Haze Occurrence Caused by High Gas-to-Particle Conversion in Moisture Air under Low Pollutant Emission in a Megacity of China
Abstract
:1. Introduction
2. Materials and Methods
2.1. Observation Site and Instruments
2.2. PMF Model
2.3. Air Mass Backward Trajectories Cluster Analysis
3. Results and Discussion
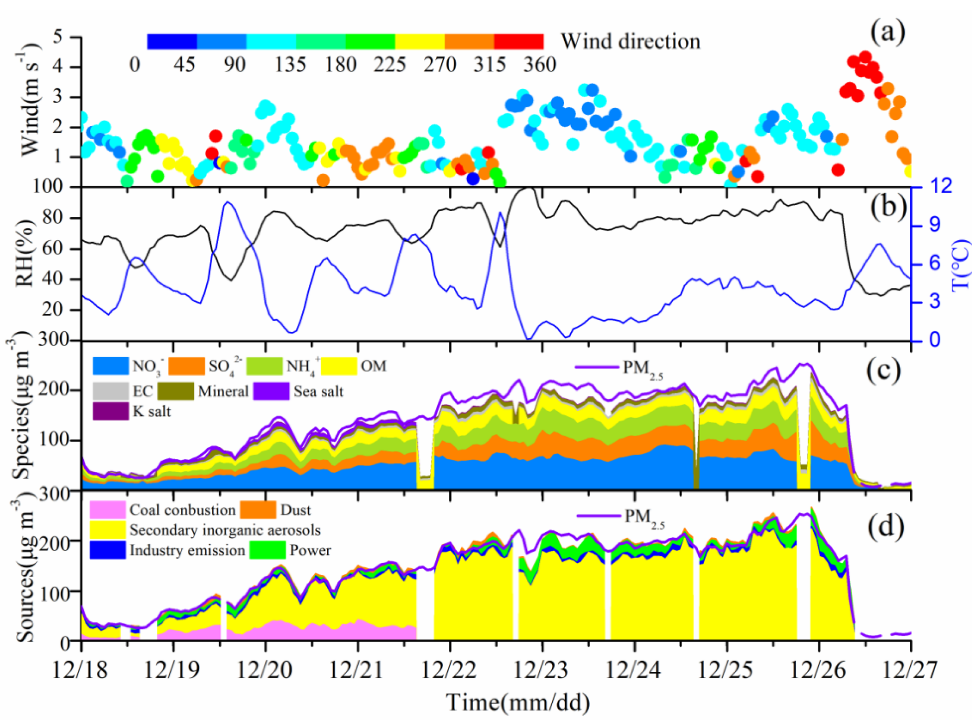
3.1. Weather Conditions and Haze Pollution
3.2. Chemical Composition of PM2.5 during the HOP
3.3. Inter-Comparison within the Whole Observation Period
3.3.1. Difference in Chemical Composition and Conversions
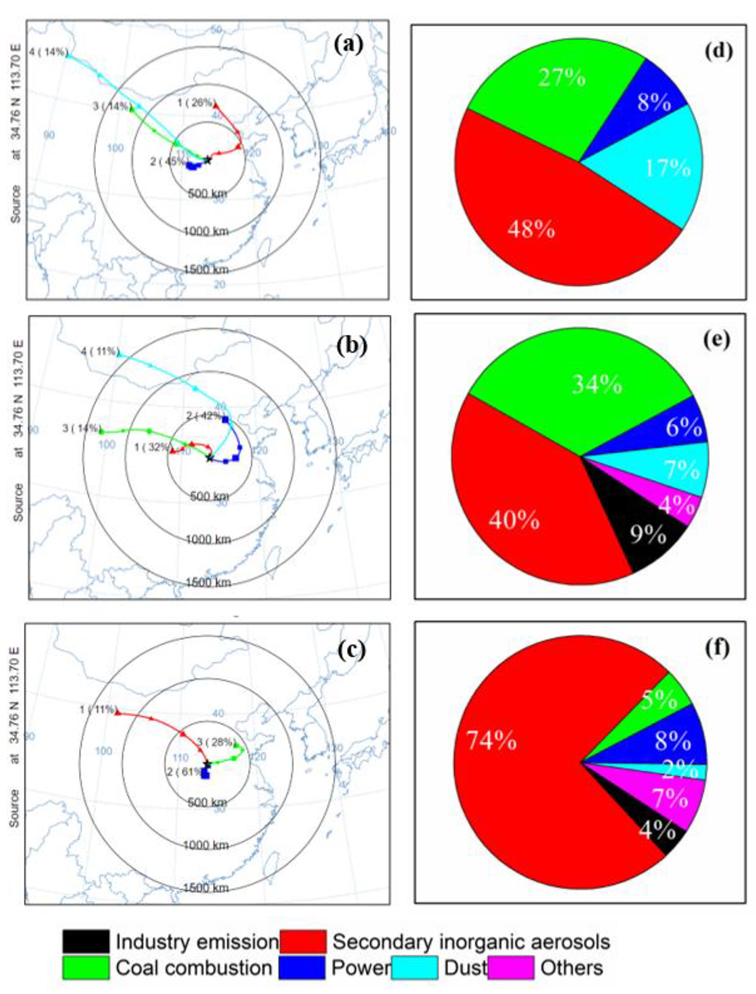
3.3.2. Difference in Source Apportionment and Trajectory Clustering
3.3.3. Significance of SIA Formation in HOP
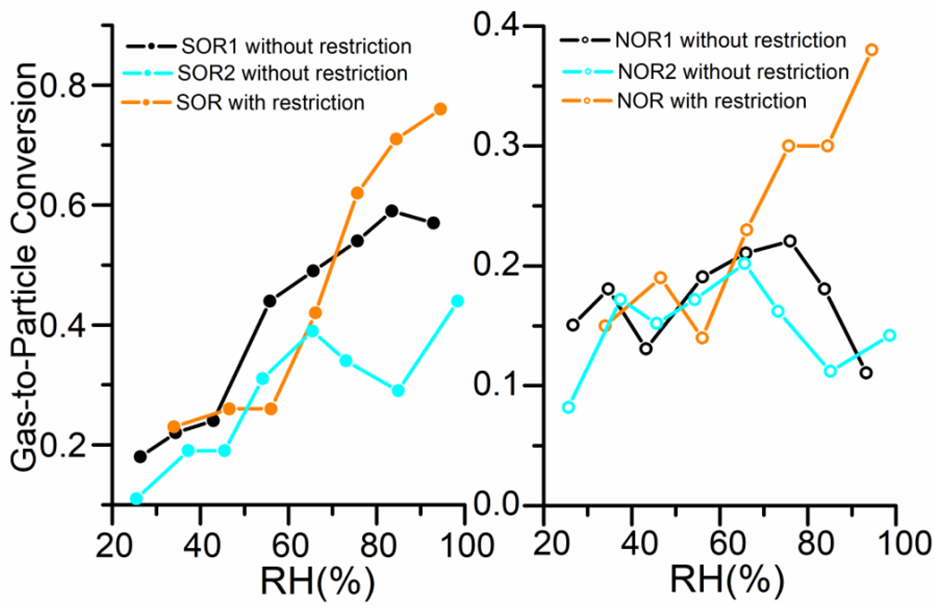
3.3.4. Inter-Comparisons of the Responses of Secondary Aerosol Formations to Emissions Reductions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Cheng, Y.F.; Zheng, G.J.; Wei, C.; Mu, Q.; Zheng, B.; Wang, Z.B.; Gao, M.; Zhang, Q.; He, K.B.; Carmichael, G.; et al. Reactive nitrogen chemistry in aerosol water as a source of sulfate during haze events in China. Sci. Adv. 2016, 2, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, Q.X.; Wu, Y.F.; Zhang, D.Z.; Wang, X.J.; Xia, Y.J.; Liu, X.Y.; Tian, P.; Han, Z.W.; Xia, X.A.; Wang, Y.; et al. Roles of regional transport and heterogeneous reactions in the PM2.5 increase during winter haze episodes in Beijing. Sci. Total Environ. 2017, 599–600, 246–253. [Google Scholar] [CrossRef] [PubMed]
- Song, X.Y.; Jia, J.; Wu, F.; Niu, H.Y.; Ma, Q.X.; Guo, B.; Shao, L.Y.; Zhang, D.Z. Local emissions and secondary pollutants cause severe PM2.5 elevation in urban air at the south edge of the North China Plain: Results from winter haze of 2017-2018 at a mega city. Sci. Total Environ. 2022, 802, 149630. [Google Scholar] [CrossRef] [PubMed]
- Ansari, T.; Wild, O.; Ryan, E.; Chen, Y.; Li, J.; Wang, Z. Temporally resolved sectoral and regional contributions to air pollution in Beijing: Informing short-term emission controls. Atmos. Chem. Phys. 2021, 21, 4471–4485. [Google Scholar] [CrossRef]
- Yan, Y.; Zhou, Y.; Kong, S.; Lin, J.; Wu, J.; Zheng, H.; Zhang, Z.; Song, A.; Bai, Y.; Ling, Z.; et al. Effectiveness of emission control in reducing PM2.5 pollution in central China during winter haze episodes under various potential synoptic controls. Atmos. Chem. Phys. 2021, 21, 3143–3162. [Google Scholar] [CrossRef]
