MRI and Adenomyosis: What Can Radiologists Evaluate?
Abstract
:1. Introduction
2. Research Method
3. Results
3.1. MRI Protocol
3.2. Junctional Zone: Physiological Changes
3.3. MR Classifications
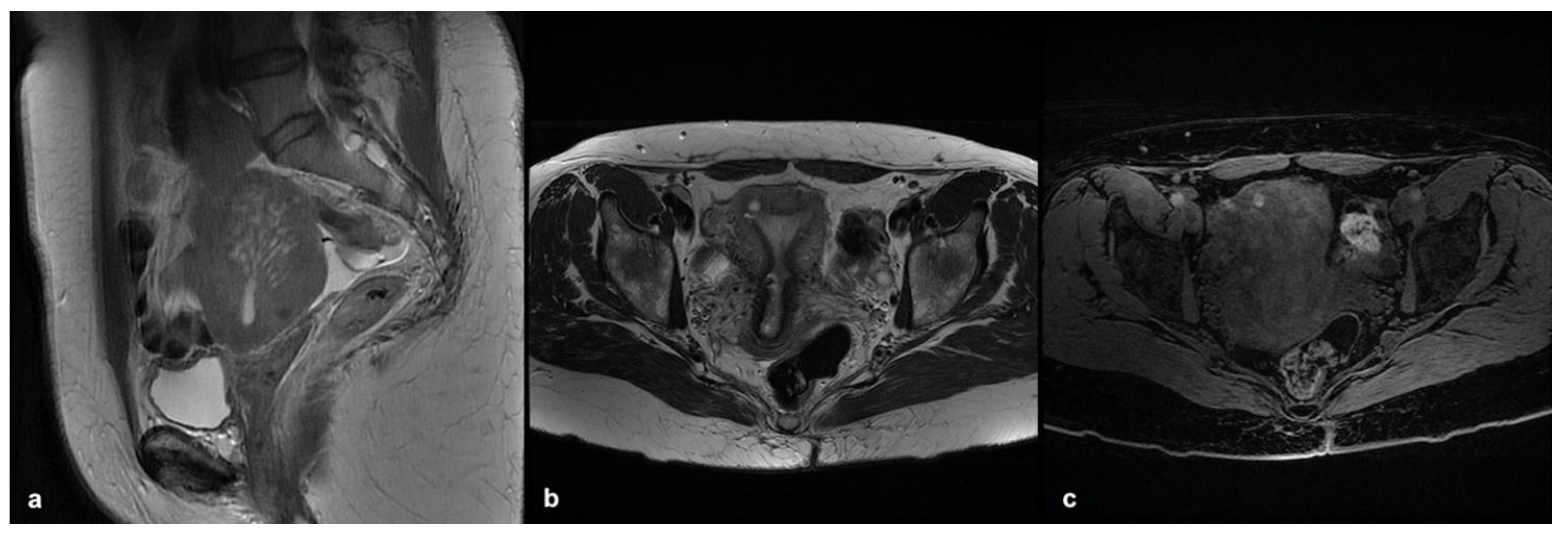
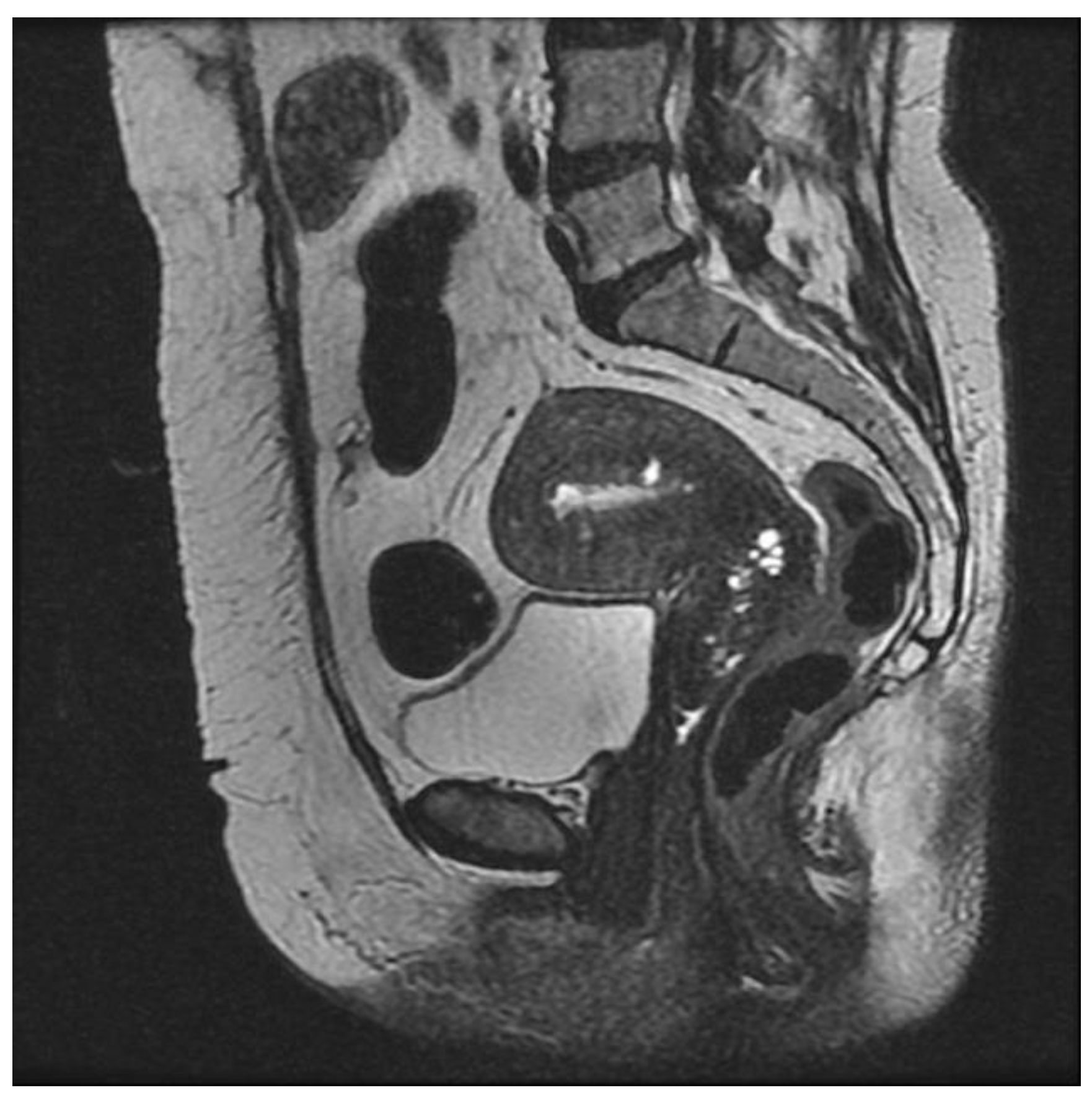
3.3.1. Internal Adenomyosis
3.3.2. External Adenomyosis
3.3.3. Adenomyoma
3.4. Pitfalls
3.5. Differential Diagnosis
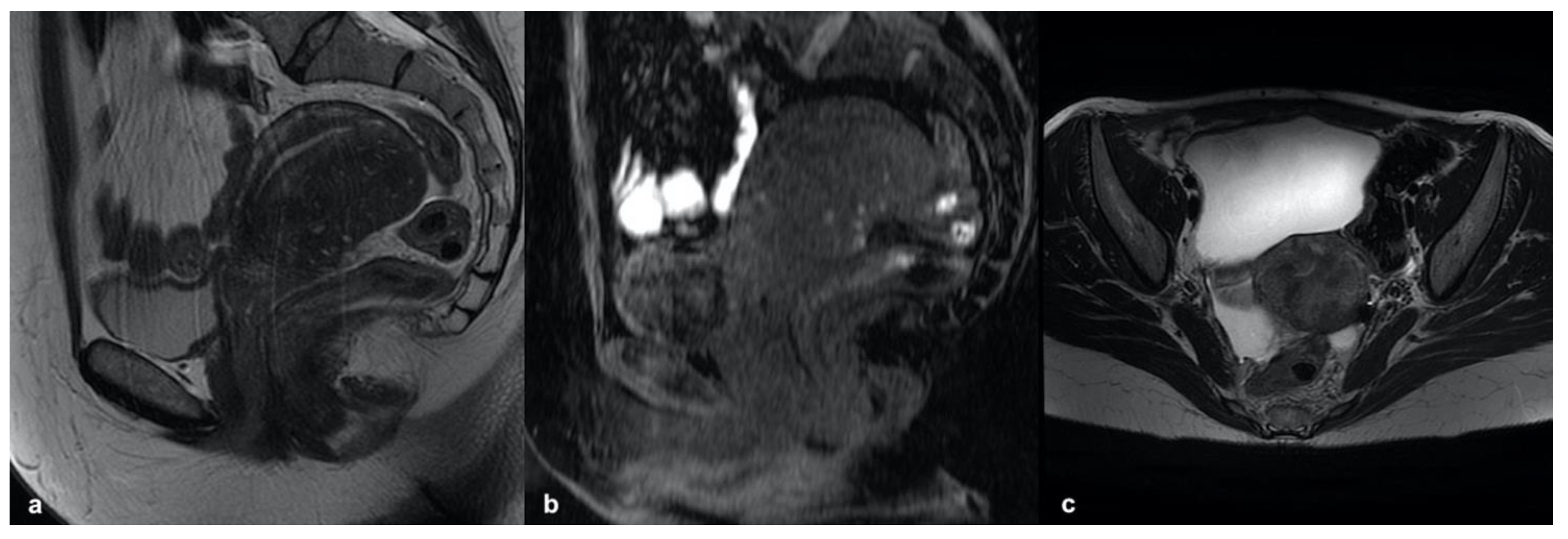
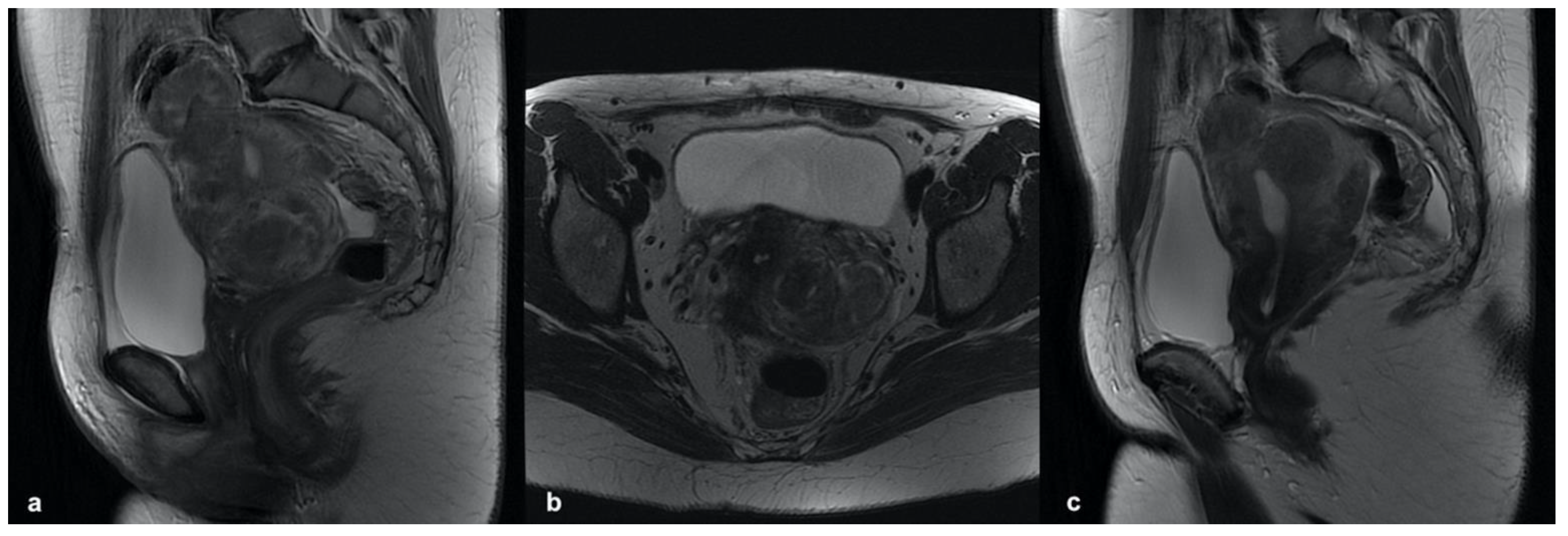
3.5.1. Leiomyoma
3.5.2. Accessory Cavitated Uterine Mass (ACUM)
3.5.3. MELF Endometrial Carcinoma
3.5.4. Low-Grade Endometrial Stroma Sarcoma (LG-ESS)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| US | Ultrasonography |
| MRI | Magnetic Resonance Imaging |
| CI | Confidence Interval |
| AUC | Area Under the Curve |
| ESUR | European Society of Urogenital Radiology |
| JZ | Junctional Zone |
| ACUM | Accessory Uterine Cavitated Mass |
| LG-ESS | Low-Grade Endometrial Stroma Sarcoma |
| DIE | Deep Infiltrating Endometriosis |
References
- Agostinho, L.; Cruz, R.; Osório, F.; Alves, J.; Setúbal, A.; Guerra, A. MRI for Adenomyosis: A Pictorial Review. Insights Imaging 2017, 8, 549–556. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tellum, T.; Matic, G.V.; Dormagen, J.B.; Nygaard, S.; Viktil, E.; Qvigstad, E.; Lieng, M. Diagnosing Adenomyosis with MRI: A Prospective Study Revisiting the Junctional Zone Thickness Cutoff of 12 Mm as a Diagnostic Marker. Eur. Radiol. 2019, 29, 6971–6981. [Google Scholar] [CrossRef] [PubMed]
- Choi, E.J.; Cho, S.B.; Lee, S.R.; Lim, Y.M.; Jeong, K.; Moon, H.-S.; Chung, H. Comorbidity of Gynecological and Non-Gynecological Diseases with Adenomyosis and Endometriosis. Obstet. Gynecol. Sci. 2017, 60, 579. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Liu, X.; Guo, S.-W. Clinical Profiles of 710 Premenopausal Women with Adenomyosis Who Underwent Hysterectomy: Clinical Profiles of Adenomyosis. J. Obstet. Gynaecol. Res. 2014, 40, 485–494. [Google Scholar] [CrossRef] [PubMed]
