A Redo Percutaneous Emergency Intervention of Left Ventricular Assist Device Graft Occlusion
Abstract
:1. Introduction
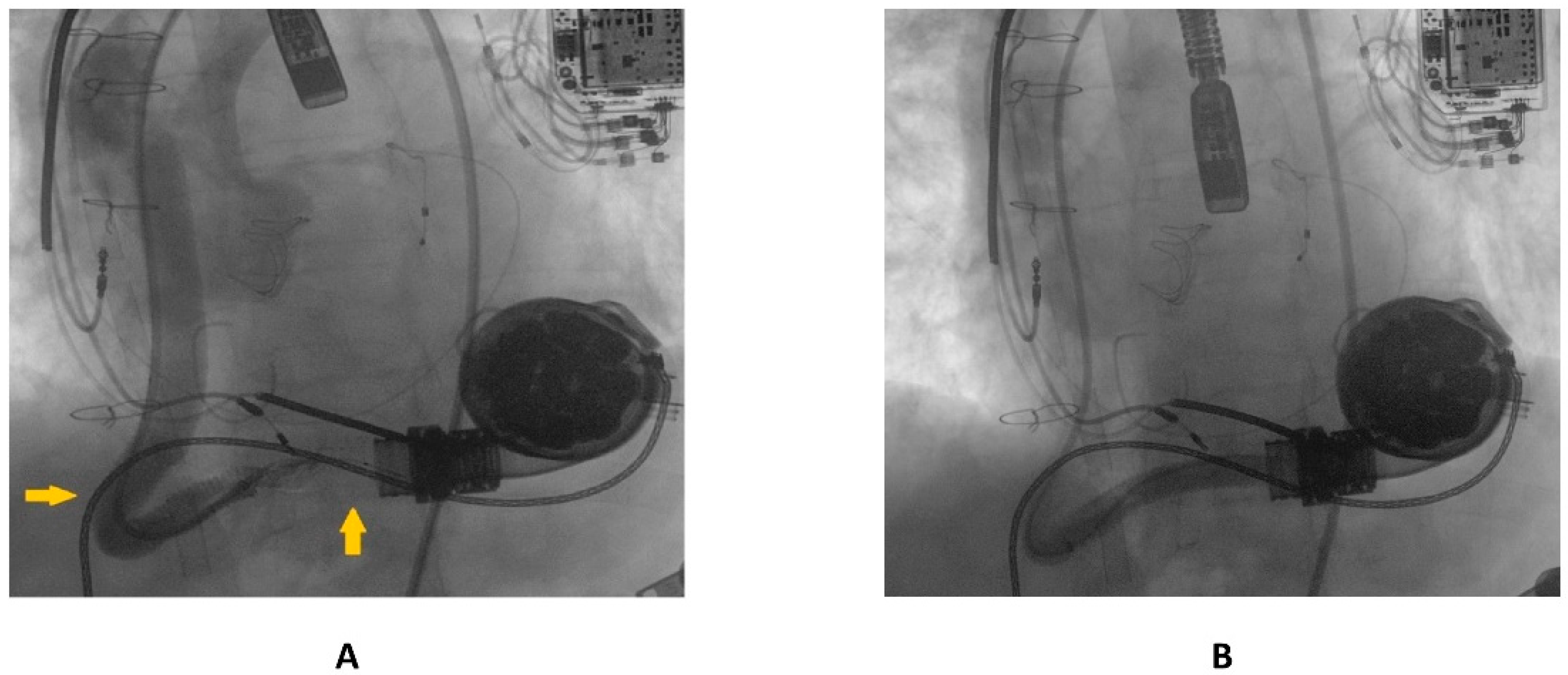
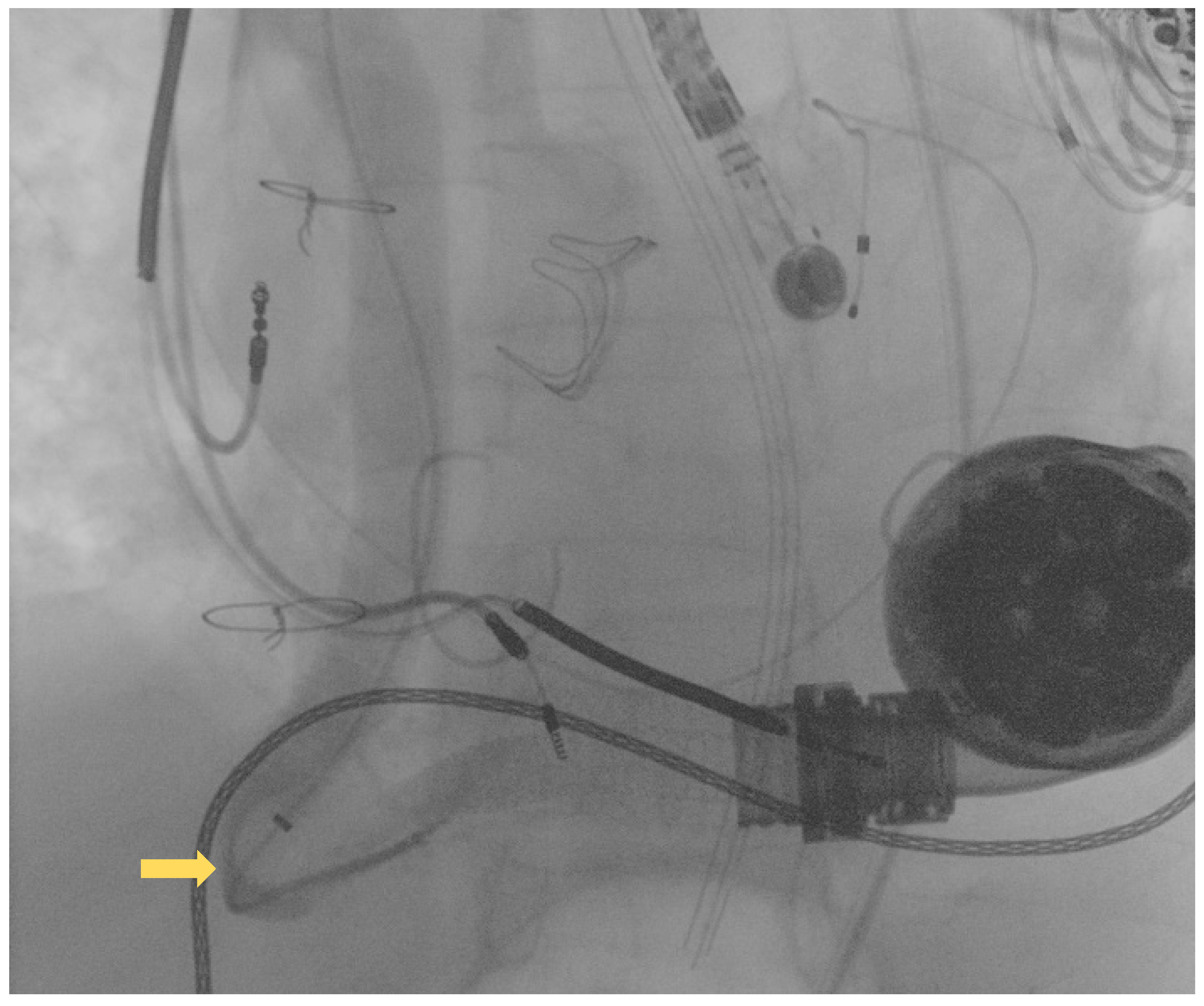
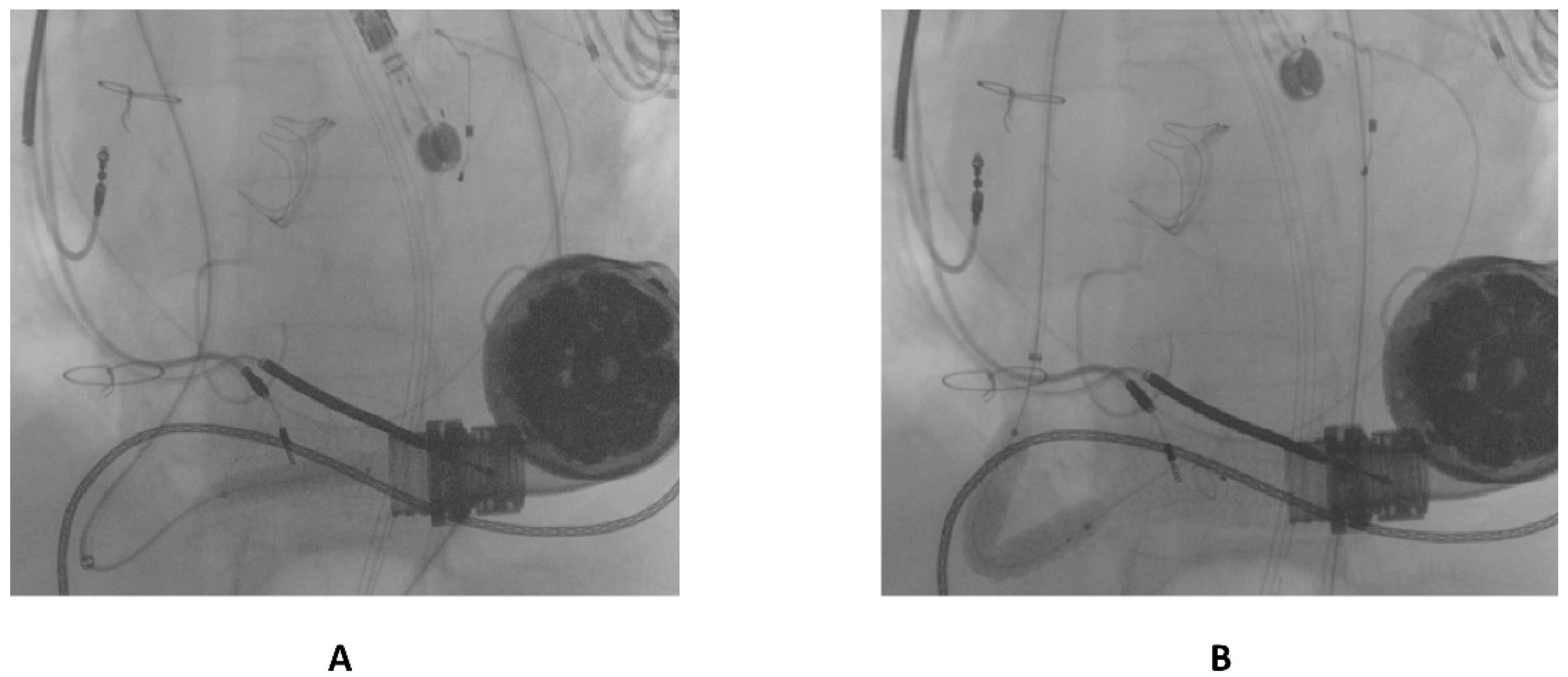
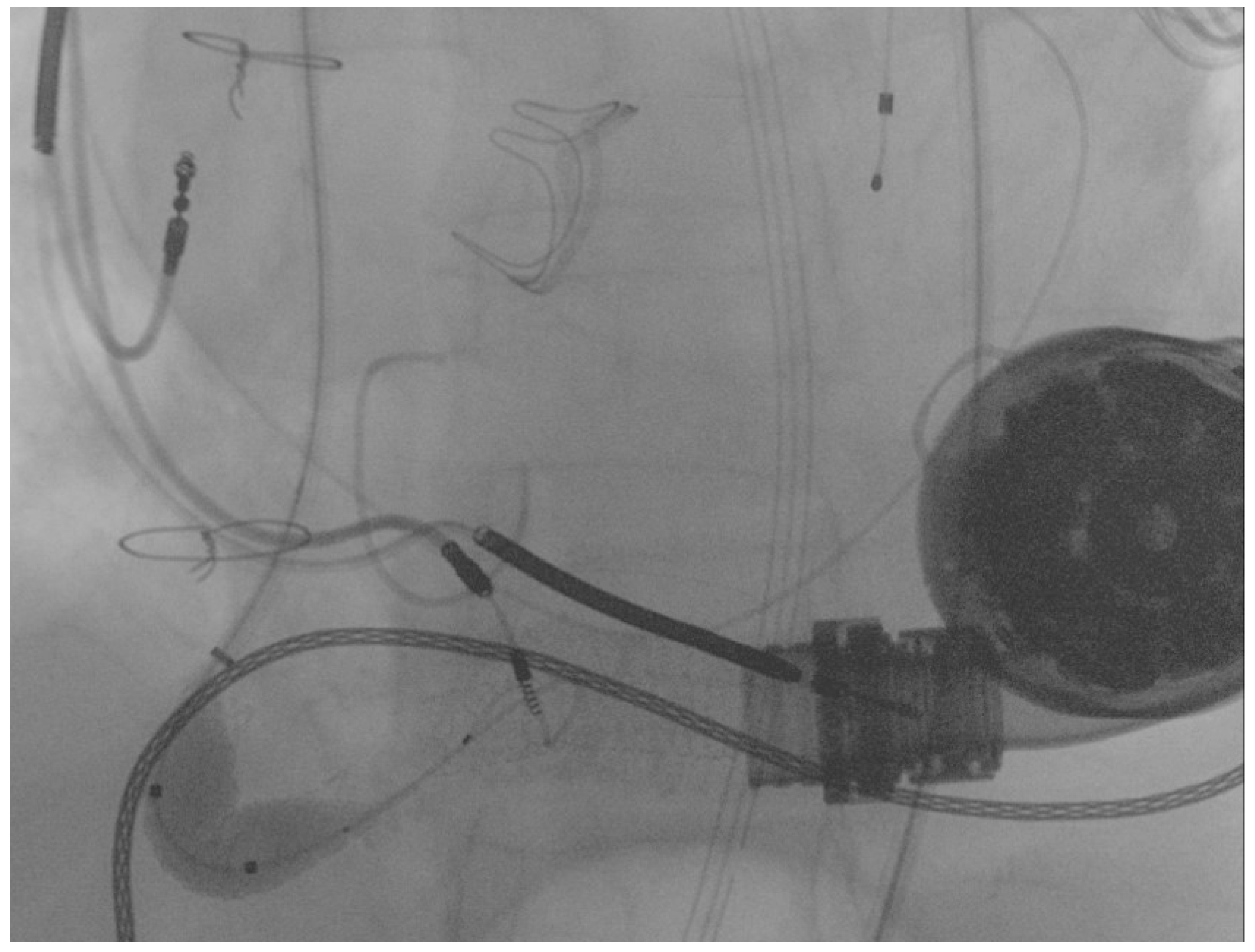
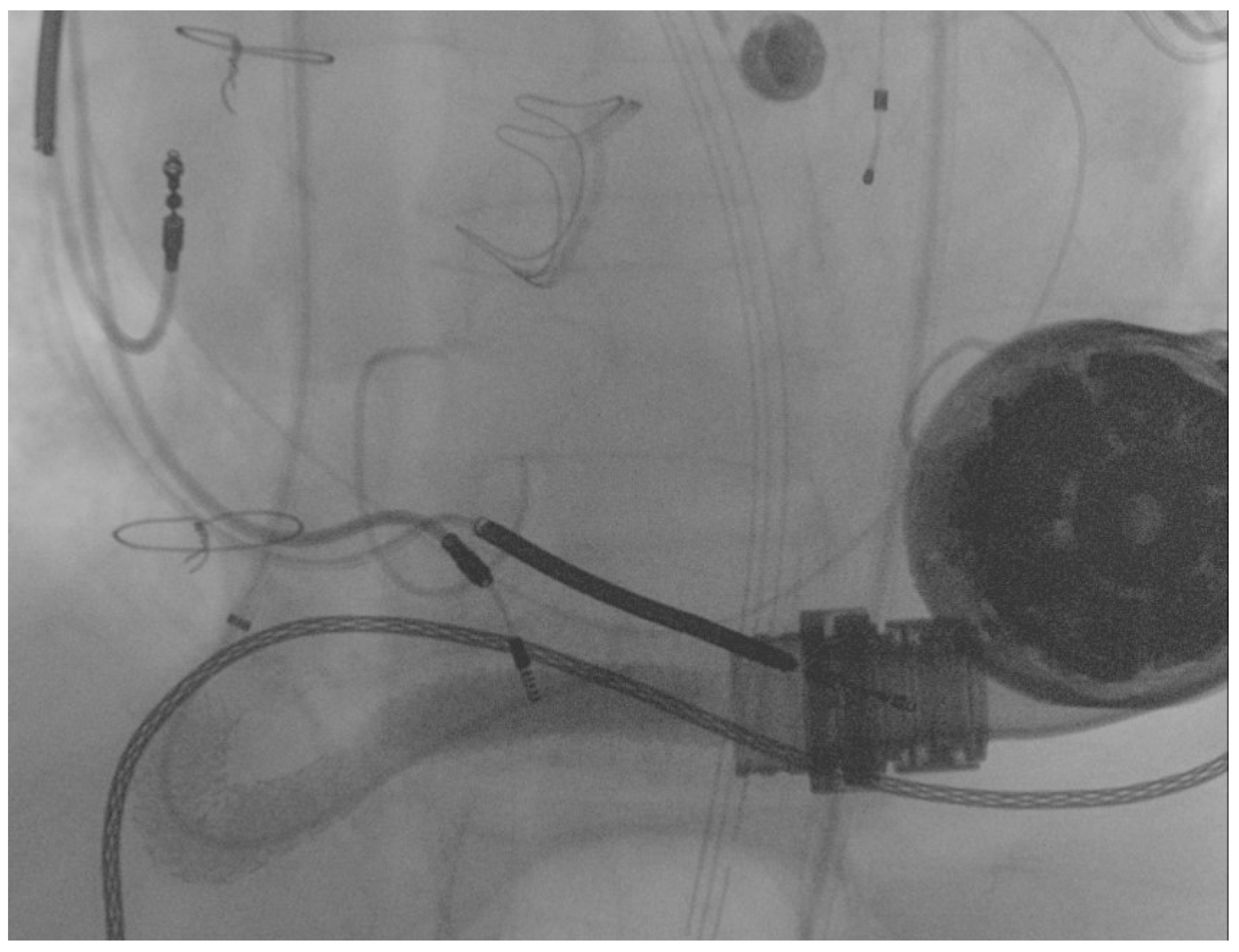
2. Case Report
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: Developed by the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) with the special contributio. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef] [PubMed]
- Truby, L.K.; Rogers, J.G. Advanced Heart Failure: Epidemiology, Diagnosis, and Therapeutic Approaches. JACC Heart Fail. 2020, 8, 523–536. [Google Scholar] [CrossRef] [PubMed]
- Gustafsson, F.; Shaw, S.; Lavee, J.; Saeed, D.; Pya, Y.; Krabatsch, T.; Schmitto, J.; Morshuis, M.; Chuang, J.; Damme, L.; et al. Six-month outcomes after treatment of advanced heart