Abstract
The present review aims to examine whether multi-component interventions for informal caregivers of people with dementia are effective on positive and negative aspects of caregiver well-being. Eleven databases were searched from inception to 8 March 2021. Only randomized controlled trials reporting the effectiveness of multi-component intervention on positive and negative aspects of caregiver well-being were eligible. Endnote X7 (Thomson ResearchSoft, Stanford, CA, USA) was used for study selection and version 5.1.0 of Cochrane Collaboration’s tool (Cochrane, London, UK) was applied for quality assessment. Review Manager (Revman) Version 5.3 (Cochrane, London, UK) was used for the meta-analysis, and if statistical synthesis was inappropriate, only narrative analysis was performed. A total of 31 RCTs with 3939 participants were included. Meta-analyses showed small to moderate effects on subjective well-being, depression, and burden of caregivers, and a moderate to high effect on caregiver anxiety. Due to insufficient data and vast heterogeneity, meta-analysis was not performed for other outcomes, such as resilience, competence, and empathy. This review suggests that individualized multi-component interventions for caregivers may be one of the ways to promote their well-being. Further research is needed to explore the impact of rigorously designed and personalized multi-component interventions on informal caregivers, especially on more positive indicators, as well as its long-term effects and sustainability.
1. Introduction
Dementia is a leading cause of disability among people aged 65 years and over [1]. It is estimated that there are approximately 50 million people living with dementia worldwide, and the number is forecasted to reach 82 million by 2030 and 152 million by 2050 [2]. As the life expectancy of the world’s population increases, the number of people with dementia continues to grow [3]. Up to 94% of people with dementia are cared for by informal caregivers, who have become the backbone of informal care [4]. Caring for people with dementia puts a significant impact on the physical condition, mental health, well-being, and social relationships of informal caregivers as evidenced by the high-level burden and high prevalence of mental health problems among caregivers, which contributes to the poor quality of care and quality of life of caregivers and those they care for [5]. Effective interventions that can support informal caregivers to manage negative emotions and enhance well-being are therefore required.
Well-being is usually described as a theoretical construct, which includes emotional (affects/feelings), psychological (positive functioning), social (relations with others), and spiritual (sense of purpose in life) aspects [6,7], and it reflects not only the relative absence of some undesirable outcomes but also the presence of positive aspects [8]. More and more evidence shows that both positive and negative feelings exist in the care process of informal caregivers [9,10]. Previous studies have focused more on the impact of interventions on negative outcomes. However, caregivers pay too much attention to negative emotions, which may result in a pessimistic spiritual outlook and secondary harm, in ignoring self-affirmation of successful performance [11]. The more negative thoughts the caregiver has, the likelier they are to experience a stronger sense of overload. Conversely, when caregivers focus on the positive aspects of care, it seems to improve their mood [12]. Positive feelings help to improve the intimate relationship between the caregiver and the people with dementia and to promote the caregiver to actively respond to the problems faced in the care process [13].
Multi-component interventions appear to have a good potential for improving caregiver well-being outcomes [14]. It means any intervention containing elements of at least two of the categories. These categories include, but are not limited to, psychoeducational interventions, psychotherapy, skills training, professional or peer support, respite care, case management, exercise, attendance at a memory clinic, meditation/mindfulness, and so on [15]. Multi-component interventions can be delivered in multiple methods (i.e., face-to-face, telephone, or online) and are conducted by the trained health professional in dyadic approaches or by the caregiver alone. These well-designed and clearly structured multi-component interventions for caregivers of people with dementia are intended to improve their positive aspects of caregiving, reduce their burden and depressive symptoms [16], and even delay the institutionalization of people with dementia [17] by individualized content for caregiver demands. Importantly, multi-component interventions have been widely used in informal caregivers of disabled elderly people [18], and similar results have been found in caregivers of people with dementia.
The recent findings regarding the effects of multi-component interventions on negative aspects of caregiver well-being of informal caregivers are inconsistent. For example, the results of some randomized controlled trials (RCTs) have indicated that the depression [19,20,21], burden [19], or anxiety [21] of caregivers was significantly ameliorated in the multi-component intervention group compared with the control group. On the contrary, some studies have shown that there were no significant differences in the depression [22,23,24], burden [20,22], or anxiety [20,24] of caregivers between the multi-component groups and control groups. Currently, there are several systematic reviews of multi-component interventions for informal caregivers of people with dementia. However, in these previous systematic reviews, the authors focused on the optimal way to combine multiple components [25], the impact on a single positive outcome such as competence in caregiving [26], or the impact on negative outcomes [27]. There is a gap in systematic evaluations exploring the effects of multicomponent interventions on both positive and negative aspects of caregiver well-being. Although the effect of multicomponent interventions on caregivers’ subjective well-being was explored in a recent systematic evaluation of a nonpharmacological intervention, no statistical significance was found. In addition, it included only a small amount of literature published in English and German and did not explore the effects on other outcomes such as self-efficacy, empathy, and resilience [28]. Therefore, it is necessary to conduct a thorough systematic review to explore the impact of multi-component interventions for dementia caregivers on both positive and negative aspects of well-being.
This review focused on RCTs investigating the effectiveness of multi-component interventions on positive and negative aspects of well-being in informal caregivers of people with dementia and compared the intervention effects of different delivery methods, including caregivers only or dyadic interventions. Findings from this review will provide evidence of effectiveness of multi-component interventions on caregiver well-being and enable health professionals to become aware of the positive aspects of caregiver well-being, which will inform the development of effective multi-component strategies and dementia care services for informal caregivers.
2. Materials and Methods
2.1. Design
A systematic review and meta-analysis were performed with reference to the Cochrane Handbook of Intervention Studies [29], and this paper was developed following the PRISMA statement (Table S1).
2.2. Search Methods
We searched the ALOIS, Medline, Embase, LILACS, PsycINFO, CINAHL, Web of Science English databases, and Wanfang, CBM, VIP, and CNKI Chinese databases for all articles published prior to July 2020. The retrieval of the databases above was updated on 8 March 2021, and a combination of the following search terms and Medical Subject Heading terms were used: ‘Alzheimer’; ‘dementia’; ‘informal caregiver’; ‘family’; ‘relative’; ‘spouse’; ‘multi-component’; ‘combinate’; ‘comprehensive’; ‘random’; and ‘RCT’. The full electronic strategy for the databases is listed in Table S2.
2.3. Inclusion and Exclusion Criteria
Studies were included if they had adult informal caregivers who provided unpaid care for people with any type of dementia in the home settings. Second, studies were RCTs in which informal caregivers were randomly assigned to a multi-component intervention group or to a control condition (e.g., usual care, wait-list control, alternative intervention, single-component intervention). Third, multi-component interventions, which means any intervention containing elements of at least two of the different intervention categories had to be included [26]. As defined in guidelines published by the National Institute for Health and Care Excellence in 2018, these categories include psychoeducational interventions, psychotherapy, skills training, professional or peer support, respite care, case management, exercise, attendance at a memory clinic, meditation/mindfulness, and so on [15]. Fourth, the intervention routes included face-to-face, telephone, and online approaches, and the form of the intervention included both individuals and groups. Both interventions that recruited caregivers only and dyadic interventions with informal caregivers as the main intervention target were included. Fifth, they had to report caregiver outcomes in any of the following categories: subjective well-being, relationship satisfaction, resilience, self-efficacy, empathy, competence, burden, anxiety, depression, and stress. Lastly, they were full-text articles published in English or Chinese.
