.; Davis, S.; Flynn, A.; Bell, R.; Dharmage, S. Impact of recent catastrophic bushfires on people with asthma in Australia: Health, social and financial burdens. Respirology 2021, 26, 296–297. [Google Scholar] [CrossRef]
- Hiles, S.A.; Harvey, E.S.; McDonald, V.M.; Peters, M.; Bardin, P.; Reynolds, P.N.; Upham, J.W.; Baraket, M.; Bhikoo, Z.; Bowden, J.; et al. Working while unwell: Workplace impairment in people with severe asthma. Clin. Exp. Allergy 2018, 48, 650–662. [Google Scholar] [CrossRef]
- Harvey, E.S.; Langton, D.; Katelaris, C.; Stevens, S.; Farah, C.S.; Gillman, A.; Harrington, J.; Hew, M.; Kritikos, V.; Radhakrishna, N.; et al. Mepolizumab effectiveness and identification of super-responders in severe asthma. Eur. Respir. J. 2020, 55, 1902420. [Google Scholar] [CrossRef]
- Chung, K.F.; Wenzel, S.E.; Brozek, J.L.; Bush, A.; Castro, M.; Sterk, P.J.; Adcock, I.M.; Bateman, E.D.; Bel, E.H.; Bleecker, E.R.; et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur. Respir. J. 2014, 43, 343–373. [Google Scholar] [CrossRef] [Green Version]
- Harris, P.A.; Taylor, R.; Thielke, R.; Payne, J.; Gonzalez, N.; Conde, J.G. Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. J. Biomed. Inform. 2009, 42, 377–381. [Google Scholar] [CrossRef] [Green Version]
- Howard, Z.L.; Carlson, S.J.; Baldwin, Z.; Johnston, F.; Durrheim, D.N.; Dalton, C.B. High community burden of smoke-related symptoms in the Hunter and New England regions during the 2019–2020 Australian bushfires. Public Health Res. Pract. 2020, 30, e30122007. [Google Scholar] [CrossRef]
- Riley, M.; Kirkwood, J.; Jiang, N.; Ross, G.; Scorgie, Y. Air quality monitoring in NSW: From long term trend monitoring to integrated urban services. Air Qual. Clim. Chang. 2020, 51, 44–51. [Google Scholar]
- Environmental Protection Authority Victoria EPA AirWatch. Available online: https://www.epa.vic.gov.au/for-community/airwatch/airwatch-table-data-page (accessed on 14 September 2021).
- Johnston, F.H.; Hanigan, I.C.; Henderson, S.B.; Morgan, G.G.; Portner, T.; Williamson, G.J.; Bowman, D.M. Creating an integrated historical record of extreme particulate air pollution events in Australian cities from 1994 to 2007. J. Air Waste Manag. Assoc. 2011, 61, 390–398. [Google Scholar] [CrossRef]
- Hanigan, I.C.; Morgan, G.G.; Williamson, G.J.; Salimi, F.; Henderson, S.B.; Turner, M.R.; Bowman, D.M.J.S.; Johnston, F.H. Extensible Database of Validated Biomass Smoke Events for Health Research. Fire 2018, 1, 50. [Google Scholar] [CrossRef] [Green Version]
- Textor, J.; van der Zander, B.; Gilthorpe, M.S.; Liskiewicz, M.; Ellison, G.T. Robust causal inference using directed acyclic graphs: The R package dagitty. Int. J. Epidemiol. 2016, 45, 1887–1894. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Westreich, D.; Greenland, S. The table 2 fallacy: Presenting and interpreting confounder and modifier coefficients. Am. J. Epidemiol. 2013, 177, 292–298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kiser, D.; Metcalf, W.J.; Elhanan, G.; Schnieder, B.; Schlauch, K.; Joros, A.; Petersen, C.; Grzymski, J. Particulate matter and emergency visits for asthma: A time-series study of their association in the presence and absence of wildfire smoke in Reno, Nevada, 2013–2018. Environ. Health 2020, 19, 92. [Google Scholar] [CrossRef] [PubMed]
