The Effects of Physical-Chemical Evolution of High-Sulfur Petroleum Coke on Hg0 Removal from Coal-Fired Flue Gas and Exploration of Its Micro-Scale Mechanism
Abstract
1. Introduction
2. The Effect of the Evolution of Pore Structure on Hg0 Removal
3. The Effect of the Evolution of Functional Groups on Hg0 Removal
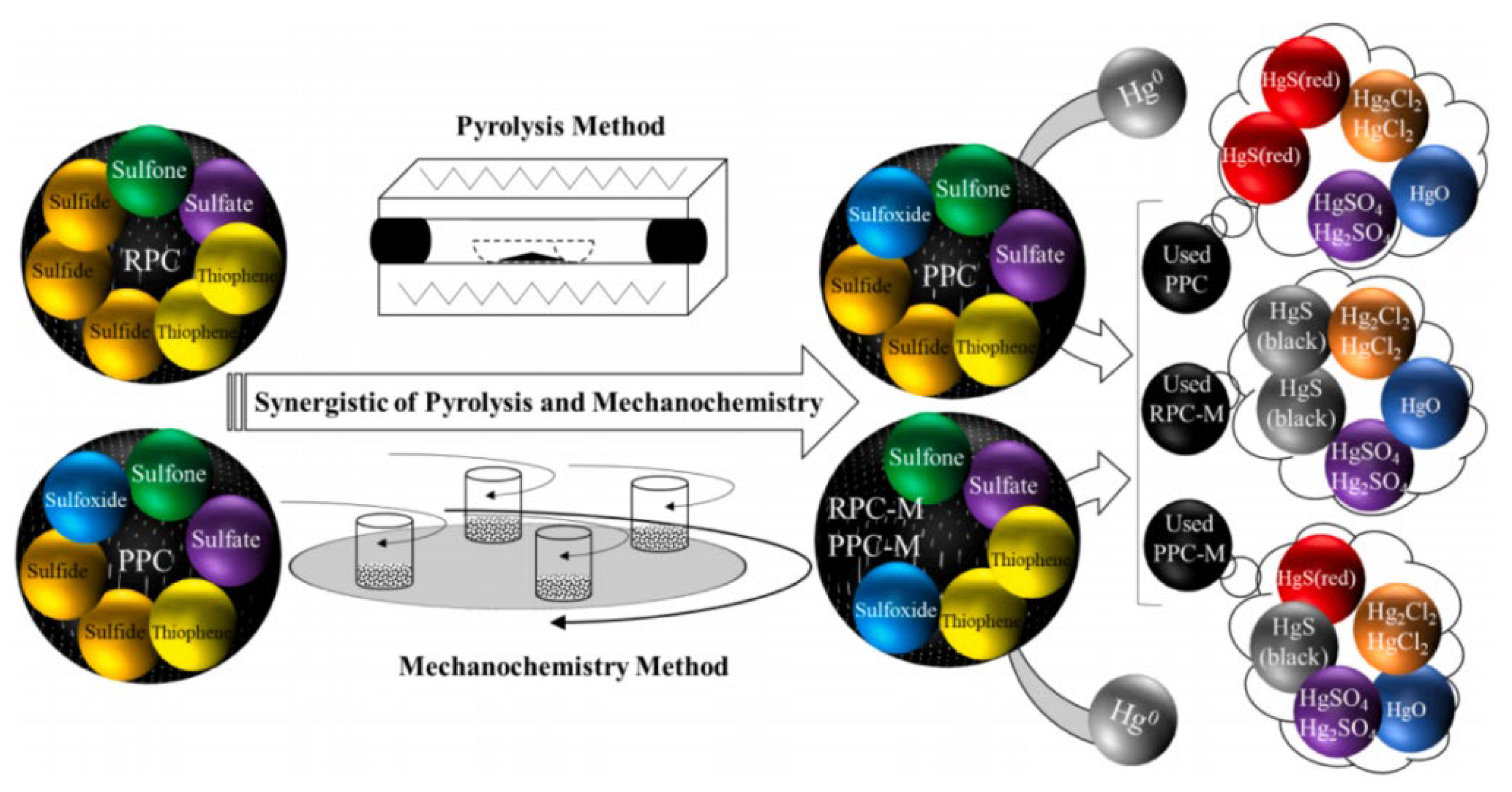
3.1. Sulfur-Containing Functional Groups for Hg0 Removal in HSPC
3.1.1. The Identification of Inherent Sulfur in HSPC
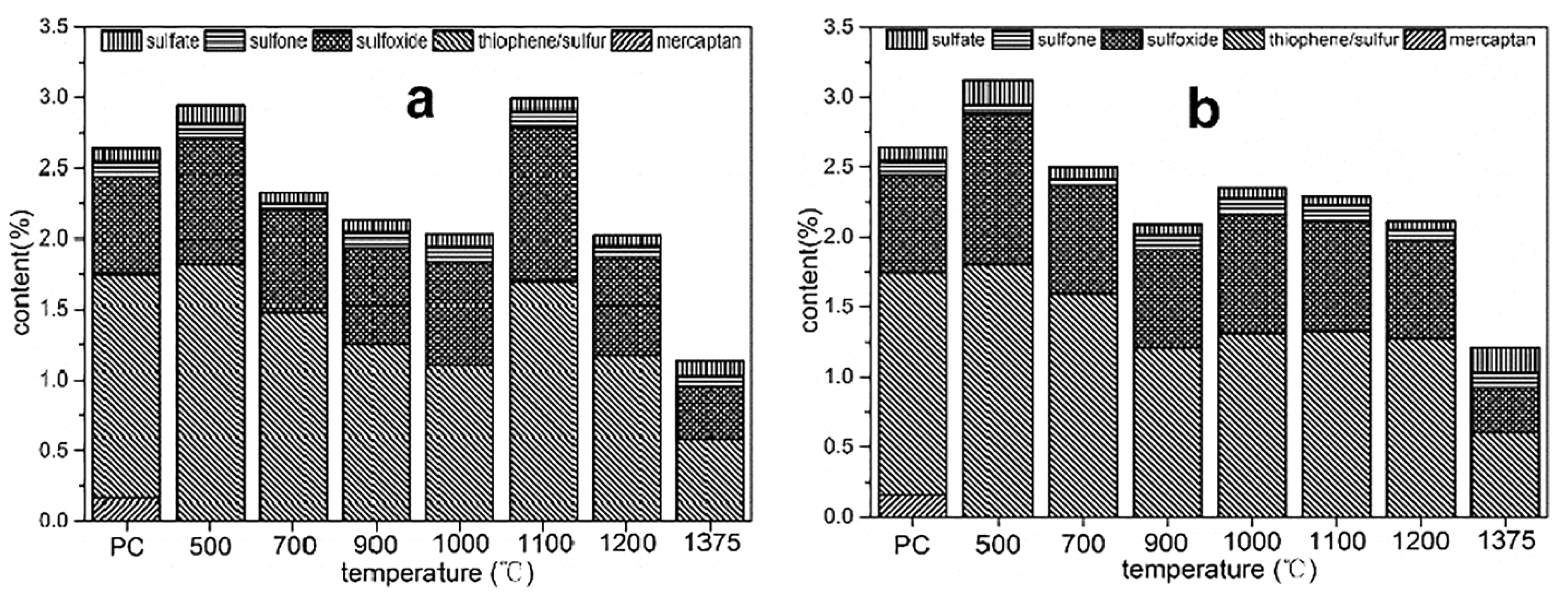
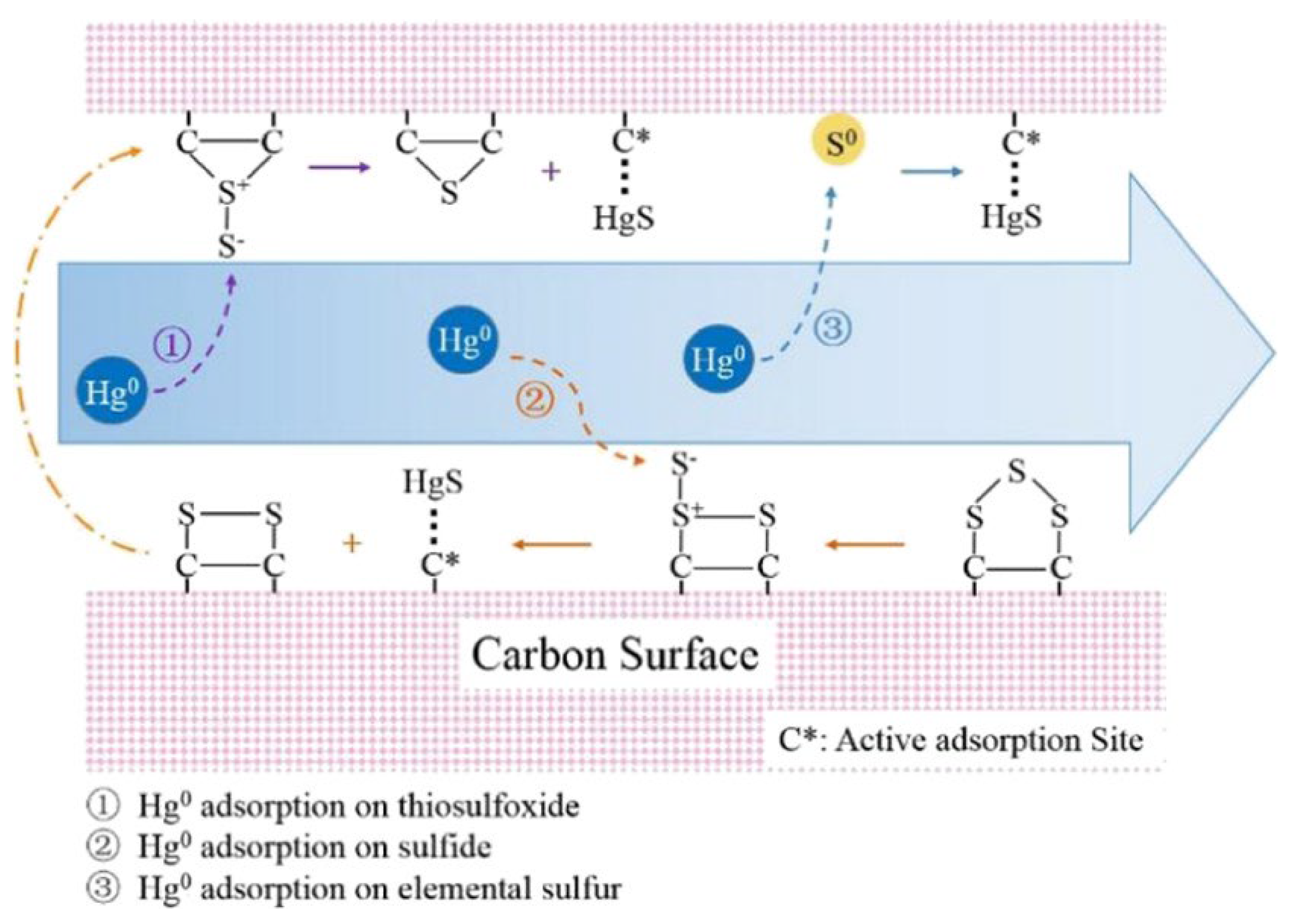
3.1.2. The Evolution and Contribution of Sulfur in HSPC
3.2. Oxygen-Containing Functional Groups for Hg0 Removal
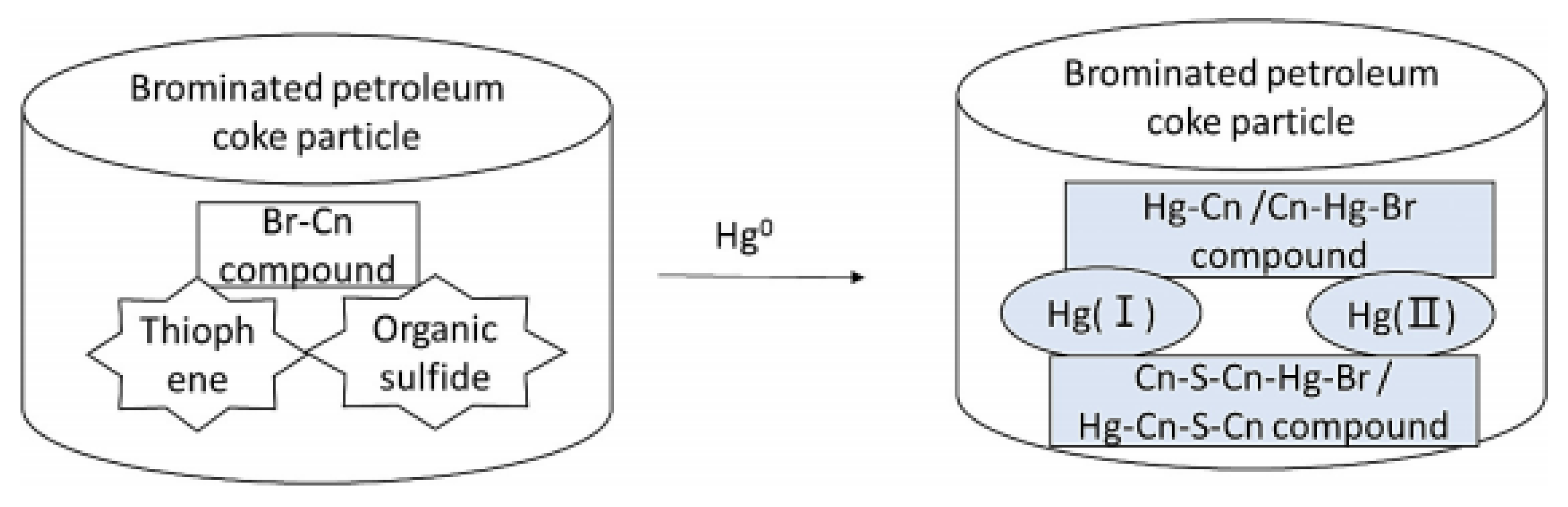
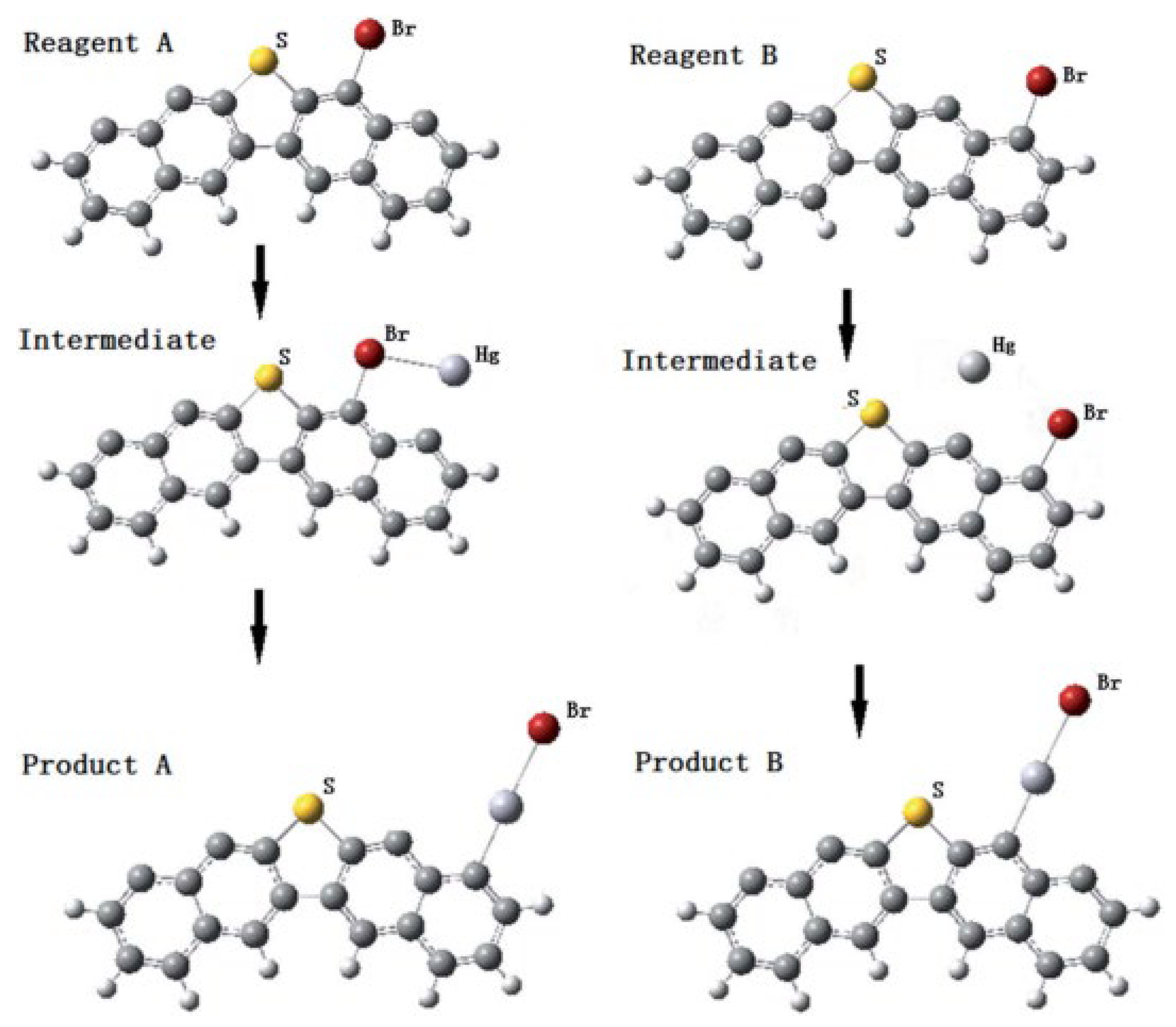
3.3. Bromine-Containing Functional Groups for Hg0 Removal
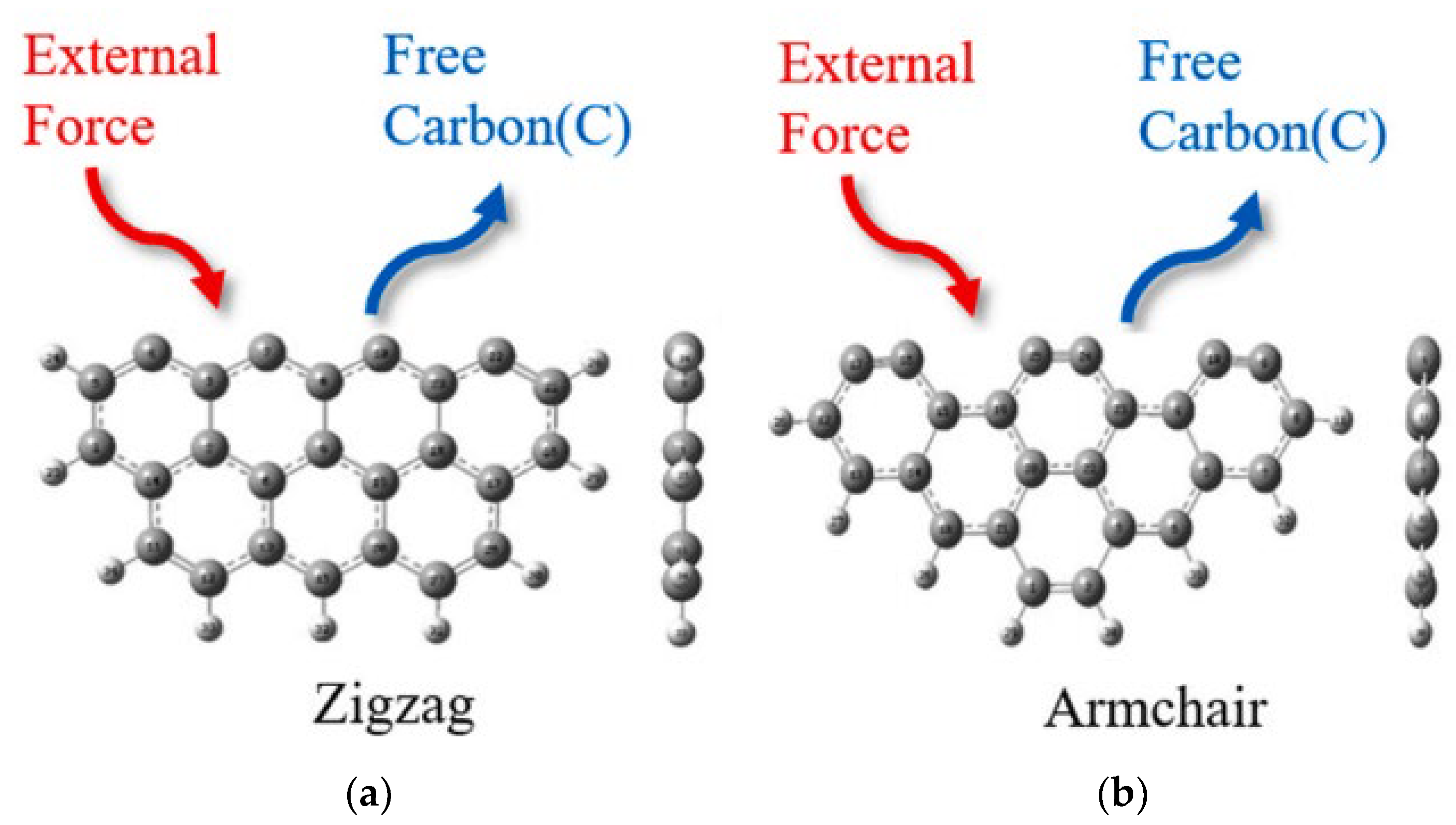
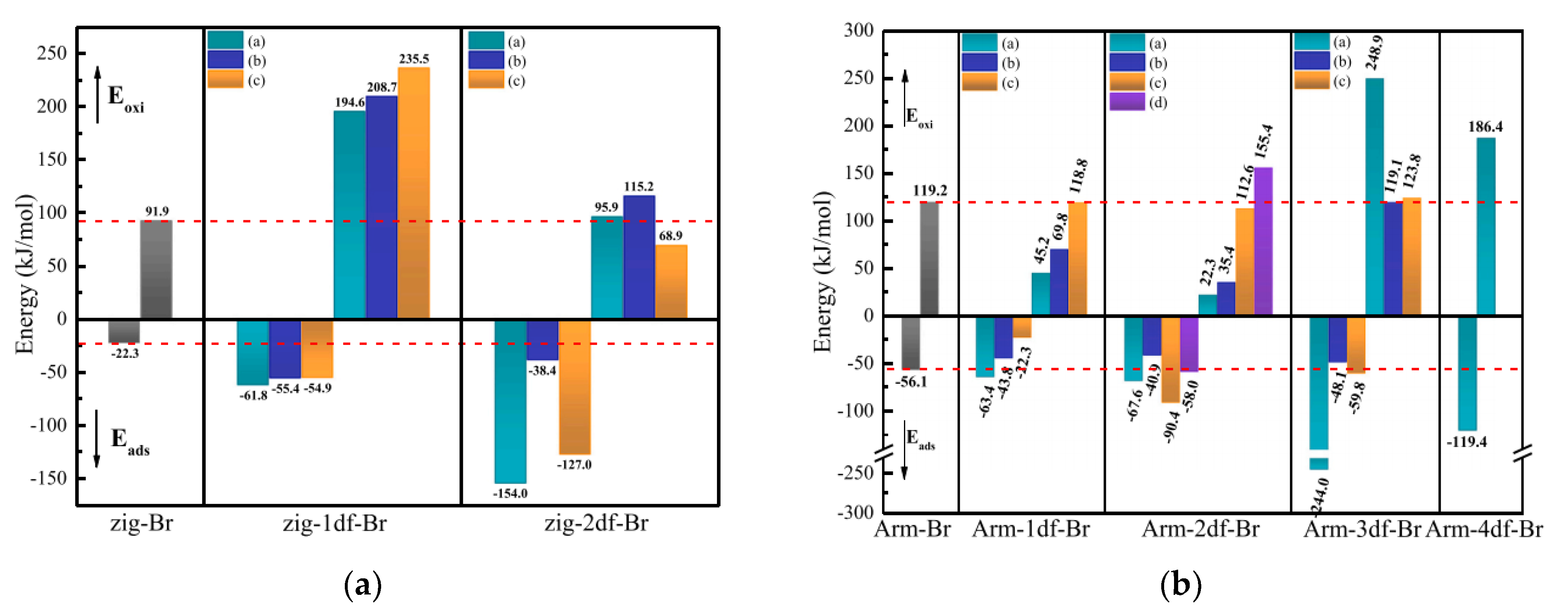
4. Micro-Scale Mechanism of Physical-Chemical Evolution of HSPC Activation and Hg0 Removal
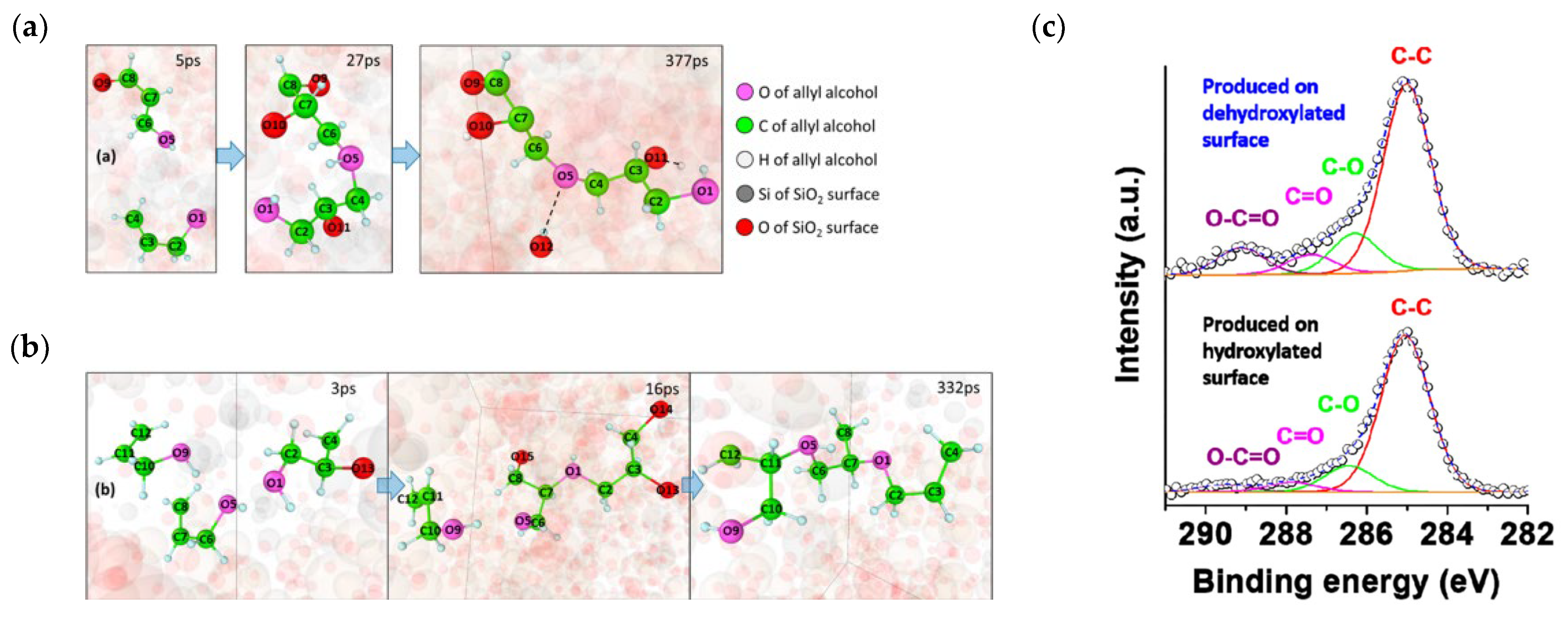
4.1. DFT Study
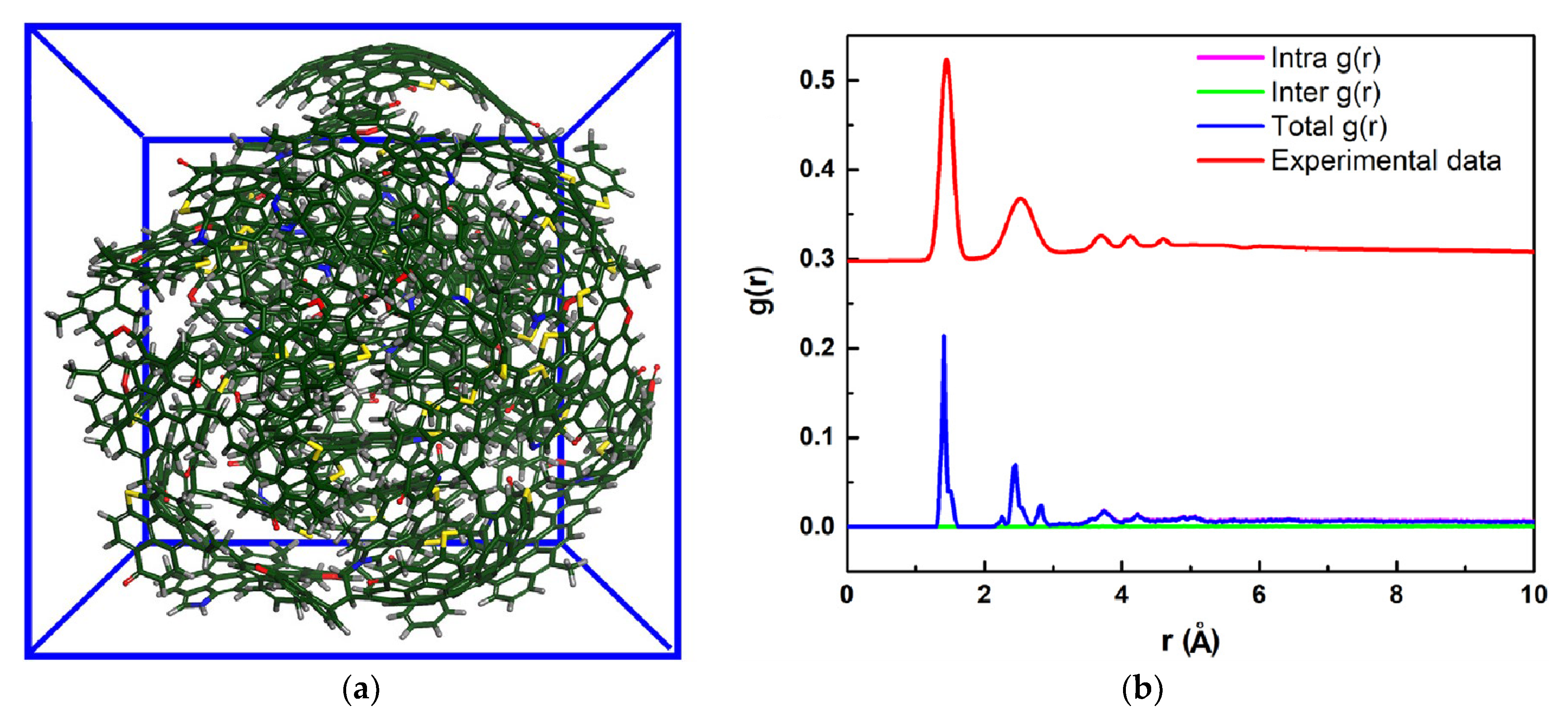
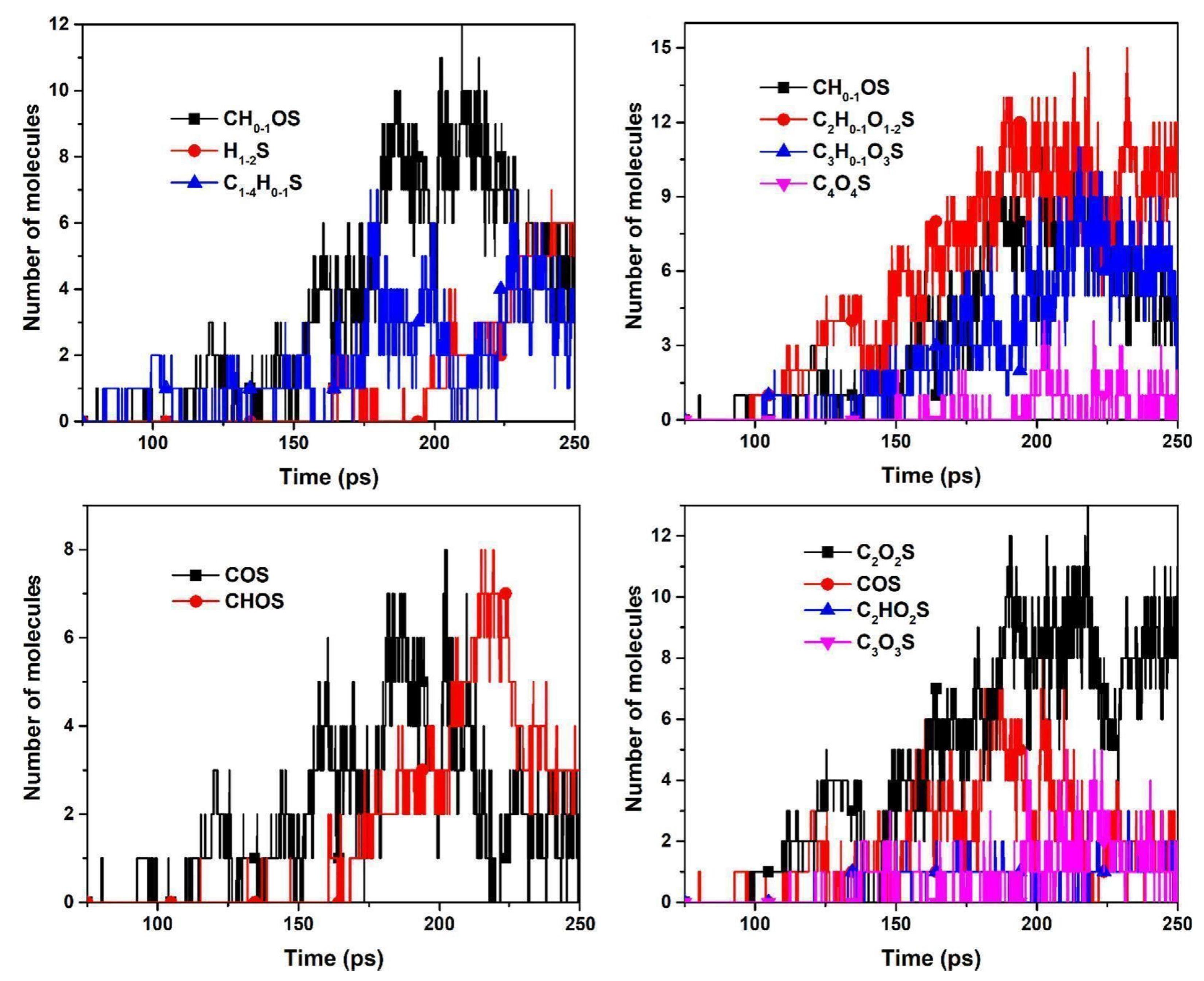
4.2. ReaxFF Study
5. Conclusions and Further Work
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wang, M.; Wan, Y.; Guo, Q.; Bai, Y.; Yu, G.; Liu, Y.; Zhang, H.; Zhang, S.; Wei, J. Brief review on petroleum coke and biomass/coal co-gasification: Syngas production, reactivity characteristics, and synergy behavior. Fuel 2021, 304, 121517. [Google Scholar] [CrossRef]
- Cai, W.; Li, K.; Jiang, K.; Lv, D.; Liu, Y.-Q.; Wang, D.; Wang, X.; Lai, C. Utilization of high-sulfur-containing petroleum coke for making sulfur-doped porous carbon composite material and its application in supercapacitors. Diam. Relat. Mater. 2021, 116, 108380. [Google Scholar] [CrossRef]
- Tyutrin, A.A.; Burdonov, A.E.; Bushuev, K.S. Expanding the Application Scope of Fine Dust from Petroleum Coke Calcining Furnaces in Aluminum Production. Mater. Sci. Forum 2022, 1052, 482–487. [Google Scholar] [CrossRef]
- Veluri, P.S.; Katchala, N.; Anandan, S.; Pramanik, M.; NarayanSrinivasan, K.; Ravi, B.; Rao, N.T. Petroleum Coke as an Efficient Single Carbon Source for High-Energy and High-Power Lithium-Ion Capacitors. Energy Fuels 2021, 35, 9010–9016. [Google Scholar] [CrossRef]
- Liu, L.; Li, Z.; Wu, S.; Li, D.; Cai, N. Conversion characteristics of lignite and petroleum coke in chemical looping combustion coupled with an annular carbon stripper. Fuel Process. Technol. 2021, 213, 106711. [Google Scholar] [CrossRef]
- Shan, Y.; Guan, D.; Meng, J.; Liu, Z.; Schroeder, H.; Liu, J.; Mi, Z. Rapid growth of petroleum coke consumption and its related emissions in China. Appl. Energy 2018, 226, 494–502. [Google Scholar] [CrossRef]
- Andrews, A.; Lattanzio, R.K. Petroleum Coke: Industry and Environmental Issues; Congressional Research Service: Washington DC, USA, 2013.
- Mackey, T.K.; Contreras, J.T.; Liang, B.A. The Minamata Convention on Mercury: Attempting to address the global controversy of dental amalgam use and mercury waste disposal. Sci. Total Environ. 2014, 472, 125–129. [Google Scholar] [CrossRef]