Studied were excluded if (1) participants were diagnosed with mild cognitive impairment, or both dementia and mild cognitive impairment, but the data could not be distinguished; if (2) interventions containing multiple forms from the same category (e.g., an intervention only including both group and individual psychoeducation intervention); and if (3) it is a letter, commentary, case report, conference abstract, literature review, systematic review, or meta-analysis.
2.4. Types of Outcome Measures
Subjective well-being was the primary outcome of this study. Subjective well-being is one of the positive indicators of intrinsic aspects of well-being, which is usually described as a positive aspect of well-being, so that individuals can fully interact with others, deal with life pressure and realize their own abilities [8]. Based on the literature review and the concept map of well-being proposed by previous studies [8,30], the secondary outcomes included self-efficacy, depression, anxiety, stress, burden, relationship satisfaction, resilience, empathy, and competence.
2.5. Study Selection
First, one reviewer merged references from different databases in Endnote X7 and deleted duplicates. Second, two reviewers independently screened titles and abstracts to select articles for full-text review. Third, two reviewers conducted a full-text review to determine which studies met inclusion criteria. Finally, we resolved disagreements through discussion or a third reviewer.
2.6. Quality Appraisal
Two reviewers independently completed the assessment of the risk of bias and any disagreements were discussed and resolved with the third reviewer in regular team meetings. The Cochrane Collaboration’s tool (version 5.1.0) was applied for quality appraisal [31], including seven domains of bias: sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective outcome reporting, and ‘other issues’. Each domain in the tool was rated as “low risk”, “high risk” or “unclear risk”. The Cochrane Handbook of Intervention Studies suggests that if multiple domains in a study are judged to be “Unclear” in a way that substantially lowers confidence in the result, the study is considered to be at high risk of bias. In addition, assessing the risk of bias due to missing results is an essential component of a Cochrane Review, so studies that were evaluated as low risk of bias in the domain of “Incomplete outcome data“ were included in this systematic review [29]. Three articles were excluded during the quality appraisal due to many unclear risks of bias and the domain of “Incomplete outcome data” was unclear [32,33,34].
2.7. Data Extraction
The family name of the first author, year of publication, country, participants, mean age, the proportion of females, condition of intervention and control group, sample size, outcomes, and follow-up time points were extracted in a standardized data extraction form and were checked for extraction accuracy for analysis in Revman Version 5.3 (Cochrane, London, UK).
2.8. Synthesis
All statistical analyses were conducted using Revman Version 5.3 when the mean and standard deviation of the outcome data were reported or calculated. Otherwise, studies were described narratively. Considering that the outcomes of the included studies were measured using different scales and that many combinations of multi-component interventions were included for review, the standardized mean difference (SMD) and 95% confidence interval (95% CI) between the scores of the two groups immediately after the intervention were calculated to estimate the overall intervention effect [29].
Cochran’s Q test and I2 statistic were used to estimate statistical heterogeneity. A p value of Cochran’s Q test < 0.1 indicated heterogeneity among included studies. I2 heterogeneity test was <40%, 40–75%, >75%, indicating low, moderate, and high consistency of the included studies, respectively. A fixed effect model was used in this review if there was no significant heterogeneity (p > 0.1, I2 < 40%). When included articles showed moderate heterogeneity (p < 0.1, 40% < I2 < 75%), we firstly checked and confirmed that the data entered into the software were accurate and then a random effect model was used to combine effect sizes [29]. If heterogeneity was high (p < 0.1 and I2 > 75%), the sources of heterogeneity were explored in terms of clinical heterogeneity and methodological heterogeneity. Subgroup analyses based on the type of participant and delivery method were performed according to the protocol design to examine the source of heterogeneity. When heterogeneity was too pronounced and unresolvable, meta-analysis was inappropriate and only narrative analysis was performed. The sensitivity analysis was performed to test the robustness of the meta-analysis results. When the number of articles included for an outcome was ≥5, publication bias of the articles were assessed visually using funnel plots and statistically by Egger’s test.
3. Results
3.1. Study Selection
The study selection process is presented in Figure 1. A total of 4946 potentially relevant citations were identified from 11 databases and imported to Endnote X7. Totally 1885 duplicate articles were excluded in the first step. After screening the titles and abstracts, 2987 articles were excluded. In all 60 articles were excluded after quality appraisal and reading the full text. Fifteen relevant articles were included by manual exploration of the reference lists of the included studies. We searched the databases again on 8 March 2021, and updated two studies. Finally, 31 studies published between 2000 and 2020 were included, 24 in English and the rest in Chinese.
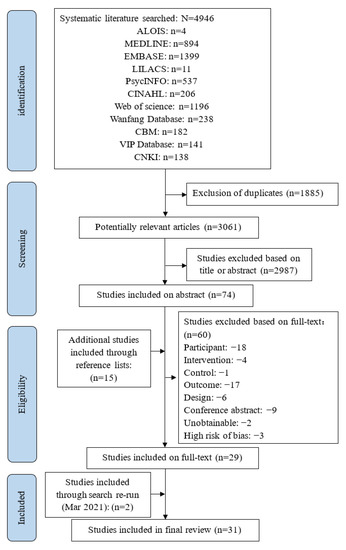
Figure 1.
Flowchart for studies selection.
3.2. Study Characteristics
In total, the 31 studies included 3939 informal caregivers, of whom 2080 were allocated to the intervention groups and 1859 to the control groups. Studies typically recruited more women (50.6–93.1%) than men, and the average age of caregivers ranged from 48.0 to 77.3 years. Most studies involved caregivers only, and 10 articles included both people with dementia and their caregivers (dyadic) [18,19,23,35,36,37,38,39,40,41]. Of the included studies, 15 studies (48.4%) were conducted or assisted by nurses, 6 studies were conducted by psychotherapists, and the remaining 10 studies were led by trained research team members or social workers. Regarding the intervention delivery methods, 21 studies conducted face-to-face interventions, three studies focused on telephone interventions, and seven studies focused on online methods. The number of interventions implemented across the included literature ranged from 4 to 16 and were usually held once a week or every two weeks, with duration ranging from 20 to 240 min. In all included studies, the outcomes were measured before and after the intervention. However, the duration of the intervention ranged from 1 to 48 weeks. Table S3 summarizes the characteristics of the included studies.
3.3. Risk of Bias
The method of random assignment sequence generation was described in 83.9% of the included studies, but the exact implementation of their allocation concealment was unclear in more than half of the studies (54.8%). Due to the nature of the intervention, only 32.3% of the studies were able to blind participants and personnel, and 54.8% of the studies were able to blind outcome assessors. All the studies reported the number and reason for dropouts. Up to 87.1% of the studies had a low risk of reporting bias, and 11 articles (35.5%) had either published protocols or were registered on clinical trial registries. In addition, one study [36] may have led to other biases due to the particular clinical setting (Figure S1).
3.4. Impact on the Positive Aspects of Caregiver Well-Being
The outcomes in this review included both positive and negative aspects of caregiver well-being. The following are outcomes for the positive aspects of well-being, including caregiver’s subjective well-being, self-efficacy, relationship satisfaction, resilience, empathy, and competence.
