Impact of Dietary Patterns on Plaque Acidogenicity and Dental Caries in Early Childhood: A Retrospective Analysis in Japan
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants and Procedures
2.2. Outcome Variables
2.3. Dietary Data
2.4. Data Analysis
3. Results
3.1. Clinical and Demographic Data
3.2. Food and Drink Frequencies
3.3. Dietary Cariogenicity
3.4. Logistic Regression
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Marsh, P.D. Are dental diseases examples of ecological catastrophes? Microbiology 2003, 149, 279–294. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Guideline: Sugars Intake for Adults and Children; World Health Organization: Geneva, Switzerland, 2015. [Google Scholar]
- Touger-Decker, R.; van Loveren, C. Sugars and dental caries. Am. J. Clin. Nutr. 2003, 78, 881S–892S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anderson, C.A.; Curzon, M.E.J.; van Loveren, C.; Tatsi, C.; Duggal, M.S. Sucrose and dental caries: A review of the evidence. Obes. Rev. 2009, 10 (Suppl. S1), 41–54. [Google Scholar] [CrossRef] [PubMed]
- Palmer, C.A.; Kent, R., Jr.; Loo, C.Y.; Hughes, C.V.; Stutius, E.; Pradhan, N.; Dahlan, M.; Kanasi, E.; Arevalo Vasquez, S.S.; Tanner, A.C.R. Diet and caries-associated bacteria in severe early childhood caries. J. Dent. Res. 2010, 89, 1224–1229. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.; Whelton, H.; Douglas, G.; Kang, J. Consumption frequency of added sugars and UK children’s dental caries. Community. Dent. Oral. Epidemiol. 2018, 46, 457–464. [Google Scholar] [CrossRef]
- Zeng, L.; Zeng, Y.; Zhou, Y.; Wen, J.; Wan, L.; Ou, X.; Zhou, X. Diet and lifestyle habits associated with caries in deciduous teeth among 3- to 5-year-old preschool children in Jiangxi Province, China. BMC Oral. Health 2018, 18, 224. [Google Scholar] [CrossRef] [Green Version]
- van Loveren, C. Sugar restriction for caries prevention: Amount and frequency. Which is more important? Caries Res. 2019, 53, 168–175. [Google Scholar] [CrossRef]
- Milgrom, P.; Riedy, C.A.; Weinstein, P.; Tanner, A.C.; Manibusan, L.; Bruss, J. Dental caries and its relationship to bacterial infection, hypoplasia, diet, and oral hygiene in 6- to 36-month-old children. Community Dent. Oral. Epidemiol. 2000, 28, 295–306. [Google Scholar] [CrossRef]
- Shinga-Ishihara, C.; Nakai, Y.; Milgrom, P.; Murakami, K.; Matsumoto-Nakano, M. Cross-cultural validity of a dietary questionnaire for studies of dental caries risk in Japanese. BMC Oral. Health 2014, 14, 1. [Google Scholar] [CrossRef] [Green Version]
- García-Closas, R.; García-Closas, M.; Serra-Majem, L. A cross-sectional study of dental caries, intake of confectionery and foods rich in starch and sugars, and salivary counts of Streptococcus mutans in children in Spain. Am. J. Clin. Nutr. 1997, 66, 1257–1263. [Google Scholar] [CrossRef] [Green Version]
- Marsh, P.D.; Martin, V.M. Oral Microbiology, 6th ed.; Churchill Livingstone Elsevier: London, UK, 2016; pp. 115–127. [Google Scholar]