- Wang, L.; Hou, D.; Cao, Y.; Ok, Y.S.; Tack, F.M.; Rinklebe, J.; O’Connor, D. Remediation of mercury contaminated soil, water, and air: A review of emerging materials and innovative technologies. Environ. Int. 2020, 134, 105281. [Google Scholar] [CrossRef]
- Streets, D.G.; Devane, M.K.; Lu, Z.; Bond, T.C.; Sunderland, E.M.; Jacob, D.J. All-time releases of mercury to the atmosphere from human activities. Environ. Sci. Technol. 2011, 45, 10485–10491. [Google Scholar] [CrossRef]
- Liu, K.; Wang, S.; Wu, Q.; Wang, L.; Ma, Q.; Zhang, L.; Li, G.; Tian, H.; Duan, L.; Hao, J. A highly resolved mercury emission inventory of Chinese coal-fired power plants. Environ. Sci. Technol. 2018, 52, 2400–2408. [Google Scholar] [CrossRef]
- Zhao, S.; Pudasainee, D.; Duan, Y.; Gupta, R.; Liu, M.; Lu, J. A review on mercury in coal combustion process: Content and occurrence forms in coal, transformation, sampling methods, emission and control technologies. Prog. Energy Combust. Sci. 2019, 73, 26–64. [Google Scholar] [CrossRef]
- Li, Y.; Yu, J.; Liu, Y.; Huang, R.; Wang, Z.; Zhao, Y. A Review on Removal of Mercury from Flue Gas Utilizing Existing Air Pollutant Control Devices (APCDs). J. Hazard. Mater. 2022, 427, 128132. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Wu, Q.; Xu, L.; Wen, M.; Liu, K.; Tang, Y.; Zou, J.; Wang, F.; Wang, Y.; Wang, S. A review on adsorption technologies for mercury emission control. Bull. Environ. Contam. Toxicol. 2019, 103, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Beckers, F.; Rinklebe, J. Cycling of mercury in the environment: Sources, fate, and human health implications: A review. Crit. Rev. Environ. Sci. Technol. 2017, 47, 693–794. [Google Scholar] [CrossRef]
- Sasmaz, E.; Kirchofer, A.; Jew, A.D.; Saha, A.; Abram, D.; Jaramillo, T.F.; Wilcox, J. Mercury chemistry on brominated activated carbon. Fuel 2012, 99, 188–196. [Google Scholar] [CrossRef]
- Hu, J.; Geng, X.; Duan, Y.; Zhao, W.; Zhu, M.; Ren, S. Effect of mechanical–chemical modification process on mercury removal of bromine modified fly ash. Energy Fuels 2020, 34, 9829–9839. [Google Scholar] [CrossRef]
- Ochedi, F.O.; Liu, Y.; Hussain, A. A review on coal fly ash-based adsorbents for mercury and arsenic removal. J. Clean. Prod. 2020, 267, 122143. [Google Scholar] [CrossRef]
- Zhu, C.; Duan, Y.; Wu, C.-Y.; Zhou, Q.; She, M.; Yao, T.; Zhang, J. Mercury removal and synergistic capture of SO2/NO by ammonium halides modified rice husk char. Fuel 2016, 172, 160–169. [Google Scholar] [CrossRef]
- Zafari, R. Synthesis and Study of Modified-Nanocrystalline Cellulose Effective for SO2 Capture; University of Ottawa: Ottawa, ON, Canada, 2021. [Google Scholar]
- Liu, Z.; Adewuyi, Y.G.; Shi, S.; Chen, H.; Li, Y.; Liu, D.; Liu, Y. Removal of gaseous Hg0 using novel seaweed biomass-based activated carbon. Chem. Eng. J. 2019, 366, 41–49. [Google Scholar] [CrossRef]
- Yang, J.; Zhao, Y.; Zhang, J.; Zheng, C. Regenerable cobalt oxide loaded magnetosphere catalyst from fly ash for mercury removal in coal combustion flue gas. Env. Sci. Technol. 2014, 48, 14837–14843. [Google Scholar] [CrossRef]