3.4.1. Impact on the Caregiver’s Subjective Well-Being
The caregiver’s subjective well-being was the primary outcome, the results of which are shown in Figure 2. The duration of the intervention varied from 2 h to 12 months. In the included studies, subjective well-being was measured by different instruments, such as two studies used the Positive Aspects of Caregiving and others used the Nottingham Health Profile, a mood improvement questionnaire, the Perceived Change Index, the Positive Affect and Negative Affect Schedule, and the Care-Related Quality of Life Instrument-Visual Analogue Scale tool, respectively. The overall pooled SMD for the seven studies was significant [SMD = 0.41, 95% CI (0.28, 0.54), p < 0.001, I2 = 25%], with effect size estimated as small to moderate. Sensitivity analysis was performed by omitting one study in each round and the results did not change. Only two studies [36,42] investigated the subjective well-being of informal caregivers at three-month follow-up and no significant difference was found (p > 0.05). However, one study reported that the intervention group’s average subjective well-being score increased during the follow-up compared with the post-intervention results, while the control group’s average score decreased [42].
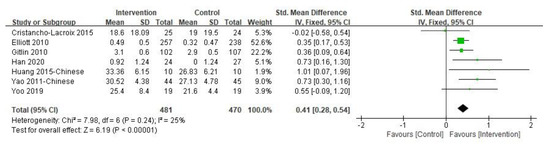
Figure 2.
Effect of multi-component interventions on informal caregiver’s subjective well-being (post-intervention).
3.4.2. Impact on the Caregiver’s Self-Efficacy
Five studies reported the effects of multi-component interventions on caregiver self-efficacy analysis. It is important to note that Duggleby et al. [43] and Possin et al. [19] used the General Self-efficacy Scale (GSES) and the Care Ecosystem Caregiver Self-efficacy Scale to assess participants’ perceived self-efficacy, respectively. Both studies found no significant improvement in self-efficacy. The other three studies [22,42,44] used the Revised Scale for Caregiving Self-efficacy (RSCSE) to measure self-efficacy in three dimensions: managing patients’ disturbing behaviors, obtaining respite and controlling upsetting thoughts. The results reported that multi-component interventions had no significant impact on the three dimensions of caregiver self-efficacy (Figure 3).
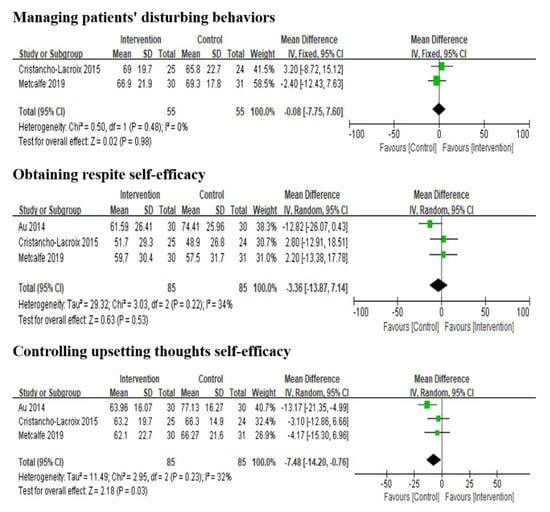
Figure 3.
Effect of multi-component interventions on informal caregiver’s self-efficacy (post-intervention).
3.4.3. Impact on Other Positive Outcomes
Relationship satisfaction was reported in two studies [SMD = 3.48, 95% CI (0.95, 6.02), p = 0.007, I2 = 0%] [42,44], and one study with a weight of 96% had statistically significant and reported higher scores in the multi-component interventions group [45].
Two studies reported the empathy and competence of caregivers, and a meta-analysis was not performed due to insufficient data [39,45]. Except for the results of Hattink et al. [45], which showed that multi-component intervention may improve caregiver empathy, other results were not statistically significant. Only Kor et al. [20] assessed the resilience of caregivers using the Brief Resilience Scale. The 10-week intervention and the third-month follow-up results showed that multi-component interventions could not improve the resilience of caregivers (p > 0.05). However, the mean score of resilience in the intervention group increased during the follow-up period, while the mean score of the control group decreased.
3.5. Impact on the Negative Aspects of Caregiver Well-Being
The following are outcomes for the negative aspects of well-being, including caregiver’s depression, burden, anxiety, and stress.
3.5.1. Impact on the Caregiver’s Depression
Eighteen of the included 31 studies reported the post-intervention depression symptoms of caregivers and 17 provided sufficient data for inclusion in meta-analysis (Figure 4). Of the 17 studies included in the analysis, six studies used the Centre for Epidemiologic Studies Depression Scale, three studies used the Self-Rating Depression Scale, three studies used the Geriatric Depression Scale, two studies used the Patient Health Questionnaire, two studies used the second version of the Beck Depression Inventory, and one study used the Symptom Checklist 90 to measure depression. A significant effect in favor of multi-component intervention over the control group was found [SMD = −0.29, 95% CI (−0.46, −0.11), p = 0.001, I2 = 0%]. The study not included in the meta-analysis also reported that multi-component intervention was statistically significant in reducing the depressive symptoms of caregivers [46].
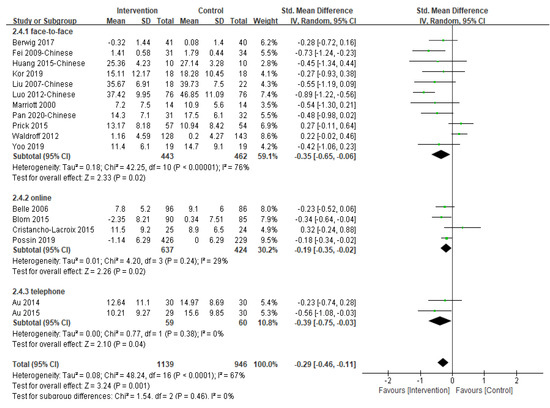
Figure 4.
Effect of multi-component interventions on informal caregiver’s depression (post-intervention).
Removing a trial [19] with the largest sample size did not change the result (p = 0.003). Furthermore, when we classified studies by the type of participants, multi-component interventions involving caregiver only as participants [SMD = −0.42, 95% CI (−0.62, −0.22), p < 0.001, I2 = 43%] were associated with slightly larger and statistically significant reductions in depressive symptoms than dyadic interventions [SMD = −0.09, 95% CI (−0.32, 0.14), p = 0.42, I2 = 68%]. Subgroup analysis according to intervention delivery methods revealed that the difference between groups was not significant (p = 0.46) (Table S4). Seven studies [20,24,36,40,41,42,47] were pooled to examine the effectiveness of the multi-component interventions on the depression of informal caregivers at three-month follow-up and no significant difference between groups was found (p = 0.26).
3.5.2. Impact on the Caregiver’s Burden
Twelve studies were pooled for the post-intervention burden of informal caregivers, with a small to moderate effect being detected [SMD = −0.34, 95% CI (−0.53, −0.16), p = 0.0003, I2 = 60%] (Figure S2). Sensitivity analysis excluding one study with the largest sample size had no significant effect on the combined effect size [19]. No significant difference was detected between studies that worked with caregivers only or that included dyadic intervention (p = 0.05). However, the difference between face-to-face and online delivery methods was significant (p = 0.02) (Table S4). Due to the extremely high heterogeneity (I2 = 95%), meta-analysis at three-month follow-up was not conducted for the six studies providing sufficient data. Four of the six studies reported caregiver burden was significantly reduced at three-month follow-up [20,24,46,47].
