Multiple Medication Adherence and Related Outcomes in Community-Dwelling Older People on Chronic Polypharmacy: A Retrospective Cohort Study on Administrative Claims Data
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
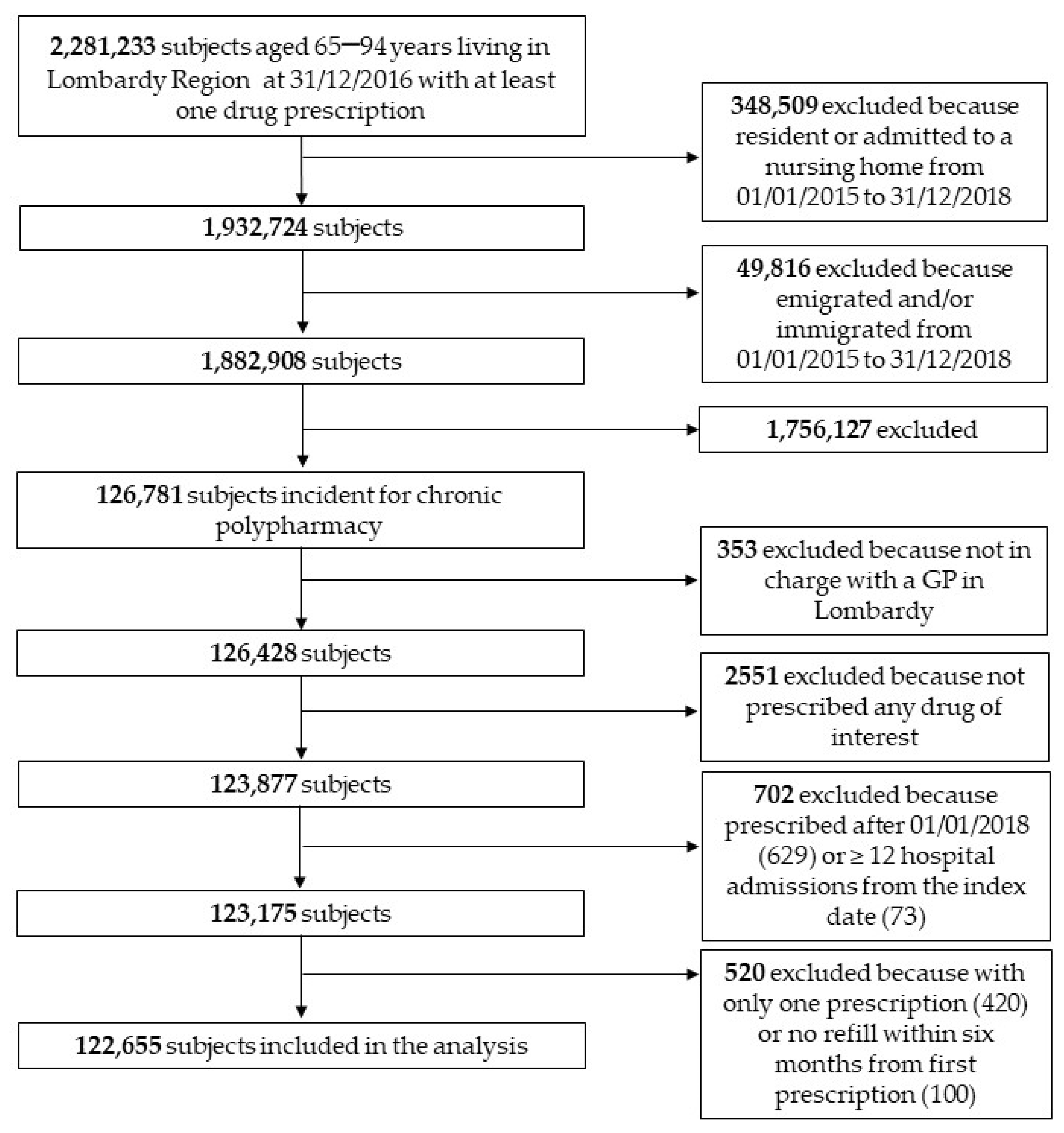
2.2. Participants and Setting
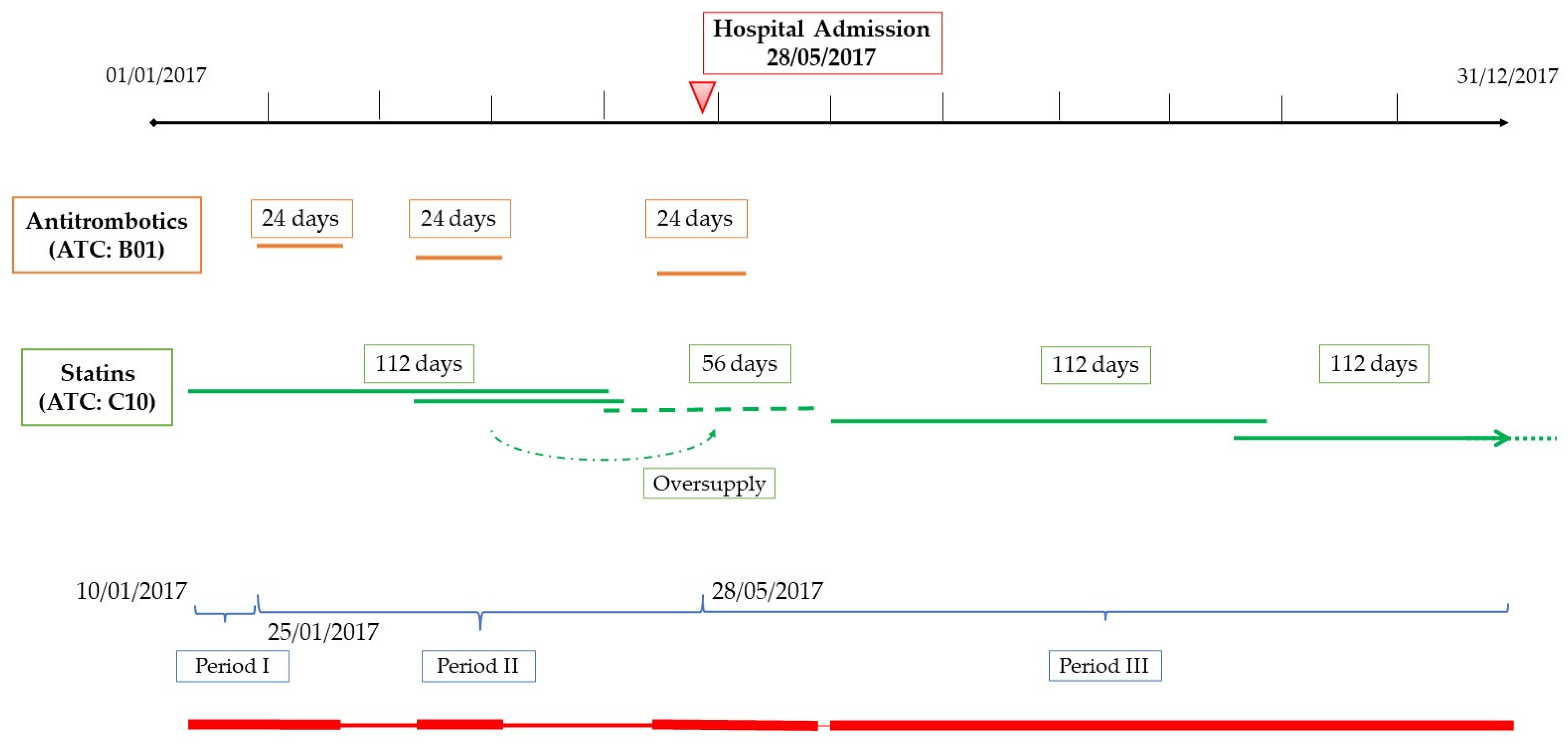
2.3. Assessment of Medication Adherence
- (1)
- Drugs for diabetes, excluding insulins and analogs (ATC: A10B-A10X);
- (2)
- Antithrombotic agents, excluding Vitamin K antagonists and heparin (ATC: B01AC-B01AX);
- (3)
- Agents acting on the renin–angiotensin system (angiotensin-converting enzyme (ACE)-inhibitors and angiotensin II receptor antagonists), hereafter referred to as antihypertensives (ATC: C09);
- (4)
- Lipid-modifying agents, referred to as statins (ATC: C10);
- (5)
- Drugs for the treatment of bone diseases, mainly bisphosphonates (ATC: M05B).