- Liu, Z.; Liu, D.; Zhao, B.; Feng, L.; Ni, M.; Jin, J. Mercury Removal Based on Adsorption and Oxidation by Fly Ash: A Review. Energy Fuels 2020, 34, 11840–11866. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, Y.; Gu, Y.; Wang, J.; Yu, X.; Wang, T.; Sun, Z.; Romero, C.E.; Pan, W.-p. Coupling of bromide and on-line mechanical modified fly ash for mercury removal at a 1000 MW coal-fired power plant. Fuel 2019, 247, 179–186. [Google Scholar] [CrossRef]
- Graydon, J.W.; Zhang, X.; Kirk, D.W.; Jia, C.Q. Sorption and stability of mercury on activated carbon for emission control. J. Hazard. Mater. 2009, 168, 978–982. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Li, Y.; Shi, S.; Chen, H.; Shan, Y.; Liu, Y. Mercury removal from flue gas by magnetic iron-copper oxide modified porous char derived from biomass materials. Fuel 2019, 256, 115977. [Google Scholar] [CrossRef]
- Shen, F.; Liu, J.; Dong, Y.; Wu, D. Mercury removal by biomass-derived porous carbon: Experimental and theoretical insights into the effect of H2S. Chem. Eng. J. 2018, 348, 409–415. [Google Scholar] [CrossRef]
- Spessato, L.; Bedin, K.C.; Cazetta, A.L.; Souza, I.P.; Duarte, V.A.; Crespo, L.H.; Silva, M.C.; Pontes, R.M.; Almeida, V.C. KOH-super activated carbon from biomass waste: Insights into the paracetamol adsorption mechanism and thermal regeneration cycles. J. Hazard. Mater. 2019, 371, 499–505. [Google Scholar] [CrossRef]
- Chen, W.; Gong, M.; Li, K.; Xia, M.; Chen, Z.; Xiao, H.; Fang, Y.; Chen, Y.; Yang, H.; Chen, H. Insight into KOH activation mechanism during biomass pyrolysis: Chemical reactions between O-containing groups and KOH. Appl. Energy 2020, 278, 115730. [Google Scholar] [CrossRef]
- Wu, M.B.; Zha, Q.F.; Qiu, J.S.; Han, X.; Guo, Y.S.; Li, Z.F.; Yuan, A.J.; Sun, X. Preparation of porous carbons from petroleum coke by different activation methods. Fuel 2005, 84, 1992–1997. [Google Scholar] [CrossRef]
- Zhu, M.; Yan, Q.; Duan, Y.; Li, J.; Zhang, X.; Han, Z.; Meng, J.; Wang, S.; Chen, C.; Wei, H. Study on Preparation and Mercury Adsorption Characteristics of Columnar Sulfur-Impregnated Activated Petroleum Coke. Energy Fuels 2020, 34, 10740–10751. [Google Scholar] [CrossRef]
- She, M.; Jia, C.Q.; Duan, Y.; Zhu, C. Influence of Different Sulfur Forms on Gas-Phase Mercury Removal by SO2-Impregnated Porous Carbons. Energy Fuels 2020, 34, 2064–2073. [Google Scholar] [CrossRef]
- Shen, C.; Wang, H.; Shen, H.; Wu, J.; Zhu, Y.; Shi, W.; Zhang, X.; Ying, Z. NH4Br-Modified Biomass Char for Mercury Removal in a Simulated Oxy-fuel Atmosphere: Mechanism Analysis by X-ray Photoelectron Spectroscopy. Energy Fuels 2020, 34, 9872–9884. [Google Scholar] [CrossRef]
- Ma, A.; Zhao, S.; Luo, H.; Sun, Z.; Xie, X.; Liao, Y.; Liang, X.; Li, H. Mercury removal from coal-fired flue gas of high-sulfur petroleum coke activated by pyrolysis and mechanochemical method. Chem. Eng. J. 2022, 429, 132154. [Google Scholar] [CrossRef]
- Sun, Z.; Ma, A.; Zhao, S.; Luo, H.; Xie, X.; Liao, Y.; Liang, X. Research progress on petroleum coke for mercury removal from coal-fired flue gas. Fuel 2022, 309, 122084. [Google Scholar] [CrossRef]