- Younes, G.; Tulandi, T. Effects of Adenomyosis on in Vitro Fertilization Treatment Outcomes: A Meta-Analysis. Fertil. Steril. 2017, 108, 483–490.e3. [Google Scholar] [CrossRef] [Green Version]
- Bruun, M.R.; Arendt, L.H.; Forman, A.; Ramlau-Hansen, C.H. Endometriosis and Adenomyosis Are Associated with Increased Risk of Preterm Delivery and a Small-for-Gestational-Age Child: A Systematic Review and Meta-Analysis. Acta Obstet. Gynecol. Scand. 2018, 97, 1073–1090. [Google Scholar] [CrossRef] [Green Version]
- Ferenczy, A. Pathophysiology of Adenomyosis. Hum. Reprod. Update 1998, 4, 312–322. [Google Scholar] [CrossRef] [Green Version]
- Matsumoto, Y. Apoptosis and Ki-67 Expression in Adenomyotic Lesions and in the Corresponding Eutopic Endometrium. Obstet. Gynecol. 1999, 94, 71–77. [Google Scholar] [CrossRef]
- Templeman, C.; Marshall, S.F.; Ursin, G.; Horn-Ross, P.L.; Clarke, C.A.; Allen, M.; Deapen, D.; Ziogas, A.; Reynolds, P.; Cress, R.; et al. Adenomyosis and Endometriosis in the California Teachers Study. Fertil. Steril. 2008, 90, 415–424. [Google Scholar] [CrossRef] [Green Version]
- Riggs, J.C.; Lim, E.K.; Liang, D.; Bullwinkel, R. Cesarean Section as a Risk Factor for the Development of Adenomyosis Uteri. J. Reprod. Med. 2014, 59, 20–24. [Google Scholar]
- Habiba, M.; Lippi, D.; Benagiano, G. The History of the Discovery of Ectopic Epithelial Cells in Lower Peritoneal Organs: The So-Called Mucosal Invasion. Reprod. Med. 2021, 2, 8. [Google Scholar] [CrossRef]
- Saba, L.; Guerriero, S.; Sulcis, R.; Ajossa, S.; Melis, G.; Mallarini, G. Agreement and Reproducibility in Identification of Endometriosis Using Magnetic Resonance Imaging. Acta Radiol. 2010, 51, 573–580. [Google Scholar] [CrossRef] [PubMed]