3.5.3. Impact on the Caregiver’s Anxiety
We pooled seven studies evaluating the effects of multi-component interventions on the anxiety of informal caregivers (Figure S2). Overall, the effect was statistically significantly different between the intervention and control groups [SMD = −0.53, 95% CI (−0.78, −0.27), p < 0.001, I2 = 48%]. Sensitivity analysis was performed by omitting one study in each round and the results did not change. However, no significant difference was detected between studies using face-to-face and online delivery methods (p = 0.35), although the only study which used online intervention, reported a much larger effect [21] (Table S4). Three highly heterogeneous (I2 = 98%) studies reported anxiety outcomes for the third month after the intervention, but no statistically significant difference was found.
3.5.4. Impact on the Caregiver’s Stress
Five studies reported the effects of multi-component interventions on caregiver stress (Figure S2), and the results revealed that the overall effect was not significant [SMD = −0.23, 95% CI (−0.47, 0.01), p = 0.06, I2 = 0%]. Subgroup analysis showed no significant difference between studies with caregivers only or dyadic studies (p = 0.95) (Table S4). Excluding a study by Cristancho-Lacroix [42], multi-component interventions tended to reduce the stress of caregivers, although this was of borderline statistical significance [SMD = −0.28, 95% CI (−0.55, −0.01), p = 0.04, I2 = 0%]. Three studies [20,41,42] extended the follow-up time by three months, but no significant changes were observed (p = 0.61).
3.5.5. Publication Bias
Publication bias were assessed using funnel plots (Figure S3 in the Supplement) and Eggers’ test, which did not indicate the presence of funnel plot asymmetry or publication bias in caregiver subjective well-being (p = 0.25), depression (p = 0.22), burden (p = 0.18), anxiety (p = 0.95) and stress (p = 0.57).
4. Discussion
Informal caregivers of people with dementia bear a heavy burden of care and increase their risk of physical and mental illnesses [48]. Caregiver support is one of the seven action areas in the Global Action Plan on the Public Health Response to Dementia 2017–2025 developed by the World Health Organization [49]. Multi-component interventions are consistently reported as the most effective intervention for maintaining caregiver health, providing caregivers with a variety of comprehensive support designed to meet their individual needs [50]. However, there is an inconclusive report on the impact of multi-component interventions on the well-being of caregivers. This systematic review synthesized all available evidence in the literature and identified 31 studies involving 3939 informal caregivers of people with dementia. Significant results were found for subjective well-being, depression symptoms, burden, and anxiety.
In contrast to a recent review [28], this review showed that multi-component interventions significantly improved the subjective well-being of the informal caregivers and the effect size was between small and moderate. One possible explanation is that the former study included randomized and nonrandomized studies, but this review only included higher-quality RCTs, and the number of studies included in this review increased, expanding the sample size. The difference may also be caused by the different emphasis on the intervention content included in the original study. The original research interventions included in the previous systematic review were psychosocial interventions and multicomponent interventions targeting the experience and/or behavior of informal caregivers. However, well-being does not only involve psychology, but also social, spiritual, and emotional factors [6]. The research included in this review can be a multi-component intervention with no limitations on the content of the intervention. The next step of research needs to develop standardized, unified, and robust subjective well-being measurement tools based on the concept map of well-being of informal caregivers proposed by existing studies [8]. Despite the promising quantitative finding of multi-component interventions on the positive aspect of well-being among caregivers, more independent replication and long-term follow-up are still needed.
Our results also showed that multi-component interventions could significantly reduce depression and anxiety symptoms of informal caregivers and alleviate care burden, which is consistent with those of previous reviews [28,51]. However, the effect was short-term, and the follow-up results were not statistically significant. Due to the small sample, the interpretation of the results should be cautious. In this study, 16 of the 17 studies that explored the impact of multi-component intervention on depression were personalized support based on the assessment results, needs or preferences of caregivers. Future research is needed, focusing on the impact of individually designed multi-component interventions on the depression and anxiety of caregivers. The care problems faced by informal caregivers of people with dementia are dynamic, varying from person to person, and cannot be generalized [48]. As caregivers become more experienced, their needs for the health of emerging issues and changing care recipients become more specific, and they are eager to obtain information about emerging issues [52]. Research has found that personally tailored activities can help increase participants’ positive emotions and have a positive impact on the burden and happiness of caregivers [53]. Therefore, it is vital to modify interventions for specific stages of the care trajectory faced by different caregivers [52,54].
Subgroup analysis found that for the subjective well-being, depression, burden, and anxiety of dementia caregivers, the effect of the face-to-face method might be better than the online method, which is inconsistent with previous studies [53]. This may be related to the fact that most of the participants included in this review were middle-aged and elderly adults who might not use computers [55]. By contrast, for the younger generation of caregivers, the independent time and place of the online intervention make it easier for them to get help [56]. Furthermore, the online intervention could expand the accessibility of the interventions, especially in the situation of COVID-19. Appropriate delivery methods should be considered according to the intervention content and target population to enhance the feasibility and effectiveness. With regard to the participants, findings of the subgroup analysis indicated that multi-component interventions recruiting caregivers only might have a better effect than dyadic interventions in terms of depression and burden. One possible reason is that intervention contents are mainly developed for caregivers [57], and caregivers can temporarily leave the caring environment and receive a short respite [25]. More detailed information about the intervention content and implementation strategies are required for exploring the best combination of intervention content and dosage in multi-component interventions.
Interventionists should have structured knowledge or professional experience in dementia [58]. Fifteen of the included 31 studies in this review were led or assisted by nurses, and nine of them were completed by multidisciplinary teams. It is reported that rapport and a higher sense of trust between the interventionist and the caregiver are key factors that facilitate the intervention, which helps to produce better results and a higher level of adherence [59]. Primary health professionals in the community are in an ideal position to provide multi-component interventions and coordinate services for informal caregivers due to the close contact, familiarity, and trust between them and caregivers [60,61]. However, some primary health professionals are not yet well prepared to undertake roles due to the lack of opportunities to engage in dementia care education [62]. It is recommended to rely on the existing public health services to deliver dementia training for health professionals and to integrate the available dementia care resources on the basis of communities or villages, which would help more healthcare professionals to provide tailored recommendations and support for people with dementia and informal caregivers [63].
The mechanisms by which multicomponent interventions improve caregiver well-being may be complex and multifactorial [64]. As previous research has illustrated, dementia care is a long-term process that forces informal caregivers to continually revise their coping strategies as the person with dementia goes through different stages of the disease, in which the caregiver’s needs are constantly changing and vary from person to person [65]. Caregiver well-being is a multiple concept that includes emotional, psychological, social, and spiritual aspects, and multi-component interventions contain a variety of components covering educational, physical, psychological, emotional, and social supports that can be selected by caregivers as needed and better meet caregiver’s needs, making it possible to personalize interventions and thus improve caregiver well-being [66]. In addition, multi-component interventions tend to have a longer intervention duration [67], and the ongoing contact between the intervention implementer and the caregiver provides continuity of care for the caregiver [68]. Caregivers reported access to ongoing care helped them seek out support services that were beneficial to them, and that “personal gains” were enhanced, such as inner strengths, self-confidence, and a sense of efficacy, which are important factors in promoting caregiver well-being [69].