- Lee, S.H.; Rhim, Y.J.; Cho, S.P.; Baek, J.I. Carbon-based novel sorbent for removing gas-phase mercury. Fuel 2006, 85, 219–226. [Google Scholar] [CrossRef]
- Huo, Q.; Wang, Y.; Chen, H.; Han, L.; Wang, J.; Bao, W.; Chang, L.; Xie, K. ZnS/AC sorbent derived from the high sulfur petroleum coke for mercury removal. Fuel Process. Technol. 2019, 191, 36–43. [Google Scholar] [CrossRef]
- Xiao, Y.; Pudasainee, D.; Gupta, R.; Xu, Z.; Diao, Y. Bromination of petroleum coke for elemental mercury capture. J. Hazard. Mater. 2017, 336, 232–239. [Google Scholar] [CrossRef]
- Fuente-Cuesta, A.; Diaz-Somoano, M.; Lopez-Anton, M.; Cieplik, M.; Fierro, J.; Martínez-Tarazona, M. Biomass gasification chars for mercury capture from a simulated flue gas of coal combustion. J. Environ. Manag. 2012, 98, 23–28. [Google Scholar] [CrossRef]
- Li, C.; Liu, X.; Zhou, Z.; Dai, Z.; Yang, J.; Wang, F. Effect of heat treatment on structure and gasification reactivity of petroleum coke. Int. J. Coal Sci. Technol. 2016, 3, 53–61. [Google Scholar] [CrossRef][Green Version]
- Zhao, S.; Luo, H.; Ma, A.; Xie, W.; Sun, K.; Sun, Z. Influence of pyrolysis conditions on the mercury removal characteristics and physicochemical properties of biomass coke. Fuel 2022, 313, 122979. [Google Scholar] [CrossRef]
- Hong, D.; Zhou, J.; Hu, C.; Zhou, Q.; Mao, J.; Qin, Q. Mercury removal mechanism of AC prepared by one-step activation with ZnCl2. Fuel 2019, 235, 326–335. [Google Scholar] [CrossRef]
- Zhao, J.; Dai, Y.; Xu, J.; Chen, S.; Xie, J. Synthesis and electrochemical characterization of mesoporous carbons prepared by chemical activation. J. Electrochem. Soc. 2008, 155, A475. [Google Scholar] [CrossRef]
- Adinata, D.; Daud, W.M.A.W.; Aroua, M.K. Preparation and characterization of activated carbon from palm shell by chemical activation with K2CO3. Bioresour. Technol. 2007, 98, 145–149. [Google Scholar] [CrossRef]
- Ma, A.; Zhao, S.; Luo, H.; Sun, Z.; Sun, K.; Li, H. Mercury removal from coal-fired flue gas by the mechanochemical S/FeS modified high sulfur petroleum coke. Fuel Processing Technol. 2022, 227, 107105. [Google Scholar] [CrossRef]
- Xiao, Y.; Pudasainee, D.; Gupta, R.; Xu, Z.; Diao, Y. Elemental mercury reaction chemistry on brominated petroleum cokes. Carbon 2017, 124, 89–96. [Google Scholar] [CrossRef]
- Zornitta, R.L.; Barcelos, K.M.; Nogueira, F.G.; Ruotolo, L.A. Understanding the mechanism of carbonization and KOH activation of polyaniline leading to enhanced electrosorption performance. Carbon 2020, 156, 346–358. [Google Scholar] [CrossRef]
- She, M.; Duan, Y.; Zhu, C.; Jia, C.Q. Impact of Nonoxidized Sulfur Species on Elemental Mercury Removal by SO2 Activated Petroleum Cokes. Energy Fuels 2020, 34, 14388–14399. [Google Scholar] [CrossRef]
- Yu, X.; Yu, D.; Yu, G.; Liu, F.; Han, J.; Wu, J.; Xu, M. Temperature-resolved evolution and speciation of sulfur during pyrolysis of a high-sulfur petroleum coke. Fuel 2021, 295, 120609. [Google Scholar] [CrossRef]
