Nutritional and Other Trace Elements and Their Associations in Raw King Bolete Mushrooms, Boletus edulis
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Essential (Zn, Cu, Mo, and Co)
3.2. Toxic Elements (Ag, As, Cd, Hg, Pb, Sb, Tl, and U)
3.3. Monovalent Elements (Cs, Li, Rb)
3.4. The Elements Ba, Sr, Ni and V
3.5. Elements Rarely Studied in B. edulis: Be, Bi, Ga, Ge, Hf, In, Nb, Sn, Ta, Th, Ti, W, Zr, ƩREEs
3.6. Principal Component Analysis
4. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Laessoe, T.; Del Conte, A.; Lincoff, G. The Mushroom Book. How to Identify, Gather and Cook Wild Mushrooms and Other Fungi; ADK Publishing Inc.: New York, NY, USA, 1996. [Google Scholar]
- Falandysz, J.; Borovička, J. Macro and trace mineral constituents and radionuclides in mushrooms: Health benefits and risks. Appl. Microbiol. Biotechnol. 2013, 97, 477–501. [Google Scholar] [CrossRef]
- Gumińska, B.; Wojewoda, W. Grzyby i Ich Oznaczanie; Państwowe Wydawnictwo Rolnicze i Leśne: Warsaw, Poland, 1985. (In Polish) [Google Scholar]
- Arora, D.; Frank, J.L. Boletus rubriceps, a new species of porcini from the southwestern USA. N. Am. Fungi 2014, 9, 1–11. [Google Scholar] [CrossRef]
- Wu, G.; Li, Y.; Zhu, X.; Zhao, K.; Han, L.; Cui, Y.; Li, F.; Xu, J.; Yang, Z.-L. One hundred noteworthy boletes from China. Fungal Div. 2016, 81, 25–188. [Google Scholar] [CrossRef]
- Domaszewicz, B.; Pac, L.; Raczkowska, J.; Wilamowska, L. Leśnictwo 2015; Informacje i Opracowania Statystyczne, GUS Departament Rolnictwa: Warsaw, Poland, 2015; ISSN 1230-574X. (In Polish)
- Falandysz, J. Selenium in edible mushrooms. J. Environ. Sci. Health Part C 2008, 26, 256–299. [Google Scholar] [CrossRef]
- Heleno, S.A.; Ferreira, R.C.; Antonio, A.L.; Queiroz, M.-J.R.P.; Barros, L.; Ferreira, I.C.F.R. Nutritional value, bioactive compounds and antioxidant properties of three edible mushrooms from Poland. Food Biosci. 2015, 11, 48–55. [Google Scholar] [CrossRef]
- Costa-Silva, F.; Marques, G.; Matos, C.C.; Barros, A.I.R.N.A.; Nunes, F.M. Selenium contents of Portuguese commercial and wild edible mushrooms. Food Chem. 2011, 126, 91–96. [Google Scholar] [CrossRef]
- Falandysz, J. Review: On published data and methods for selenium in mushrooms. Food Chem. 2013, 138, 242–250. [Google Scholar] [CrossRef]
- Jaworska, G.; Pogoń, K.; Bernaś, E.; Skrzypczak, A. Effect of different drying methods and 24-month storage on water activity, rehydration capacity and antioxidants in Boletus edulis mushrooms. Dry. Technol. 2014, 32, 291–300. [Google Scholar] [CrossRef]
- Jaworska, G.; Pogoń, K.; Skrzypczak, A.; Bernaś, E. Composition and antioxidant properties of wild mushrooms Boletus edulis and Xerocomus badius prepared for consumption. J. Food Sci. Technol. 2015, 52, 7944–7953. [Google Scholar] [CrossRef]
- Falandysz, J.; Saba, M.; Rutkowska, M.; Konieczka, P. Total mercury and methylmercury (MeHg) in braised and crude Boletus edulis carpophores during various developmental stages. Environ. Sci. Pollut. Res. 2021. [Google Scholar] [CrossRef]
- Saba, M.; Falandysz, J. The effects of different cooking modes on the 137Cs, 40K, and total K content in Boletus edulis (King Bolete) mushrooms. Environ. Sci. Pollut. Res. 2021, 28, 12441–12446. [Google Scholar] [CrossRef] [PubMed]
- Falandysz, J.; Meloni, D.; Fernandes, A.R.; Saniewski, M. Effect of drying, blanching, pickling and maceration on fate of 40K, total K and 137Cs in bolete mushrooms and dietary intake. Environ. Sci. Pollut. Res. 2021. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, N.; Solmaz, M.; Türkekul, I.; Elmastaş, M. Fatty acid composition in some wild edible mushrooms growing in the middle Black Sea region of Turkey. Food Chem. 2006, 99, 168–174. [Google Scholar] [CrossRef]
- Pietrzak-Fiećko, R.; Gałgowska, M.; Bakuła, S. Fatty acid composition in wild Boletus edulis from Poland. Ital. J. Food Sci. 2016, 28, 402–411. [Google Scholar]