- Bianek-Bodzak, A.; Szurowska, E.; Sawicki, S.; Liro, M. The Importance and Perspective of Magnetic Resonance Imaging in the Evaluation of Endometriosis. BioMed Res. Int. 2013, 2013, 436589. [Google Scholar] [CrossRef] [PubMed]
- Atri, M.; Reinhold, C.; Mehio, A.R.; Chapman, W.B.; Bret, P.M. Adenomyosis: US Features with Histologic Correlation in an in Vitro Study. Radiology 2000, 215, 783–790. [Google Scholar] [CrossRef]
- Bazot, M.; Cortez, A.; Darai, E.; Rouger, J.; Chopier, J.; Antoine, J.-M.; Uzan, S. Ultrasonography Compared with Magnetic Resonance Imaging for the Diagnosis of Adenomyosis: Correlation with Histopathology. Hum. Reprod. 2001, 16, 2427–2433. [Google Scholar] [CrossRef] [Green Version]
- Dueholm, M.; Lundorf, E.; Hansen, E.S.; Sørensen, J.S.; Ledertoug, S.; Olesen, F. Magnetic Resonance Imaging and Transvaginal Ultrasonography for the Diagnosis of Adenomyosis. Fertil. Steril. 2001, 76, 588–594. [Google Scholar] [CrossRef]
- Champaneria, R.; Abedin, P.; Daniels, J.; Balogun, M.; Khan, K.S. Ultrasound Scan and Magnetic Resonance Imaging for the Diagnosis of Adenomyosis: Systematic Review Comparing Test Accuracy. Acta Obstet. Gynecol. Scand. 2010, 89, 1374–1384. [Google Scholar] [CrossRef]
- Van den Bosch, T.; Dueholm, M.; Leone, F.P.G.; Valentin, L.; Rasmussen, C.K.; Votino, A.; Van Schoubroeck, D.; Landolfo, C.; Installé, A.J.F.; Guerriero, S.; et al. Terms, Definitions and Measurements to Describe Sonographic Features of Myometrium and Uterine Masses: A Consensus Opinion from the Morphological Uterus Sonographic Assessment (MUSA) Group. Ultrasound Obstet. Gynecol. 2015, 46, 284–298. [Google Scholar] [CrossRef]
- Van den Bosch, T.; de Bruijn, A.M.; de Leeuw, R.A.; Dueholm, M.; Exacoustos, C.; Valentin, L.; Bourne, T.; Timmerman, D.; Huirne, J.A.F. Sonographic Classification and Reporting System for Diagnosing Adenomyosis. Ultrasound Obstet. Gynecol. 2019, 53, 576–582. [Google Scholar] [CrossRef]
