Toxic Characteristics and Action Mode of the Mixotrophic Dinoflagellate Akashiwo sanguinea on Co-Occurring Phytoplankton and Zooplankton
Abstract
:1. Introduction
2. Materials and Methods
2.1. Algal Cultures and Conditions
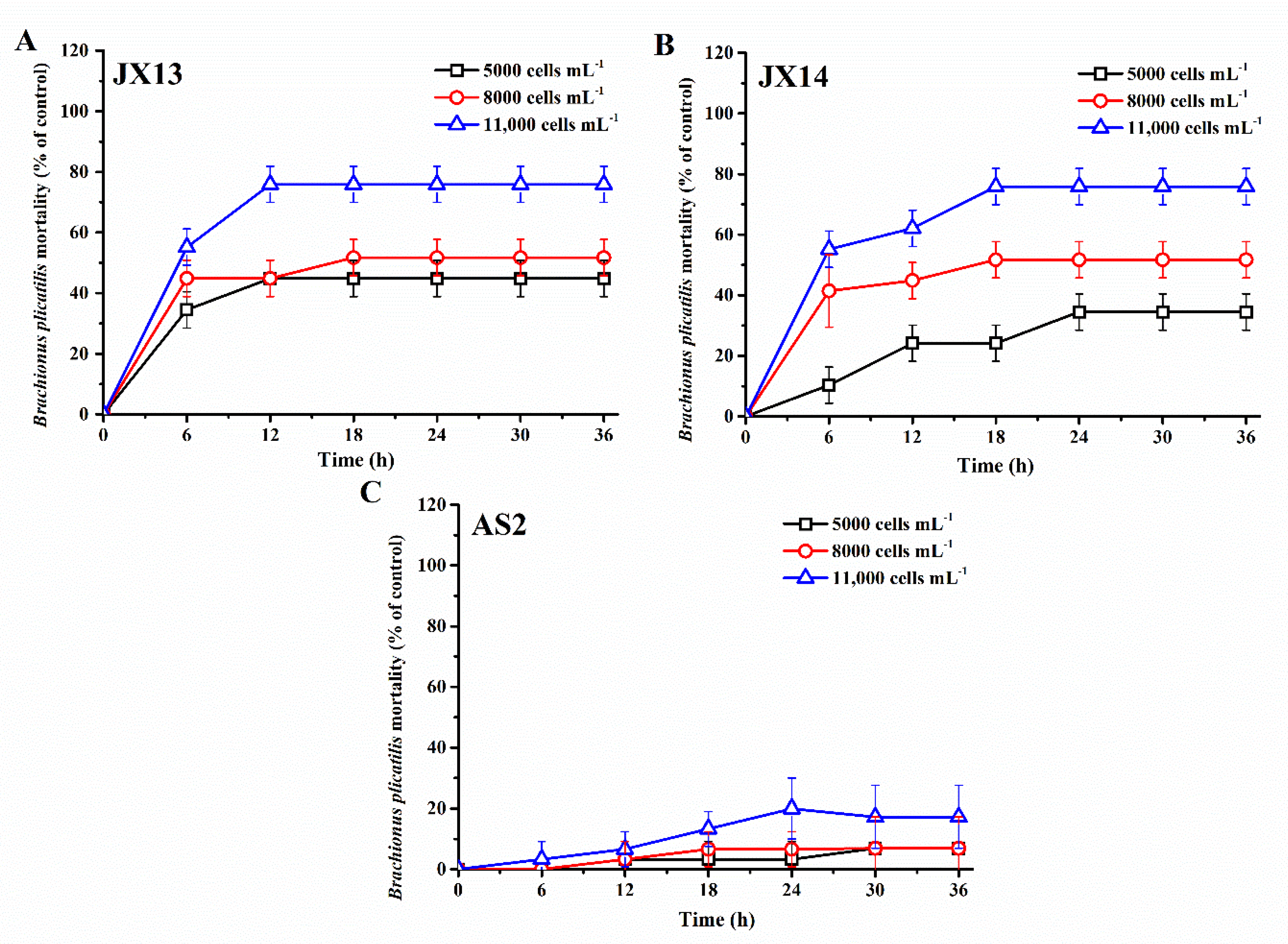
2.2. Study 1: Toxic Effects of A. sanguinea on Co-Occurring Zooplankton
2.3. Study 2: Toxic Effects of A. sanguinea on Co-Occurring Phytoplankton
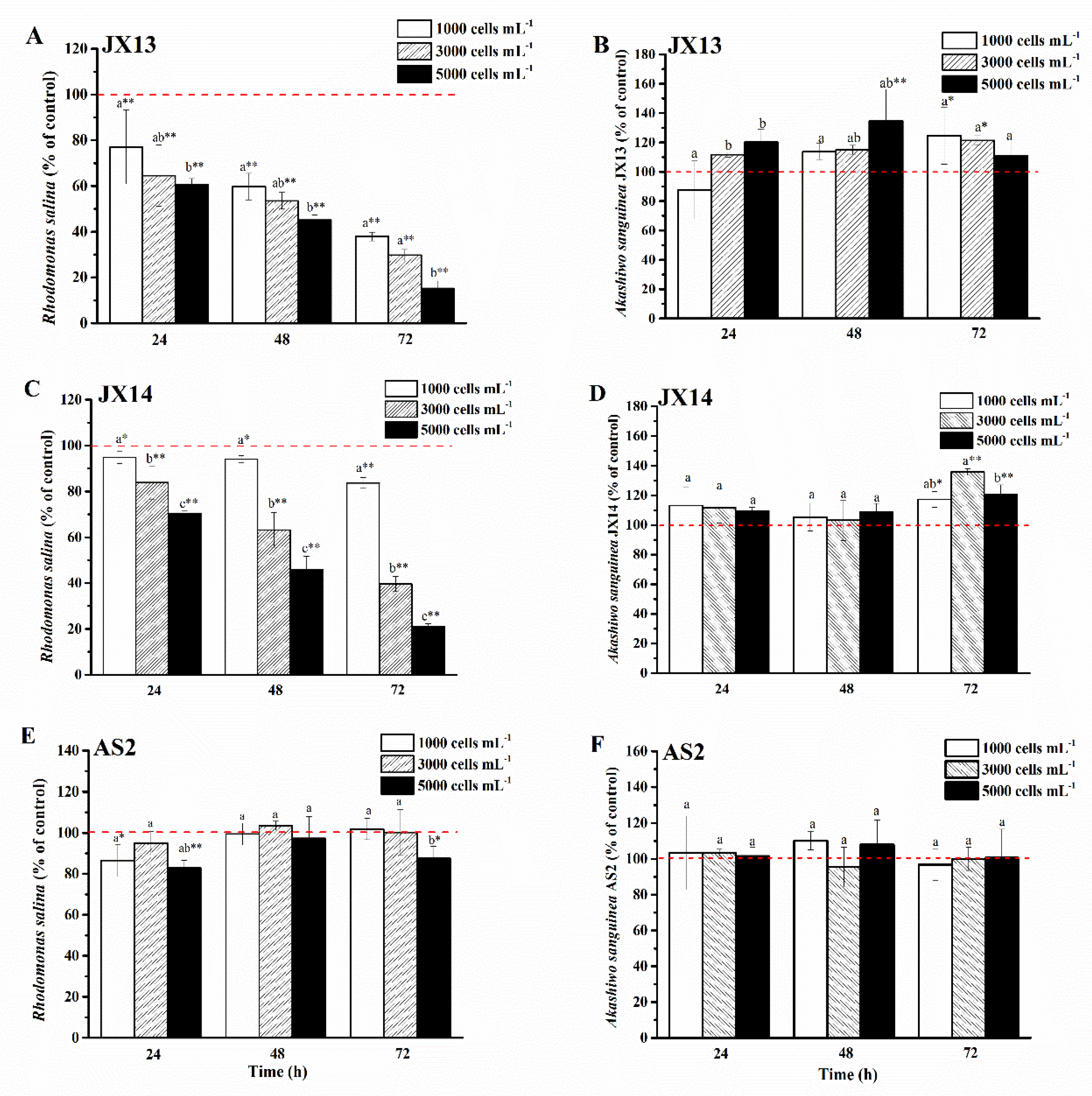
2.3.1. Variations of Toxicity among A. sanguinea Strains
2.3.2. Variations of A. sanguinea Toxicity among Different Growth Stages and between Extra- and Intra-Cellular Fractions
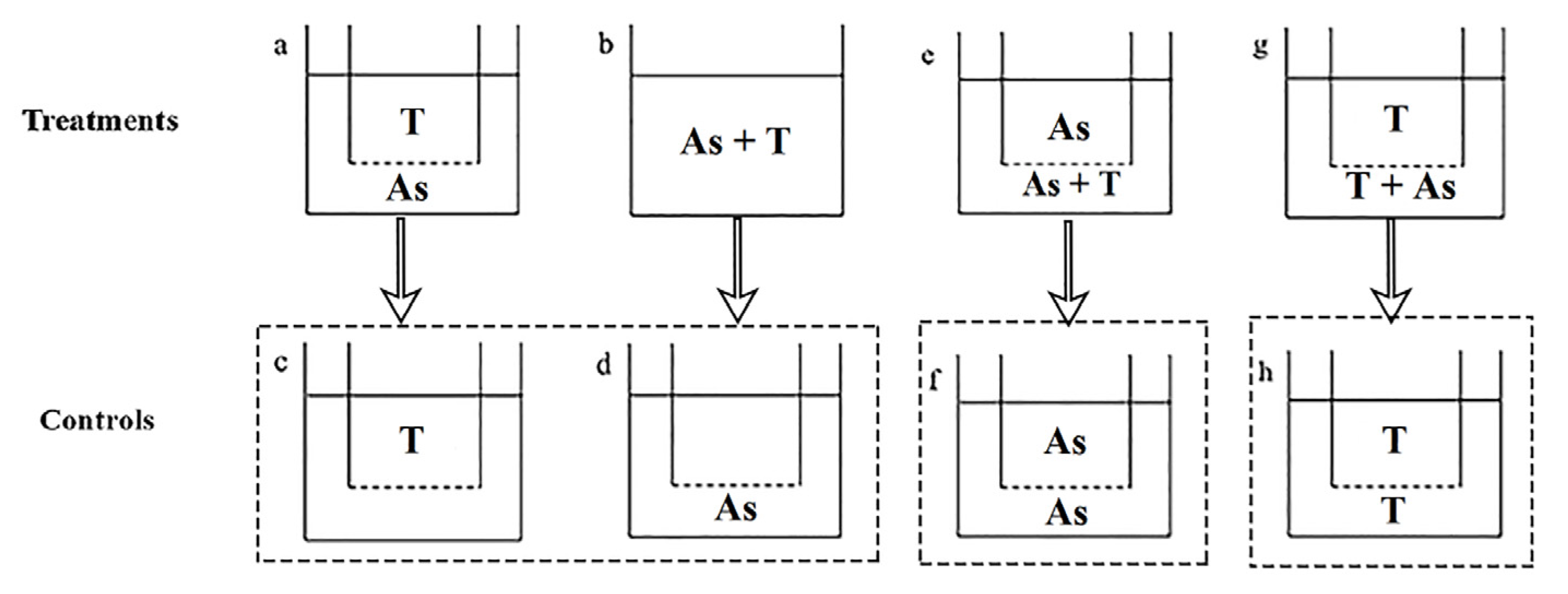
2.4. Study 3: Action Mode of A. sanguinea Toxicity on Phytoplankton and Zooplankton
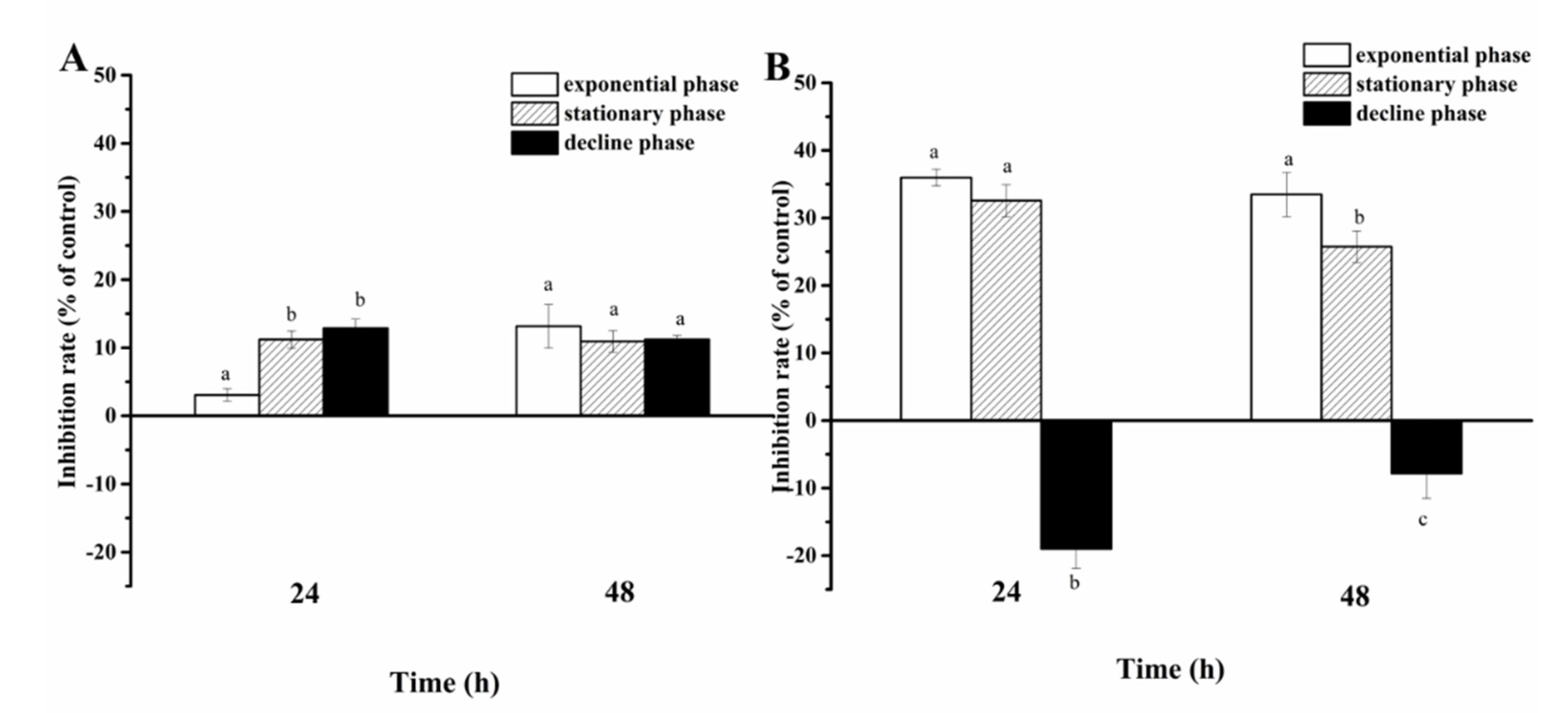
2.4.1. Action Mode of A. sanguinea Toxicity on Phytoplankton
2.4.2. Action Mode of A. sanguinea Toxicity on Zooplankton
2.5. Statistical Analysis
3. Results