- Zhong, Q.; Tao, S.; Ma, J.; Liu, J.; Shen, H.; Shen, G.; Guan, D.; Yun, X.; Meng, W.; Yu, X.; et al. PM2.5 reductions in Chinese cities from 2013 to 2019 remain significant despite the inflating effects of meteorological conditions. One Earth 2021, 4, 448–458. [Google Scholar] [CrossRef]
- Peng, J.F.; Hu, M.; Shang, D.J. Explosive Secondary Aerosol Formation during Severe Haze in the North China Plain. Environ. Sci. Technol. 2021, 55, 2189–2207. [Google Scholar] [CrossRef]
- Ma, Q.X.; Wu, Y.F.; Fu, S.L.; Zhang, D.Z.; Han, Z.W.; Zhang, R.J. Pollution severity-dependent aerosol light scattering enhanced by inorganic species formation in Beijing haze. Sci. Total Environ. 2020, 719, 137545. [Google Scholar] [CrossRef]
- Wang, G.; Zhang, R.; Gomez, M.E.; Yang, L.; Zamora, M.L.; Hu, M.; Lin, Y.; Peng, J.; Guo, S.; Meng, J.; et al. Persistent sulfate formation from London fog to Chinese haze. Proc. Natl. Acad. Sci. USA 2016, 113, 13630–13635. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meng, F.; Zhang, Y.; Kang, J.; Heal, M.R.; Reis, S.; Wang, M.; Liu, L.; Wang, K.; Yu, S.; Li, P.; et al. Trends in secondary inorganic aerosol pollution in China and its responses to emission controls of precursors in wintertime. Atmos. Chem. Phys. 2022, 22, 6291–6308. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, J.; Du, R.G.; Teng, X.M.; Hu, R.; Yuan, Q.; Tang, S.S.; Ren, C.H.; Huang, X.; Xu, L.; et al. Chemistry of Atmospheric Fine Particles during the COVID-19 Pandemic in a Megacity of Eastern China. Geophys. Res. Lett. 2021, 48, e2020GL091611. [Google Scholar] [CrossRef]
- Gen, M.S.; Zhang, R.F.; Huang, D.D.; Li, Y.J.; Chan, C.K. Heterogeneous Oxidation of SO2 in Sulfate Production during Nitrate Photolysis at 300 nm: Effect of pH, Relative Humidity, Irradiation Intensity, and the Presence of Organic Compounds. Environ. Sci. Technol. 2019, 53, 8757–8766. [Google Scholar] [CrossRef]
- Lin, Y.C.; Zhang, Y.L.; Fan, M.Y.; Bao, M.Y. Heterogeneous formation of particulate nitrate under ammonium-rich regimes during the high-PM2.5 events in Nanjing, China. Atmos. Chem. Phys. 2020, 20, 3999–4011. [Google Scholar] [CrossRef] [Green Version]
- Fu, X.; Wang, T.; Gao, J.; Wang, P.; Liu, Y.; Wang, S.; Zhao, B.; Xue, L. Persistent heavy winter nitrate pollution driven by increased photochemical oxidants in northern China. Environ. Sci. Technol. 2020, 54, 3881–3889. [Google Scholar] [CrossRef]