- Pietrzak-Fiećko, R.; Gałgowska, M.M.; Falandysz, J. Impact of mushrooms’ vegetative places and morphological parts of a fruiting body on the fatty acids profile of wild Leccinum aurantiacum and Leccinum versipelle. Chem. Biodivers. 2020, 17, e2000032. [Google Scholar] [CrossRef]
- Mirończuk-Chodakowska, I.; Witkowska, A.M. Evaluation of Polish wild mushrooms as beta-glucan sources. Int. J. Environ. Res. Public Health 2020, 17, 7299. [Google Scholar] [CrossRef]
- Kumar, A.; Zhang, K.Y.J. Human chitinases: Structure, function, and inhibitor discovery. In Targeting Chitin-Containing Organisms; Yang, Q., Fukamizo, T., Eds.; Advances in Experimental Medicine and Biology; Springer: Singapore, 2019; Volume 1142, pp. 221–251. [Google Scholar]
- Mutanen, M. Bioavailability of selenium in mushrooms Boletus edulis to young women. Int. J. Vitam. Nutr. Res. 1986, 56, 297–301. [Google Scholar] [PubMed]
- Cocchi, L.; Vescovi, L.; Petrini, L.; Petrini, O. Heavy metals in edible mushrooms in Italy. Food Chem. 2006, 98, 277–284. [Google Scholar] [CrossRef]
- Giannaccini, G.; Betti, L.; Palego, L.; Mascia, G.; Schmid, L.; Lanza, M.; Mela, A.; Fabbrini, L.; Biondi, L.; Lucacchini, A. The trace element content of top-soil and wild edible mushroom samples collected in Tuscany, Italy. Environ. Monit. Assess. 2012, 184, 7579–7595. [Google Scholar] [CrossRef]
- Quinche, J.P. Phosphorus and heavy metals in some species of fungi. Rev. Suisse D’agric. 1997, 29, 151–156. [Google Scholar]
- Malinowski, R.; Sotek, Z.; Stasińska, M.; Malinowska, K.; Radke, P.; Malinowska, A. Bioaccumulation of macronutrients in edible mushrooms in various habitat conditions of NW Poland—Role in the human diet. Int. J. Environ. Res. Public Health 2021, 18, 8881. [Google Scholar] [CrossRef]
- Řanda, Z.; Kučera, J. Trace elements in higher fungi (mushrooms) determined by activation analysis. J. Radioanal. Nucl. Chem. 2004, 259, 99–107. [Google Scholar] [CrossRef]
- Nasr, M.; Malloch, D.W.; Arp, P.A. Quantifying Hg within ectomycorrhizal fruiting bodies, from emergence to senescence. Fungal Biol. 2012, 116, 1163–1177. [Google Scholar] [CrossRef]
- Varo, P.; Lähelmä, O.; Nuurtamo, M.; Saari, E.; Koivistoinen, P. Mineral element composition of Finnish foods. VII. Potato, vegetables, fruits, berries, nuts and mushrooms. Acta Agric. Scand. 1980, 22, 89–113. [Google Scholar]
- Falandysz, J.; Szymczyk, K.; Ichihashi, H.; Bielawski, L.; Gucia, M.; Frankowska, A.; Yamasaki, S.-I. ICP/MS and ICP/AES elemental analysis (38 elements) of edible wild mushrooms growing in Poland. Food Addit. Contam. 2001, 18, 503–513. [Google Scholar] [CrossRef]
- Řanda, Z.; Soukal, L.; Mizera, J. Possibilities of the short-term thermal and epithermal neutron activation for analysis of macromycetes (mushrooms). J. Radioanal. Nucl. Chem. 2005, 264, 67–76. [Google Scholar] [CrossRef]
- Falandysz, J.; Frankowska, A. Niektóre pierwiastki metaliczne i ich współczynniki biokoncentracji w borowiku szlachetnym (Boletus edulis) z Puszczy Świętokrzyskiej. Bromatol. Chem. Toksykol. 2007, 40, 257–260. (In Polish) [Google Scholar]
- Jorhem, L.; Sundstrōm, B. Levels of some trace elements in edible fungi. Z. Lebensm. Unters. Forsch. 1995, 201, 311–316. [Google Scholar] [CrossRef] [PubMed]
- Nikkarinen, M.; Mertanen, E. Impact of geological origin on traces element composition of edible mushrooms. J. Food Compos. Anal. 2004, 17, 301–310. [Google Scholar] [CrossRef]
- Falandysz, J.; Kunito, T.; Kubota, R.; Bielawski, L.; Mazur, A.; Falandysz, J.J.; Tanabe, S. Multivariate characterization of elements accumulated in King Bolete Boletus edulis mushroom at lowland and high mountain regions. J. Environ. Sci. Health Part A 2008, 43, 1692–1699. [Google Scholar] [CrossRef]
- Falandysz, J.; Frankowska, A.; Jarzyńska, G.; Dryżałowska, A.; Kojta, A.K.; Zhang, D. Survey on composition and bioconcentration potential of 12 metallic elements in King Bolete (Boletus edulis) mushroom that emerged at 11 spatially distant sites. J. Environ. Sci. Health Part B 2011, 46, 231–246. [Google Scholar] [CrossRef]