3.1. Toxic Effects of A. sanguinea on Co-Occurring Zooplankton
3.2. Toxic Effects of A. sanguinea on Co-Occurring Phytoplankton
3.2.1. Variations of Toxicity among A. sanguinea Strains
3.2.2. Variations of A. sanguinea Toxicity among Different Growth Stages and between Extra- and Intra-Cellular Fractions
3.3. Action Mode of A. sanguinea Toxicity on Phytoplankton and Zooplankton
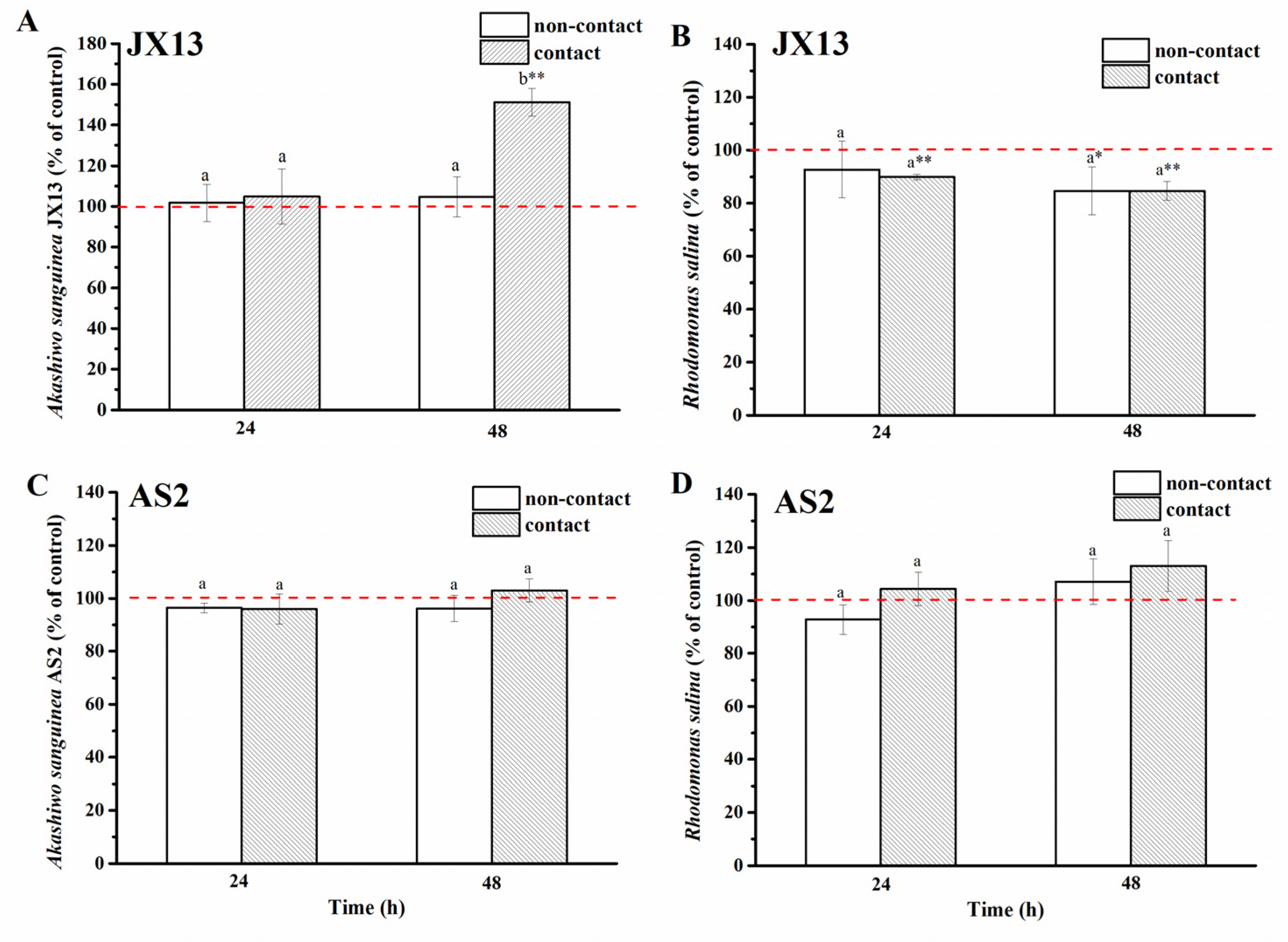
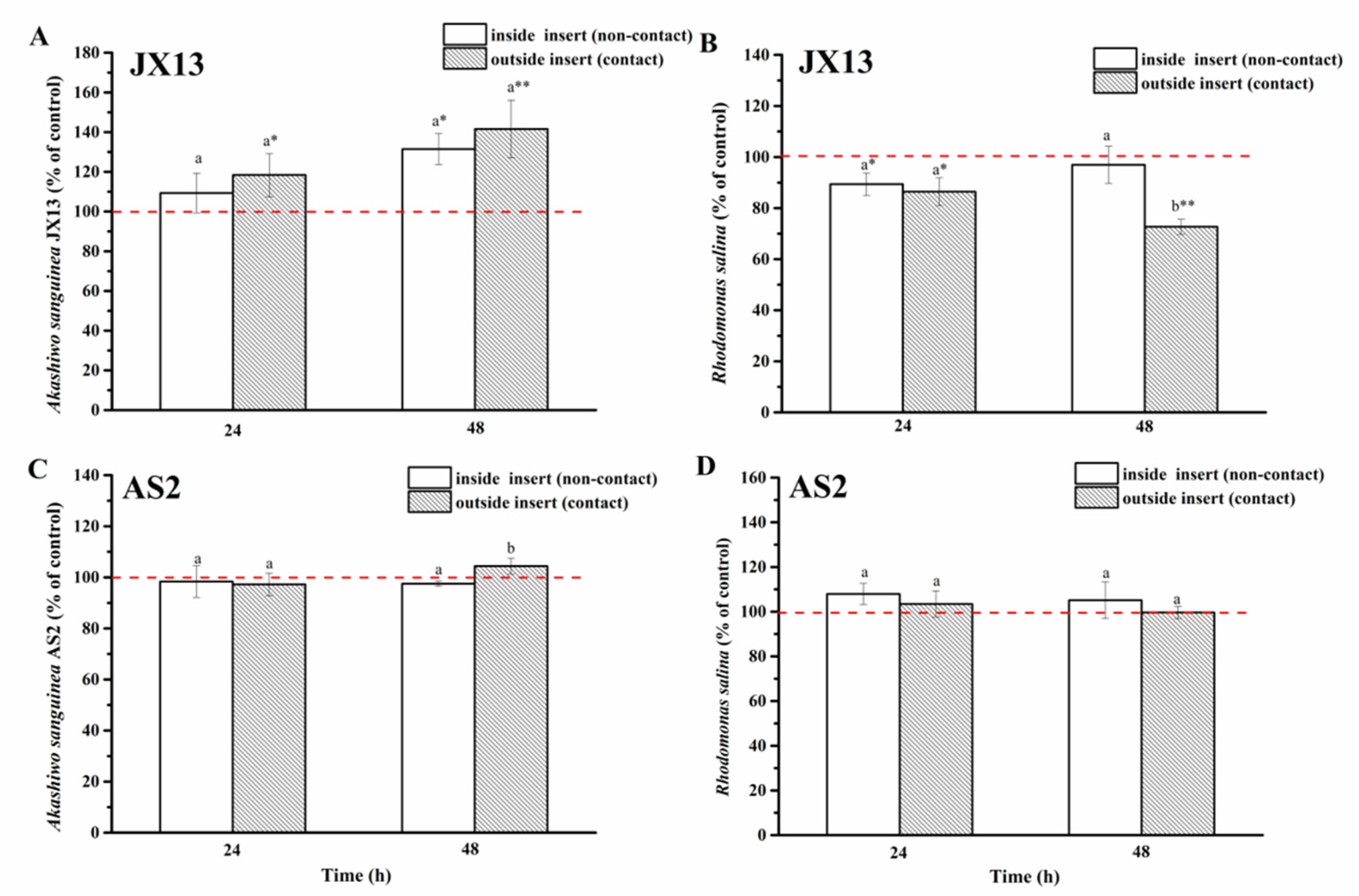
3.3.1. Action Mode of A. sanguinea Toxicity on Phytoplankton
- (1)
- Contact and non-contact coculture
- (2)
- Half-contact coculture
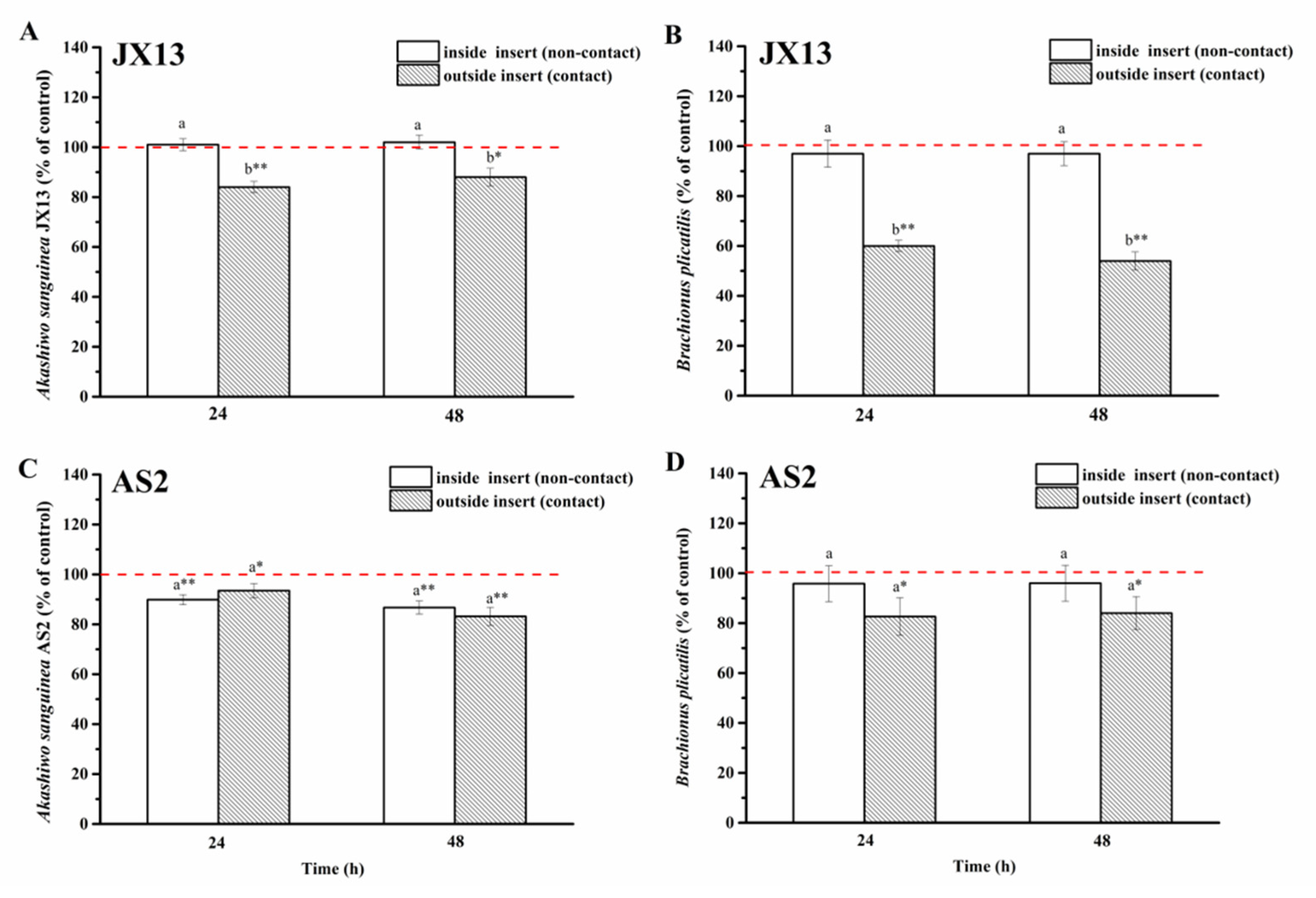
3.3.2. Action Mode of A. sanguinea Toxicity on Zooplankton
- (1)
- Contact and non-contact coculture
- (2)
- Half-contact coculture
4. Discussion
4.1. Toxic Effects of A. sanguinea on Co-Occurring Plankton
4.2. Variation Characteristics of A. sanguinea Toxicity
4.3. Action Mode of A. sanguinea Toxicity on Phytoplankton and Zooplankton