- Jin, Q.-F.Z.J.X. Desulfurization of Petroleum Coke by Calcination in Ammonia Atmosphere below 1000 °C. China Pet. Process. Petrochem. Technol. 2016, 18, 41. [Google Scholar]
- Wang, L.; Feng, X.; Shen, L.; Jiang, S.; Gu, H. Carbon and sulfur conversion of petroleum coke in the chemical looping gasification process. Energy 2019, 179, 1205–1216. [Google Scholar] [CrossRef]
- Tripathi, N.; Singh, R.S.; Hills, C.D. Microbial removal of sulphur from petroleum coke (petcoke). Fuel 2019, 235, 1501–1505. [Google Scholar] [CrossRef]
- Shan, J.; Huang, J.-j.; Li, J.-z.; Li, G.; Zhao, J.-t.; Fang, Y.-t. Insight into transformation of sulfur species during KOH activation of high sulfur petroleum coke. Fuel 2018, 215, 258–265. [Google Scholar] [CrossRef]
- Huang, Q.; Schafranski, A.S.; Hazlett, M.J.; Xiao, Y.; Hill, J.M. Nitric acid functionalization of petroleum coke to access inherent sulfur. Catalysts 2020, 10, 259. [Google Scholar] [CrossRef]
- Xiao, Y.; Liu, X.; Diao, Y. Understanding the effect of thiophene sulfur on brominated petroleum coke for elemental mercury capture from flue gases. RSC Adv. 2021, 11, 4515–4522. [Google Scholar] [CrossRef] [PubMed]
- D’Alessandro, N.; Tonucci, L.; Bonetti, M.; Di Deo, M.; Bressan, M.; Morvillo, A. Oxidation of dibenzothiophene by hydrogen peroxide or monopersulfate and metal–sulfophthalocyanine catalysts: An easy access to biphenylsultone or 2-(2′-hydroxybiphenyl) sulfonate under mild conditions. New J. Chem. 2003, 27, 989–993. [Google Scholar] [CrossRef]
- Kumar Reddy, K.S.; Prabhu, A.; Al Shoaibi, A.; Srinivasakannan, C. Application of sulfonated carbons for mercury removal in gas processing. Energy Fuels 2016, 30, 3227–3232. [Google Scholar] [CrossRef]
- Reddy, K.S.K.; Al Shoaibi, A.; Srinivasakannan, C. Mercury removal using metal sulfide porous carbon complex. Process Saf. Environ. Prot. 2018, 114, 153–158. [Google Scholar] [CrossRef]
- Abraham, A.M.; Kumar, S.V.; Alhassan, S.M. Porous sulphur copolymer for gas-phase mercury removal and thermal insulation. Chem. Eng. J. 2018, 332, 1–7. [Google Scholar] [CrossRef]
- Gu, Y.; Zhang, Y.; Lin, L.; Xu, H.; Orndorff, W.; Pan, W.-P. Evaluation of elemental mercury adsorption by fly ash modified with ammonium bromide. J. Therm. Anal. Calorim. 2015, 119, 1663–1672. [Google Scholar] [CrossRef]
- Chalkidis, A.; Jampaiah, D.; Amin, M.H.; Hartley, P.G.; Sabri, Y.M.; Bhargava, S.K. CeO2-Decorated-MnO2 Nanotubes: A Highly Efficient and Regenerable Sorbent for Elemental Mercury Removal from Natural Gas. Langmuir 2019, 35, 8246–8256. [Google Scholar] [CrossRef]
- Dranga, B.-A.; Lazar, L.; Koeser, H. Oxidation Catalysts for Elemental Mercury in Flue Gases—A Review. Catalysts 2012, 2, 139–170. [Google Scholar] [CrossRef]
- Hong, Y.; Duan, Y.; Zhu, C.; Zhou, Q.; She, M.; Wei, H.; Hong, Y. Experimental study on mercury removal of high-sulfur petroleum coke activated carbon impregnated with bromine. Proc. CSEE 2014, 34, 1762–1768. [Google Scholar]
- Marczak-Grzesik, M.; Budzyń, S.; Tora, B.; Szufa, S.; Kogut, K.; Burmistrz, P. Low-Cost Organic Adsorbents for Elemental Mercury Removal from Lignite Flue Gas. Energies 2021, 14, 2174. [Google Scholar] [CrossRef]