- Haikerwal, A.; Akram, M.; Sim, M.R.; Meyer, M.; Abramson, M.J.; Dennekamp, M. Fine particulate matter (PM2.5) exposure during a prolonged wildfire period and emergency department visits for asthma. Respirology 2016, 21, 88–94. [Google Scholar] [CrossRef] [PubMed]
- Morgan, G.; Sheppeard, V.; Khalaj, B.; Ayyar, A.; Lincoln, D.; Jalaludin, B.; Beard, J.; Corbett, S.; Lumley, T. Effects of bushfire smoke on daily mortality and hospital admissions in Sydney, Australia. Epidemiology 2010, 21, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Johnston, F.H. Understanding and managing the health impacts of poor air quality from landscape fires. Med. J. Aust. 2017, 207, 229–230. [Google Scholar] [CrossRef] [PubMed]
- Carlsten, C.; Salvi, S.; Wong, G.W.K.; Chung, K.F. Personal strategies to minimise effects of air pollution on respiratory health: Advice for providers, patients and the public. Eur. Respir. J. 2020, 55, 1902056. [Google Scholar] [CrossRef] [PubMed]
| Variables | Number (%) |
|---|---|
| Respondents n | 240 |
| Age, years | 63.47 (53.76, 71.30) |
| Sex (female) | 145 (60.0) |
| Smoking status | |
| Never (%)/current (%)/ex-smoker (%) | 62.7/2.1/35.2 |
| Pack years (current/ex-smoker) | 15.0 (5.3, 30.0) |
| Currently in paid employment | 76 (31.7) |
| ASTHMA CHARACTERISTICS | |
| Severe asthma | 222 (92.5) |
| Asthma duration, years | 34.93 (18.76, 52.53) |
| Atopy, n = 191 | 143 (74.9) |
| PRE-BUSHFIRE VISIT * | |
| Asthma control | |
| ACQ-5 score | 1.4 (0.6, 2.2) |
| Post-bronchodilator lung function, n = 179 | |
| FEV1 % predicted | 69.40 (21.52) |
| FVC % predicted | 86.10 (16.50) |
| FEV1/FVC | 0.62 (0.15) |
| Exacerbations, n = 208 | |
| Requiring OCS, n = 208 | 83 (39.9) |
| Requiring hospital admission, n = 207 | 15 (7.2) |
| Requiring emergency department visit, n = 208 | 8 (3.8) |
| Requiring IV corticosteroids, n = 207 | 4 (1.9) |
| Requiring unscheduled Dr visit, n = 208 | 24 (11.5) |
| Standardized Juniper Asthma Quality of Life Questionnaire, n = 198 | |
| AQLQ(S) overall score | 5.45 (4.50, 6.34) |
| AQLQ(S) activity limitations | 5.50 (4.36, 6.45) |
| AQLQ(S) symptoms | 5.41 (4.50, 6.40) |
| AQLQ(S) emotional function | 5.60 (4.40, 6.60) |
| AQLQ(S) environmental stimuli | 5.75 (4.50, 6.50) |
| Asthma treatments | |
| Using maintenance OCS, n = 232 | 49 (21.12) |
| Using low dose macrolides, n = 231 | 27 (11.7) |
| Using monoclonal antibody, n = 234 | 156 (66.7) |
| Using ICS, n = 232 | 39 (16.81) |
| Using LABA, n = 232 | 3 (1.3) |
| Using LAMA, n = 232 | 101 (43.5) |
| Using ICS/LABA, n = 232 | 203 (87.5) |
| Using ICS/LABA/LAMA, n = 232 | 12 (5.1) |
| Using Theophylline, n = 232 | 11 (4.7) |
| Using Montelukast, n = 232 | 23 (9.9) |
| Variables | Symptoms during the Bushfire Period | |||
|---|---|---|---|---|
| Crude RR (95%CI) | p-Value | Adjusted RR (95%CI) | p-Value | |
| Fire day > 41 days | 1.02 (0.89–1.16) | 0.78 | 0.98 (0.87–1.11) | 0.82 |
| Consecutive fire days (>10 days) | 1.03 (0.90–1.17) | 0.70 | 1.0 (0.89–1.12) | 0.97 |
| Mean PM2.5 (>16 µg/m3) | 0.98 (0.86–1.11) | 0.70 | 0.95 (0.85–1.07) | 0.43 |
