Use of Biological Drugs for Psoriasis: A Drug-Utilization Study Using Tuscan Administrative Databanks
Abstract
:1. Introduction
2. Materials and Methods
- the inhabitant registry, including information on gender, date of birth, and subject’s date of entry and of exit;
- the drug dispensing registry, including information on date, type, dose and number of packages of dispensed drugs by community or hospital pharmacies to subjects; drug is coded according to the Anatomical Therapeutic Chemical classification system (ATC);
- the exemption from co-payment registry, containing the release date and disease of subjects; exemption is coded according to the Italian exemption code;
- the hospital discharge registry, including information on date of hospital admission, date of hospital discharge, primary and secondary diagnoses and procedures of subjects admitted to the hospital; diagnosis is coded according to the ICD-9-CM;
- the emergency department (ED) registry, containing information on date of ED admission, date of ED discharge, primary and secondary diagnoses of subjects admitted to ED; diagnosis is coded according to the ICD-9-CM;
- the outpatient services registry, collecting date of visit/test, record of specialist encounters, without diagnosis code, and diagnostic tests without results of subjects attending visits;
- the certificates of childbirth assistance, terminations, and miscarriages records, including information on duration of gestation, date of delivery, duration of amenorrhea, date of termination of pregnancy.
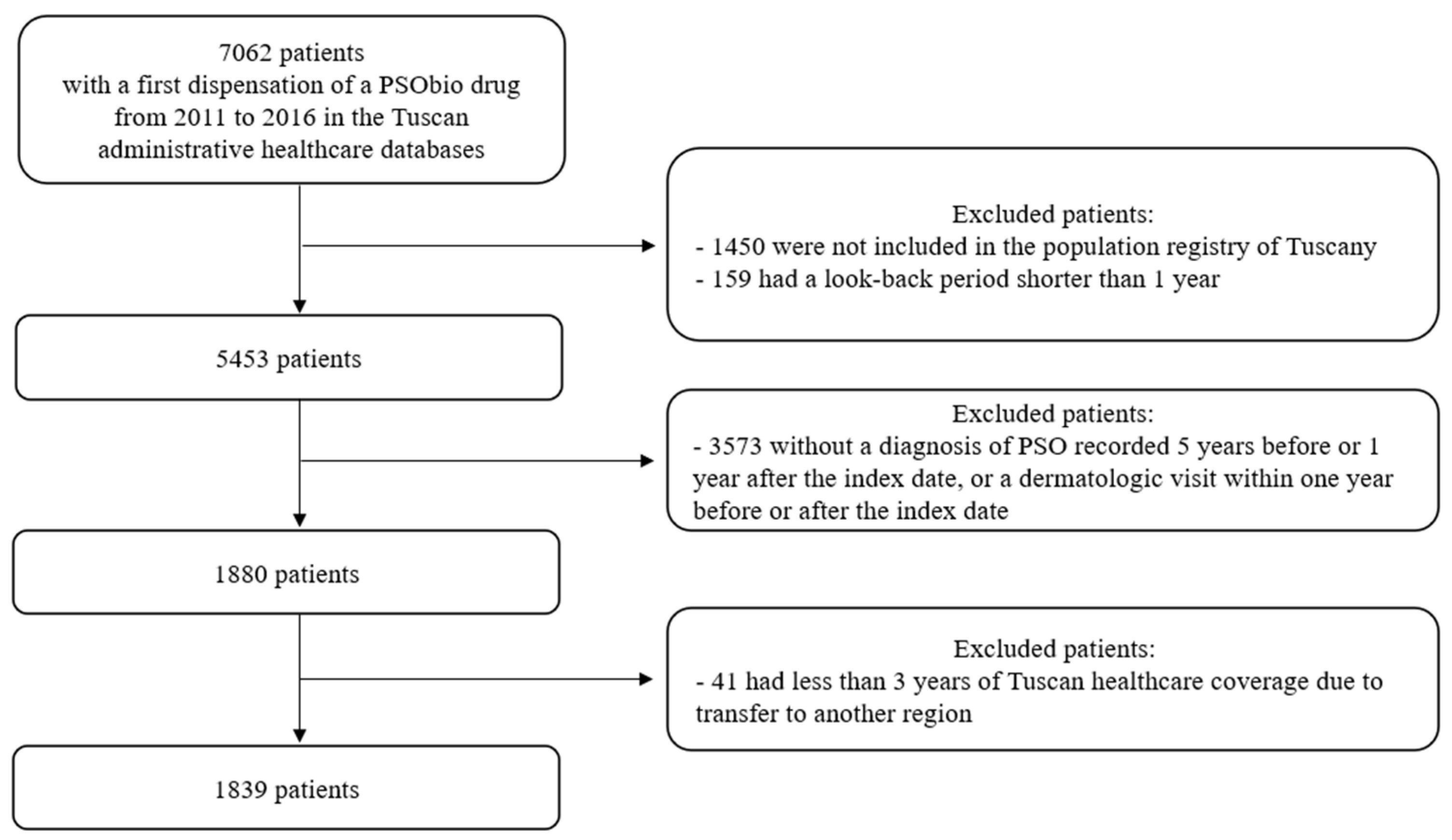
2.1. Study Population and Cohort Definition
2.2. Follow-Up
2.3. Study Period
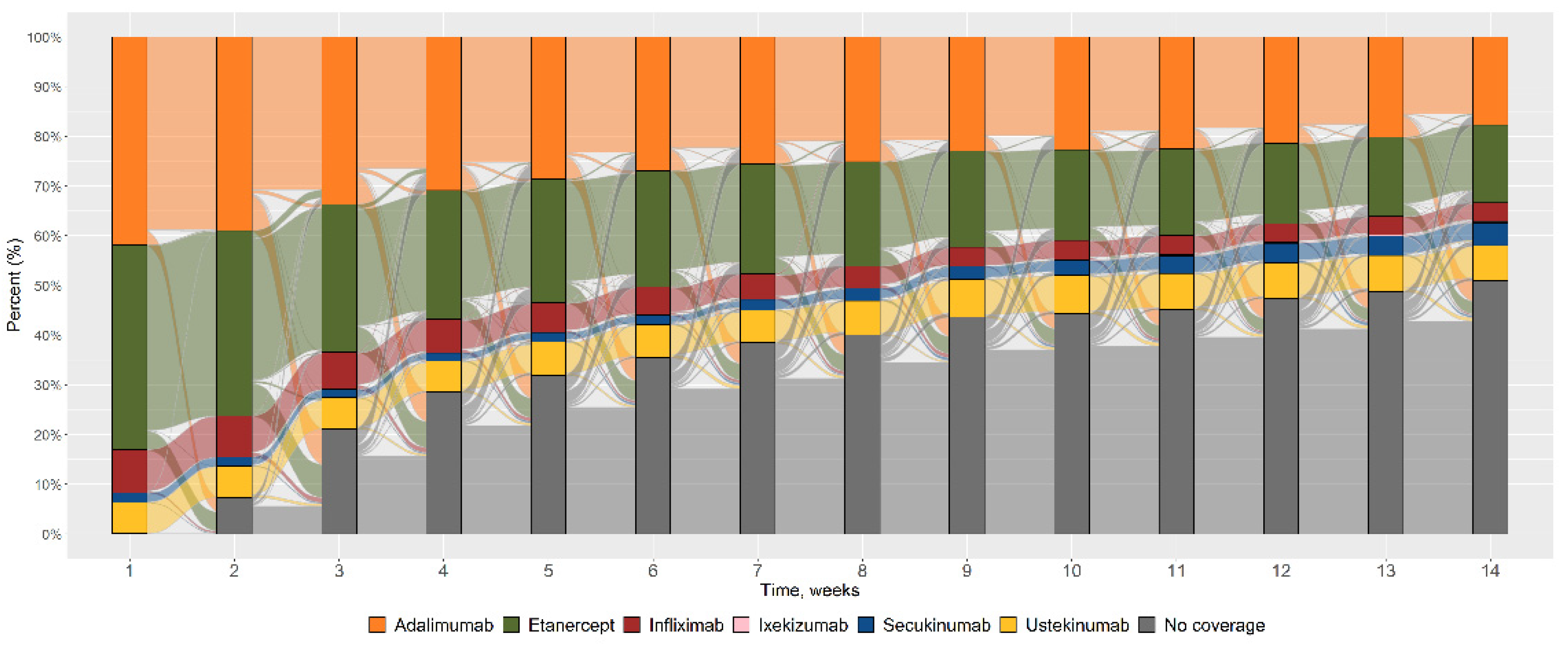
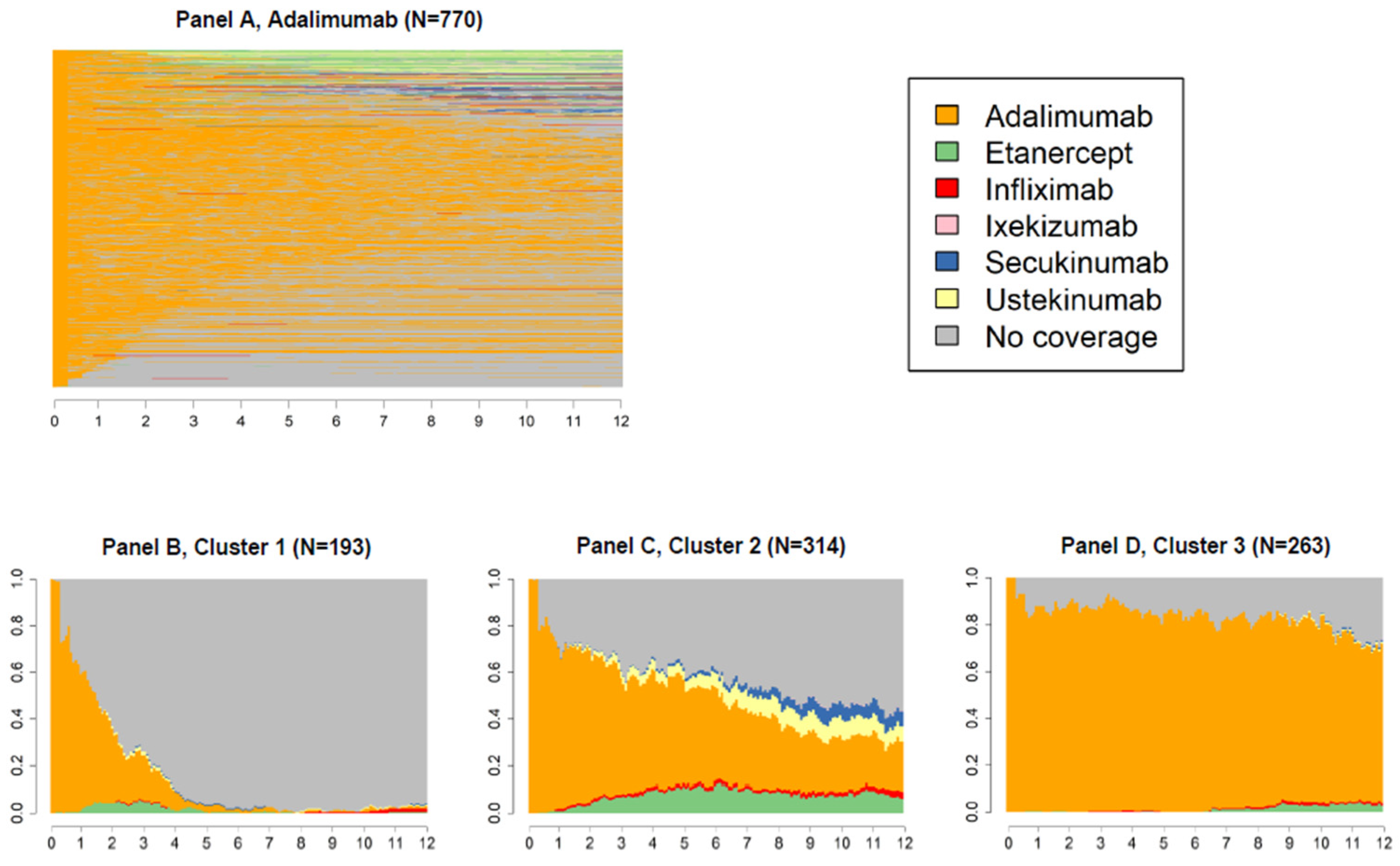
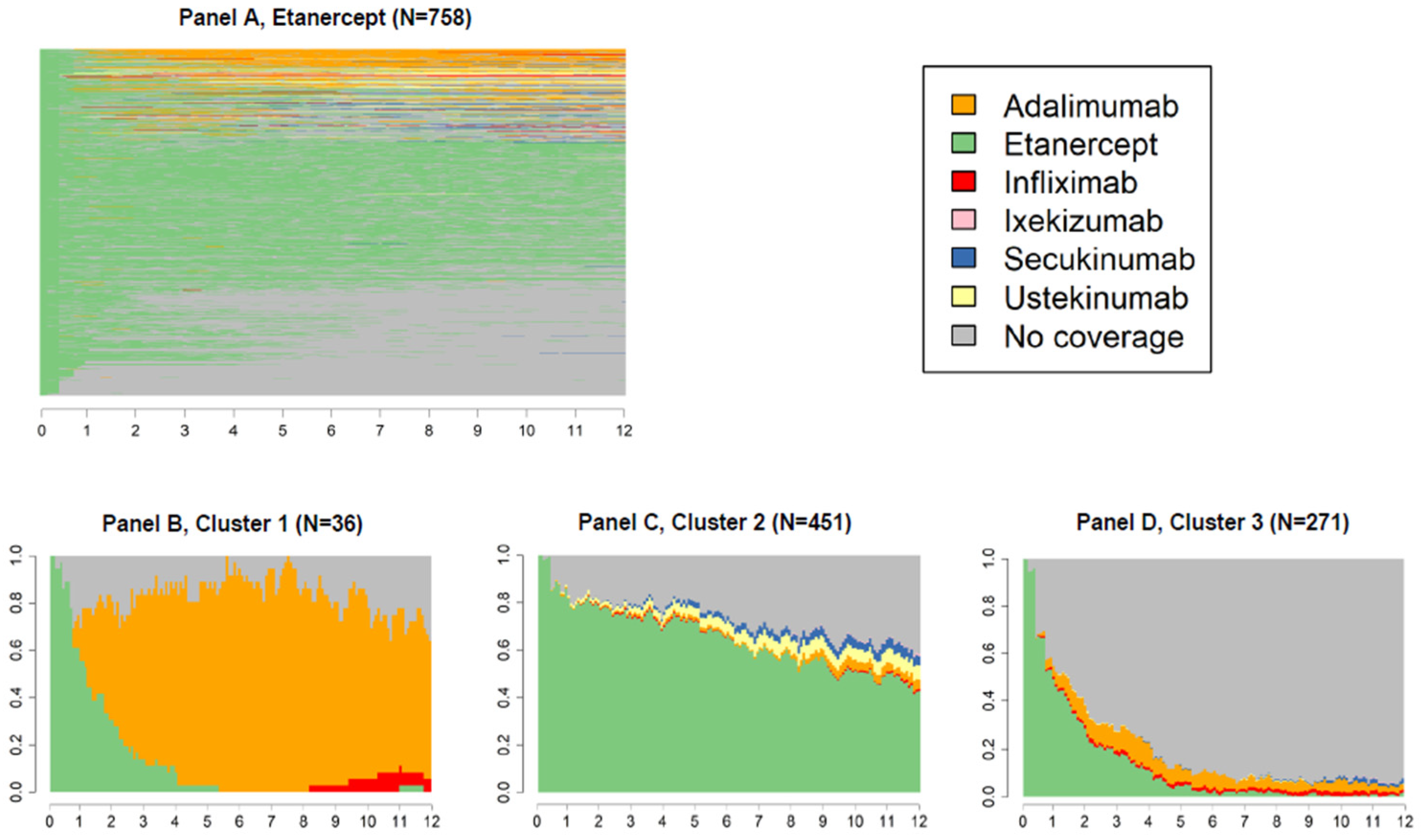
2.4. Variable of Interest: Switch
2.5. Covariates
2.6. Data Analysis