This systematic evaluation extends previous research by providing evidence that supports the efficacy of multi-component interventions in improving positive and negative aspects of well-being for informal caregivers of people with dementia. As the aging of the global population accelerates, there is an urgent need for healthcare providers to identify effective ways to support informal dementia caregivers in community settings. Trained primary health professionals who integrate community resources and provide multifaceted support for caregivers can maximize the coverage of factors that meet the individual needs of most caregivers and help informal caregivers caring for people with dementia to effectively cope with the caregiving issues they face. This would help to address the concerns of the growing strain on the dementia-related health and social care systems in countries facing a rapidly aging population.
5. Limitations and Implications for Future Research
There are several limitations to this systematic review. The duration and content of the interventions in the included studies are diverse. What we extracted was the data measured immediately after the intervention and did not account for differences between groups at baseline. However, because the duration of the interventions ranged from 2 h to 48 weeks, the time point for the outcome measurement varied from study to study. This may have led to the different effect sizes reported by studies. Furthermore, most of the included studies failed to report follow-up data and a small number of trials were included in the meta-analysis to estimate the pooled follow-up effect, reducing the power of the analysis. Future studies exploring the best intervention dosage involving cost-effectiveness analysis and the most appropriate combination of different components in multi-component interventions with longer follow-up are therefore highly recommended. Due to the high heterogeneity and inadequate data, we were unable to estimate the overall effects of multi-component intervention on relationship satisfaction, empathy, and competence. Future research should incorporate more positive outcome measures.
6. Conclusions
This systematic review suggests that the integrated and diverse intervention components of multi-component interventions may help meet the changing needs of different caregivers during the progressive stages of the disease and effectively improve subjective well-being and reduce depression, anxiety, and burden among informal caregivers of people with dementia. Primary health professionals are important for dementia caregiving support, and they should be trained to better leverage their strengths of close contact and trust with caregivers to apply these effective multi-component interventions to informal caregivers of people with dementia. It is strongly suggested that intervention practice should focus on pre-intervention assessment and delivery methods should be tailored according to caregivers’ personal situations and preferences. Considering the lack of studies exploring the impact of multi-component interventions on caregiver well-being, especially the positive aspects, more rigorous RCTs incorporating more positive outcomes and with longer follow-up time are needed. It is also recommended that future studies have detailed information on the interventions to explore the best combination of the different components when practiced in the community.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijerph19126973/s1, Table S1: PRISMA 2020 Checklist; Table S2: The full electronic search strategy for the databases; Table S3: Characteristics of included studies, References [70,71,72,73,74,75,76,77,78,79,80] are cited in the supplementary materials; Table S4: The results of subgroup analysis by delivery methods and participant types; Figure S1: Risk of bias in included studies; Figure S2: Effect of multi-component interventions on informal caregiver’s burden, anxiety and stress (post-intervention); Figure S3: Funnel plots assessing publication bias on the meta-analysis of multicomponent intervention on informal caregiver outcomes (i.e., subjective well-being, depression, burden, anxiety, stress).
Author Contributions
J.H. and J.W. contributed to the design of the study. J.H., H.Z. and C.G. undertook the searches and screened studies for eligibility, assessed the quality of the papers, and conducted statistical analyzes. J.H. drafted the original manuscript. J.W. reviewed and edited the manuscript. J.W. completed fund acquisition. All authors revised the important contents of the manuscript and approved the final version. All authors have read and agreed to the published version of the manuscript.
Funding
The National Social Science Fund of China (Grant No. 20XRK004).
Institutional Review Board Statement
A review protocol was published with PROSPERO (registration number: CRD42020197120).
Informed Consent Statement
Not applicable.
Data Availability Statement
All data generated or analyzed during this study are included in this article and supplementary materials. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
No conflict of interest has been declared by the authors.
References
- World Health Organization (WHO). Dementia: A Public Health Priority; World Health Organization: Geneva, Switzerland, 2012.
- Alzheimer’s Disease International. World Alzheimer Report 2018: The State of the Art of Dementia Research; Alzheimer’s Disease International: London, UK, 2018; pp. 1–46. [Google Scholar]
- Wang, H.; Xie, H.; Qu, Q.; Chen, W.; Sun, Y.; Zhang, N.; Liu, Y.; Li, T.; Chan, K.Y.; Gauthier, S.; et al. The Continuum of Care for Dementia: Needs, Resources and Practice in China. J. Glob. Health 2019, 9, 020321. [Google Scholar] [CrossRef]
- Alzheimer’s Disease International (ADI). World Alzheimer Report 2015: The Global Impact of Dementia; Alzheimer’s Disease International: London, UK, 2015. [Google Scholar]
- Collins, R.N.; Kishita, N. Prevalence of Depression and Burden among Informal Caregivers of People with Dementia: A Meta-Analysis. Ageing Soc. 2020, 40, 2355–2392. [Google Scholar] [CrossRef] [Green Version]
- Lindert, J.; Bain, P.A.; Kubzansky, L.D.; Stein, C. Well-Being Measurement and the WHO Health Policy Health 2010: Systematic Review of Measurement Scales. Eur. J. Public Health 2015, 25, 731–740. [Google Scholar] [CrossRef] [Green Version]