- Wang, J.F.; Li, J.Y.; Ye, J.H.; Zhao, Y.; Wu, J.Z.; Hu, J.L.; Liu, D.T.; Nie, D.Y.; Shen, F.Z.; Huang, X.P.; et al. Fast sulfate formation from oxidation of SO2 by NO2 and HONO observed in Beijing haze. Nat. Commun. 2020, 11, 2844. [Google Scholar] [CrossRef]
- Ye, S.Q.; Ma, T.; Duan, F.K.; Li, H.; He, K.B.; Xia, J.; Yang, S.; Zhu, L.D.; Ma, Y.L.; Huang, T.; et al. Characteristics and formation mechanisms of winter haze in Changzhou, a highly polluted industrial city in the Yangtze River Delta, China. Environ. Pollut. 2019, 253, 377–383. [Google Scholar] [CrossRef]
- George, C.; Ammann, M.; D’Anna, B.; Donaldson, D.J.; Nizkorodov, S.A. Heterogeneous photochemistry in the atmosphere. Chem. Rev. 2015, 115, 4218–4258. [Google Scholar] [CrossRef] [Green Version]
- An, Z.; Huang, R.; Zhang, R.; Tie, X.; Li, G.; Cao, J.; Zhou, W.; Shi, Z.; Han, Y.; Gu, Z.; et al. Severe haze in northern China: A synergy of anthropogenic emissions and atmospheric processes. Proc. Natl. Acad. Sci. USA 2019, 116, 8657–8666. [Google Scholar] [CrossRef] [Green Version]
- Xia, Y.J.; Wu, Y.F.; Huang, R.J.; Xia, X.A.; Tang, J.; Wang, M.; Li, J.W.; Wang, C.Y.; Zhou, C.; Zhang, R.J. Variation in black carbon concentration and aerosol optical properties in Beijing: Role of emission control and meteorological transport variability. Chemosphere 2020, 254, 126849. [Google Scholar] [CrossRef]
- Li, W.; Shao, L.; Wang, W.; Li, H.; Wang, X.; Li, Y.; Li, W.; Jones, T.; Zhang, D. Air quality improvement in response to intensified control strategies in Beijing during 2013–2019. Sci. Total Environ. 2020, 744, 140776. [Google Scholar] [CrossRef]
- Li, X.; Zhao, B.; Zhou, W.; Shi, H.; Yin, R.; Cai, R.; Yang, D.; Dallenbach, K.; Deng, C.; Fu, Y.; et al. Responses of gaseous sulfuric acid and particulate sulfate to reduced SO2 concentration: A perspective from long–term measurements in Beijing. Sci. Total Environ. 2020, 721, 137700. [Google Scholar] [CrossRef]
- Zhang, Z.; Guan, H.; Luo, L.; Zheng, N.; Xiao, H. Response of fine aerosol nitrate chemistry to Clean Air Action in winter Beijing: Insights from the oxygen isotope signatures. Sci. Total Environ. 2020, 746, 141210. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, H.; Zhang, X.; Peng, Y.; Zhong, J.; Wang, Y.; Zhao, Y. Evaluating the contributions of changed meteorological conditions and emission to substantial reductions of PM2.5 concentration from winter 2016 to 2017 in Central and Eastern China. Sci. Total Environ. 2020, 716, 136892. [Google Scholar] [CrossRef]