- Obot, I.; Macdonald, D.; Gasem, Z. Density functional theory (DFT) as a powerful tool for designing new organic corrosion inhibitors. Part 1: An overview. Corros. Sci. 2015, 99, 1–30. [Google Scholar] [CrossRef]
- Makkar, P.; Ghosh, N.N. A review on the use of DFT for the prediction of the properties of nanomaterials. RSC Adv. 2021, 11, 27897–27924. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Diao, Y.; Lu, Y.; Chen, S.; Tian, L. Complete Reaction Mechanisms of Mercury Binding on Petroleum Coke and Brominated Petroleum Coke. Energy Fuels 2019, 33, 5488–5497. [Google Scholar] [CrossRef]
- Frisch, A. Gaussian 09W Reference; Gaussian Inc.: Wallingford, CT, USA, 2009; 25p. [Google Scholar]
- Xiao, J.; Zhong, Q.; Li, F.; Huang, J.; Zhang, Y.; Wang, B. Modeling the change of green coke to calcined coke using Qingdao high-sulfur petroleum coke. Energy Fuels 2015, 29, 3345–3352. [Google Scholar] [CrossRef]
- Hodge, S.A.; Bayazit, M.K.; Coleman, K.S.; Shaffer, M.S. Unweaving the rainbow: A review of the relationship between single-walled carbon nanotube molecular structures and their chemical reactivity. Chem. Soc. Rev. 2012, 41, 4409–4429. [Google Scholar] [CrossRef]
- Geng, X.; Liu, X.; Ding, X.; Zhou, Q.; Huang, T.; Duan, Y. Mechanochemical bromination of unburned carbon in fly ash and its mercury removal mechanism: DFT study. J. Hazard. Mater. 2022, 423, 127198. [Google Scholar] [CrossRef]
- Padak, B.; Wilcox, J. Understanding mercury binding on activated carbon. Carbon 2009, 47, 2855–2864. [Google Scholar] [CrossRef]
- Rungnim, C.; Promarak, V.; Hannongbua, S.; Kungwan, N.; Namuangruk, S. Complete reaction mechanisms of mercury oxidation on halogenated activated carbon. J. Hazard. Mater. 2016, 310, 253–260. [Google Scholar] [CrossRef]
- Vakili, F.; Rashidi, A.; Taghavi, L.; Mansouri, N. Conversion of biomass to N, S co-doped porous graphene as an adsorbent for mercury vapor removal: Optimization and DFT study. J. Environ. Health Sci. Eng. 2021, 19, 1569–1582. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Li, J.; Xiang, K.; He, S.; Shen, F. DFT and Experimental Studies on the Mechanism of Mercury Adsorption on O2-/NO-Codoped Porous Carbon. ACS Omega 2021, 6, 12343–12350. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zhang, Y.; Wang, B.; Cheng, H.; Cheng, X.; Huang, Z. DFT study on Al-doped defective graphene towards adsorption of elemental mercury. Appl. Surf. Sci. 2018, 427, 547–553. [Google Scholar] [CrossRef]
- Yan, G.; Gao, Z.; Zhao, M.; Yang, W.; Ding, X. A comprehensive exploration of mercury adsorption sites on the carbonaceous surface: A DFT study. Fuel 2020, 282, 118781. [Google Scholar] [CrossRef]
- Yang, S.; Fan, X.; Liu, J.; Zhao, W.; Hu, B.; Lu, Q. Mechanism insight into the formation of H2S from thiophene pyrolysis: A theoretical study. Front. Environ. Sci. Eng. 2021, 15, 120. [Google Scholar] [CrossRef]
- Li, H.; Liu, S.; Yang, J.; Liu, Y.; Hu, Y.; Feng, S.; Yang, Z.; Zhao, J.; Qu, W. Role of SO2 and H2O in the mercury adsorption on ceria surface: A DFT study. Fuel 2020, 260, 116289. [Google Scholar] [CrossRef]