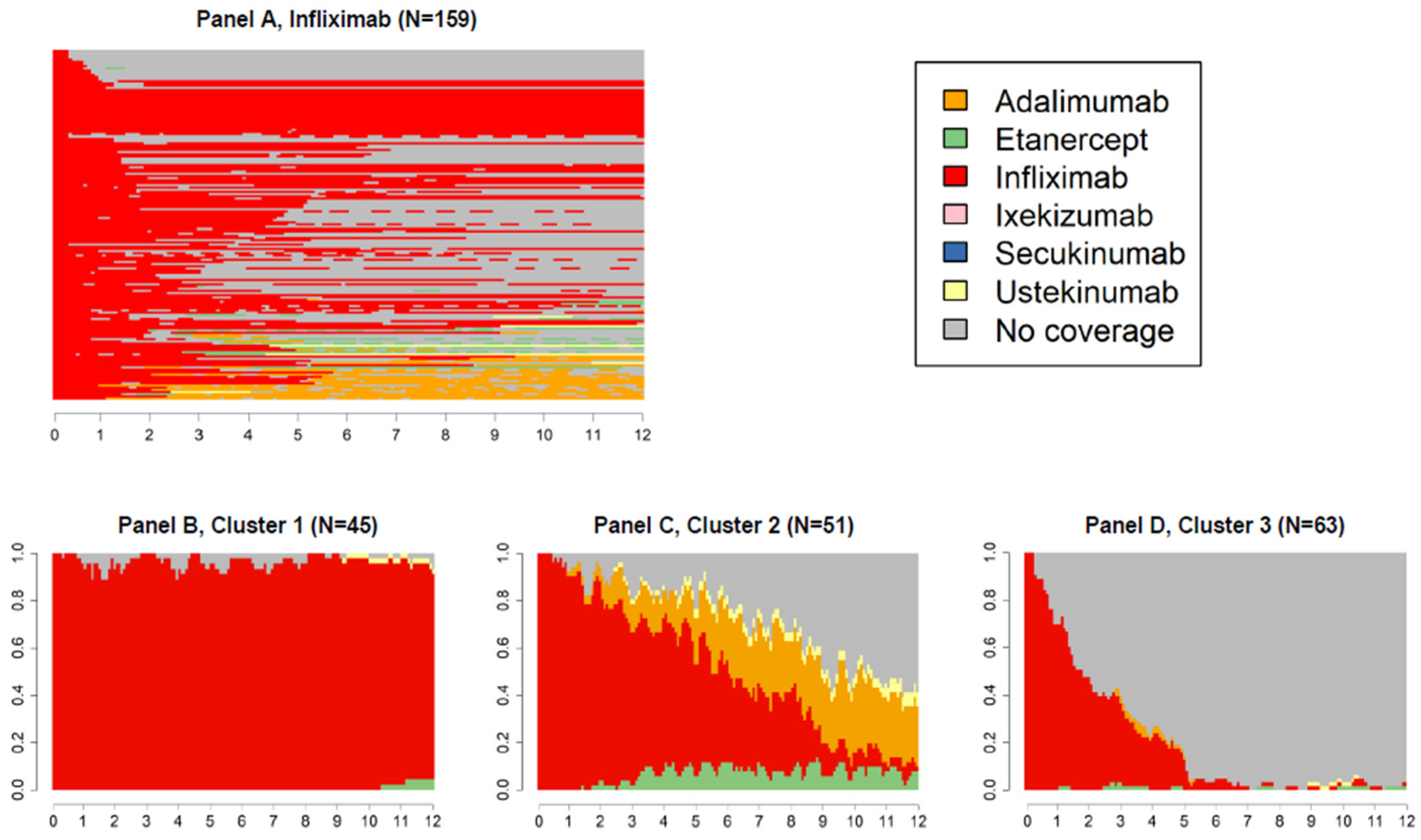
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Griffiths, C.E.M.; Armstrong, A.W.; Gudjonsson, J.E.; Barker, J.N.W.N. Psoriasis. Lancet 2021, 397, 1301–1315. [Google Scholar] [CrossRef]
- Boehncke, W.H.; Schön, M.P. Psoriasis. Lancet 2015, 386, 983–994. [Google Scholar] [CrossRef]
- Nast, A.; Smith, C.; Spuls, P.I.; Avila Valle, G.; Bata-Csörgö, Z.; Boonen, H.; de Jong, E.; Garcia-Doval, I.; Gisondi, P.; Kaur-Knudsen, D.; et al. EuroGuiDerm Guideline on the Systemic Treatment of Psoriasis Vulgaris-Part 1: Treatment and Monitoring Recommendations. J. Eur. Acad. Dermatol. Venereol. 2020, 34, 2461–2498. [Google Scholar] [CrossRef] [PubMed]
- Gordon, P.M.; Diffey, B.L.; Matthews, J.N.S.; Farr, P.M. A Randomized Comparison of Narrow-Band TL-01 Phototherapy and PUVA Photochemotherapy for Psoriasis. J. Am. Acad. Dermatol. 1999, 41, 728–732. [Google Scholar] [CrossRef]
- Diette, K.M.; Momtaz, K.; Stern, R.S.; Arndt, K.A.; Parrish, J.A. Psoralens and UV-A and UV-B Twice Weekly for the Treatment of Psoriasis. Arch. Dermatol. 1984, 120, 1169–1173. [Google Scholar] [CrossRef] [PubMed]
- Calzavara-Pinton, P.; Ortel, B.; Carlino, A.; Honigsmann, H.; de Panfilis, G. A Reappraisal of the Use of 5-methoxypsoralen in the Therapy of Psoriasis. Exp. Dermatol. 1992, 1, 46–51. [Google Scholar] [CrossRef]
- Henseler, T.; Hönigsmann, H.; Wolff, K.; Christophers, E. ORAL 8-METHOXYPSORALEN PHOTOCHEMOTHERAPY OF PSORIASIS. The European PUVA Study: A Cooperative Study among 18 European Centres. Lancet 1981, 317, 853–857. [Google Scholar] [CrossRef]
- Amoruso, G.F.; Nisticò, S.P.; Iannone, L.; Russo, E.; Rago, G.; Patruno, C.; Bennardo, L. Ixekizumab May Improve Renal Function in Psoriasis. Healthcare 2021, 9, 543. [Google Scholar] [CrossRef]