- Huber, M.; Knottnerus, J.A.; Green, L.; Van der Horst, H.; Jadad, A.R.; Kromhout, D.; Leonard, B.; Lorig, K.; Loureiro, M.I.; Van der Meer, J.W.; et al. How Should We Define Health? BMJ 2011, 343, d4163. [Google Scholar] [CrossRef] [Green Version]
- Cunningham, N.A.; Cunningham, T.R.; Roberston, J.M. Understanding and Measuring the Wellbeing of Carers of People with Dementia. Gerontologist 2019, 59, e552–e564. [Google Scholar] [CrossRef]
- Farhadi, A.; Foroughan, M.; Mohammadi, F.; Rassouli, M.; Moghadam, L.S.; Nazari, S.; Sadeghi, N. Caregiving Appraisal in Family Caregivers of Older Adults. Salmand-Iran. J. Ageing 2016, 11, 8–18. [Google Scholar] [CrossRef] [Green Version]
- Stansfeld, J.; Stoner, C.R.; Wenborn, J.; Vernooij-Dassen, M.; Moniz-Cook, E.; Orrell, M. Positive Psychology Outcome Measures for Family Caregivers of People Living with Dementia: A Systematic Review. Int. Psychogeriatr. 2017, 29, 1281–1296. [Google Scholar] [CrossRef] [Green Version]
- Yu, D.S.F.; Cheng, S.-T.; Wang, J. Unravelling Positive Aspects of Caregiving in Dementia: An Integrative Review of Research Literature. Int. J. Nurs. Stud. 2018, 79, 1–26. [Google Scholar] [CrossRef]
- Kajiwara, K.; Noto, H.; Yamanaka, M. Changes in Caregiving Appraisal among Family Caregivers of Persons with Dementia: A Longitudinal Study Over 12 Months. Psychogeriatrics 2018, 18, 460–467. [Google Scholar] [CrossRef]
- Yoon, H.K.; Kim, G.S. An Empowerment Program for Family Caregivers of People with Dementia. Public Health Nurs. 2020, 37, 222–233. [Google Scholar] [CrossRef]
- Elvish, R.; Lever, S.-J.; Johnstone, J.; Cawley, R.; Keady, J. Psychological Interventions for Carers of People with Dementia: A Systematic Review of Quantitative and Qualitative Evidence. Couns. Psychother. Res. 2013, 13, 106–125. [Google Scholar] [CrossRef]
- National Institute for Health and Care Excellence (UK). Dementia: Assessment, Management and Support for People Living with Dementia and Their Carers; National Institute for Health and Care Excellence: London, UK, 2018. [Google Scholar]
- Cheung, K.S.-L.; Lau, B.H.-P.; Wong, P.W.-C.; Leung, A.Y.-M.; Lou, V.W.Q.; Chan, G.M.-Y.; Schulz, R. Multicomponent Intervention on Enhancing Dementia Caregiver Well-Being and Reducing Behavioral Problems among Hong Kong Chinese: A Translational Study Based on REACH II. Int. J. Geriatr. Psychiatry 2015, 30, 460–469. [Google Scholar] [CrossRef] [PubMed]
- Dickinson, C.; Dow, J.; Gibson, G.; Hayes, L.; Robalino, S.; Robinson, L. Psychosocial Intervention for Carers of People with Dementia: What Components are Most Effective and When? A Systematic Review of Systematic Reviews. Int. Psychogeriatr. 2017, 29, 31–43. [Google Scholar] [CrossRef]
- Waldorff, F.B.; Buss, D.V.; Eckermann, A.; Rasmussen, M.L.H.; Keiding, N.; Rishøj, S.; Siersma, V.; Sørensen, J.; Sørensen, L.V.; Vogel, A.; et al. Efficacy of Psychosocial Intervention in Patients with Mild Alzheimer’s Disease: The Multicentre, Rater Blinded, Randomised Danish Alzheimer Intervention Study (DAISY). BMJ Br. Med. J. 2012, 345, e4693. [Google Scholar] [CrossRef] [Green Version]
- Possin, K.L.; Merrilees, J.J.; Dulaney, S.; Bonasera, S.J.; Chiong, W.; Lee, K.; Hooper, S.M.; Allen, I.E.; Braley, T.; Bernstein, A.; et al. Effect of Collaborative Dementia Care via Telephone and Internet on Quality of Life, Caregiver Well-being, and Health Care Use: The Care Ecosystem Randomized Clinical Trial. JAMA Intern. Med. 2019, 179, 1658–1667. [Google Scholar] [CrossRef]
- Kor, P.P.K.; Liu, J.Y.W.; Chien, W.T. Effects of a Modified Mindfulness-Based Cognitive Therapy for Family Caregivers of People with Dementia: A Pilot Randomized Controlled Trial. Int. J. Nurs. Stud. 2019, 98, 107–117. [Google Scholar] [CrossRef]
- Blom, M.M.; Zarit, S.H.; Groot Zwaaftink, R.B.; Cuijpers, P.; Pot, A.M. Effectiveness of an Internet Intervention for Family Caregivers of People with Dementia: Results of a Randomized Controlled Trial. PLoS ONE 2015, 10, e0116622. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Metcalfe, A.; Jones, B.; Mayer, J.; Gage, H.; Oyebode, J.; Boucault, S.; Aloui, S.; Schwertel, U.; Böhm, M.; Tezenas du Montcel, S.; et al. Online Information and Support for Carers of People with Young-Onset Dementia: A Multi-Site Randomised Controlled Pilot Study. Int. J. Geriatr. Psychiatry 2019, 34, 1455–1464. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.X.; Zhang, H.Y. Study of the Effect of Psychosocial Intervention on Depressive Psychology and Care Burden of Family Caregivers of Dementia Patients. Shanghai Med. Pharm. J. 2020, 41, 13–15, 49. (In Chinese) [Google Scholar]
- Berwig, M.; Heinrich, S.; Spahlholz, J.; Hallensleben, N.; Brahler, E.; Gertz, H.-J. Individualized Support for Informal Caregivers of People with Dementia-Effectiveness of the German Adaptation of REACH II. BMC Geriatr. 2017, 17, 286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liew, T.M.; Lee, C.S. Reappraising the Efficacy and Acceptability of Multicomponent Interventions for Caregiver Depression in Dementia: The Utility of Network Meta-Analysis. Gerontologist 2019, 59, e380–e392. [Google Scholar] [CrossRef]
- Ying, J.; Wang, Y.; Zhang, M.; Wang, S.; Shi, Y.; Li, H.; Li, Y.; Xing, Z.; Sun, J. Effect of Multicomponent Interventions on Competence of Family Caregivers of People with Dementia: A Systematic Review. J. Clin. Nurs. 2018, 27, 1744–1758. [Google Scholar] [CrossRef] [PubMed]
- Laver, K.; Milte, R.; Dyer, S.; Crotty, M. A Systematic Review and Meta-Analysis Comparing Carer Focused and Dyadic Multicomponent Interventions for Carers of People with Dementia. J. Aging Health 2017, 29, 1308–1349. [Google Scholar] [CrossRef] [Green Version]
- Walter, E.; Pinquart, M. How Effective Are Dementia Caregiver Interventions? An Updated Comprehensive Meta-Analysis. Gerontologist 2020, 60, e609–e619. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.T.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. (Eds.) Cochrane Handbook for Systematic Reviews of Interventions Version 6.3; Cochrane: London, UK, 2022. [Google Scholar]
- Lomas, T.; Medina, J.C.; Ivtzan, I.; Rupprecht, S.; Eiroa-Orosa, F.J. A Systematic Review and Meta-Analysis of the Impact of Mindfulness-Based Interventions on the Well-Being of Healthcare Professionals. Mindfulness 2019, 10, 1193–1216. [Google Scholar] [CrossRef] [Green Version]
- Higgins, J.P.T.; Green, S. (Eds.) Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0; Cochrane: London, UK, 2011. [Google Scholar]