- Frankowska, A.; Ziółkowska, J.; Bielawski, L.; Falandysz, J. Profile and bioconcentration of minerals by King Bolete (Boletus edulis) from the Płocka Dale in Poland. Food Addit. Contam. Part B 2010, 3, 1–6. [Google Scholar] [CrossRef]
- Zhang, D.; Frankowska, A.; Jarzyńska, G.; Kojta, A.K.; Drewnowska, M.; Wydmańska, D.; Bielawski, L.; Wang, J.; Falandysz, J. Metals of King Bolete (Boletus edulis) collected at the same site over two years. Afr. J. Agric. Res. 2010, 5, 3050–3055. [Google Scholar]
- Mleczek, M.; Siwulski, M.; Stuper-Szablewska, K.; Rissmann, I.; Sobieralski, K.; Goliński, P. Accumulation of elements by edible mushroom species: Part I. Problem of trace element toxicity in mushrooms. J. Environ. Sci. Health Part B 2013, 48, 69–81. [Google Scholar] [CrossRef]
- Mirończuk-Chodakowska, I.; Socha, K.; Zujko, M.E.; Terlikowska, K.M.; Borawska, M.H.; Witkowska, A.M. Copper, manganese, selenium and zinc in wild-growing edible mushrooms from the eastern territory of “Green Lungs of Poland”: Nutritional and toxicological implications. Int. J. Environ. Res. Public Health 2019, 16, 3614. [Google Scholar] [CrossRef]
- Falandysz, J.; Bona, H.; Danisiewicz, D. Silver content of wild-grown mushrooms from Northern Poland. Z. Lebensm. Unters. Forsch. 1994, 199, 222–224. [Google Scholar] [CrossRef]
- Falandysz, J.; Lipka, K.; Kawano, M.; Brzostowski, A.; Dadej, M.; Jędrusiak, A.; Puzyn, T. Mercury content and its bioconcentration factors at Łukta and Morąg, North-eastern Poland. J. Agric. Food Chem. 2003, 51, 2832–2836. [Google Scholar] [CrossRef]
- Falandysz, J.; Frankowska, A.; Mazur, A. Mercury and its bioconcentration factors in King Bolete (Boletus edulis) Bull. Fr. J. Environ. Sci. Health Part A 2007, 42, 2089–2095. [Google Scholar] [CrossRef]
- Petkovšek, S.A.S.; Pokorny, B. Lead and cadmium in mushrooms from the vicinity of two large emission sources in Slovenia. Sci. Total Environ. 2013, 443, 944–954. [Google Scholar] [CrossRef] [PubMed]
- Falandysz, J.; Krasińska, G.; Pankavec, S.; Nnorom, I.C. Mercury in certain Boletus mushrooms from Poland and Belarus. J. Environ. Sci. Health Part B 2014, 49, 690–695. [Google Scholar] [CrossRef]
- Falandysz, J.; Zalewska, T.; Krasińska, G.; Apanel, A.; Wang, Y.; Pankavec, S. Evaluation of the radioactive contamination in Fungi genus Boletus in the region of Europe and Yunnan Province in China. Appl. Microbiol. Biotechnol. 2015, 99, 8217–8224. [Google Scholar] [CrossRef][Green Version]
- Dimitrijevic, M.V.; Mitic, V.D.; Cvetkovic, J.S.; Stankov Jovanovic, V.P.; Mutic, J.J.; Nikolic Mandic, S.D. Update on element content profiles in eleven wild edible mushrooms from family Boletaceae. Eur. Food Res. Technol. 2016, 242, 1–10. [Google Scholar] [CrossRef]
- Collin-Hansen, C.; Yttri, K.F.; Andersen, R.A.; Berthelsen, B.O.; Steinnes, E. Mushrooms from two metal-contaminated areas in Norway: Occurrence of metals and metallothionein-like proteins. Geochem. Explor. Environ. Anal. 2002, 2, 121–130. [Google Scholar] [CrossRef]
- Brzezicha-Cirocka, J.; Mędyk, M.; Falandysz, J.; Szefer, P. Bio- and toxic elements in edible wild mushrooms from two regions of potentially different environmental conditions in eastern Poland. Environ. Sci. Pollut. Res. 2016, 23, 21517–21522. [Google Scholar] [CrossRef]
- Strumińska-Parulska, D.; Falandysz, J. A review of the occurrence of alpha-emitting radionuclides in wild mushrooms. Int. J. Environ. Res. Public Health 2020, 17, 8220. [Google Scholar] [CrossRef]
- Strumińska-Parulska, D.; Falandysz, J.; Moniakowska, A. Beta-emitting radionuclides in wild mushrooms and potential radiotoxicity for their consumers. Trends Food Sci. Technol. 2021, 114, 672–683. [Google Scholar] [CrossRef]
- Falandysz, J.; Zalewska, D.; Saniewski, M.; Fernandes, A.R. An evaluation of the occurrence and trends in 137Cs and 40K radioactivity in King Bolete Boletus edulis mushrooms in Poland during 1995–2019. Environ. Sci. Pollut. Res. 2021, 28, 32405–32415. [Google Scholar] [CrossRef]