- Becker, M.R.; Paster, B.J.; Leys, E.J.; Moeschberger, M.L.; Kenyon, S.G.; Galvin, J.L.; Boches, S.K.; Dewhirst, F.E.; Griffen, A.L. Molecular analysis of bacterial species associated with childhood caries. J. Clin. Microbiol. 2002, 40, 1001–1009. [Google Scholar] [CrossRef] [Green Version]
- Aas, J.A.; Griffen, A.L.; Dardis, S.R.; Lee, A.M.; Olsen, I.; Dewhirst, F.E.; Leys, E.J.; Paster, B.J. Bacteria of dental caries in primary and permanent teeth in children and young adults. J. Clin. Microbiol. 2008, 46, 1407–1417. [Google Scholar] [CrossRef] [Green Version]
- Nakajo, K.; Takahashi, N.; Beighton, D. Resistance to acidic environments of caries-associated bacteria: Bifidobacterium dentium and Bifidobacterium longum. Caries Res. 2010, 44, 431–437. [Google Scholar] [CrossRef]
- Marsh, P.D. Dental plaque as a biofilm and a microbial community—Implications for health and disease. BMC Oral Health 2006, 6, S14. [Google Scholar] [CrossRef] [Green Version]
- Vandenbroucke, J.P.; von Elm, E.; Altman, D.G.; Gøtzsche, P.C.; Mulrow, C.D.; Pocock, S.J.; Poole, C.; Schlesselman, J.J.; Egger, M. STROBE Initiative. Strengthening the reporting of observational studies in epidemiology (STROBE): Explanation and elaboration. Epidemiology 2007, 18, 805–835. [Google Scholar] [CrossRef] [Green Version]
- Tsubouchi, J.; Yamamoto, S.; Shimono, T.; Domoto, P.K. A longitudinal assessment of predictive value of a caries activity test in young children. ASDC J. Dent. Child. 1995, 62, 34–37. [Google Scholar]
- Koroluk, L.; Hoover, J.N.; Komiyama, K. The sensitivity and specificity of a colorimetric microbiological caries activity test (Cariostat) in preschool children. Pediatr. Dent. 1994, 16, 276–281. [Google Scholar]
- Weinstein, P.; Smith, W.F.; Fraser-Lee, N.; Shimono, T.; Tsubouchi, J. Epidemiologic study of 19-month-old Edmonton, Alberta children: Caries rates and risk factors. ASDC J. Dent. Child. 1996, 63, 426–433. [Google Scholar]
- Abdelaziz, W.E.; Dowidar, K.M.; El Tantawi, M.M. Association of healthy eating, juice consumption, and bacterial counts with early childhood caries. Pediatr. Dent. 2015, 37, 462–467. [Google Scholar]
- World Health Organization. Oral Health Surveys: Basic Methods, 5th ed.; World Health Organization: Geneva, Switzerland, 2013. [Google Scholar]
- Drury, T.F.; Horowits, A.M.; Ismail, A.I.; Maertens, M.P.; Rozier, R.G.; Selwitz, R.H. Diagnosing and reporting early childhood caries for research purposes. A report of a workshop sponsored by the National Institute of Dental and Craniofacial Research, the Health Resources and Services Administration, and the Health Care Financing Administration. J. Public Health Dent. 1999, 59, 192–197. [Google Scholar]
- Papas, A.S.; Palmer, C.A.; Rounds, M.C.; Herman, J.; McGandy, R.B.; Hartz, S.C.; Russell, R.M.; DePaola, P. Longitudinal relationships between nutrition and oral health. Ann. N. Y. Acad. Sci. 1989, 561, 124–142. [Google Scholar] [CrossRef] [PubMed]
- Organization for Economic Cooperation and Development. Sugar Projections: Consumption, Food. In OECD-FAO Agricultural Outlook 2017–2026; Table A.14.2; OECD Publishing: Paris, France, 2017; Available online: https://doi.org/10.1787/agr_outlook-2017-table147-en (accessed on 13 June 2022).