- Ghosal, R.; Saha, E. Impact of the COVID-19 induced lockdown measures on PM2.5 concentration in USA. Atmos. Environ. 2021, 254, 118388. [Google Scholar] [CrossRef]
- Jeong, C.; Yousif, M.; Evans, G. Impact of the COVID-19 lockdown on the chemical composition and sources of urban PM2.5. Environ. Pollut. 2022, 292, 118417. [Google Scholar] [CrossRef]
- Du, H.Y.; Li, J.; Wang, Z.F.; Yang, W.Y.; Chen, X.S.; Wei, Y. Sources of PM2.5 and its responses to emission reduction strategies in the Central Plains Economic Region in China: Implications for the impacts of COVID-19. Environ. Pollut. 2021, 288, 117783. [Google Scholar] [CrossRef]
- Liu, S.; Hua, S.; Wang, K.; Qiu, P.; Liu, H.; Wu, B.; Shao, P.; Liu, X.; Wu, Y.; Xue, Y.; et al. Spatial-temporal variation characteristics of air pollution in Henan of China: Localized emission inventory, WRF/Chem simulations and potential source contribution analysis. Sci. Total Environ. 2018, 624, 396–406. [Google Scholar] [CrossRef]
- Yang, L.; Ren, Q.; Ge, S.J.; Jiao, Z.Q.; Zhan, W.H.; Hou, R.X.; Ruan, X.L.; Pan, Y.F.; Wang, Y.Y. Metal(loid)s Spatial Distribution, Accumulation, and Potential Health Risk Assessment in Soil-Wheat Systems near a Pb/Zn Smelter in Henan Province, Central China. Int. J. Environ. Res. Public Health 2022, 19, 2527. [Google Scholar] [CrossRef]
- Yang, W.Y.; Li, J.; Wang, Z.F.; Wang, L.L.; Dao, X.; Zhu, L.L.; Pan, X.L.; Li, Y.Y.; Sun, Y.L.; Ma, S.L.; et al. Source apportionment of PM2.5 in the most polluted Central Plains Economic Region in China: Implications for joint prevention and control of atmospheric pollution. J. Clean. Prod. 2021, 283, 124557. [Google Scholar] [CrossRef]
- Wang, H.; Li, J.H.; Peng, Y.; Zhang, M.; Che, H.Z.; Zhang, X.Y. The impacts of the meteorology features on PM2.5 levels during a severe haze episode in central-east China. Atmos. Environ. 2019, 197, 177–189. [Google Scholar] [CrossRef]
- Zhengzhou Municipal People’s Government. Available online: http://public.zhengzhou.gov.cn/10K/3525671.jhtml:2019-12-18 (accessed on 12 December 2019).