2.4. Statistical Analysis
3. Results
3.1. Overall Patient Characteristics and Medication Adherence
3.2. Factors Associated with Multiple Medication Adherence
3.3. Clinical Outcomes
One-Year Mortality and Nursing Home Admission
3.4. Emergency Department and Hospital Admissions
4. Discussion
Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sabate, E. Adherence to Long-Term Therapies: Evidence for Action; World Health Organisation (WHO): Geneva, Switzerland, 2003; Available online: http://www.who.int/chp/knowledge/publications/adherence_report/en/ (accessed on 1 February 2022).
- Marcucci, M.; Franchi, C.; Nobili, A.; Mannucci, P.M.; Ardoino, I. REPOSI Investigators. Defining Aging Phenotypes and Related Outcomes: Clues to Recognize Frailty in Hospitalized Older Patients. J. Gerontol. A Biol. Sci. Med. Sci. 2017, 72, 395–402. [Google Scholar] [CrossRef] [PubMed]
- Franchi, C.; Lancellotti, G.; Bertolotti, M.; Di Salvatore, S.; Nobili, A.; Mannucci, P.M.; Mussi, C.; Ardoino, I. Use of Lipid-Lowering Drugs and Associated Outcomes According to Health State Profiles in Hospitalized Older Patients. Clin. Interv. Aging 2021, 16, 1251–1264. [Google Scholar] [CrossRef] [PubMed]
- Franchi, C.; Marcucci, M.; Mannucci, P.M.; Tettamanti, M.; Pasina, L.; Fortino, I.; Bortolotti, A.; Merlino, L.; Nobili, A. Changes in clinical outcomes for community-dwelling older people exposed to incident chronic polypharmacy: A comparison between 2001 and 2009. Pharmacoepidemiol. Drug Saf. 2016, 25, 204–211. [Google Scholar] [CrossRef] [PubMed]
- Franchi, C.; Ardoino, I.; Ludergnani, M.; Cukay, G.; Merlino, L.; Nobili, A. Medication adherence in community-dwelling older people exposed to chronic polypharmacy. J. Epidemiol. Community Health 2021, 75, 854–859. [Google Scholar] [CrossRef] [PubMed]
- Kardas, P.; Lewek, P.; Matyjaszczyk, M. Determinants of patient adherence: A review of systematic reviews. Front. Pharmacol. 2013, 4, 91. [Google Scholar] [CrossRef] [Green Version]
- Sinha, A.; Mukherjee, S.; Tripathi, S.; Dutta, S. Issues and challenges of polypharmacy in the elderly: A review of contemporary Indian literature. J. Family Med. Prim. Care 2021, 10, 3544–3547. [Google Scholar] [CrossRef]
- Kurczewska-Michalak, M.; Lewek, P.; Jankowska-Polańska, B.; Giardini, A.; Granata, N.; Maffoni, M.; Costa, E.; Midão, L.; Kardas, P. Polypharmacy Management in the Older Adults: A Scoping Review of Available Interventions. Front. Pharmacol. 2021, 12, 734045. [Google Scholar] [CrossRef]
- Owsiany, M.T.; Hawley, C.E.; Paik, J.M. Differential Diagnoses and Clinical Implications of Medication Nonadherence in Older Patients with Chronic Kidney Disease: A Review. Drugs Aging 2020, 37, 875–884. [Google Scholar] [CrossRef]