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Matsubara, T.; Nagasoe, S.; Yamasaki, Y.; Shikata, T.; Shimasaki, Y.; Oshima, Y.; Honjo, T. Effects of temperature, salinity, and irradiance on the growth of the dinoflagellate Akashiwo sanguinea. J. Exp. Mar. Biol. Ecol. 2007, 342, 226–230. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, T.T.; Song, S.Q.; Li, C.W. Effects of nitrogenous nutrition on growth and nitrogen assimilation enzymes of dinoflagellate Akashiwo sanguinea. Harmful Algae 2015, 50, 99–106. [Google Scholar] [CrossRef]
- Tang, Y.Z.; Gobler, C.J. Sexual resting cyst production by the dinoflagellate Akashiwo sanguinea: A potential mechanism contributing to the ubiquitous distribution of a harmful alga. J. Phycol. 2015, 51, 298–309. [Google Scholar] [CrossRef] [PubMed]
- Hallegraeff, G.M. Harmful algal blooms in the Australian region. Mar. Poll. Bull. 1992, 25, 186–190. [Google Scholar] [CrossRef]
- Horner, R.A.; Garrison, D.L.; Plumley, F.G. Harmful algal blooms and red tide problems on the US west coast. Limnol. Oceanogr. 1997, 42, 1076–1088. [Google Scholar] [CrossRef]
- Gómez, F.; Souissi, S. The impact of the 2003 summer heat wave and the 2005 late cold wave on the phytoplankton in the north-eastern English Channel. Comptes Rendus Biologies 2008, 331, 678–685. [Google Scholar] [CrossRef]
- Kahru, M.; Michell, B.G.; Diaz, A.; Miura, M. MODIS detects a devastating algal bloom in Paracas Bay, Peru. Eos Trans. AGU 2004, 85, 465–472. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.L.; Zhou, C.X.; Zhang, Y.S.; Pu, X.M.; Li, W.H. Evolution and causes of formation of Gymnodinium sanguineum bloom in Yantai Sishili Bay. Oceanol. Limnol. Sin. 2000, 32, 159–167. (In Chinese) [Google Scholar] [CrossRef]
- Lu, S.H.; Hodgkiss, I.J. Harmful algal bloom causative collected from Hong Kong waters. Hydrobiologia 2004, 512, 231–238. [Google Scholar] [CrossRef]