- Chen, H.; Huo, J.; Fu, Q.; Duan, Y.; Xiao, H.; Chen, J. Impact of quarantine measures on chemical compositions of PM2.5 during the COVID-19 epidemic in Shanghai, China. Sci. Total Environ. 2020, 743, 140758. [Google Scholar] [CrossRef] [PubMed]
- Stieger, B.; Spindler, G.; Fahlbusch, B.; Müller, K.; Grüner, A.; Poulain, L.; Thöni, L.; Seitler, E.; Wallasch, M.; Herrmann, H. Measurements of PM10 ions and trace gases with the online system MARGA at the research station Melpitz in Germany—A five-year study. J. Atmos. Chem. 2018, 75, 33–70. [Google Scholar] [CrossRef]
- Yang, Y.R.; Liu, X.G.; Qu, Y.; Wang, J.L.; An, J.L.; Zhang, Y.H.; Zhang, F. Formation mechanism of continuous extreme haze episodes in the megacity Beijing, China, in January 2013. Atmos. Res. 2015, 155, 192–203. [Google Scholar] [CrossRef]
- Zhang, R.J.; Jing, J.; Tao, J.; Hsu, S.-C.; Wang, G.; Cao, J.; Lee, C.S.L.; Zhu, L.; Chen, Z.; Zhao, Y.; et al. Chemical characterization and source apportionment of PM2.5 in Beijing: Seasonal perspective. Atmos. Chem. Phys. 2013, 13, 7053–7074. [Google Scholar] [CrossRef] [Green Version]
- Malm, W.C.; Sisler, J.F.; Huffman, D.; Eldred, R.A.; Cahill, T.A. Spatial and seasonal trends in particle concentration and optical extinction in the United States. J. Geophys. Res. 1994, 99, 1347–1370. [Google Scholar] [CrossRef]
- Chow, J.C.; Watson, J.G.; Fujita, E.M.; Lu, Z.; Lawson, D.R. Temporal and spatial variation of PM2.5 and PM10 aerosol in the Southern California air quality study. Atmos. Environ. 1994, 28, 2061–2080. [Google Scholar] [CrossRef]
- Hsu, S.C.; Liu, S.C.; Tsai, F.; Engling, G.; Lin, I.I.; Chou, C.K.C.; Kao, S.J.; Lung, S.C.C.; Chan, C.Y.; Lin, S.C.; et al. High wintertime particulate matter pollution over an offshore island (Kinmen) off southeastern China: An overview. J. Geophys. Res. 2010, 115, D17309. [Google Scholar] [CrossRef] [Green Version]
- Pósfai, M.; Gelencsér, A.; Simonics, R.; Arató, K.; Li, J.; Hobbs, P.V.; Buseck, P.R. Atmospheric tar balls: Particles from biomass and biofuel burning. J. Geophys. Res. 2004, 109, D06213. [Google Scholar] [CrossRef] [Green Version]
- Han, T.; Liu, X.G.; Zhang, Y.H.; Qu, Y.; Zeng, L.M.; Hu, M.; Zhu, T. Role of secondary aerosols in haze formation in summer in the Megacity Beijing. J. Environ. Sci. 2015, 31, 51–60. [Google Scholar] [CrossRef]
- Zhang, Q.; Quan, J.N.; Tie, X.X.; Li, X.; Liu, Q.; Gao, Y.; Zhao, D.L. Effects of meteorology and secondary particle formation on visibility during heavy haze events in Beijing, China. Sci. Total Environ. 2015, 502, 578–584. [Google Scholar] [CrossRef]