- Wimmer, B.C.; Cross, A.J.; Jokanovic, N.; Wiese, M.D.; George, J.; Johnell, K.; Diug, B.; Bell, J.S. Clinical Outcomes Associated with Medication Regimen Complexity in Older People: A Systematic Review. J. Am. Geriatr. Soc. 2017, 65, 747–753. [Google Scholar] [CrossRef]
- Hess, L.M.; Raebel, M.A.; Conner, D.A.; Malone, D.C. Measurement of adherence in pharmacy administrative databases: A proposal for standard definitions and preferred measures. Ann. Pharmacother. 2006, 40, 1280–1288. [Google Scholar] [CrossRef]
- Arnet, I.; Abraham, I.; Messerli, M.; Hersberger, K.E. A method for calculating adherence to polypharmacy from dispensing data records. Int. J. Clin. Pharm. 2014, 36, 192–201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arnet, I.; Greenland, M.; Knuiman, M.W.; Rankin, J.M.; Hung, J.; Nedkoff, L.; Briffa, T.G.; Sanfilippo, F.M. Operationalization and validation of a novel method to calculate adherence to polypharmacy with refill data from the Australian pharmaceutical benefits scheme (PBS) database. Clin. Epidemiol. 2018, 10, 1181–1194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Franchi, C.; Cartabia, M.; Risso, P.; Mari, D.; Tettamanti, M.; Parabiaghi, A.; Pasina, L.; Djignefa Djade, C.; Fortino, I.; Bortolotti, A.; et al. Geographical differences in the prevalence of chronic polypharmacy in older people: Eleven years of the EPIFARM-Elderly Project. Eur. J. Clin. Pharmacol. 2013, 69, 1477–1483. [Google Scholar] [CrossRef] [PubMed]
- Ospina, R.; Ferrari, S.L.P. A general class of zero-or-one inflated beta regression models. Comp. Stat. Data Anal. 2012, 56, 1609–1623. [Google Scholar] [CrossRef] [Green Version]
- Marubini, E.; Valsecchi, M.G. Analysing Survival Data from Clinical Trials and Observational Studies; Wiley: Chichester, UK, 1995. [Google Scholar]
- Liu, W.S.; Cela, J. Count data model in SAS®. In Proceedings of the 2008 SAS Global Forum, San Antonio, TX, USA, 16–19 March 2008. [Google Scholar]
- Marcum, Z.A.; Gellad, W.F. Medication adherence to multidrug regimens. Clin. Geriatr. Med. 2012, 28, 287–300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manteuffel, M.; Williams, S.; Chen, W.; Verbrugge, R.R.; Pittman, D.G.; Steinkellner, A. Influence of patient sex and gender on medication use, adherence, and prescribing alignment with guidelines. J. Womens Health 2014, 23, 112–119. [Google Scholar] [CrossRef]
- Gomes, D.; Placido, A.I.; Mó, R.; Simões, J.L.; Amaral, O.; Fernandes, I.; Lima, F.; Morgado, M.; Figueiras, A.; Herdeiro, M.T.; et al. Daily Medication Management and Adherence in the Polymedicated Elderly: A Cross-Sectional Study in Portugal. Int. J. Environ. Res. Public Health 2019, 17, 200. [Google Scholar] [CrossRef] [Green Version]
- Rea, F.; Cantarutti, A.; Merlino, L.; Ungar, A.; Corrao, G.; Mancia, G. Antihypertensive Treatment in Elderly Frail Patients: Evidence from a Large Italian Database. Hypertension 2020, 76, 442–449. [Google Scholar] [CrossRef]