- Jessup, D.A.; Miller, M.A.; Ryan, J.P.; Nevins, H.M.; Kerkering, H.A.; Mekebri, A.; Crane, D.B.; Johnson, T.A.; Kudela, R.M. Mass stranding of marine birds caused by a surfactant-producing red tide. PLoS ONE 2009, 4, e4550. [Google Scholar] [CrossRef]
- White, A.E.; Watkins-Brandt, K.S.; McKibben, S.M.; Wood, A.M.; Hunter, M.; Forster, Z.; Du, X.; Peterson, W.T. Large-scale bloom of Akashiwo sanguinea in the Northern California current system in 2009. Harmful Algae 2014, 37, 38–46. [Google Scholar] [CrossRef]
- Chen, B.H.; Kang, W.; Hui, L. Akashiwo sanguinea blooms in Chinese waters in 1998–2017. Mar. Poll. Bull. 2019, 149, 110652. [Google Scholar] [CrossRef]
- Badylak, S.; Phlips, E.J.; Mathews, A.L.; Kelley, K. Akashiwo sanguinea (Dinophyceae) extruding mucous from pores on the cell surface. Algae 2014, 29, 197. [Google Scholar] [CrossRef] [Green Version]
- Shumway, S.E. A review of the effects of algal blooms on shellfish and aquaculture. J. World Aquacult. Soc. 1990, 21, 65–104. [Google Scholar] [CrossRef]