- Dastoli, S.; Nisticò, S.P.; Morrone, P.; Patruno, C.; Leo, A.; Citraro, R.; Gallelli, L.; Russo, E.; de Sarro, G.; Bennardo, L. Colchicine in Managing Skin Conditions: A Systematic Review. Pharmaceutics 2022, 14, 294. [Google Scholar] [CrossRef]
- Woo, Y.R.; Cho, D.H.; Park, H.J. Molecular Mechanisms and Management of a Cutaneous Inflammatory Disorder: Psoriasis. Int. J. Mol. Sci. 2017, 18, 2684. [Google Scholar] [CrossRef] [Green Version]
- del Duca, E.; Morelli, P.; Bennardo, L.; di Raimondo, C.; Nisticò, S.P. Cytokine Pathways and Investigational Target Therapies in Hidradenitis Suppurativa. Int. J. Mol. Sci. 2020, 21, 8436. [Google Scholar] [CrossRef] [PubMed]
- Gabadinho, A.; Ritschard, G.; Müller, N.S.; Studer, M. Analyzing and Visualizing State Sequences in R with TraMineR. J. Stat. Softw. 2011, 40, 1–37. [Google Scholar] [CrossRef] [Green Version]
- Doshi, J.A.; Takeshita, J.; Pinto, L.; Li, P.; Yu, X.; Rao, P.; Viswanathan, H.N.; Gelfand, J.M. Biologic Therapy Adherence, Discontinuation, Switching, and Restarting among Patients with Psoriasis in the US Medicare Population. J. Am. Acad. Dermatol. 2016, 74, 1057–1065. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hsu, D.Y.; Gniadecki, R. Patient Adherence to Biologic Agents in Psoriasis. Dermatology 2016, 232, 326–333. [Google Scholar] [CrossRef]
- Wu, B.; Muser, E.; Teeple, A.; Pericone, C.D.; Feldman, S.R. Treatment Adherence and Persistence of Five Commonly Prescribed Medications for Moderate to Severe Psoriasis in a U.S. Commercially Insured Population. J. Dermatol. Treat. 2020, 32, 595–602. [Google Scholar] [CrossRef]
- Piragine, E.; Petri, D.; Martelli, A.; Janowska, A.; Dini, V.; Romanelli, M.; Calderone, V.; Lucenteforte, E. Adherence and Persistence to Biological Drugs for Psoriasis: Systematic Review with Meta-Analysis. J. Clin. Med. 2022, 11, 1506. [Google Scholar] [CrossRef]