- Falandysz, J.; Saba, M.; Strumińska-Parulska, D. 137Caesium, 40K and total K in Boletus edulis at different maturity stages: Effect of braising and estimated radiation dose intake. Chemosphere 2021, 268, 129336. [Google Scholar] [CrossRef] [PubMed]
- Falandysz, J.; Saniewski, M.; Zalewska, T.; Zhang, J. Radiocaesium pollution of fly agaric Amanita muscaria in fruiting bodies decreases with developmental stage. Isot. Environ. Health Stud. 2019, 55, 317–324. [Google Scholar] [CrossRef]
- Falandysz, J.; Sapkota, A.; Dryżałowska, A.; Mędyk, M.; Feng, X. Analysis of some metallic elements and metalloids composition and relationships in parasol mushroom Macrolepiota procera. Environ. Sci. Pollut. Res. 2017, 24, 15528–15537. [Google Scholar] [CrossRef] [PubMed]
- Falandysz, J.; Sapkota, A.; Mędyk, M.; Feng, X. Rare earth elements in parasol mushroom Macrolepiota procera. Food Chem. 2017, 221, 24–28. [Google Scholar] [CrossRef]
- Wyrzykowska, B.; Szymczyk, K.; Ichihashi, H.; Falandysz, J.; Yamasaki, S. Application of HR/ICP/MS and principal component analysis for studying interdependences among 23 trace elements in Polish beers. J. Agric. Food Chem. 2001, 49, 3425–3431. [Google Scholar] [CrossRef]
- Liang, Q.; Grégoire, D.C. Determination of trace elements in twenty six Chinese geochemistry reference materials by inductively coupled plasma-mass spectrometry. Geostand. Newsl. 2000, 24, 51–63. [Google Scholar] [CrossRef]
- Shi, W.; Feng, X.; Zhang, G.; Ming, L.; Yin, R.; Zhao, Z.; Wang, J. High-precision measurement of mercury isotope ratios of atmospheric deposition over the past 150 years recorded in a peat core taken from Hongyuan, Sichuan Province, China. Chin. Sci. Bull. 2011, 56, 877–882. [Google Scholar] [CrossRef]
- Collin-Hansen, C.; Andersen, R.F.; Steinnes, E. Molecular defense systems are expressed in the king bolete (Boletus edulis) growing near metal smelters. Mycologia 2005, 97, 973–983. [Google Scholar] [CrossRef] [PubMed]
- Oszczepalski, S.; Speczik, S.; Zieliński, K.; Chmielewski, A. The Kupferschiefer deposits and prospects in SW Poland: Past, present and future. Minerals 2019, 9, 592. [Google Scholar] [CrossRef]
- European Commission. Commission Regulation (EU) 2018/73 of 16 January 2018 amending Annexes II and III to Regulation (EC) No 396/2005 of the European Parliament and of the Council as regards maximum residue levels for mercury compounds in or on certain products. Off. J. Eur. Union 2018, l13, 8–20. [Google Scholar]
- Stegemeier, J.P.; Schwab, F.; Colman, B.P.; Webb, S.M.; Newville, M.; Lanzirotti, A.; Winkler, C.; Wiesner, M.R.; Lowry, G.V. Speciation matters: Bioavailability of silver and silver sulfide nanoparticles to Alfalfa (Medicago sativa). Environ. Sci. Technol. 2015, 49, 8451–8460. [Google Scholar] [CrossRef]
- Colman, B.P.; Espinasse, B.; Richardson, C.J.; Matson, C.W.; Lowry, G.V.; Hunt, D.E.; Wiesner, M.R.; Bernhardt, E.S. Emerging contaminant or an old toxin in disguise? Silver nanoparticle impacts on ecosystems. Environ. Sci. Technol. 2014, 48, 5229–5236. [Google Scholar] [CrossRef] [PubMed]
- Voelker, D.; Schlich, K.; Hohndorf, L.; Koch, W.; Kuehnen, U.; Polleichtner, C.; Kussatz, C.; Hund-Rinke, K. Approach on environmental risk assessment of nanosilver released from textiles. Environ. Res. 2015, 140, 661–672. [Google Scholar] [CrossRef]
- Rutkowska, M.; Falandysz, J.; Saba, M.; Szefer, P.; Misztal-Szkudlińska, M.; Konieczka, P. Determination of total mercury and mono-methylmercury in samples of fungi from different regions of the world. J. Trace Elem. Med. Biol. 2021. submitted. [Google Scholar]
- Kautmanová, I.; Brachtýr, O.; Gbúrová Štubňová, E.; Szabóová, D.; Šottník, P.; Lalinská-Voleková, B. Potentially toxic elements in macromycetes and plants from areas affected by antimony mining. Biologia 2021, 76, 2133–2159. [Google Scholar] [CrossRef]