- Tian, Y.; Zhang, Y.; Liang, Y.; Niu, Z.; Xue, Q.; Feng, Y. PM2.5 source apportionment during severe haze episodes in a Chinese megacity based on a 5-month period by using hourly species measurements: Explore how to better conduct PMF during haze episodes. Atmos. Environ. 2020, 224, 117364. [Google Scholar] [CrossRef]
- Tian, J.; Wang, Q.; Zhang, Y.; Yan, M.; Liu, H.; Zhang, N.; Ran, W.; Cao, J. Impacts of primary emissions and secondary aerosol formation on air pollution in an urban area of China during the COVID-19 lockdown. Environ. Int. 2021, 150, 106426. [Google Scholar] [CrossRef]
- Zhang, D.; He, B.; Yuan, M.; Yu, S.; Yin, S.; Zhang, R. Characteristics, sources and health risks assessment of VOCs in Zhengzhou, China during haze pollution season. J. Environ. Sci. 2021, 108, 44–57. [Google Scholar] [CrossRef]
- Wang, S.B.; Wang, L.L.; Wang, N.; Ma, S.L.; Su, F.C.; Zhang, R.Q. Formation of droplet-mode secondary inorganic aerosol dominated the increased PM2.5 during both local and transport haze episodes in Zhengzhou, China. Chemosphere 2021, 269, 128744. [Google Scholar] [CrossRef]
- Fang, Y.; Ye, C.; Wang, J.; Wu, Y.; Hu, M.; Lin, W.; Xu, F.; Zhu, T. Relative humidity and O3 concentration as two prerequisites for sulfate formation. Atmos. Chem. Phys. 2019, 19, 12295–12307. [Google Scholar] [CrossRef] [Green Version]
- Liu, P.F.; Ye, C.; Xue, C.Y.; Zhang, C.L.; Mu, Y.J.; Sun, X. Formation mechanisms of atmospheric nitrate and sulfate during the winter haze pollution periods in Beijing: Gas–phase, heterogeneous and aqueous–phase chemistry. Atmos. Chem. Phys. 2020, 20, 4153–4165. [Google Scholar] [CrossRef] [Green Version]
- Liu, T.; Clegg, S.L.; Abbatt, J.P.D. Fast oxidation of sulfur dioxide by hydrogen peroxide in deliquesced aerosol particles. PNAS 2020, 117, 1354–1359. [Google Scholar] [CrossRef]
- Li, G.; Bei, N.; Cao, J.; Huang, R.; Wu, J.; Feng, T.; Wang, Y.; Liu, S.; Zhang, Q.; Tie, X.; et al. A possible pathway for rapid growth of sulfate during haze days in China. Atmos. Chem. Phys. 2017, 17, 3301–3316. [Google Scholar] [CrossRef] [Green Version]
- Zang, L.; Wang, Z.; Zhu, B.; Zhang, Y. Roles of Relative Humidity in Aerosol Pollution Aggravation over Central China during Wintertime. Int. J. Environ. Res. Public Health 2019, 16, 4422. [Google Scholar] [CrossRef] [Green Version]