- Gordts, S.; Grimbizis, G.; Campo, R. Symptoms and Classification of Uterine Adenomyosis, Including the Place of Hysteroscopy in Diagnosis. Fertil. Steril. 2018, 109, 380–388.e1. [Google Scholar] [CrossRef] [Green Version]
- Kobayashi, H.; Matsubara, S. A Classification Proposal for Adenomyosis Based on Magnetic Resonance Imaging. Gynecol. Obstet. Investig. 2020, 85, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Bazot, M.; Bharwani, N.; Huchon, C.; Kinkel, K.; Cunha, T.M.; Guerra, A.; Manganaro, L.; Buñesch, L.; Kido, A.; Togashi, K.; et al. European Society of Urogenital Radiology (ESUR) Guidelines: MR Imaging of Pelvic Endometriosis. Eur. Radiol. 2017, 27, 2765–2775. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coutinho, A.; Bittencourt, L.K.; Pires, C.E.; Junqueira, F.; de Oliveira Lima, C.M.A.; Coutinho, E.; Domingues, M.A.; Domingues, R.C.; Marchiori, E. MR Imaging in Deep Pelvic Endometriosis: A Pictorial Essay. RadioGraphics 2011, 31, 549–567. [Google Scholar] [CrossRef] [PubMed]
- Hricak, H.; Alpers, C.; Crooks, L.; Sheldon, P. Magnetic Resonance Imaging of the Female Pelvis: Initial Experience. Am. J. Roentgenol. 1983, 141, 1119–1128. [Google Scholar] [CrossRef] [PubMed]
- Kishi, Y.; Suginami, H.; Kuramori, R.; Yabuta, M.; Suginami, R.; Taniguchi, F. Four Subtypes of Adenomyosis Assessed by Magnetic Resonance Imaging and Their Specification. Am. J. Obstet. Gynecol. 2012, 207, 114.e1–114.e7. [Google Scholar] [CrossRef] [PubMed]
- Bazot, M.; Daraï, E. Role of Transvaginal Sonography and Magnetic Resonance Imaging in the Diagnosis of Uterine Adenomyosis. Fertil. Steril. 2018, 109, 389–397. [Google Scholar] [CrossRef] [Green Version]
- Gordts, S.; Brosens, J.J.; Fusi, L.; Benagiano, G.; Brosens, I. Uterine Adenomyosis: A Need for Uniform Terminology and Consensus Classification. Reprod. BioMed Online 2008, 17, 244–248. [Google Scholar] [CrossRef]