- Rea, F.; Mancia, G.; Corrao, G. Statin treatment reduces the risk of death among elderly frail patients: Evidence from a large population-based cohort. Eur. J. Prev. Cardiol. 2020, 22, zwaa126. [Google Scholar] [CrossRef]
- Dalli, L.L.; Kim, J.; Cadilhac, D.A.; Greenland, M.; Sanfilippo, F.M.; Andrew, N.E.; Thrift, A.G.; Grimley, R.; Lindley, R.I.; Sundararajan, V.; et al. Greater Adherence to Secondary Prevention Medications Improves Survival After Stroke or Transient Ischemic Attack: A Linked Registry Study. Stroke 2021, 52, 3569–3577. [Google Scholar] [CrossRef]
- Kim, Y.Y.; Lee, J.S.; Kang, H.J.; Park, S.M. Effect of medication adherence on long-term all-cause-mortality and hospitalization for cardiovascular disease in 65,067 newly diagnosed type 2 diabetes patients. Sci. Rep. 2018, 8, 12190. [Google Scholar] [CrossRef] [PubMed]
| VARIABLE | N (%) | ||
|---|---|---|---|
| SEX | |||
| Male | 58,592 (47.8) | ||
| Female | 64,063 (52.2) | ||
| AGE | |||
| Median (IQR) | 77 (72–82) | ||
| 65–69 | 19,495 (15.9) | ||
| 70–74 | 24,588 (20.0) | ||
| 75–79 | 31,999 (26.1) | ||
| 80–84 | 26,695 (21.8) | ||
| 85–89 | 15,390 (12.5) | ||
| 90–94 | 4488 (3.7) | ||
| PAI | 5036 (4.1) | ||
| HOSPITAL ADMISSIONS | |||
| 0 | 66,574 (54.3) | ||
| 1 | 26,127 (21.3) | ||
| 2 | 14,350 (11.7) | ||
| 3 | 7287 (5.9) | ||
| ≥4 | 8317 (6.8) | ||
| NUMBER OF CHRONIC DISEASES | Median (IQR) | 2 (2–3) | |
| NUMBER OF DRUGS | |||
| 1 | 15,466 (12.6) | ||
| 2 | 35,250 (28.7) | ||
| 3 | 49,644 (40.5) | ||
| 4 | 21,579 (17.6) | ||
| 5 | 716 (0.6) | ||
| DRUG CATEGORY | |||
| Drugs used for diabetes | 39,730 (32.1) | ||
| Antithrombotic agents | 85,404 (69.6) | ||
| Antihypertensives | 101,804 (83.0) | ||
| Statins | 84,915 (69.2) | ||
| Bisphosphonates | 12,941 (10.6) |
| BETA REGRESSION | ONE INFLATION | |
|---|---|---|
| OR (95%CI) | OR (95%CI) | |
| SEX | ||
| MALE | 1 | 1 |
| FEMALE | 0.85 (0.84–0.86) | 0.82 (0.78–0.85) |
| AGE GROUPS | ||
| >65 | 1 | 1 |
| >75 | 0.87 (0.86–0.88) | 0.86 (0.82–0.90) |
| >85 | 0.77 (0.76–0.79) | 0.90 (0.84–0.96) |
| NUMBER OF DISEASES | 1 | 1 |
| 1 | ||
| 2/3 | 0.96 (0.94–0.97) | 0.68 (0.65–0.72) |
| 4+ | 0.88 (0.86–0.90) | 0.57 (0.53–0.61) |
| SURVIVAL | NURSING HOME ADMISSION | |
|---|---|---|
| HR (95%CI) | SDHR (95%CI) | |
| SEX | ||
| MALE | 1 | 1 |
| FEMALE | 0.74 (0.70–0.77) | 1.38 (1.27–1.50) |
| AGE GROUPS | ||
| >65 | 1 | 1 |
| >75 | 2.20 (2.08–2.34) | 3.64 (3.18–4.16) |
| >85 | 5.85 (5.49–6.23) | 9.51 (8.30–10.91) |
| NUMBER OF DISEASES | ||
| 1 | 1 | 1 |
| 2/3 | 1.39 (1.29–1.49) | 0.98 (0.88–1.08) |
| 4+ | 2.51 (2.33–2.70) | 1.30 (1.16–1.47) |
| PAI | ||
| NO | 1 | 1 |
| YES | 0.94 (0.83–1.05) | 0.80 (0.65–1.01) |
| DPPR | 0.93 (0.92–0.94) | 0.95 (0.93–0.97) |
| POISSON | ZERO INFLATION | |
|---|---|---|