- Marshall, T.A.; Broffitt, B.; Eichenberger-Gilmore, J.; Warren, J.J.; Cunningham, M.A.; Levy, S.M. The roles of meal, snack, and daily total food and beverage exposures on caries experience in young children. J. Public Health Dent. 2005, 65, 166–173. [Google Scholar] [CrossRef] [PubMed]
- Arcella, D.; Ottolenghi, L.; Polimeni, A.; Leclercq, C. The relationship between frequency of carbohydrates intake and dental caries: A cross-sectional study in Italian teenagers. Public Health Nutr. 2002, 5, 553–560. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dusseldorp, E.; Kamphuis, M.; Schuller, A. Impact of lifestyle factors on caries experience in three different age groups: 9, 15, and 21-year-olds. Community Dent. Oral. Epidemiol. 2015, 43, 9–16. [Google Scholar] [CrossRef]
- Bowen, W.H.; Lawrence, R.A. Comparison of the cariogenicity of Cola, honey, cow milk, human milk, and sucrose. Pediatrics 2005, 116, 921–926. [Google Scholar] [CrossRef]
- Peres, R.C.; Coppi, L.C.; Volpato, M.C.; Groppo, F.C.; Cury, J.A.; Rosalen, P.L. Cariogenic potential of cows’, human and infant formula milks and effect of fluoride supplementation. Br. J. Nutr. 2009, 101, 376–382. [Google Scholar] [CrossRef] [Green Version]
- Sheikh, C.; Erickson, P.R. Evaluation of plaque pH changes following oral rinse with eight infant formulas. Pediatr. Dent. 1996, 18, 200–204. [Google Scholar]
- Tan, S.F.; Tong, H.J.; Lin, X.Y.; Mok, B.; Hong, C.H. The cariogenicity of commercial infant formulas: A systematic review. Eur. Arch. Paediatr. Dent. 2016, 17, 145–156. [Google Scholar] [CrossRef]
- Avila, W.M.; Pordeus, I.A.; Paiva, S.M.; Martins, C.C. Breast and bottle feeding as risk factors for dental caries: A systematic review and meta-analysis. PLoS ONE 2015, 10, e0142922. [Google Scholar] [CrossRef]