- Novellas, S.; Chassang, M.; Delotte, J.; Toullalan, O.; Chevallier, A.; Bouaziz, J.; Chevallier, P. MRI Characteristics of the Uterine Junctional Zone: From Normal to the Diagnosis of Adenomyosis. Am. J. Roentgenol. 2011, 196, 1206–1213. [Google Scholar] [CrossRef]
- Kissler, S.; Zangos, S.; Wiegratz, I.; Kohl, J.; Rody, A.; Gaetje, R.; Doebert, N.; Wildt, L.; Kunz, G.; Leyendecker, G.; et al. Utero-Tubal Sperm Transport and Its Impairment in Endometriosis and Adenomyosis. Ann. N. Y. Acad. Sci. 2007, 1101, 38–48. [Google Scholar] [CrossRef]
- Fusi, L.; Cloke, B.; Brosens, J.J. The Uterine Junctional Zone. Best Pract. Res. Clin. Obstet. Gynaecol. 2006, 20, 479–491. [Google Scholar] [CrossRef]
- Masui, T.; Katayama, M.; Kobayashi, S.; Nakayama, S.; Nozaki, A.; Kabasawa, H.; Ito, T.; Sakahara, H. Changes in Myometrial and Junctional Zone Thickness and Signal Intensity: Demonstration with Kinematic T2-Weighted MR Imaging. Radiology 2001, 221, 75–85. [Google Scholar] [CrossRef] [PubMed]
- Bazot, M.; Lafont, C.; Rouzier, R.; Roseau, G.; Thomassin-Naggara, I.; Daraï, E. Diagnostic Accuracy of Physical Examination, Transvaginal Sonography, Rectal Endoscopic Sonography, and Magnetic Resonance Imaging to Diagnose Deep Infiltrating Endometriosis. Fertil. Steril. 2009, 92, 1825–1833. [Google Scholar] [CrossRef] [PubMed]
- Rees, C.O.; Nederend, J.; Mischi, M.; Vliet, H.A.A.M.; Schoot, B.C. Objective Measures of Adenomyosis on MRI and Their Diagnostic Accuracy—A Systematic Review & Meta-analysis. Acta Obstet. Gynecol. Scand. 2021, 100, 1377–1391. [Google Scholar] [CrossRef] [PubMed]
- Monte, G.L.; Wenger, J.M.; Petignat, P.; Marci, R. Role of Imaging in Endometriosis. Clevel. Clin. J. Med. 2014, 81, 361–366. [Google Scholar] [CrossRef] [PubMed]