failure with a full magnetically levitated continuous flow left ventricular assist device: Report from the ELEVATE registry. Eur. Heart J. 2018, 39, 3454–3460. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burke, M.A.; Givertz, M.M. Assessment and management of heart failure after left ventricular assist device implantation. Circulation 2014, 129, 1161–1166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Milwidsky, A.; Alvarez Villela, M.; Wiley, J.; Sanina, C.; Patel, S.R.; Sutton, N.; Latib, A.; Sims, D.B.; Forest, S.J.; Shin, J.J.; et al. Outflow graft obstruction in patients with the HM 3 LVAD: A percutaneous approach. Catheter. Cardiovasc. Interv. Off. J. Soc. Card. Angiogr. Interv. 2021, 98, 1383–1390. [Google Scholar] [CrossRef] [PubMed]
- Stio, R.E.; Montalto, A.; Feccia, M.; Intorcia, A.; Buffa, V.; Cesario, V.; Petroni, G.; De Felice, F.; Musumeci, F. Extracorporeal membrane oxygenation-assisted emergency percutaneous treatment of left ventricular assist device graft occlusion. ESC Heart Fail. 2021, 8, 1627–1630. [Google Scholar] [CrossRef] [PubMed]
- Scandroglio, A.M.; Kaufmann, F.; Pieri, M.; Kretzschmar, A.; Müller, M.; Pergantis, P.; Dreysse, S.; Falk, V.; Krabatsch, T.; Potapov, E.V. Diagnosis and Treatment Algorithm for Blood Flow Obstructions in Patients With Left Ventricular Assist Device. J. Am. Coll. Cardiol. 2016, 67, 2758–2768. [Google Scholar] [CrossRef] [PubMed]
- Stio, R.E.; Montalto, A.; Intorcia, A.; Polizzi, V.; Feccia, M.; Musto, C.; Pennacchi, M.; Paolucci, L.; Stumpo, R.; D’Avino, E.; et al. A Case of Successful Use of the “Anchoring Technique” for Percutaneous Treatment of Left Ventricular Assist Device Graft Occlusion. Int. J. Environ. Res. Public Health 2022, 19, 2441. [Google Scholar] [CrossRef] [PubMed]