- Brownstone, N.D.; Hong, J.; Mosca, M.; Hadeler, E.; Liao, W.; Bhutani, T.; Koo, J. Biologic Treatments of Psoriasis: An Update for the Clinician. Biol. Targets Ther. 2021, 15, 39. [Google Scholar] [CrossRef]
- Piaserico, S.; Cazzaniga, S.; Chimenti, S.; Giannetti, A.; MacCarone, M.; Picardo, M.; Peserico, A.; Naldi, L.; Griseta, V.; Miracapillo, A.; et al. Efficacy of Switching between Tumor Necrosis Factor-Alfa Inhibitors in Psoriasis: Results from the Italian Psocare Registry. J. Am. Acad. Dermatol. 2014, 70, 257–262.e3. [Google Scholar] [CrossRef]
- Ortonne, J.P.; Chimenti, S.; Reich, K.; Gniadecki, R.; Sprøgel, P.; Unnebrink, K.; Kupper, H.; Goldblum, O.; Thaçi, D. Efficacy and Safety of Adalimumab in Patients with Psoriasis Previously Treated with Anti-Tumour Necrosis Factor Agents: Subanalysis of BELIEVE. J. Eur. Acad. Dermatol. Venereol. 2011, 25, 1012–1020. [Google Scholar] [CrossRef]
- Papoutsaki, M.; Chimenti, M.S.; Costanzo, A.; Talamonti, M.; Zangrilli, A.; Giunta, A.; Bianchi, L.; Chimenti, S. Adalimumab for Severe Psoriasis and Psoriatic Arthritis: An Open-Label Study in 30 Patients Previously Treated with Other Biologics. J. Am. Acad. Dermatol. 2007, 57, 269–275. [Google Scholar] [CrossRef]
- Talamonti, M.; Galluzzo, M.; Bernardini, N.; Caldarola, G.; Persechino, S.; Cantoresi, F.; Egan, C.G.; Potenza, C.; Peris, K.; Bianchi, L. Psoriasis Area and Severity Index Response in Moderate-Severe Psoriatic Patients Switched to Adalimumab: Results from the OPPSA Study. J. Eur. Acad. Dermatol. Venereol. 2018, 32, 1737–1744. [Google Scholar] [CrossRef] [PubMed]
- Bissonnette, R.; Bolduc, C.; Poulin, Y.; Guenther, L.; Lynde, C.W.; Maari, C. Efficacy and Safety of Adalimumab in Patients with Plaque Psoriasis Who Have Shown an Unsatisfactory Response to Etanercept. J. Am. Acad. Dermatol. 2010, 63, 228–234. [Google Scholar] [CrossRef] [PubMed]