- Peres, K.G.; Nascimento, G.G.; Peres, M.A.; Mittinty, M.N.; Demarco, F.F.; Santos, I.S.; Matijasevich, A.; Barros, A.J.D. Impact of prolonged breastfeeding on dental caries: A population-based birth cohort study. Pediatrics 2017, 140, e20162943. [Google Scholar] [CrossRef] [Green Version]
- Peres, K.G.; Chaffee, B.W.; Feldens, C.A.; Flores-Mir, C.; Moynihan, P.; Rugg-Gunn, A. Breastfeeding and oral health: Evidence and methodological challenges. J. Dent. Res. 2018, 97, 251–258. [Google Scholar] [CrossRef] [Green Version]
- Bingham, S.A.; Gill, C.; Welch, A.; Day, K.; Cassidy, A.; Khaw, K.T.; Sneyd, M.J.; Key, T.J.; Roe, L.; Day, N.E. Comparison of dietary assessment methods in nutritional epidemiology: Weighed records v. 24 h recalls, food-frequency questionnaires and estimated-diet records. Br. J. Nutr. 1994, 72, 619–643. [Google Scholar] [CrossRef] [Green Version]
- Burrows, T.L.; Martin, R.J.; Collins, C.E. A systematic review of the validity of dietary assessment methods in children when compared with the method of doubly labeled water. J. Am. Diet. Assoc. 2010, 110, 1501–1510. [Google Scholar] [CrossRef]
- Evans, E.W.; Hayes, C.; Palmer, C.A.; Bermudez, O.I.; Naumova, E.N.; Cohen, S.A.; Must, A. Development of a pediatric cariogenicity index. J. Public Health Dent. 2013, 73, 179–186. [Google Scholar] [CrossRef]
- Feldens, C.A.; Rodrigues, P.H.; Rauber, F.; Chaffee, B.W.; Vitolo, M.R. Food expenditures, cariogenic dietary practices and childhood dental caries in southern Brazil. Caries Res. 2013, 47, 373–381. [Google Scholar] [CrossRef]
- Hirakawa, T. Dental fear in Japanese school children. Jpn. J. Pediatr. Dent. 2003, 41, 843–854. [Google Scholar]


| Total Sample (n = 118) | Caries Status | p-Value | Plaque Acidogenicity | p-Value | |||
|---|---|---|---|---|---|---|---|
| ECC (n = 30) | Caries-Free (n = 88) | High (n = 51) | Low (n = 67) | ||||