- Mehra, M.R.; Uriel, N.; Naka, Y.; Cleveland, J.C.J.; Yuzefpolskaya, M.; Salerno, C.T.; Walsh, M.N.; Milano, C.A.; Patel, C.B.; Hutchins, S.W.; et al. A Fully Magnetically Levitated Left Ventricular Assist Device-Final Report. N. Engl. J. Med. 2019, 380, 1618–1627. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, A.; Alexy, T.; Kamioka, N.; Shafi, T.; Stowe, J.; Morris, A.A.; Vega, J.D.; Babaliaros, V.; Burke, M.A. Outflow graft obstruction after left ventricular assist device implantation: A retrospective, single-centre case series. ESC Heart Fail. 2021, 8, 2349–2353. [Google Scholar] [CrossRef] [PubMed]
- Barac, Y.D.; Nevo, A.; Schroder, J.N.; Milano, C.A.; Daneshmand, M.A. LVAD Outflow Graft Role in Pump Thrombosis. ASAIO J. 2020, 66, 128–131. [Google Scholar] [CrossRef] [PubMed]
- Duero Posada, J.G.; Moayedi, Y.; Alhussein, M.; Rodger, M.; Alvarez, J.; Wintersperger, B.J.; Ross, H.J.; Butany, J.; Billia, F.; Rao, V. Outflow Graft Occlusion of the HeartMate 3 Left Ventricular Assist Device. Circ. Heart Fail. 2017, 10, e004275. [Google Scholar] [CrossRef] [PubMed]