| Peak PM2.5 (>115 µg/m3) | 0.98 (0.86–1.12) | 0.80 | 1.01 (0.90–1.14) | 0.85 |
| Variables | During 2019/2020 Bushfire Period (1 October 2019 to 29 February 2020) | During the 2018/2019 Bushfire Period (1 October 2018 to 1 March 2019) | p-Value |
|---|---|---|---|
| Exposure data | |||
| Bushfire day †, median (Q1, Q3), n = 165 | 42 (5,43) | 2 (0,2) | <0.001 |
| Maximum consecutive bushfire days †, median (Q1, Q3), n = 165 | 11 (0,11) | 1 (0,1) | <0.001 |
| Mean PM2.5 (µg/m3) †, median (Q1, Q3), n = 165 | 16.4 (11.3, 16.7) | 7.7 (7.0, 8.3) | <0.001 |
| Peak PM2.5 (µg/m3) †, median (Q1, Q3), n = 165 | 115.0 (101.3, 191.7) | 18.3 (17.4, 22.0) | <0.001 |
| Exposure data categories | |||
| Bushfire days > 41 §, n (%), n = 165 | 90 (54.5) | 0 (0.0) | <0.001 |
| Maximum consecutive bushfire days >10 §, n (%), n = 165 | 85 (51.5) | 0 (0.0) | <0.001 |
| Mean PM2.5 > 16 µg/m3 §, n (%), n = 165 | 100 (60.6) | 0 (0.0) | <0.001 |
| Peak PM2.5 > 115 µg/m3 §, n (%), n = 165 | 82 (49.7) | 0 (0.0) | <0.001 |
| Experienced an attack of asthma that resulted in: | |||
| OCS started or increased for at least 3 days n (%), n = 92 | 37 (40.2) | 28 (30.4) | 0.11 |
| Unscheduled Dr visits ‡, n (%) n = 92 | 23 (25.0) | 9 (9.8) | 0.008 |
| OCS started or increased, courses † median (Q1, Q3) n = 88 | 0 (0, 2) | 0 (0, 1) | 0.13 |
| Unscheduled Dr visits †, median (Q1, Q3), n = 91 | 0 (0, 1) | 0 (0, 0) | 0.016 |
| Variables | Total | Persistent Symptoms following Bushfire Period n (%) | Crude RR (95% CI) | p-Value | |
|---|---|---|---|---|---|
| Yes (156) | No (84) | ||||
| Age (years) during the bushfire† | 240 | 63 (50.5, 71.5) | 64 (56.0, 70.0) | 1.00 (0.99–1.01) | 0.45 |
| Sex | 240 | ||||
| Male | 95 | 52 (33.3) | 43 (51.2) | ||
| Female | 145 | 104 (66.7) | 41 (48.8) | 1.31 (1.06–1.62) | 0.012 |
| Smoking status | 236 | ||||
| Never smoker | 148 | 89 (57.8) | 59 (72.0) | ||
| Smoker (Ex and current) | 88 | 65 (42.2) | 23 (28.0) | 1.23 (1.02–1.47) | 0.026 |
| Missing | 4 | 2 | 2 | ||
| Asthma severity | 240 | ||||
| Severe asthma | 222 | 145 (93.0) | 77 (91.7) | 1.07 (0.73–1.56) | 0.73 |
| Non-severe asthma | 18 | 11 (7.0) | 7 (8.3) | ||
| Using maintenance OCS | 232 | ||||
| Yes | 49 | 34 (22.5) | 15 (18.5) | 1.08 (0.87–1.35) | 0.46 |
| No | 183 | 117 (77.5) | 66 (81.5) | ||
| Missing | 8 | 5 | 3 | ||
| Monoclonal antibody use | 234 | ||||
| Yes | 156 | 97 (63.8) | 59 (72.0) | 0.88 (0.73–1.06) | 0.19 |
| No | 78 | 55 (36.2) | 23 (28.0) | ||
| Missing | 6 | 4 | 2 | ||
| Macrolide use | 231 | ||||
| Yes | 27 | 21 (14.0) | 6 (7.4) | 1.23 (0.98–1.54) | 0.07 |
| No | 204 | 129 (86.0) | 75 (92.6) | ||
| Missing | 6 | 3 | |||
| ACQ-5 score at pre-bushfire visit | 231 | ||||
| Uncontrolled asthma (ACQ ≥ 1.5) | 103 | 78 (52.7) | 25 (30.1) | 1.38 (1.14–1.68) | 0.001 |
| Controlled asthma (ACQ < 1.5) | 128 | 70 (47.3) | 58 (69.9) | ||
| Missing | 9 | 8 | 1 | ||
| Exacerbation at pre-bushfire visit | |||||
| Yes | 87 | 60 (45.1) | 27 (36.0) | 1.14 (0.93–1.40) | 0.19 |
| No | 121 | 73 (54.9) | 48 (64.0) | ||
| Missing | 32 | 23 | 9 | ||
| Lung function | |||||
| FEV1 % predicted preB2 < 80 | 125 | 83 (72.8) | 42 (71.2) | 1.03 (0.80–1.31) | 0.82 |