- Chang, Y.; Huang, R.; Ge, X.; Huang, X.; Hu, J.; Duan, Y.; Zou, Z.; Liu, X.; Lehmann, M.F. Puzzling haze events in China during the coronavirus (COVID-19) shutdown. Geophys. Res. Lett. 2020, 47, e2020GL088533. [Google Scholar] [CrossRef]
- Duan, J.; Huang, R.J.; Chang, Y.H.; Zhong, H.B.; Gu, Y.F.; Lin, C.S.; Hoffmann, T.; O’Dowd, C. Measurement report of the change of PM2.5 composition during the COVID-19 lockdown in urban Xi’an: Enhanced secondary formation and oxidation. Sci. Total Environ. 2021, 791, 148126. [Google Scholar] [CrossRef]
- Ministry of Ecology and Environment of the People’s Republic of China (MEE), China Environmental State Bulletin 2020. 2020. Available online: https://www.mee.gov.cn/hjzl/sthjzk/zghjzkgb/ (accessed on 20 March 2021). (In Chinese)
- Ye, Z.L.; Li, J.; Pan, Y.P.; Wang, Z.F.; Guo, X.R.; Cheng, L.; Tang, X.; Zhu, J.; Kong, L.; Song, Y.; et al. Synergistic effect of reductions in multiple gaseous precursors on secondary inorganic aerosols in winter under a meteorology-based redistributed daily NH3 emission inventory within the Beijing-Tianjin-Hebei region, China. Sci. Total Environ. 2022, 821, 153383. [Google Scholar] [CrossRef]
- Lu, M.M.; Tang, X.; Feng, Y.C.; Wang, Z.F.; Chen, X.S.; Kong, L.; Ji, D.S.; Liu, Z.R.; Liu, K.X.; Wu, H.J.; et al. Nonlinear response of SIA to emission changes and chemical processes over eastern and central China during a heavy haze month. Sci. Total Environ. 2021, 788, 147747. [Google Scholar] [CrossRef]
- Shaha, V.; Jaeglé, L.; Thorntona, J.A.; Lopez-Hilfikera, F.D.; Lee, B.H.; Schroder, J.C.; Campuzano-Jost, P.; Green, J.R.; Fiddler, M.N.; Bililigng, S.; et al. Chemical feedbacks weaken the wintertime response of particulate sulfate and nitrate to emissions reductions over the eastern United States. Proc. Natl. Acad. Sci. USA 2018, 115, 8110–8115. [Google Scholar] [CrossRef] [Green Version]
- Feng, J.; Chan, E.; Vet, R. Air quality in the eastern United States and Eastern Canada for 1990–2015: 25 years of change in response to emission reductions of SO2 and NOx in the region. Atmos. Chem. Phys. 2020, 20, 3107–3134. [Google Scholar] [CrossRef] [Green Version]


| Components or Their Conversions | Formulas | References |
|---|---|---|
| Secondary inorganic aerosols (SIAs) | SO42− + NO3− + NH4+ | [6,34] |
| Organic matter (OM) | 1.6 × OC | [7,35] |