- Levy, G.; Dehaene, A.; Laurent, N.; Lernout, M.; Collinet, P.; Lucot, J.-P.; Lions, C.; Poncelet, E. An Update on Adenomyosis. Diagn. Interv. Imaging 2013, 94, 3–25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bourdon, M.; Oliveira, J.; Marcellin, L.; Santulli, P.; Bordonne, C.; Maitrot Mantelet, L.; Millischer, A.E.; Plu Bureau, G.; Chapron, C. Adenomyosis of the Inner and Outer Myometrium Are Associated with Different Clinical Profiles. Hum. Reprod. 2021, 36, 349–357. [Google Scholar] [CrossRef]
- Bazot, M.; Darai, E.; Hourani, R.; Thomassin, I.; Cortez, A.; Uzan, S.; Buy, J.-N. Deep Pelvic Endometriosis: MR Imaging for Diagnosis and Prediction of Extension of Disease. Radiology 2004, 232, 379–389. [Google Scholar] [CrossRef] [Green Version]
- Chapron, C.; Fauconnier, A.; Vieira, M.; Barakat, H.; Dousset, B.; Pansini, V.; Vacher-Lavenu, M.C.; Dubuisson, J.B. Anatomical Distribution of Deeply Infiltrating Endometriosis: Surgical Implications and Proposition for a Classification. Hum. Reprod. 2003, 18, 157–161. [Google Scholar] [CrossRef]
- Scardapane, A.; Lorusso, F.; Scioscia, M.; Ferrante, A.; Stabile Ianora, A.A.; Angelelli, G. Standard High-Resolution Pelvic MRI vs. Low-Resolution Pelvic MRI in the Evaluation of Deep Infiltrating Endometriosis. Eur. Radiol. 2014, 24, 2590–2596. [Google Scholar] [CrossRef]
- Bazot, M.; Daraï, E.; de Givry, S.C.; Boudghène, F.; Uzan, S.; Le Blanche, A.F. Fast Breath-Hold T2-Weighted MR Imaging Reduces Interobserver Variability in the Diagnosis of Adenomyosis. Am. J. Roentgenol. 2003, 180, 1291–1296. [Google Scholar] [CrossRef]
- Johnson, W.; Taylor, M.B.; Carrington, B.M.; Bonington, S.C.; Swindell, R. The Value of Hyoscine Butylbromide in Pelvic MRI. Clin. Radiol. 2007, 62, 1087–1093. [Google Scholar] [CrossRef] [PubMed]
- Nakai, A.; Koyama, T.; Fujimoto, K.; Togashi, K. Functional MR Imaging of the Uterus. Magn. Reson. Imaging Clin. N. Am. 2008, 16, 673–684. [Google Scholar] [CrossRef] [PubMed]