- van Lümig, P.P.M.; Lecluse, L.L.A.; Driessen, R.J.B.; Spuls, P.I.; Boezeman, J.B.; van de Kerkhof, P.C.M.; de Jong, E.M.G.J. Switching from Etanercept to Adalimumab Is Effective and Safe: Results in 30 Patients with Psoriasis with Primary Failure, Secondary Failure or Intolerance to Etanercept. Br. J. Dermatol. 2010, 163, 838–846. [Google Scholar] [CrossRef]
- Ayala, F.; Lambert, J.; Lembo, S. Efficacy, Tolerability and Safety of Switching from Etanercept to Infliximab for the Treatment of Moderate-to-Severe Psoriasis: A Multicenter, Open-Label Trial (TANGO). J. Dermatol. Treat. 2015, 26, 304–311. [Google Scholar] [CrossRef] [PubMed]
- Chiricozzi, A.; Conti, A.; Burlando, M.; Odorici, G.; Gaiani, F.; Panduri, S.; Malagoli, P. Switching from Secukinumab to Ustekinumab in Psoriasis Patients: Results from a Multicenter Experience. Dermatology 2019, 235, 213–218. [Google Scholar] [CrossRef]
- Damiani, G.; Conic, R.R.Z.; de Vita, V.; Costanzo, A.; Regazzini, R.; Pigatto, P.D.M.; Bragazzi, N.L.; Pacifico, A.; Malagoli, P. When IL-17 Inhibitors Fail: Real-Life Evidence to Switch from Secukinumab to Adalimumab or Ustekinumab. Dermatol. Ther. 2019, 32, e12793. [Google Scholar] [CrossRef]
- Haitz, K.A.; Kalb, R.E. Infliximab in the Treatment of Psoriasis in Patients Previously Treated with Etanercept. J. Am. Acad. Dermatol. 2007, 57, 120–125. [Google Scholar] [CrossRef]
- Gottlieb, A.B.; Kalb, R.E.; Blauvelt, A.; Heffernan, M.P.; Sofen, H.L.; Ferris, L.K.; Kerdel, F.A.; Calabro, S.; Wang, J.; Kerkmann, U.; et al. The Efficacy and Safety of Infliximab in Patients with Plaque Psoriasis Who Had an Inadequate Response to Etanercept: Results of a Prospective, Multicenter, Open-Label Study. J. Am. Acad. Dermatol. 2012, 67, 642–650. [Google Scholar] [CrossRef]