- Chenoweth, K.; Van Duin, A.C.; Goddard, W.A. ReaxFF reactive force field for molecular dynamics simulations of hydrocarbon oxidation. J. Phys. Chem. A 2008, 112, 1040–1053. [Google Scholar] [CrossRef]
- Senftle, T.P.; Hong, S.; Islam, M.M.; Kylasa, S.B.; Zheng, Y.; Shin, Y.K.; Junkermeier, C.; Engel-Herbert, R.; Janik, M.J.; Aktulga, H.M. The ReaxFF reactive force-field: Development, applications and future directions. Npj Comput. Mater. 2016, 2, 15011. [Google Scholar] [CrossRef]
- Sharma, S. Molecular Dynamics Simulation of Nanocomposites Using BIOVIA Materials Studio, Lammps and Gromacs; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Sharma, S.; Kumar, P.; Chandra, R. Applications of BIOVIA materials studio, LAMMPS, and GROMACS in various fields of science and engineering. In Molecular Dynamics Simulation of Nanocomposites Using BIOVIA Materials Studio, Lammps and Gromacs; Elsevier: Amsterdam, The Netherlands, 2019; pp. 329–341. [Google Scholar]
- Zhong, Q.; Mao, Q.; Xiao, J.; van Duin, A.C.; Mathews, J.P. ReaxFF simulations of petroleum coke sulfur removal mechanisms during pyrolysis and combustion. Combust. Flame 2018, 198, 146–157. [Google Scholar] [CrossRef]
- Zhong, Q.; Mao, Q.; Xiao, J.; van Duin, A.; Mathews, J.P. Sulfur removal from petroleum coke during high-temperature pyrolysis. Analysis from TG-MS data and ReaxFF simulations. J. Anal. Appl. Pyrolysis 2018, 132, 134–142. [Google Scholar] [CrossRef]
- Zhong, Q.; Zhang, Y.; Shabnam, S.; Mao, Q.; Xiao, J.; van Duin, A.C.; Mathews, J.P. ReaxFF MD simulations of petroleum coke CO2 gasification examining the S/N removal mechanisms and CO/CO2 reactivity. Fuel 2019, 257, 116051. [Google Scholar] [CrossRef]
- Zhong, Q.; Zhang, Y.; Shabnam, S.; Xiao, J.; Van Duin, A.C.; Mathews, J.P. Reductive gaseous (H2/NH3) desulfurization and gasification of high-sulfur petroleum coke via reactive force field molecular dynamics simulations. Energy Fuels 2019, 33, 8065–8075. [Google Scholar] [CrossRef]
- Yeon, J.; He, X.; Martini, A.; Kim, S.H. Mechanochemistry at solid surfaces: Polymerization of adsorbed molecules by mechanical shear at tribological interfaces. ACS Appl. Mater. Interfaces 2017, 9, 3142–3148. [Google Scholar] [CrossRef]
- Khajeh, A.; He, X.; Yeon, J.; Kim, S.H.; Martini, A. Mechanochemical association reaction of interfacial molecules driven by shear. Langmuir 2018, 34, 5971–5977. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zheng, M.; Ren, C.; Guo, L. ReaxFF molecular dynamics simulations of thermal reactivity of various fuels in pyrolysis and combustion. Energy Fuels 2021, 35, 11707–11739. [Google Scholar] [CrossRef]
- Mo, Z.; Li, X.; Li, G. Algorithms of GPU-enabled reactive force field (ReaxFF) molecular dynamics. J. Mol. Graph. Model. 2013, 41, 1–11. [Google Scholar]




| Samples | Origins | Sulfur Content wt% | Year and Ref. |
|---|---|---|---|
| HSPC1 | Shandong, China | 7.06 | 2019 [37] |
| HSPC2 | Tianjing, China | 3.5 | 2020 [48] |
| HSPC3 | China | 6.233 | 2020 [31] |
| HSPC4 | Jiangxi, China | 4.3 | 2021 [49] |