- Xu, N.; Wang, M.; Tang, Y.Z.; Zhang, Q.; Duan, S.S.; Gobler, C.J. Acute toxicity of the cosmopolitan bloom-forming dinoflagellate Akashiwo sanguinea to finfish, shellfish, and zooplankton. Aquat. Microb. Ecol. 2017, 80, 209–222. [Google Scholar] [CrossRef]
- Yang, Y.Y.; Huang, B.Z.; Tang, Y.Z.; Xu, N. Allelopathic effects of mixotrophic dinoflagellate Akashiwo sanguinea on co-occurring phytoplankton: The significance of nutritional ecology. J. Ocean. Limnol. 2021, 39, 903–917. [Google Scholar] [CrossRef]
- Ding, X.F.; Wang, Y.F.; Zhang, Q.; Xu, N. Hemolytic toxicity of three important harmful microalgae isolated from Pearl River Estuary. Asian J. Ecotoxicol. 2018, 13, 66–76, in Chinese. [Google Scholar] [CrossRef]
- Tang, Y.Z.; Gobler, C.J. Allelopathic effects of Cochlodinium polykrikoides isolates and blooms from the estuaries of Long Island, New York, on co-occurring phytoplankton. Mar. Ecol. Prog. Ser. 2010, 406, 19–31. [Google Scholar] [CrossRef]