- Woolf, R.T.; Smith, C.H.; Robertson, K.; Barker, J.N.W.N. Switching to Adalimumab in Patients with Moderate to Severe Psoriasis Who Have Failed on Etanercept: A Retrospective Case Cohort Study. Br. J. Dermatol. 2010, 163, 889–892. [Google Scholar] [CrossRef]
- Fonseca, E.; Iglesias, R.; Paradela, S.; Fernández-Torres, R.M.; Elberdín, L. Efficacy and Safety of Adalimumab in Psoriatic Patients Previously Treated with Etanercept in a Real-World Setting. J. Dermatol. Treat. 2014, 26, 217–222. [Google Scholar] [CrossRef]
- Honda, H.; Umezawa, Y.; Kikuchi, S.; Yanaba, K.; Fukuchi, O.; Ito, T.; Nobeyama, Y.; Asahina, A.; Nakagawa, H. Switching of Biologics in Psoriasis: Reasons and Results. J. Dermatol. 2017, 44, 1015–1019. [Google Scholar] [CrossRef] [PubMed]
- Bayaraa, B.; Imafuku, S. Sustainability and Switching of Biologics for Psoriasis and Psoriatic Arthritis at Fukuoka University Psoriasis Registry. J. Dermatol. 2019, 46, 389–398. [Google Scholar] [CrossRef] [PubMed]
- Menter, A.; Papp, K.A.; Gooderham, M.; Pariser, D.M.; Augustin, M.; Kerdel, F.A.; Fakharzadeh, S.; Goyal, K.; Calabro, S.; Langholff, W.; et al. Drug Survival of Biologic Therapy in a Large, Disease-based Registry of Patients with Psoriasis: Results from the Psoriasis Longitudinal Assessment and Registry (PSOLAR). J. Eur. Acad. Dermatol. Venereol. 2016, 30, 1148. [Google Scholar] [CrossRef] [PubMed]
- Gooderham, M.J.; Lynde, C.; Turchin, I.; Avadisian, M.; Labelle, M.; Papp, K.A. Real-World, Long-Term Treatment Patterns of Commonly Used Biologics in Canadian Patients with Moderate-to-Severe Chronic Plaque Psoriasis. J. Dermatol. 2022, 49, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Tokuyama, M.; Ota, M.; Saitoh, R.; Sawamura, M.; Okitsu, N.; Shimizu, T.; Kondoh, A.; Yamaoka, H.; Mabuchi, T. Biological Retention Rates, the Reasons of Switching, and Prognostic Factors in Patients with Psoriasis Treated Biologics. Tokai J. Exp. Clin. Med. 2020, 45, 230–235. [Google Scholar] [PubMed]