| HSPC5 | Fushun, Liaoning, China | 4.74 | 2022 [34] |
| HSPC6 | Alberta, Canada | 5.85 | 2017 [38] |
| HSPC7 | Saskatchewan, Canada | 2.14 | 2017 [46] |
| HSPC8-1 | Tianjing, China | 7.21 | 2016 [50] |
| HSPC8-2 | Qingdao, China | 6.53 | 2016 [50] |
| HSPC8-3 | Zhenhai, China | 5.21 | 2016 [50] |
| HSPC8-4 | Dongming, China | 2.25 | 2016 [50] |
| HSPC9-1 | Nanjing, China | 2.08 | 2016 [40] |
| HSPC9-2 | America | 6.01 | 2016 [40] |
| HSPC10 | Jiangsu, China | 6.81 | 2019 [51] |
| HSPC11 | Jamnagar, Gujarat, India | 6.31 | 2019 [52] |
| HSPC12-1 | Zibo, China | 3.6 | 2020 [53] |
| HSPC12-2 | America | 3.81 | 2020 [53] |
| HSPC13 | Canada | 6.5 | 2020 [54] |
| HSPC14 | Xiamen, China | 8.92 | 2021 [2] |
| Samples | Sulfide | Thiophene/ Elemental Sulfur | Sulfoxide | Sulfone | Sulfonate | Sulfate |
|---|---|---|---|---|---|---|
| RPC | 3.05 | 1.56 | 0.00 | 0.08 | 0.00 | 0.04 |
| S600–60-17 | 0.61 | 6.33 | 3.23 | 0.72 | 0.00 | 0.00 |
| FeS600–60-17 | 0.52 | 4.62 | 2.36 | 0.00 | 0.87 | 0.44 |
| FeS600–60-25 | 2.82 | 3.66 | 1.86 | 0.66 | 2.86 | 1.46 |
| Sample | Content of Carbon Functional Groups (Atom %) | Beneficial Oxygen Functional Groups (Atom %) | |||
|---|---|---|---|---|---|
| C–C/C–H | C–O | C=O | COOH/C(O)–O–C | ||
| RPC | 59.54 | 29.97 | 0.00 | 0.00 | 0.00 |
| CSAC | 38.34 | 11.93 | 18.93 | 5.81 | 24.74 |
| SCAC | 16.51 | 15.24 | 10.62 | 19.04 | 29.66 |
| Sample | Total Amount (at. %) | Absolute Content (at. %) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| C | O | N | C–H/C–C | Peak | C=O | Peak | C–O–O– | Peak | |
| BC−N2−600−10 | 87.13 | 10.61 | 2.26 | 75.77 | 284.80 | 6.82 | 286.92 | 4.55 | 288.72 |
| BC−N2 + 7%O2−800−10 | 88.76 | 9.55 | 1.68 | 73.97 | 284.80 | 8.88 | 286.97 | 5.92 | 288.77 |
| BC−N2 + 15%CO2−700−10 | 89.21 | 8.97 | 1.81 | 74.97 | 284.8 | 8.25 | 286.88 | 6.00 | 288.69 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, J.; Diao, Y. The Effects of Physical-Chemical Evolution of High-Sulfur Petroleum Coke on Hg0 Removal from Coal-Fired Flue Gas and Exploration of Its Micro-Scale Mechanism. Int. J. Environ. Res. Public Health 2022, 19, 7082. https://doi.org/10.3390/ijerph19127082
Jiang J, Diao Y. The Effects of Physical-Chemical Evolution of High-Sulfur Petroleum Coke on Hg0 Removal from Coal-Fired Flue Gas and Exploration of Its Micro-Scale Mechanism. International Journal of Environmental Research and Public Health. 2022; 19(12):7082. https://doi.org/10.3390/ijerph19127082
Chicago/Turabian StyleJiang, Jie, and Yongfa Diao. 2022. "The Effects of Physical-Chemical Evolution of High-Sulfur Petroleum Coke on Hg0 Removal from Coal-Fired Flue Gas and Exploration of Its Micro-Scale Mechanism" International Journal of Environmental Research and Public Health 19, no. 12: 7082. https://doi.org/10.3390/ijerph19127082
APA StyleJiang, J., & Diao, Y. (2022). The Effects of Physical-Chemical Evolution of High-Sulfur Petroleum Coke on Hg0 Removal from Coal-Fired Flue Gas and Exploration of Its Micro-Scale Mechanism. International Journal of Environmental Research and Public Health, 19(12), 7082. https://doi.org/10.3390/ijerph19127082