- Jang, Y.; Clay, O.J.; Roth, D.L.; Haley, W.E.; Mittelman, M.S. Neuroticism and Longitudinal Change in Caregiver Depression: Impact of a Spouse-Caregiver Intervention Program. Gerontologist 2004, 44, 311–317. [Google Scholar] [CrossRef] [Green Version]
- Shang, S.M.; Wang, Z.W.; Deng, S.H.; Liu, Q.S.; Fu, Y.; Yue, P.; Zhang, H. Effects of the Nursing Intervention on Caregivers’ Burden for Dementia Patients in Community. J. Nurs. Adm. 2011, 11, 88–90, 101. (In Chinese) [Google Scholar]
- Mittelman, M.S.; Ferris, S.H.; Shulman, E.; Steinberg, G.; Ambinder, A.; Mackell, J.A.; Cohen, J. A Comprehensive Support Program: Effect on Depression in Spouse-Caregivers of AD Patients. Gerontologist 1995, 35, 792–802. [Google Scholar] [CrossRef] [PubMed]
- Belle, S.H.; Burgio, L.; Burns, R.; Coon, D.; Czaja, S.J.; Gallagher-Thompson, D.; Gitlin, L.N.; Klinger, J.; Koepke, K.M.; Lee, C.C.; et al. Enhancing the Quality of Life of Dementia Caregivers from Different Ethnic or Racial Groups—A Randomized, Controlled Trial. Ann. Intern. Med. 2006, 145, 727–738. [Google Scholar] [CrossRef]
- Birkenhäger-Gillesse, E.G.; Achterberg, W.P.; Janus, S.I.M.; Kollen, B.J.; Zuidema, S.U. Effects of Caregiver Dementia Training in Caregiver-Patient Dyads: A Randomized Controlled Study. Int. J. Geriatr. Psychiatry 2020, 35, 1376–1384. [Google Scholar] [CrossRef]
- Elliott, A.F.; Burgio, L.D.; Decoster, J. Enhancing Caregiver Health: Findings from the Resources for Enhancing Alzheimer’s Caregiver Health II Intervention. J. Am. Geriatr. Soc. 2010, 58, 30–37. [Google Scholar] [CrossRef]
- Gitlin, L.N.; Winter, L.; Dennis, M.P.; Hodgson, N.; Hauck, W.W. A Biobehavioral Home-Based Intervention and the Well-Being of Patients with Dementia and Their Caregivers: The COPE Randomized Trial. JAMA 2010, 304, 983–991. [Google Scholar] [CrossRef]
- Laakkonen, M.-L.; Kautiainen, H.; Holtta, E.; Savikko, N.; Tilvis, R.S.; Strandberg, T.E.; Pitkala, K.H. Effects of Self-Management Groups for People with Dementia and Their Spouses-Randomized Controlled Trial. J. Am. Geriatr. Soc. 2016, 64, 752–760. [Google Scholar] [CrossRef] [PubMed]
- Marriott, A.; Donaldson, C.; Tarrier, N.; Burns, A. Effectiveness of Cognitive-Behavioural Family Intervention in Reducing the Burden of Care in Carers of Patients with Alzheimer’s Disease. Br. J. Psychiatry 2000, 176, 557–562. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prick, A.-E.; De Lange, J.; Twisk, J.; Pot, A.M. The Effects of a Multi-Component Dyadic Intervention on the Psychological Distress of Family Caregivers Providing Care to People with Dementia: A Randomized Controlled Trial. Int. Psychogeriatr. 2015, 27, 2031–2044. [Google Scholar] [CrossRef] [Green Version]
- Cristancho-Lacroix, V.; Wrobel, J.; Cantegreil-Kallen, I.; Dub, T.; Rouquette, A.; Rigaud, A.-S. A Web-Based Psychoeducational Program for Informal Caregivers of Patients with Alzheimer’s Disease: A Pilot Randomized Controlled Trial. J. Med. Internet Res. 2015, 17, e117. [Google Scholar] [CrossRef] [PubMed]
- Duggleby, W.; Ploeg, J.; McAiney, C.; Peacock, S.; Fisher, K.; Ghosh, S.; Markle-Reid, M.; Swindle, J.; Williams, A.; Triscott, J.A.; et al. Web-Based Intervention for Family Carers of Persons with Dementia and Multiple Chronic Conditions (My Tools 4 Care): Pragmatic Randomized Controlled Trial. J. Med. Internet Res. 2018, 20, e10484. [Google Scholar] [CrossRef]
- Au, A.; Wong, M.K.; Leung, L.M.; Leung, P.; Wong, A. Telephone-Assisted Pleasant-Event Scheduling to Enhance Well-Being of Caregivers of People with Dementia: A Randomised Controlled Trial. Hong Kong Med. J. 2014, 20, 30–33. [Google Scholar]
- Hattink, B.; Meiland, F.; Van der Roest, H.; Kevern, P.; Abiuso, F.; Bengtsson, J.; Giuliano, A.; Duca, A.; Sanders, J.; Basnett, F.; et al. Web-Based STAR E-Learning Course Increases Empathy and Understanding in Dementia Caregivers: Results from a Randomized Controlled Trial in the Netherlands and the United Kingdom. J. Med. Internet Res. 2015, 17, 15. [Google Scholar] [CrossRef] [PubMed]
- Shata, Z.N.; Amin, M.R.; El-Kady, H.M.; Abu-Nazel, M.W. Efficacy of a Multi-Component Psychosocial Intervention Program for Caregivers of Persons Living with Neurocognitive Disorders, Alexandria, Egypt: A randomized Controlled Trial. Avicenna J. Med. 2017, 7, 54–63. [Google Scholar] [CrossRef]
- Hepburn, K.W.; Tornatore, J.; Center, B.; Ostwald, S.W. Dementia Family Caregiver Training: Affecting Beliefs about Caregiving and Caregiver Outcomes. J. Am. Geriatr. Soc. 2001, 49, 450–457. [Google Scholar] [CrossRef]
- Chiao, C.Y.; Wu, H.S.; Hsiao, C.Y. Caregiver Burden for Informal Caregivers of Patients with Dementia: A Systematic Review. Int. Nurs. Rev. 2015, 62, 340–350. [Google Scholar] [CrossRef]
- World Health Organization. Global Action Plan on the Public Health Response to Dementia 2017–2025. Available online: https://www.who.int/mental_health/neurology/dementia/action_plan_2017_2025/en/ (accessed on 31 March 2017).
- Moreno-Camara, S.; Palomino-Moral, P.A.; Moral-Fernandez, L.; Frias-Osuna, A.; Parra-Anguita, L.; Del-Pino-Casado, R. Perceived Needs of the Family Caregivers of People with Dementia in a Mediterranean Setting: A Qualitative Study. Int. J. Environ. Res. Public Health 2019, 16, 993. [Google Scholar] [CrossRef] [Green Version]
- Abrahams, R.; Liu, K.P.Y.; Bissett, M.; Fahey, P.; Cheung, K.S.L.; Bye, R.; Chaudhary, K.; Chu, L.W. Effectiveness of Interventions for Co-residing Family Caregivers of People with Dementia: Systematic Review and Meta-analysis. Aust. Occup. Ther. J. 2018, 65, 208–224. [Google Scholar] [CrossRef] [PubMed]
- Wasilewski, M.B.; Stinson, J.N.; Cameron, J.I. Web-based Health Interventions for Family Caregivers of Elderly Individuals: A Scoping Review. Int. J. Med. Inform. 2017, 103, 109–138. [Google Scholar] [CrossRef] [PubMed]
- Pink, J.; O’Brien, J.; Robinson, L.; Longson, D. Dementia: Assessment, Management and Support: Summary of Updated NICE Guidance. BMJ 2018, 361, k2438. [Google Scholar] [CrossRef] [PubMed]
- Kishita, N.; Hammond, L.; Dietrich, C.M.; Mioshi, E. Which Interventions Work for Dementia Family Carers?: An Updated Systematic Review of Randomized Controlled Trials of Carer Interventions. Int. Psychogeriatr. 2018, 30, 1679–1696. [Google Scholar] [CrossRef]
- Levinson, A.J.; Ayers, S.; Butler, L.; Papaioannou, A.; Marr, S.; Sztramko, R. Barriers and Facilitators to Implementing Web-Based Dementia Caregiver Education from the Clinician’s Perspective: Qualitative Study. JMIR Aging 2020, 3, e21264. [Google Scholar] [CrossRef] [PubMed]