- Borovička, J.; Kubrová, J.; Rohovec, J.; Řanda, Z.; Dunn, C.E. Uranium, thorium and rare earth elements in macrofungi: What are the genuine concentrations? Biometals 2011, 24, 837–845. [Google Scholar] [CrossRef] [PubMed]
- Mochnacka, K.; Bernaś, M. Occurrence and genetic relationships of uranium and thorium mineralization in the Karkonosze Izera Block (the Sudety Mts, SW Poland). Ann. Soc. Geol. Pol. 2000, 70, 137–150. [Google Scholar]
- Pankavec, S.; Falandysz, J.; Hanć, A.; Komorowicz, I.; Fernandes, A.R.; Barałkiewicz, D. Lithiation of Agaricus bisporus mushrooms using compost fortified with LiOH: Effect of fortification levels on Li uptake and co-accumulation of other trace elements. J. Environ. Sci. Health Part B 2021, 56, 761–770. [Google Scholar] [CrossRef] [PubMed]
- Pankavec, S.; Falandysz, J.; Hanć, A.; Komorowicz, I.; Barałkiewicz, D.; Fernandes, A.R. Enhancing the Lithium content of white button mushrooms Agaricus bisporus using LiNO3 fortified compost: Effects on the uptake of Li and other trace elements. Food Addit. Contam. Part A 2021, 38, 1193–1205. [Google Scholar] [CrossRef] [PubMed]
- Pankavec, S.; Falandysz, J.; Komorowicz, I.; Hanć, A.; Barałkiewicz, D.; Fernandes, A.R. Lithiation of white button mushrooms (Agaricus bisporus) using lithium-fortified substrate: Effect of fortification levels on Li uptake and on other trace elements. Environ. Sci. Pollut. Res. 2021, 28, 48905–48920. [Google Scholar] [CrossRef] [PubMed]
- Pankavec, S.; Falandysz, J.; Komorowicz, I.; Fernandes, A.R.; Hanć, A.; Barałkiewicz, D. The use of Li2O fortified growing compost to enhance lithiation in white Agaricus bisporus mushrooms: Li uptake and co-accumulation of other trace elements. Eur. Food. Res. Technol. 2021, 247, 2239–2252. [Google Scholar] [CrossRef]
- Falandysz, J.; Fernandes, A.; Meloni, D. An overview of the lithium content and lithiation of the cultivable macrofungal species, Agaricus bisporus and Pleurotus spp. Trends Food Sci. Technol. 2022, 119, 338–347. [Google Scholar] [CrossRef]
- Filippini, T.; Tancredi, S.; Malagoli, C.; Malavolti, M.; Bargellini, A.; Vescovi, L.; Nicolini, F.; Vinceti, M. Dietary estimated intake of trace elements: Risk assessment in an Italian population. Expo. Health 2020, 12, 641–655. [Google Scholar] [CrossRef]
- Thibon, F.; Weppe, L.; Vigier, N.; Churlaud, C.; Lacoue-Labarthe, T.; Metian, M.; Cherel, Y.; Bustamante, P. Large-scale survey of lithium concentrations in marine organisms. Sci. Total Environ. 2021, 751, 141453. [Google Scholar] [CrossRef]
- Falandysz, J.; Zalewska, T.; Fernandes, A. 137Cs and 40K in Cortinarius caperatus mushrooms (1996–2016) in Poland—bioconcentration and estimated intake: 137Cs in Cortinarius spp. from the Northern Hemisphere from 1974–2016. Environ. Pollut. 2019, 255, 113208. [Google Scholar] [CrossRef] [PubMed]
- Falandysz, J.; Saniewski, M.; Fernandes, A.R.; Meloni, D.; Cocchi, L.; Strumińska-Parulska, D.; Zalewska, T. Radiocaesium in Tricholoma spp. from the Northern Hemisphere in 1971–2016. Sci. Total Environ. 2022, 802, 149829. [Google Scholar] [CrossRef] [PubMed]
- Orita, M.; Nakashima, K.; Taira, Y.; Fukuda, T.; Fukushima, Y.; Kudo, T.; Endo, Y.; Yamashita, S.; Takamura, N. Radiocesium concentrations in wild mushrooms after the accident at the Fukushima Daiichi Nuclear Power Station: Follow-up study in Kawauchi village. Sci. Rep. 2017, 7, 6744. [Google Scholar] [CrossRef] [PubMed]
- Alaimo, M.G.; Saitta, A.; Ambrosio, E. Bedrock and soil geochemistry influence the content of chemical elements in wild edible mushrooms (Morchella group) from South Italy (Sicily). Acta Mycol. 2019, 54, 11–22. [Google Scholar] [CrossRef]
- Falandysz, J.; Barałkiewicz, D.; Hanć, A.; Zhang, J.; Treu, R. Metallic and metalloid elements in various developmental stages of Amanita muscaria (L.) Lam. Fungal Biol. 2020, 124, 174–182. [Google Scholar] [CrossRef]
- Meisch, H.-U.; Reinle, W.; Schmitt, J.A. High vanadium content in mushrooms is not restricted to the Fly Agaric (Amanita muscaria). Naturwissenschaften 1979, 66, 620–621. [Google Scholar] [CrossRef]