| mean ± SD | mean ± SD | mean ± SD | |||||
| Age (years) | 1.76 ± 0.97 | 2.7 ± 1.1 | 1.4 ± 0.7 | <0.001 | 2.0 ± 1.0 | 1.6 ± 0.9 | 0.01 |
| No. of erupted teeth | 15.1 ± 5.1 | 18.1 ± 3.3 | 14.1 ± 5.2 | <0.001 | 16.2 ± 4.4 | 14.2 ± 5.4 | 0.03 |
| n (%) | n (%) | n (%) | |||||
| Sex | |||||||
| Girl | 59 (50) | 14 (46.7) | 45 (51.1) | 0.83 | 22 (43.1) | 37 (55.2) | 0.27 |
| Boy | 59 (50) | 16 (53.3) | 43 (48.9) | 29 (56.9) | 30 (44.8) | ||
| Total Sample (n = 118) | Caries Status | p | Plaque Acidogenicity | p | |||
|---|---|---|---|---|---|---|---|
| ECC (n = 30) | Caries-Free (n = 88) | High (n = 51) | Low (n = 67) | ||||
| Mean ± SD | Mean ± SD | Mean ± SD | |||||
| Food intake for 3 days of survey | |||||||
| Caries-protective (cario 00) | 4.5 ± 4.6 | 4.2 ± 4.4 | 4.6 ± 4.6 | 0.65 | 4.6 ± 4.7 | 4.5 ± 4.5 | 0.83 |
| Non-cariogenic (cario 0) | 9.4 ± 4.9 | 11.2 ± 5.6 | 8.8 ± 4.5 | 0.02 | 9.4 ± 5.0 | 9.5 ± 4.9 | 0.92 |
| Low cariogenic (cario 1) | 19.5 ± 7.4 | 19.0 ± 7.2 | 19.6 ± 7.5 | 0.71 | 19.1 ± 7.5 | 19.8 ± 7.4 | 0.61 |
| Liquids (cario 2) | 4.6 ± 3.6 | 6.1 ± 3.9 | 4.1 ± 3.6 | 0.009 | 5.1 ± 3.7 | 4.2 ± 3.5 | 0.17 |
| Solid/retentive food (cario 3) | 8.3 ± 3.4 | 8.0 ± 4.1 | 8.4 ± 3.1 | 0.52 | 8.5 ± 4.0 | 8.2 ± 2.9 | 0.60 |
| Total food and drink items consumed | 46.3 ± 11.5 | 48.5 ± 12.8 | 45.6 ± 11.0 | 0.23 | 46.7 ± 11.9 | 46.0 ± 11.3 | 0.760 |
| Dietary cariogenicity score a | 95.4 ± 23.0 | 99.4 ± 27.5 | 94.0 ± 21.2 | 0.27 | 96.9 ± 25.7 | 94.2 ± 20.8 | 0.540 |
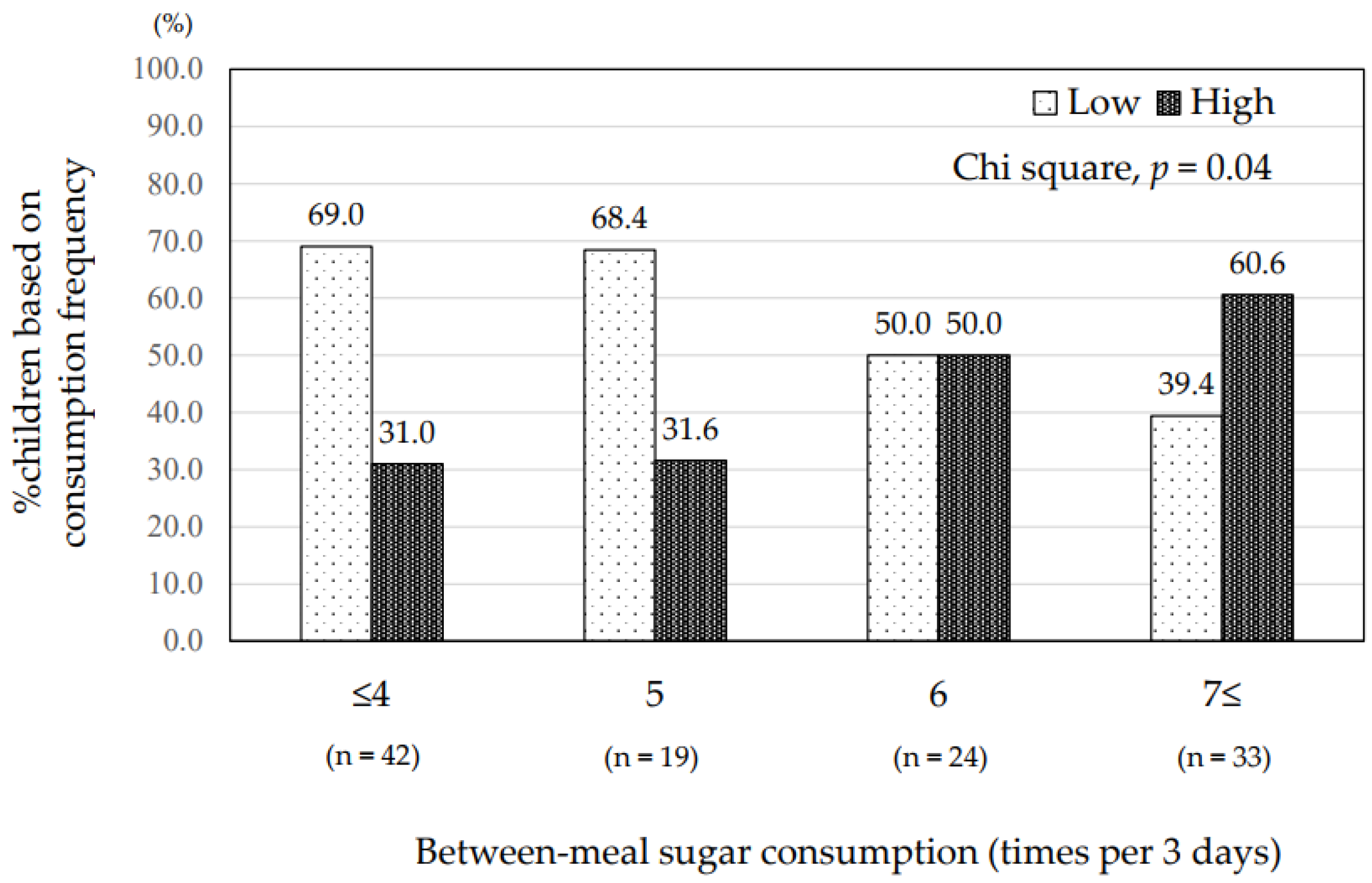
| Frequency of between-meal sugar consumption | 5.7 ± 3.2 | 7.6 ± 4.1 | 5.0 ± 2.6 | 0.002 | 6.7 ± 3.8 | 4.9 ± 2.5 | 0.006 |
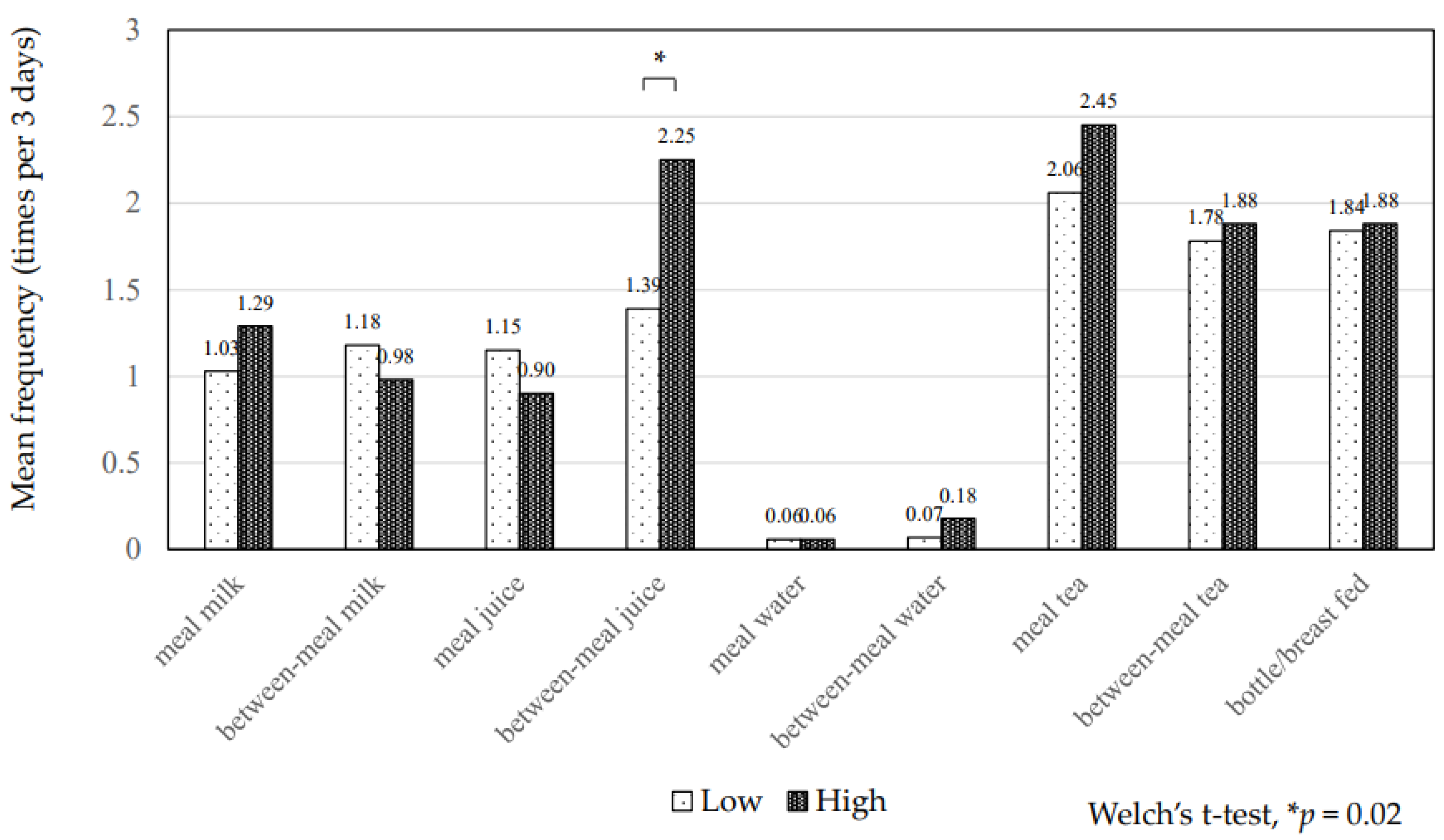
| Frequency of breast/bottle fed | 1.9 ± 4.7 | 1.8 ± 5.7 | 1.9 ± 4.3 | 0.904 | 1.9 ± 5.5 | 1.8 ± 3.9 | 0.957 |
| Predictive Variables | Outcome Variables | |||||