- Deeken, F.; Rezo, A.; Hinz, M.; Discher, R.; Rapp, M.A. Evaluation of Technology-Based Interventions for Informal Caregivers of Patients with Dementia-A Meta-Analysis of Randomized Controlled Trials. Am. J. Geriatr. Psychiatry 2019, 27, 426–445. [Google Scholar] [CrossRef] [PubMed]
- Bayly, M.; Morgan, D.; Elliot, V.; Kosteniuk, J.; Froehlich Chow, A.; Peacock, S.; O’Connell, M.E. Does Early-Stage Intervention Improve Caregiver Well-Being or Their Ability to Provide Care to Persons with Mild Dementia or Mild Cognitive impairment? A systematic review and meta-analysis. Psychol. Aging 2021, 36, 834–854. [Google Scholar] [CrossRef]
- Tang, W.K.; Chan, C.Y.J. Effects of Psychosocial Interventions on Self-Efficacy of Dementia Caregivers: A Literature Review. Int. J. Geriatr. Psychiatry 2016, 31, 475–493. [Google Scholar] [CrossRef]
- Cheung, D.S.K.; Tang, S.K.; Ho, K.H.M.; Jones, C.; Tse, M.M.Y.; Kwan, R.Y.C.; Chan, K.Y.; Chiang, V.C.L. Strategies to Engage People with Dementia and Their Informal Caregivers in Dyadic Intervention: A Scoping Review. Geriatr. Nurs. 2021, 42, 412–420. [Google Scholar] [CrossRef] [PubMed]
- Evripidou, M.; Charalambous, A.; Middleton, N.; Papastavrou, E. Nurses’ Knowledge and Attitudes about Dementia Care: Systematic Literature Review. Perspect. Psychiatr. Care 2019, 55, 48–60. [Google Scholar] [CrossRef] [Green Version]
- Dening, K.; Hibberd, P. Exploring the Community Nurse Role in Family-Centred Care for Patients with Dementia. Br. J. Community Nurs. 2016, 21, 198–202. [Google Scholar] [CrossRef]
- Wang, Y.; Xiao, L.D.; Ullah, S.; He, G.-P.; De Bellis, A. Evaluation of a Nurse-Led Dementia Education and Knowledge Translation Programme in Primary Care: A Cluster Randomized Controlled Trial. Nurse Educ. Today 2017, 49, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Van’t Leven, N.; Van der Ploeg, E.; De Lange, J.; Pot, A.M. Indicators to Estimate the Appropriateness of Activating Interventions for People Living with Dementia and for Their Informal Caregivers. Aging Ment. Health 2018, 22, 1416–1423. [Google Scholar] [CrossRef]
- Sun, Y.; Ji, M.; Leng, M.; Li, X.; Zhang, X.; Wang, Z. Comparative Efficacy of 11 Non-Pharmacological Interventions on Depression, Anxiety, Quality of Life, and Caregiver Burden for Informal Caregivers of People with Dementia: A Systematic Review and Network Meta-Analysis. Int. J. Nurs. Stud. 2022, 129, 104204. [Google Scholar] [CrossRef]
- Etxeberria, I.; Salaberria, K.; Gorostiaga, A. Online Support for Family Caregivers of People with Dementia: A Systematic Review and Meta-Analysis of RCTs and Quasi-Experimental Studies. Aging Ment. Health 2021, 25, 1165–1180. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.-T.; Zhang, F. A Comprehensive Meta-Review of Systematic Reviews and Meta-Analyses on Nonpharmacological Interventions for Informal Dementia Caregivers. BMC Geriatr. 2020, 20, 137. [Google Scholar] [CrossRef] [Green Version]
- Lee, M.; Ryoo, J.H.; Crowder, J.; Byon, H.D.; Wiiliams, I.C. A Systematic Review and Meta-Analysis on Effective Interventions for Health-Related Quality of Life among Caregivers of People with Dementia. J. Adv. Nurs. 2020, 76, 475–489. [Google Scholar] [CrossRef]
- Lapid, M.I.; Atherton, P.J.; Kung, S.; Sloan, J.A.; Shahi, V.; Clark, M.M.; Rummans, T.A. Cancer Caregiver Quality of Life: Need for Targeted Intervention. Psychooncology 2016, 25, 1400–1407. [Google Scholar] [CrossRef] [PubMed]
- Phillipson, L.; Johnson, K.; Fielding, E.; Cridland, E.; Hall, D.; Neville, C.; Hasan, H. Rethinking Respite in Australia: A Naturalistic Effect Study of a Multicomponent Community Program to Promote Respite Knowledge, Attitudes and Behaviours of Carers of People with Dementia. Health Soc. Care Community 2021, 29, 1566–1583. [Google Scholar] [CrossRef]
- Au, A.; Gallagher-Thompson, D.; Wong, M.K.; Leung, J.; Chan, W.C.; Chan, C.C.; Lu, H.J.; Lai, M.K.; Chan, K. Behavioral activation for dementia caregivers: Scheduling pleasant events and enhancing communications. Clin. Interv. Aging 2015, 10, 611–619. [Google Scholar] [CrossRef] [Green Version]
- Chien, W.T.; Lee, I.Y.M. Randomized controlled trial of a dementia care programme for families of home-resided older people with dementia. J. Adv. Nurs. 2011, 67, 774–787. [Google Scholar] [CrossRef]
- Fei, J.X.; Zhao, X.P.; Shen, L.Z. Investigation on nursing intervention improving coping style and emotional disorder of relatives of the senile dementia in hospital. Chin. J. Mod. Nurs. 2009, 15, 2249–2251. [Google Scholar] [CrossRef]
- Gonyea, J.G.; O’Connor, M.K.; Boyle, P.A. Project CARE: A randomized controlled trial of a behavioral intervention group for Alzheimer’s disease caregivers. Gerontologist 2006, 46, 827–832. [Google Scholar] [CrossRef] [Green Version]
- Han, A.; Kim, T.H.; Hong, H. A factorial randomized controlled trial to examine separate and combined effects of a simulation-based empathy enhancement program and a lecture-based education program on family caregivers of people with dementia. Aging Ment. Health 2020, 25, 1930–1940. [Google Scholar] [CrossRef]
- Huang, Y.N. Impact of comprehensive intervention on caregivers of patients with Alzheimer’s disease. Health Guide Med. Res. 2015, 7, 66–67. (In Chinese) [Google Scholar]
- Liu, Q.S.; Shang, S.M.; Wang, Z.W.; Fu, Y.; Yue, P.; Zhang, H.; Liu, Y. Effects of a one-year nursing intervention on depression and anxiety in family caregivers of homebound patients with dementia. Chin. J. Behav. Med. Sci. 2007, 16, 172–174. (In Chinese) [Google Scholar] [CrossRef]
- Luo, L.C.; Wang, J.A. Effect evaluation of nursing intervention on alleviating depression and anxiety among caregivers with Alzheimer’s disease at home. Today Nurse 2012, 10, 22–24. (In Chinese) [Google Scholar]
- Yao, A.H. Effect of cognitive-behavior intervention on caregivers of alzheimer’s disease patients. Chin. J. Mod. Nurs. 2011, 17, 373–375. (In Chinese) [Google Scholar] [CrossRef]
- Yoo, R.; Yeom, J.; Kim, G.H.; Park, H.K.; Kang, Y.; Hwang, J.; Choi, S.H.; Na, H.R.; Cho, S.J.; Yu, K.H.; et al. A multicenter, randomized clinical trial to assess the efficacy of a therapeutic intervention program for caregivers of people with dementia. J. Clin. Neurol. 2019, 15, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.; Lin, Z.; He, X.J.; Shao, Z.M. Influence of collaborative care model on the burden of family caregivers of senile dementia patients in the community. Mod. Med. J. 2016, 44, 1780–1784. (In Chinese) [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).