| N (%) | |
|---|---|
| At index date | |
| Gender | |
| Female | 973 (52.9) |
| Male | 866 (47.1) |
| Age | |
| Mean (SD) | 51.6 (15.2) |
| Class of ages | |
| 0–20 | 61 (3.3) |
| 21–40 | 352 (19.1) |
| 41–50 | 421 (22.9) |
| 51–60 | 443 (24.1) |
| 61–70 | 375 (20.4) |
| 71–80 | 167 (9.1) |
| 81–100 | 20 (1.1) |
| Index drug | |
| Adalimumab | 770 (41.9) |
| Etanercept | 758 (41.2) |
| Infliximab | 159 (8.6) |
| Secukinumab | 37 (2.0) |
| Ustekinumab | 115 (6.3) |
| One year before ID (look-back period) | |
| Dermatologic visits | |
| Mean (SD) | 3.7 (3.7) |
| Median (1° quartile–3° quartile) | 2 (1–5) |
| Comorbidities | |
| Lung disease | 24 (1.3) |
| Myocardial infarction | 4 (0.2) |
| Other CV disease | 37 (2.0) |
| Stroke | 11 (0.6) |
| Hypertension | 59 (3.2) |
| Diabetes | 36 (2.0) |
| Fracture (of hip/spine/leg) | 16 (0.9) |
| Depression | 8 (0.4) |
| Gastrointestinal ulcer | 0 (0.0) |
| Other gastrointestinal disorders | 8 (0.4) |
| Sjögren’s syndrome | 1 (0.1) |
| Rheumatoid nodules | 0 (0.0) |
| Rheumatoid lung disease | 4 (0.2) |
| Myopathies | 1 (0.1) |
| Polyneuropathy | 0 (0.0) |
| Cancer | 39 (2.1) |
| None 1 | 1652 (89.8) |
| Concomitant therapies | |
| Non-biological drugs for psoriasis | 1148 (62.5) |
| Acitretin | 113 (6.1) |
| Anti-psoriatic for topical use | 480 (26.1) |
| Apremilast | 0 (0.0) |
| Cyclosporin | 276 (15.0) |
| Methotrexate | 713 (38.8) |
| Psoralens for systemic use | 0 (0.0) |
| Psoralens for topical use | 0 (0.0) |
| Retinoids for treatment of psoriasis | 113 (6.1) |
| At least one systemic treatment | 981 (53.3) |
| None 2 | 690 (37.5) |
| Glucocorticoid for systemic use | 963 (52.4) |
| Non-Steroidal Anti-Inflammatory Drugs (NSAIDs) | 968 (52.7) |
| Opioid analgesics | 391 (21.3) |
| None 3 | 173 (9.4) |
|
Overall (N = 1839) |
Etanercept (N = 758) |
Adalimumab (N = 770) |