| RR (95%CI) | OR (95%CI) | |
| SEX | ||
| MALE | 1 | 1 |
| FEMALE | 0.94 (0.92–0.96) | 0.88 (0.84–0.91) |
| AGE GROUPS | ||
| >65 | 1 | 1 |
| >75 | 1.04 (1.02–1.06) | 0.71 (0.68–0.73) |
| >85 | 1.04 (1.02–1.07) | 0.51 (0.45–0.57) |
| NUMBER OF DISEASES | 1 | 1 |
| 1 | ||
| 2/3 | 1.09 (1.06–1.12) | 0.95 (0.90–1.01) |
| 4+ | 1.24 (1.21–1.29) | 0.74 (0.69–0.79) |
| DPPR (10 POINT) | 0.98 (0.97–0.99) | 1.02 (1.01–1.03) |
| PAI | ||
| NO | 1 | 1 |
| YES | 0.97 (0.92–1.01) | 1.10 (1.01–1.21) |
| PREVIOUS ED VISITS | ||
| NO | 1 | 1 |
| YES | 1.29 (1.26–1.31) | 0.69 (0.66–0.71) |
| POISSON | ZERO INFLATION | |
|---|---|---|
| RR (95%CI) | OR (95%CI) | |
| SEX | 1 | 1 |
| MALE | ||
| FEMALE | 0.94 (0.91–0.96) | 1.16 (1.11–1.22) |
| AGE GROUPS | ||
| >65 | 1 | 1 |
| >75 | 0.97 (0.95–1.00) | 0.79 (0.76–0.83) |
| >85 | 0.84 (0.81–0.88) | 0.51 (0.47–0.55) |
| NUMBER OF DISEASES | ||
| 1 | 1 | |
| 2/3 | 1.11 (1.06–1.17) | 0.85 (0.79–0.91) |
| 4+ | 1.28 (1.21–1.33) | 0.62 (0.57–0.67) |
| DPPR (10 POINT) | 0.99 (0.98–1.00) | 1.03 (1.01–1.04) |
| PAI | ||
| NO | 1 | 1 |
| YES | 0.89 (0.83–0.96) | 0.98 (0.87–1.11) |
| PREVIOUS HOSPITAL ADMISSIONS | ||
| NO | 1 | 1 |
| YES | 1.32 (1.28–1.36) | 0.49 (0.46–0.51) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Franchi, C.; Ludergnani, M.; Merlino, L.; Nobili, A.; Fortino, I.; Leoni, O.; Ardoino, I. Multiple Medication Adherence and Related Outcomes in Community-Dwelling Older People on Chronic Polypharmacy: A Retrospective Cohort Study on Administrative Claims Data. Int. J. Environ. Res. Public Health 2022, 19, 5692. https://doi.org/10.3390/ijerph19095692
Franchi C, Ludergnani M, Merlino L, Nobili A, Fortino I, Leoni O, Ardoino I. Multiple Medication Adherence and Related Outcomes in Community-Dwelling Older People on Chronic Polypharmacy: A Retrospective Cohort Study on Administrative Claims Data. International Journal of Environmental Research and Public Health. 2022; 19(9):5692. https://doi.org/10.3390/ijerph19095692
Chicago/Turabian StyleFranchi, Carlotta, Monica Ludergnani, Luca Merlino, Alessandro Nobili, Ida Fortino, Olivia Leoni, and Ilaria Ardoino. 2022. "Multiple Medication Adherence and Related Outcomes in Community-Dwelling Older People on Chronic Polypharmacy: A Retrospective Cohort Study on Administrative Claims Data" International Journal of Environmental Research and Public Health 19, no. 9: 5692. https://doi.org/10.3390/ijerph19095692
APA StyleFranchi, C., Ludergnani, M., Merlino, L., Nobili, A., Fortino, I., Leoni, O., & Ardoino, I. (2022). Multiple Medication Adherence and Related Outcomes in Community-Dwelling Older People on Chronic Polypharmacy: A Retrospective Cohort Study on Administrative Claims Data. International Journal of Environmental Research and Public Health, 19(9), 5692. https://doi.org/10.3390/ijerph19095692