- Davila, C.D.; Kiernan, M.S.; Kapur, N.K. Percutaneous Management of Outflow Graft Obstruction in Patients With Continuous Flow Left Ventricular Assist Devices. JACC. Case Rep. 2020, 2, 400–405. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stio, R.E.; Comisso, M.; Paolucci, L.; Coletta, S.; Cesario, V.; Gioia, M.; Nazzaro, M.S.; Saitto, G.; Contento, C.; D’Avino, E.; et al. A Redo Percutaneous Emergency Intervention of Left Ventricular Assist Device Graft Occlusion. Int. J. Environ. Res. Public Health 2022, 19, 5976. https://doi.org/10.3390/ijerph19105976
Stio RE, Comisso M, Paolucci L, Coletta S, Cesario V, Gioia M, Nazzaro MS, Saitto G, Contento C, D’Avino E, et al. A Redo Percutaneous Emergency Intervention of Left Ventricular Assist Device Graft Occlusion. International Journal of Environmental Research and Public Health. 2022; 19(10):5976. https://doi.org/10.3390/ijerph19105976
Chicago/Turabian StyleStio, Rocco Edoardo, Marina Comisso, Luca Paolucci, Silvio Coletta, Vincenzo Cesario, Michele Gioia, Marco Stefano Nazzaro, Guglielmo Saitto, Carlo Contento, Emilio D’Avino, and et al. 2022. "A Redo Percutaneous Emergency Intervention of Left Ventricular Assist Device Graft Occlusion" International Journal of Environmental Research and Public Health 19, no. 10: 5976. https://doi.org/10.3390/ijerph19105976
APA StyleStio, R. E., Comisso, M., Paolucci, L., Coletta, S., Cesario, V., Gioia, M., Nazzaro, M. S., Saitto, G., Contento, C., D’Avino, E., De Felice, F., Gabrielli, D., & Musumeci, F. (2022). A Redo Percutaneous Emergency Intervention of Left Ventricular Assist Device Graft Occlusion. International Journal of Environmental Research and Public Health, 19(10), 5976. https://doi.org/10.3390/ijerph19105976