Infliximab (N = 159) |
Ustekinumab (N = 115) |
Secukinumab (N = 37) | |
|---|---|---|---|---|---|---|
|
No switch, n (%) | 1345 (73.1) | 527 (69.5) | 575 (74.7) | 115 (72.3) | 94 (81.7) | 34 (91.9) |
| One switch, n (%) | 378 (20.6) | 170 (22.4) | 153 (19.9) | 37 (23.3) | 16 (13.9) | 2 (5.4) |
| Two or more switches, n (%) | 116 (6.3) | 61 (8.0) | 42 (5.5) | 7 (4.4) | 5 (4.3) | 1 (2.7) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giometto, S.; Tillati, S.; Baglietto, L.; De Bortoli, N.; Mosca, M.; Conte, M.; Tuccori, M.; Gini, R.; Lucenteforte, E. Use of Biological Drugs for Psoriasis: A Drug-Utilization Study Using Tuscan Administrative Databanks. Int. J. Environ. Res. Public Health 2022, 19, 6799. https://doi.org/10.3390/ijerph19116799
Giometto S, Tillati S, Baglietto L, De Bortoli N, Mosca M, Conte M, Tuccori M, Gini R, Lucenteforte E. Use of Biological Drugs for Psoriasis: A Drug-Utilization Study Using Tuscan Administrative Databanks. International Journal of Environmental Research and Public Health. 2022; 19(11):6799. https://doi.org/10.3390/ijerph19116799
Chicago/Turabian StyleGiometto, Sabrina, Silvia Tillati, Laura Baglietto, Nicola De Bortoli, Marta Mosca, Marco Conte, Marco Tuccori, Rosa Gini, and Ersilia Lucenteforte. 2022. "Use of Biological Drugs for Psoriasis: A Drug-Utilization Study Using Tuscan Administrative Databanks" International Journal of Environmental Research and Public Health 19, no. 11: 6799. https://doi.org/10.3390/ijerph19116799
APA StyleGiometto, S., Tillati, S., Baglietto, L., De Bortoli, N., Mosca, M., Conte, M., Tuccori, M., Gini, R., & Lucenteforte, E. (2022). Use of Biological Drugs for Psoriasis: A Drug-Utilization Study Using Tuscan Administrative Databanks. International Journal of Environmental Research and Public Health, 19(11), 6799. https://doi.org/10.3390/ijerph19116799