- Falandysz, J.; Kunito, T.; Kubota, R.; Lipka, K.; Mazur, A.; Falandysz, J.J.; Tanabe, S. Selected elements in Fly agaric Amanita muscaria. J. Environ. Sci. Health Part A 2007, 42, 1615–1623. [Google Scholar] [CrossRef] [PubMed]
- Bau, M.; Schmidt, K.; Pack, A.; Bendel, V.; Kraemer, D. The European shale: An improved data set for normalisation of rare earth element and yttrium concentrations in environmental and biological samples from Europe. Appl. Geochem. 2018, 90, 142–149. [Google Scholar] [CrossRef]
- Đurđić, S.; Stanković, V.; Ražić, S.; Mutić, J. Lead isotope ratios as tool for elucidation of chemical environment in a system of Macrolepiota procera (Scop.) Singer-soil. Environ. Sci. Pollut. Res. 2021, 28, 59003–59014. [Google Scholar] [CrossRef] [PubMed]
- Jäppinen, J.-P. Wild mushrooms as food in Finland. Mycologist 1988, 2, 99–101. [Google Scholar] [CrossRef]
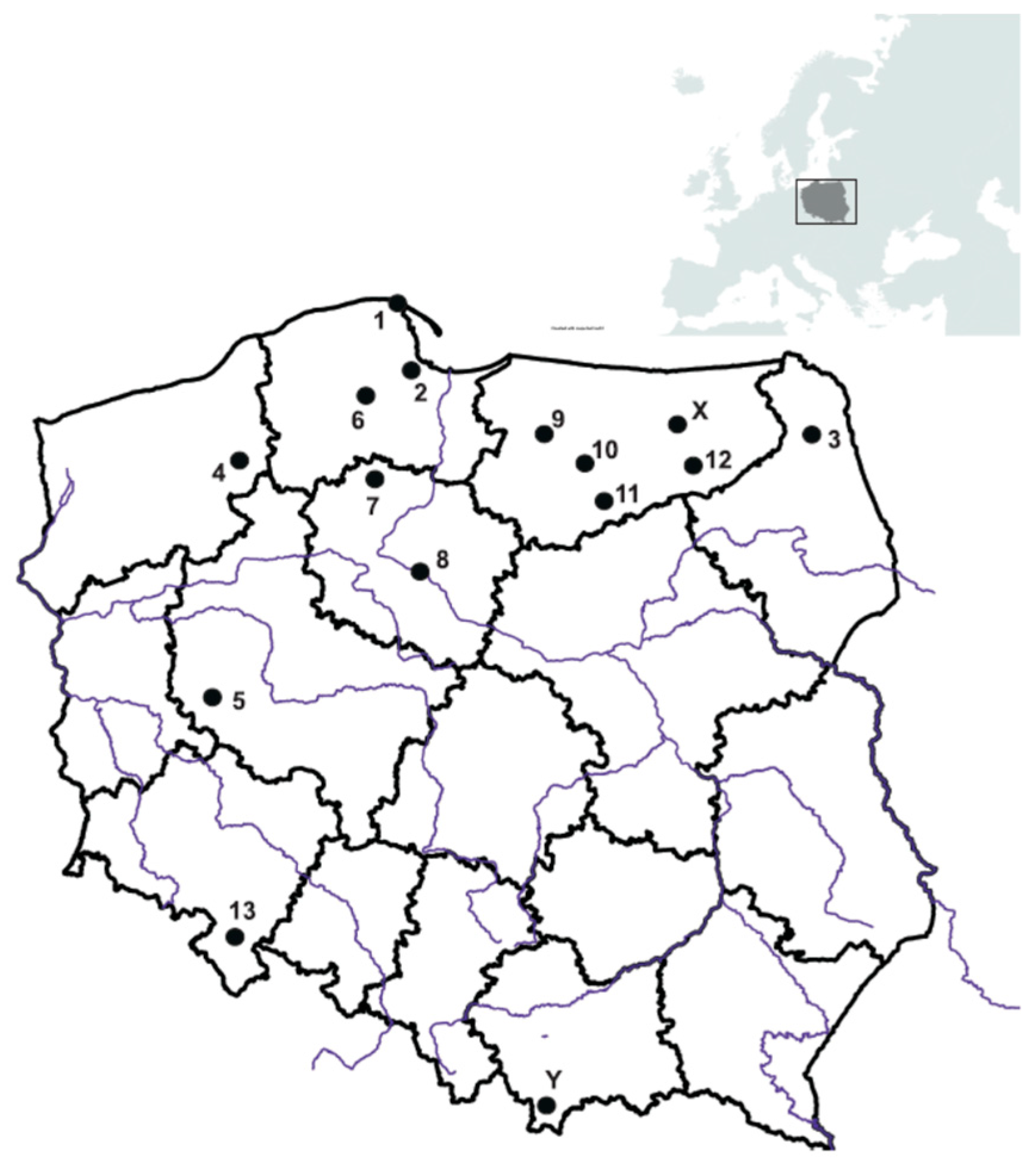
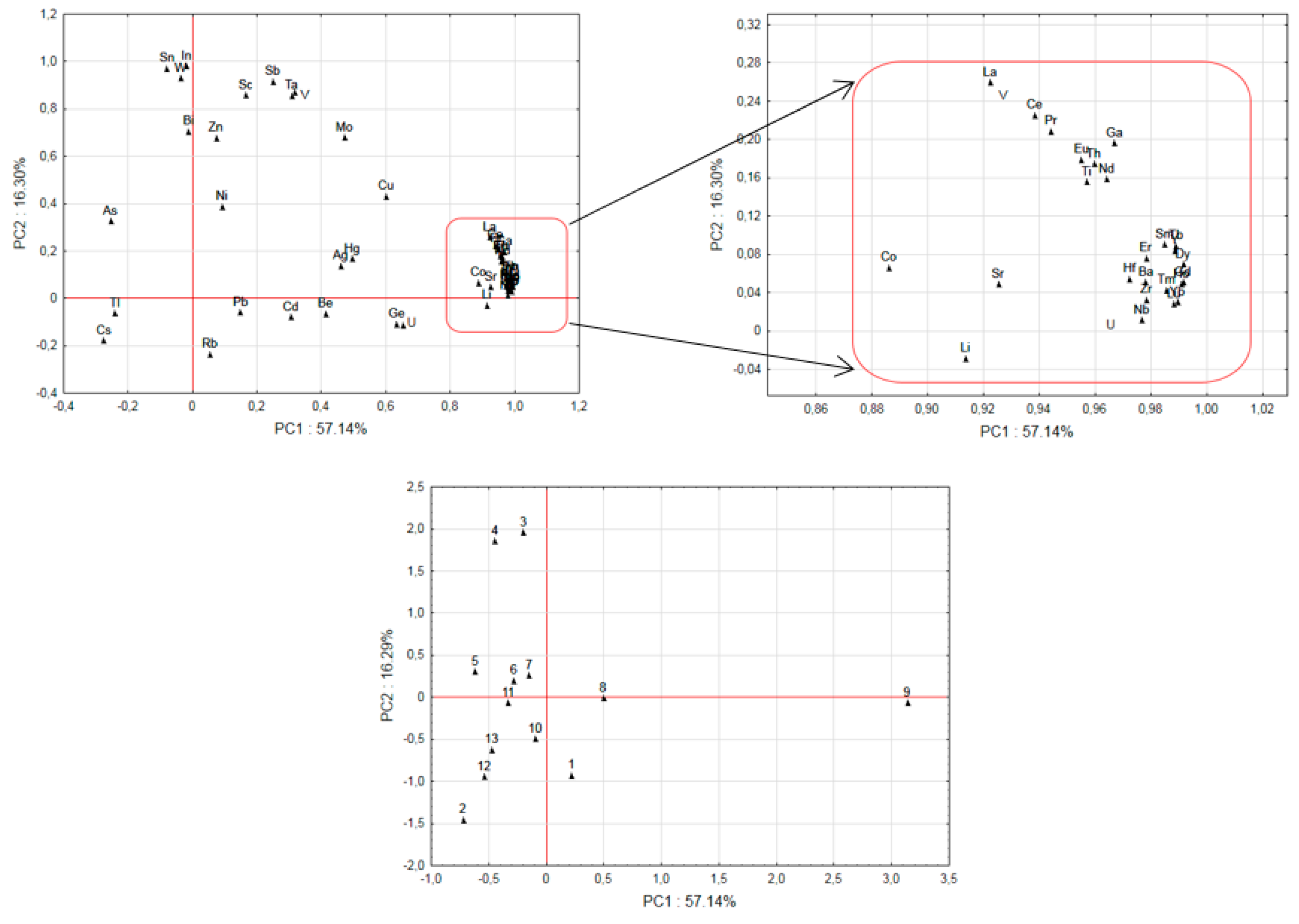

| Place (Number of Specimens and Morphological Part) * | Ag | As | Ba | Be | Bi | Cd | Co | Cu | Cs | Ga | Ge | Hf | Hg | In | Li | Mo | |
| (1) Coastal Landscape Park (15; c) | 3.1 | 0.58 | 1.2 | 0.026 | 0.0063 | 2.0 | 0.044 | 30 | 1.2 | 0.074 | 0.080 | 0.024 | 1.4 | 0.0016 | 0.14 | 0.082 | |
| (1) Coastal Landscape Park (15; s) | 1.4 | 0.44 | 3.0 | 0.021 | 0.0086 | 0.82 | 0.028 | 26 | 2.8 | 0.065 | 0.057 | 0.059 | 1.3 | 0.0014 | 0.036 | 0.054 | |
| (1) Coastal Landscape Park (15; w) | 2.2 | 0.51 | 2.1 | 0.023 | 0.0074 | 1.4 | 0.036 | 28 | 1.5 | 0.070 | 0.068 | 0.041 | 1.3 | 0.0015 | 0.088 | 0.068 | |
| (2) TLP, Osowa (10; w) | 1.1 | 0.43 | 1.1 | 0.016 | 0.0013 | 1.2 | 0.055 | 20 | 2.3 | 0.050 | 0.097 | 0.0022 | 1.6 | 0.0011 | 0.033 | 0.068 | |
| (X) Mazury, Giżycko (15; c) | 4.8 | 0.48 | 1.4 | 0.031 | 0.0072 | 2.6 | 0.56 | 27 | 2.0 | 0.073 | 0,093 | 0.0073 | 2.9 | 0.0072 | 0.060 | 0.13 | |
| (X) Mazury, Giżycko (15; s) | 1.7 | 0.43 | 1.9 | 0.022 | 0.0038 | 0.98 | 0.71 | 12 | 0.95 | 0.055 | 0.057 | 0.017 | 1.5 | 0.0069 | 0.11 | 0.069 | |
| (X) Mazury, Giżycko (15; w) | 3.2 | 0.45 | 1.6 | 0.026 | 0.0055 | 1.8 | 0.63 | 19 | 1.5 | 0.064 | 0.075 | 0.012 | 2.2 | 0.0070 | 0.085 | 0.10 | |
| (3) Augustów Primeval Forest (16; w) | 3.1 | 0.67 | 2.7 | 0.024 | 0.026 | 2.2 | 0.13 | 25 | 1.7 | 0.089 | 0.079 | 0.015 | 1.8 | 0.021 | 0.081 | 0.12 | |
| (4) Pomerania, Szczecinek (22; w) | 3.4 | 0.65 | 1.2 | 0.020 | 0.013 | 1.5 | 0.11 | 29 | 2.2 | 0.072 | 0.071 | 0.026 | 1.1 | 0.020 | 0.043 | 0.092 | |
| (5) Greater Poland, Porażyn (13; w) | 2.8 | 0.55 | 0.64 | 0.011 | 0.017 | 1.4 | 0.021 | 21 | 1.3 | 0.046 | 0.056 | 0.010 | 1.5 | 0.012 | 0.014 | 0.063 | |
| (6) WLP, Kościerzyna (45; w) | 3.4 | 0.47 | 1.5 | 0.033 | 0.0046 | 2.0 | 0.11 | 25 | 2.0 | 0.066 | 0.080 | 0.016 | 1.7 | 0.012 | 0.14 | 0.078 | |
| (7) Tuchola Pinewoods, Osiek (15; w) | 2.1 | 0.44 | 1.5 | 0.017 | 0.025 | 1.2 | 0.087 | 22 | 1.2 | 0.073 | 0.064 | 0.020 | 1.1 | 0.0090 | 0.15 | 0.084 | |
| (8) Kujawy region, Toruń forests (16; w) | 3.2 | 0.51 | 3.3 | 0.029 | 0.0022 | 2.9 | 0.12 | 27 | 2.3 | 0.086 | 0.086 | 0.046 | 1.3 | 0.010 | 0.069 | 0.083 | |
| (9) Warmia, Morąg (30; w) | 1.4 | 0.41 | 3.0 | 0.035 | 0.0084 | 0.82 | 0.85 | 31 | 1.1 | 0.18 | 0.11 | 0.11 | 2.0 | 0.0014 | 0.49 | 0.054 | |
| (10) Warmia, Olsztyn (19; w) | 2.4 | 0.43 | 1.6 | 0.021 | 0.0021 | 2.1 | 0.028 | 26 | 2.8 | 0.065 | 0.057 | 0.026 | 2.0 | 0.0056 | 0.036 | 0.082 | |
| (11) Warmia, Puchałowo (15; w) | 1.5 | 0.48 | 1.3 | 0.027 | 0.0041 | 2.2 | 0.073 | 21 | 2.5 | 0.075 | 0.09 | 0.0047 | 1.4 | 0.010 | 0.026 | 0.094 | |
| (12) Mazury, Piska Forest (15; c) | 4.3 | 0.39 | 0.44 | 0.010 | 0.0004 | 3.0 | 0.047 | 24 | 2.2 | 0.057 | 0.088 | 0.0011 | 1.3 | 0.0050 | 0.050 | 0.080 | |
| (12) Mazury, Piska Forest (15; s) | 1.4 | 0.39 | 0.69 | 0.017 | 0.00044 | 0.82 | 0.079 | 11 | 1.2 | 0.028 | 0.046 | 0.0027 | 0.57 | 0.0053 | 0.040 | 0.038 | |