- Tamai, K.; Koyama, T.; Umeoka, S.; Saga, T.; Fujii, S.; Togashi, K. Spectrum of MR Features in Adenomyosis. Best Pract. Res. Clin. Obstet. Gynaecol. 2006, 20, 583–602. [Google Scholar] [CrossRef] [PubMed]
- Acién, P.; Acién, M.; Fernández, F.; José Mayol, M.; Aranda, I. The Cavitated Accessory Uterine Mass: A Müllerian Anomaly in Women With an Otherwise Normal Uterus. Obstet. Gynecol. 2010, 116, 1101–1109. [Google Scholar] [CrossRef]
- Acien, P.; Bataller, A.; Fernandez, F.; Acien, M.I.; Rodriguez, J.M.; Mayol, M.J. New Cases of Accessory and Cavitated Uterine Masses (ACUM): A Significant Cause of Severe Dysmenorrhea and Recurrent Pelvic Pain in Young Women. Hum. Reprod. 2012, 27, 683–694. [Google Scholar] [CrossRef] [Green Version]
- Peyron, N.; Jacquemier, E.; Charlot, M.; Devouassoux, M.; Raudrant, D.; Golfier, F.; Rousset, P. Accessory Cavitated Uterine Mass: MRI Features and Surgical Correlations of a Rare but under-Recognised Entity. Eur. Radiol. 2019, 29, 1144–1152. [Google Scholar] [CrossRef]
- Murray, S.K.; Young, R.H.; Scully, R.E. Unusual Epithelial and Stromal Changes in Myoinvasive Endometrioid Adenocarcinoma: A Study of Their Frequency, Associated Diagnostic Problems, and Prognostic Significance. Int. J. Gynecol. Pathol. 2003, 22, 324–333. [Google Scholar] [CrossRef]
- Kuwahara, R.; Kido, A.; Yajima, R.; Nishio, N.; Nakao, K.; Kurata, Y.; Tanaka, S.; Minamiguchi, S.; Baba, T.; Mandai, M.; et al. Microcystic, Elongated and Fragmented Pattern Invasion Can Adversely Influence Preoperative Staging for Low-Grade Endometrial Carcinoma. Magn. Reson. Med. Sci. 2021, 20, 20–27. [Google Scholar] [CrossRef] [Green Version]
- Tamai, K.; Togashi, K.; Ito, T.; Morisawa, N.; Fujiwara, T.; Koyama, T. MR Imaging Findings of Adenomyosis: Correlation with Histopathologic Features and Diagnostic Pitfalls. RadioGraphics 2005, 25, 21–40. [Google Scholar] [CrossRef]