|---|---|---|---|---|---|---|
| ECC | High Plaque Acidogenicity | |||||
| Adjusted OR | 95% CI | p-Value | Adjusted OR | 95% CI | p-Value | |
| Frequency of between-meal sugar | ||||||
| ≤6 times per 3 days | 1 | reference | 1 | reference | ||
| >6 times per 3 days | 4.2 | 1.1–15.8 | 0.03 | 3.9 | 1.3–11.1 | 0.01 |
| Frequency of breast/bottle fed | ||||||
| <8 times per 3 days | 1 | reference | 1 | reference | ||
| ≥8 times per 3 days | 10.7 | 1.1–102.6 | 0.04 | 3.40 | 0.6–20.4 | 0.18 |
| Dietary cariogenicity score | ||||||
| <110 per 3 days | 1 | reference | 1 | reference | ||
| ≥110 per 3 days | 0.6 | 0.2–2.4 | 0.50 | 0.38 | 0.1–1.1 | 0.08 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nakai, Y.; Mori-Suzuki, Y. Impact of Dietary Patterns on Plaque Acidogenicity and Dental Caries in Early Childhood: A Retrospective Analysis in Japan. Int. J. Environ. Res. Public Health 2022, 19, 7245. https://doi.org/10.3390/ijerph19127245
Nakai Y, Mori-Suzuki Y. Impact of Dietary Patterns on Plaque Acidogenicity and Dental Caries in Early Childhood: A Retrospective Analysis in Japan. International Journal of Environmental Research and Public Health. 2022; 19(12):7245. https://doi.org/10.3390/ijerph19127245
Chicago/Turabian StyleNakai, Yukie, and Yukako Mori-Suzuki. 2022. "Impact of Dietary Patterns on Plaque Acidogenicity and Dental Caries in Early Childhood: A Retrospective Analysis in Japan" International Journal of Environmental Research and Public Health 19, no. 12: 7245. https://doi.org/10.3390/ijerph19127245
APA StyleNakai, Y., & Mori-Suzuki, Y. (2022). Impact of Dietary Patterns on Plaque Acidogenicity and Dental Caries in Early Childhood: A Retrospective Analysis in Japan. International Journal of Environmental Research and Public Health, 19(12), 7245. https://doi.org/10.3390/ijerph19127245