| (12) Mazury, Piska Forest (15; w) | 2.8 | 0.39 | 0.56 | 0.013 | 0.00042 | 1.9 | 0.063 | 17 | 1.7 | 0.042 | 0.067 | 0.0019 | 0.93 | 0.0051 | 0.045 | 0.059 | |
| (13) Sudety Mts., Kłodzka Dale (15; c) | 9.5 | 1.0 | 1.8 | 0.044 | 0.0009 | 6.9 | 0.070 | 34 | 7.2 | 0.065 | 0.088 | 0.0064 | 1.9 | 0.0052 | 0.19 | 0.095 | |
| (13) Sudety Mts., Kłodzka Dale (15; s) | 3.5 | 0.76 | 2.4 | 0.046 | 0.0037 | 2.7 | 0.12 | 19 | 4.3 | 0.051 | 0.064 | 0.012 | 1.0 | 0.0058 | 0.095 | 0.053 | |
| (13) Sudety Mts., Kłodzka Dale (15; w) | 6.5 | 0.88 | 2.2 | 0.042 | 0.0063 | 4.8 | 0.095 | 26 | 5.7 | 0.058 | 0.076 | 0.0092 | 1.4 | 0.0055 | 0.14 | 0.074 | |
| (Y) Tatra Mountains, Chochołowska Valley (12; c) | 9.2 | 0.51 | 0.84 | 0.012 | 0.056 | 3.6 | 0.37 | 28 | 1.7 | 0.055 | 0.039 | 0.045 | 1.4 | 0.011 | 0.12 | 0.10 | |
| Mean caps (5 composites) | 6.2 | 0.59 | 1.1 | 0.025 | 0.014 | 3.6 | 0.22 | 29 | 2.9 | 0.065 | 0.078 | 0.017 | 1.8 | 0.0060 | 0.11 | 0.097 | |
| SD | 3.0 | 0.24 | 0.5 | 0.014 | 0.024 | 1.9 | 0.23 | 4 | 2.4 | 0.009 | 0.022 | 0.018 | 0.7 | 0.0030 | 0.06 | 0.020 | |
| Median | 4.8 | 0.51 | 1.2 | 0.026 | 0.006 | 3.0 | 0.07 | 28 | 2.0 | 0.065 | 0.088 | 0.007 | 1.4 | 0.0052 | 0.12 | 0.095 | |
| Mean stipes (4 composites) | 2.0 | 0.50 | 2.0 | 0.026 | 0.0041 | 1.3 | 0.23 | 17 | 2.3 | 0.050 | 0.056 | 0.023 | 1.1 | 0.0048 | 0.070 | 0.054 | |
| SD | 0.9 | 0.15 | 0.8 | 0.011 | 0.0029 | 0.8 | 0.28 | 6 | 1.3 | 0.013 | 0.006 | 0.022 | 0.3 | 0.0021 | 0.032 | 0.011 | |
| Median | 1.5 | 0.44 | 2.1 | 0.021 | 0.0037 | 0.9 | 0.10 | 15 | 2.0 | 0.053 | 0.052 | 0.014 | 1.1 | 0.0055 | 0.067 | 0.054 | |
| Mean whole (14 composites) | 2.8 | 0.52 | 1.7 | 0.024 | 0.0088 | 2.0 | 0.17 | 24 | 2.1 | 0.074 | 0.077 | 0.024 | 1.5 | 0.009 | 0.10 | 0.080 | |
| SD | 1.3 | 0.13 | 0.8 | 0.009 | 0.0084 | 1.0 | 0.25 | 4 | 1.1 | 0.033 | 0.015 | 0.028 | 0.4 | 0.006 | 0.12 | 0.018 | |
| Median | 2.9 | 0.47 | 1.5 | 0.023 | 0.0059 | 1.8 | 0.091 | 25 | 1.8 | 0.068 | 0.075 | 0.015 | 1.4 | 0.008 | 0.075 | 0.080 | |
| Place (Number of Specimens and Morphological Part) * | Nb | Ni | Pb | Rb | Sb | Sn | Sr | Ta | Th | Ti | Tl | U | V | W | Zn | Zr | ƩREEs |
| (1) Coastal Landscape Park (15; c) | 0.031 | 1.6 | 0.36 | 250 | 0.012 | 0.15 | 0.37 | 0.0041 | 0.0076 | 21 | 0.052 | 0.0071 | 1.3 | 0.012 | 99 | 1.2 | 0.20 |
| (1) Coastal Landscape Park (15; s) | 0.041 | 1.3 | 0.51 | 180 | 0.015 | 0.15 | 0.44 | 0.012 | 0.016 | 37 | 0.14 | 0.012 | 1.4 | 0.016 | 80 | 1.2 | 0.43 |
| (1) Coastal Landscape Park (15; w) | 0.036 | 1.4 | 0.43 | 210 | 0.013 | 0.15 | 0.40 | 0.008 | 0.012 | 29 | 0.096 | 0.0095 | 1.3 | 0.014 | 89 | 1.2 | 0.31 |
| (2) TLP, Osowa (10; w) | 0.0053 | 2.0 | 1.8 | 190 | 0.011 | 0.075 | 0.34 | 0.0007 | 0.0024 | 8.1 | 0.088 | 0.0027 | 0.77 | 0.014 | 91 | 0.11 | 0.12 |
| (X) Mazury, Giżycko (15; c) | 0.016 | 2.0 | 0.72 | 290 | 0.026 | 0.15 | 0.55 | 0.013 | 0.0090 | 15 | 0.052 | 0.0055 | 1.4 | 0.043 | 87 | 0.33 | 0.35 |
| (X) Mazury, Giżycko (15; s) | 0.033 | 1.4 | 0.81 | 120 | 0.022 | 0.14 | 0.65 | 0.016 | 0.011 | 15 | 0.14 | 0.0066 | 1.5 | 0.036 | 45 | 0.73 | 0.48 |
| (X) Mazury, Giżycko (15; w) | 0.024 | 1.7 | 0.76 | 200 | 0.024 | 0.14 | 0.60 | 0.015 | 0.010 | 15 | 0.096 | 0.0060 | 1.4 | 0.039 | 66 | 0.53 | 0.47 |
| (3) Augustów Primeval Forest (16; w) | 0.034 | 2.5 | 1.3 | 160 | 0.057 | 0.43 | 0.77 | 0.034 | 0.026 | 22 | 0.13 | 0.0071 | 1.9 | 0.14 | 110 | 0.71 | 0.76 |
| (4) Pomerania, Szczecinek (22; w) | 0.021 | 1.5 | 0.82 | 190 | 0.045 | 0.42 | 0.39 | 0.035 | 0.0081 | 20 | 0.089 | 0.0049 | 2.2 | 0.16 | 110 | 0.99 | 0.34 |
| (5) Greater Poland, Porażyn (13; w) | 0.0098 | 1.3 | 1.1 | 120 | 0.026 | 0.26 | 0.20 | 0.022 | 0.0039 | 11 | 0.096 | 0.0037 | 1.6 | 0.084 | 69 | 0.44 | 0.17 |
| (6) WLP, Kościerzyna (45; w) | 0.017 | 1.4 | 1.1 | 210 | 0.025 | 0.23 | 0.51 | 0.034 | 0.0088 | 16 | 0.13 | 0.0053 | 1.7 | 0.061 | 86 | 0.70 | 0.25 |
| (7) Tuchola Pinewoods, Osiek (15; w) | 0.024 | 1.7 | 0.63 | 200 | 0.022 | 0.18 | 0.46 | 0.022 | 0.010 | 20 | 0.069 | 0.0071 | 1.7 | 0.062 | 95 | 1.0 | 0.40 |