- Takeuchi, M.; Matsuzaki, K.; Nishitani, H. Hyperintense Uterine Myometrial Masses on T2-Weighted Magnetic Resonance Imaging: Differentiation With Diffusion-Weighted Magnetic Resonance Imaging. J. Comput. Assist. Tomogr. 2009, 33, 834–837. [Google Scholar] [CrossRef]


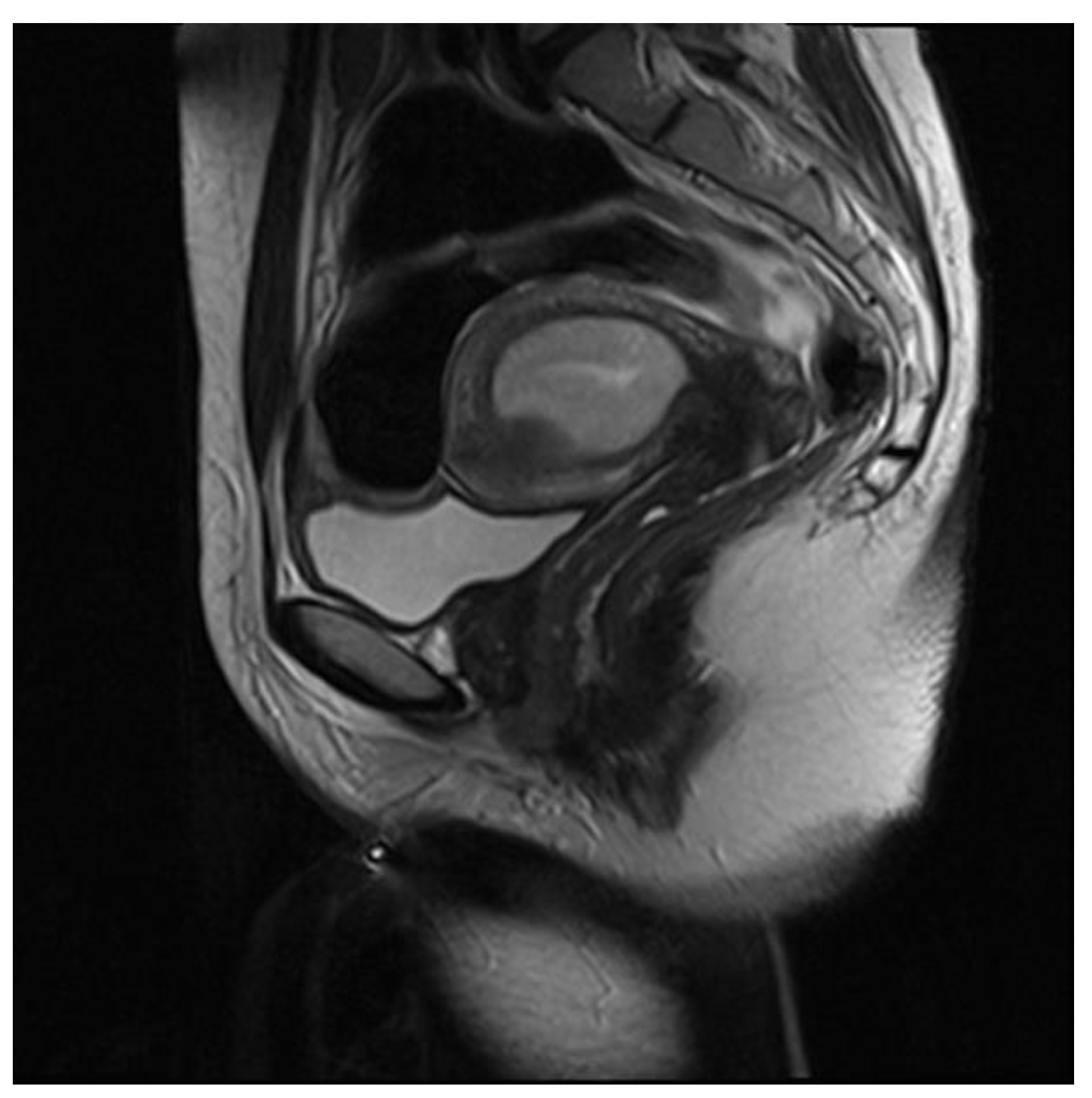
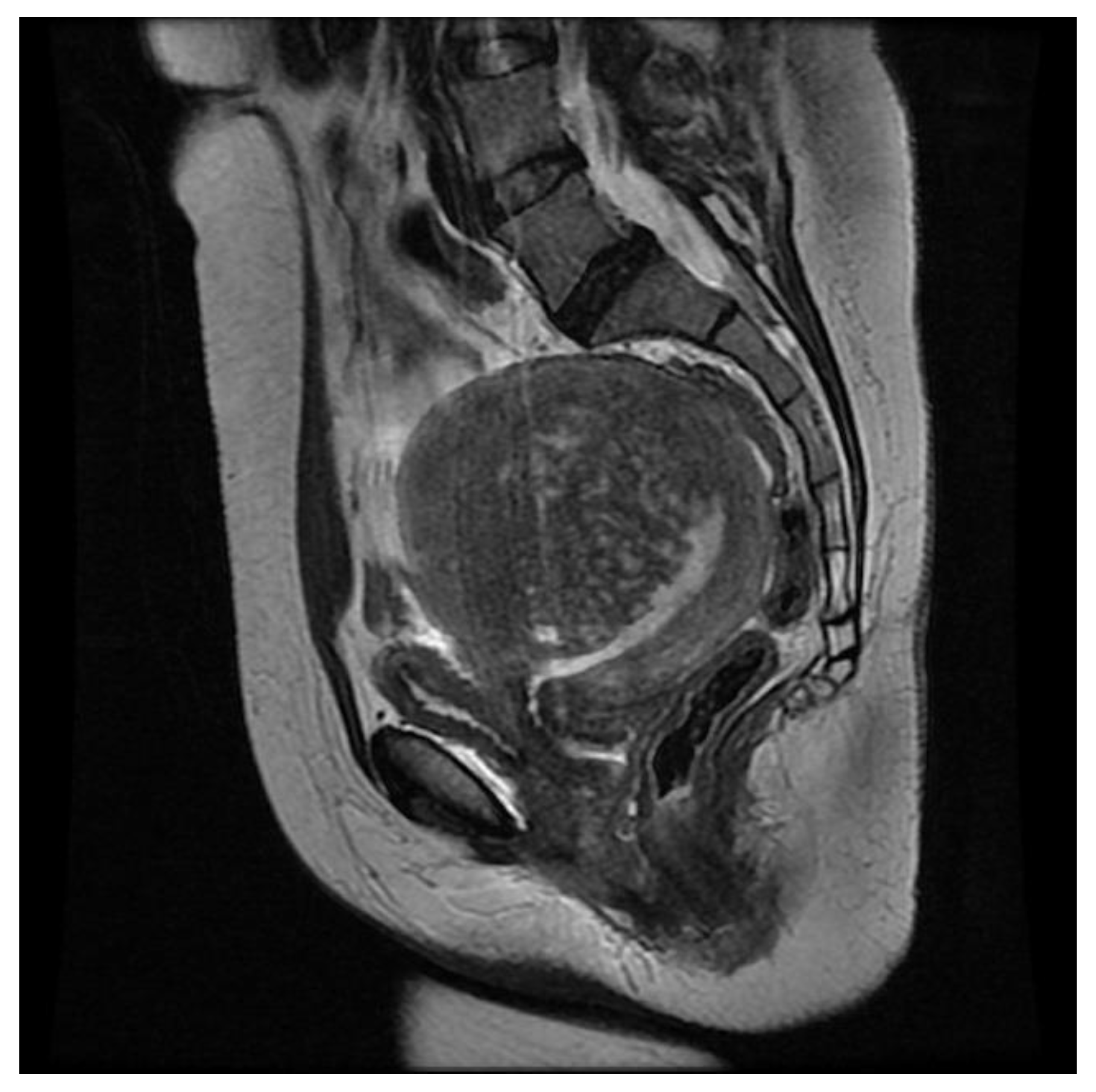



| Sequence | TR (msec) | TE (msec) | FOV (mm) | Matrix | Thickness (mm) |
|---|---|---|---|---|---|
| T2 TSE | 3000 | 68 | 260 × 260 | 320 × 256 | 4 |
| T1 TSE with and without FS | 500 | Min | 320 × 280 | 192 × 256 | 4 |
| Non-CE and CE T1 VIBE | 150 | Min | 320 × 280 | 256 × 256 | 4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Celli, V.; Dolciami, M.; Ninkova, R.; Ercolani, G.; Rizzo, S.; Porpora, M.G.; Catalano, C.; Manganaro, L. MRI and Adenomyosis: What Can Radiologists Evaluate? Int. J. Environ. Res. Public Health 2022, 19, 5840. https://doi.org/10.3390/ijerph19105840
Celli V, Dolciami M, Ninkova R, Ercolani G, Rizzo S, Porpora MG, Catalano C, Manganaro L. MRI and Adenomyosis: What Can Radiologists Evaluate? International Journal of Environmental Research and Public Health. 2022; 19(10):5840. https://doi.org/10.3390/ijerph19105840
Chicago/Turabian StyleCelli, Veronica, Miriam Dolciami, Roberta Ninkova, Giada Ercolani, Stefania Rizzo, Maria Grazia Porpora, Carlo Catalano, and Lucia Manganaro. 2022. "MRI and Adenomyosis: What Can Radiologists Evaluate?" International Journal of Environmental Research and Public Health 19, no. 10: 5840. https://doi.org/10.3390/ijerph19105840
APA StyleCelli, V., Dolciami, M., Ninkova, R., Ercolani, G., Rizzo, S., Porpora, M. G., Catalano, C., & Manganaro, L. (2022). MRI and Adenomyosis: What Can Radiologists Evaluate? International Journal of Environmental Research and Public Health, 19(10), 5840. https://doi.org/10.3390/ijerph19105840