| Mineral | 2.20 Al + 2.49 Si + 1.63 Ca + 2.42 Fe + 1.94 Ti | [36,37] |
| Sea salt | Mg2+ + Na+ + F− + Cl− | [38] |
| K salt | K+ | [39] |
| SOR | [SO42−]/([SO42−] + [SO2]) molar concentration | [40] |
| NOR | [NO3−]/([NO3−] + [NO] + [NO2]) | [41] |
| PP1 before Restriction | PP2 before Restriction | HOP with Restriction | |
|---|---|---|---|
| PM2.5 | 136.35 ± 37.53 | 104.162 ± 2.17 | 172.63 ± 43.10 |
| NO3− | 37.07 ± 10.10 | 29.43 ± 7.08 | 57.42 ± 14.98 |
| SO42− | 18.02 ± 5.39 | 11.41 ± 4.54 | 30.66 ± 11.80 |
| NH4+ | 20.36 ± 5.68 | 14.67 ± 3.36 | 30.88 ± 8.93 |
| OM | 23.63 ± 8.43 | 21.18 ± 4.96 | 22.10 ± 5.07 |
| EC | 3.27 ± 1.29 | 3.13 ± 1.02 | 4.83 ± 1.49 |
| NOx | 122.59 ± 59.43 | 104.48 ± 44.42 | 107.12 ± 52.64 |
| SO2 | 12.27 ± 6.29 | 16.23 ± 5.00 | 10.23 ± 2.43 |
| O3 | 16.37 ± 22.35 | 15.81 ± 19.55 | 9.81 ± 11.62 |
| NOR | 0.21 ± 0.08 | 0.19 ± 0.07 | 0.30 ± 0.08 |
| SOR | 0.50 ± 0.13 | 0.33 ± 0.14 | 0.64 ± 0.12 |
| RH | 67.86 ± 17.40 | 51.37 ± 9.99 | 78.12 ± 10.05 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, Q.; Wang, W.; Liu, D.; Zhao, R.; Zhao, J.; Li, W.; Pan, Y.; Zhang, D. Haze Occurrence Caused by High Gas-to-Particle Conversion in Moisture Air under Low Pollutant Emission in a Megacity of China. Int. J. Environ. Res. Public Health 2022, 19, 6405. https://doi.org/10.3390/ijerph19116405
Ma Q, Wang W, Liu D, Zhao R, Zhao J, Li W, Pan Y, Zhang D. Haze Occurrence Caused by High Gas-to-Particle Conversion in Moisture Air under Low Pollutant Emission in a Megacity of China. International Journal of Environmental Research and Public Health. 2022; 19(11):6405. https://doi.org/10.3390/ijerph19116405
Chicago/Turabian StyleMa, Qingxia, Weisi Wang, Dexin Liu, Rongke Zhao, Jingqi Zhao, Wanlong Li, Yanfang Pan, and Daizhou Zhang. 2022. "Haze Occurrence Caused by High Gas-to-Particle Conversion in Moisture Air under Low Pollutant Emission in a Megacity of China" International Journal of Environmental Research and Public Health 19, no. 11: 6405. https://doi.org/10.3390/ijerph19116405
APA StyleMa, Q., Wang, W., Liu, D., Zhao, R., Zhao, J., Li, W., Pan, Y., & Zhang, D. (2022). Haze Occurrence Caused by High Gas-to-Particle Conversion in Moisture Air under Low Pollutant Emission in a Megacity of China. International Journal of Environmental Research and Public Health, 19(11), 6405. https://doi.org/10.3390/ijerph19116405