| (8) Kujawy region, Toruń forests (16; w) | 0.039 | 1.5 | 0.79 | 160 | 0.029 | 0.20 | 0.83 | 0.022 | 0.021 | 22 | 0.16 | 0.010 | 1.6 | 0.082 | 83 | 2.1 | 0.56 |
| (9) Warmia, Morąg (30; w) | 0.15 | 1.7 | 1.5 | 230 | 0.015 | 0.15 | 2.0 | 0.031 | 0.070 | 57 | 0.061 | 0.028 | 1.9 | 0.058 | 91 | 5.0 | 1.8 |
| (10) Warmia, Olsztyn (19; w) | 0.041 | 1.3 | 1.2 | 180 | 0.017 | 0.14 | 0.44 | 0.018 | 0.013 | 20 | 0.082 | 0.0073 | 1.5 | 0.033 | 80 | 1.2 | 0.31 |
| (11) Warmia, Puchałowo (15; w) | 0.018 | 1.6 | 0.79 | 230 | 0.030 | 0.25 | 0.52 | 0.019 | 0.0076 | 16 | 0.094 | 0.0045 | 1.6 | 0.18 | 130 | 0.21 | 0.23 |
| (12) Mazury, Piska Forest (15; c) | 0.010 | 1.5 | 0.41 | 240 | 0.013 | 0.10 | 0.23 | 0.013 | 0.0027 | 11 | 0.051 | 0.0032 | 1.2 | 0.024 | 98 | 0.083 | 0.074 |
| (12) Mazury, Piska Forest (15; s) | 0.0064 | 0.86 | 0.35 | 110 | 0.012 | 0.13 | 0.30 | 0.013 | 0.0018 | 5.9 | 0.14 | 0.0025 | 1.4 | 0.032 | 39 | 0.12 | 0.10 |
| (12) Mazury, Piska Forest (15; w) | 0.0082 | 1.2 | 0.38 | 170 | 0.012 | 0.11 | 0.26 | 0.013 | 0.0022 | 8.4 | 0.095 | 0.0028 | 1.3 | 0.028 | 68 | 0.10 | 0.086 |
| (13) Sudety Mts., Kłodzka Dale (15; c) | 0.014 | 1.9 | 1.5 | 580 | 0.029 | 0.15 | 0.57 | 0.014 | 0.0071 | 13 | 0.16 | 0.032 | 1.3 | 0.033 | 110 | 0.29 | 0.19 |
| (13) Sudety Mts., Kłodzka Dale (15; s) | 0.023 | 1.5 | 1.7 | 270 | 0.020 | 0.10 | 1.2 | 0.011 | 0.013 | 12 | 0.40 | 0.025 | 1.2 | 0.011 | 54 | 0.49 | 0.28 |
| (13) Sudety Mts., Kłodzka Dale (15; w) | 0.018 | 1.7 | 1.6 | 420 | 0.024 | 0.12 | 0.88 | 0.012 | 0.010 | 12 | 0.28 | 0.028 | 1.2 | 0.022 | 82 | 0.39 | 0.23 |
| (Y) Tatra Mountains (12; c) | 0.0092 | 1.3 | 1.1 | 150 | 0.042 | 0.25 | 0.30 | 0.021 | 0.049 | 12 | 0.026 | 0.038 | 1.7 | 0.082 | 94 | 0.22 | 0.13 |
| Mean caps (5 composites) | 0.016 | 1.7 | 0.82 | 300 | 0.024 | 0.16 | 0.40 | 0.013 | 0.015 | 14 | 0.068 | 0.017 | 1.4 | 0.039 | 98 | 0.42 | 0.19 |
| SD | 0.009 | 0.3 | 0.48 | 160 | 0.012 | 0.05 | 0.16 | 0.006 | 0.019 | 4 | 0.052 | 0.016 | 0.2 | 0.027 | 8 | 0.44 | 0.10 |
| Median | 0.014 | 1.6 | 0.72 | 250 | 0.026 | 0.15 | 0.37 | 0.013 | 0.008 | 13 | 0.052 | 0.0071 | 1.3 | 0.033 | 98 | 0.29 | 0.19 |
| Mean stipes (4 composites) | 0.026 | 1.3 | 0.84 | 170 | 0.017 | 0.13 | 0.65 | 0.013 | 0.010 | 17 | 0.20 | 0.011 | 1.4 | 0.024 | 54 | 0.53 | 0.34 |
| SD | 0.013 | 0.2 | 0.52 | 64 | 0.004 | 0.02 | 0.34 | 0.002 | 0.001 | 12 | 0.11 | 0.008 | 0.1 | 0.010 | 16 | 0.39 | 0.15 |
| Median | 0.028 | 1.3 | 0.66 | 150 | 0.017 | 0.13 | 0.54 | 0.012 | 0.012 | 13 | 0.14 | 0.009 | 1.4 | 0.024 | 49 | 0.61 | 0.36 |
| Mean whole (14 composites) | 0.032 | 1.6 | 1.0 | 200 | 0.025 | 0.20 | 0.61 | 0.020 | 0.014 | 20 | 0.11 | 0.0091 | 1.5 | 0.070 | 89 | 1.1 | 0.43 |
| SD | 0.036 | 0.3 | 0.4 | 69 | 0.012 | 0.11 | 0.45 | 0.010 | 0.017 | 12 | 0.05 | 0.0083 | 0.3 | 0.054 | 18 | 1.3 | 0.43 |
| Median | 0.022 | 1.5 | 0.96 | 190 | 0.024 | 0.16 | 0.48 | 0.020 | 0.010 | 18 | 0.095 | 0.0065 | 1.6 | 0.059 | 87 | 0.70 | 0.31 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Falandysz, J. Nutritional and Other Trace Elements and Their Associations in Raw King Bolete Mushrooms, Boletus edulis. Int. J. Environ. Res. Public Health 2022, 19, 417. https://doi.org/10.3390/ijerph19010417
Falandysz J. Nutritional and Other Trace Elements and Their Associations in Raw King Bolete Mushrooms, Boletus edulis. International Journal of Environmental Research and Public Health. 2022; 19(1):417. https://doi.org/10.3390/ijerph19010417
Chicago/Turabian StyleFalandysz, Jerzy. 2022. "Nutritional and Other Trace Elements and Their Associations in Raw King Bolete Mushrooms, Boletus edulis" International Journal of Environmental Research and Public Health 19, no. 1: 417. https://doi.org/10.3390/ijerph19010417
APA StyleFalandysz, J. (2022). Nutritional and Other Trace Elements and Their Associations in Raw King Bolete Mushrooms, Boletus edulis. International Journal of Environmental Research and Public Health, 19(1), 417. https://doi.org/10.3390/ijerph19010417