| FEV1 % predicted preB2 ≥ 80 | 48 | 31 (27.2) | 17 (28.8) | ||
| Missing | 67 | 42 | 25 | ||
| FVC % predicted preB2 < 100 | 143 | 98 (86.7) | 45 (76.3) | 1.32 (0.91–1.92) | 0.14 |
| FVC % predicted preB2 ≥ 100 | 29 | 15 (13.3) | 14 (23.7) | ||
| Missing | 68 | 43 | 25 | ||
| Quality of life, AQLQ(S) pre-bushfire visit† | 198 | 5.1 (4.1, 6.1) | 6.1 (5.1, 6.8) | 0.83 (0.77–0.90) | <0.001 |
| Missing | 42 | 29 | 13 | ||
| Asthma triggers total * | 225 | 5.8 (2.1) | 5.0 (2.2) | 1.06 (1.01–1.10) | 0.010 |
| Missing | 15 | 8 | 7 | ||
| Variables | Persistent Symptoms | ||||
| (a). | mAb User | mAb Non-User | |||
| aRR | p-Value | aRR | p-Value | P for Interaction | |
| Fire day > 41 days | 1.09 (0.84–1.42) | 0.51 | 0.65 (0.47–0.90) | 0.009 | 0.015 |
| Consecutive fire day (>10 days) | 1.08 (0.83–1.40) | 0.59 | 0.77 (0.53–1.13) | 0.18 | 0.24 |
| Mean PM2.5 (>16 µg/m3) | 0.90 (0.69–1.17) | 0.44 | 0.67 (0.48–0.95) | 0.023 | 0.33 |
| Peak PM2.5 (>115 µg/m3) | 0.77 (0.60–0.99) | 0.046 | 1.69 (1.26–2.26) | <0.001 | <0.001 |
| Variables | Persistent symptoms | ||||
| (b). | Controlled asthma | Uncontrolled asthma | |||
| aRR | p-value | aRR | p-value | P for Interaction | |
| Fire day > 41 days | 1.25 (0.87–1.79) | 0.23 | 0.84 (0.64–1.10) | 0.21 | 0.07 |
| Consecutive fire day (>10 days) | 1.18 (0.83–1.68) | 0.36 | 1.0 (0.79–1.26) | 0.99 | 0.26 |
| Mean PM2.5 (>16 µg/m3) | 1.0 (0.71–1.40) | 0.99 | 0.86 (0.67–1.11) | 0.25 | 0.44 |
| Peak PM2.5 (>115 µg/m3) | 0.66 (0.45–0.96) | 0.031 | 1.15 (0.91–1.46) | 0.24 | 0.009 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Beyene, T.; Harvey, E.S.; Van Buskirk, J.; McDonald, V.M.; Jensen, M.E.; Horvat, J.C.; Morgan, G.G.; Zosky, G.R.; Jegasothy, E.; Hanigan, I.; et al. ‘Breathing Fire’: Impact of Prolonged Bushfire Smoke Exposure in People with Severe Asthma. Int. J. Environ. Res. Public Health 2022, 19, 7419. https://doi.org/10.3390/ijerph19127419
Beyene T, Harvey ES, Van Buskirk J, McDonald VM, Jensen ME, Horvat JC, Morgan GG, Zosky GR, Jegasothy E, Hanigan I, et al. ‘Breathing Fire’: Impact of Prolonged Bushfire Smoke Exposure in People with Severe Asthma. International Journal of Environmental Research and Public Health. 2022; 19(12):7419. https://doi.org/10.3390/ijerph19127419
Chicago/Turabian StyleBeyene, Tesfalidet, Erin S. Harvey, Joseph Van Buskirk, Vanessa M. McDonald, Megan E. Jensen, Jay C. Horvat, Geoffrey G. Morgan, Graeme R. Zosky, Edward Jegasothy, Ivan Hanigan, and et al. 2022. "‘Breathing Fire’: Impact of Prolonged Bushfire Smoke Exposure in People with Severe Asthma" International Journal of Environmental Research and Public Health 19, no. 12: 7419. https://doi.org/10.3390/ijerph19127419
APA StyleBeyene, T., Harvey, E. S., Van Buskirk, J., McDonald, V. M., Jensen, M. E., Horvat, J. C., Morgan, G. G., Zosky, G. R., Jegasothy, E., Hanigan, I., Murphy, V. E., Holliday, E. G., Vertigan, A. E., Peters, M., Farah, C. S., Jenkins, C. R., Katelaris, C. H., Harrington, J., Langton, D., ... Gibson, P. G. (2022). ‘Breathing Fire’: Impact of Prolonged Bushfire Smoke Exposure in People with Severe Asthma. International Journal of Environmental Research and Public Health, 19(12), 7419. https://doi.org/10.3390/ijerph19127419