- Guillard, R.R.L. Culture of phytoplankton for feeding marine invertebrates. In Culture of Marine Invertebrate Animals; Smith, W.L., Chanley, M.H., Eds.; Springer: Boston, MA, USA, 1975; pp. 29–60. [Google Scholar] [CrossRef]
- Landsberg, J.H. The effects of harmful algal blooms on aquatic organisms. Rev. Fish Sci. 2002, 10, 113–390. [Google Scholar] [CrossRef]
- Shen, A.L.; Ouyang, L.L.; Yin, Y.E.; Zhou, Q.; Ma, Z.L. Effects of Akashiwo sanguinea-dominated algal blooms on the plankton community as observed from the coastal waters of southern Zhejiang, China. Mar. Environ. Sci. 2018, 37, 625–630. [Google Scholar] [CrossRef]
- Cao, J.R.; Huan, Q.L.; Wu, N.; Jiang, T.J. Effects of temperature, light intensity and nutrient condition on the growth and hemolytic activity of six species of typical ichthyotoxic algae. Mar. Environ. Sci. 2015, 34, 321–329. [Google Scholar] [CrossRef]
- Xu, N.; Tang, Y.Z.; Qin, J.L.; Duan, S.S.; Gobler, C.J. Ability of the marine diatoms Pseudo-nitzschia multiseries and P. pungens to inhibit the growth of co-occurring phytoplankton via allelopathy. Aquat. Microb. Ecol. 2015, 74, 29–41. [Google Scholar] [CrossRef]
- Bockstahler, K.; Coats, D. Grazing of the mixotrophic dinoflagellate Gymnodinium sanguineum on ciliate populations of Chesapeake Bay. Mar. Biol. 1993, 116, 477–487. [Google Scholar] [CrossRef]
- Kudela, R.M.; Lane, J.Q.; Cochlan, W.P. The potential role of anthropogenically derived nitrogen in the growth of harmful algae in California, USA. Harmful Algae 2008, 8, 103–110. [Google Scholar] [CrossRef]
- Jeong, H.J.; Yoo, Y.; Park, J.Y.; Kim, K. Feeding by phototrophic red-tide dinoflagellates: Five species newly revealed and six species previously known to be mixotrophic. Aquat. Microb. Ecol. 2005, 40, 133–150. [Google Scholar] [CrossRef] [Green Version]
- Berge, T.; Poulsen, L.K.; Moldrup, M.; Daugbjerg, N.; Hansen, P.J. Marine microalgae attack and feed on metazoans. ISME J. 2012, 6, 1926–1936. [Google Scholar] [CrossRef]
- Song, X.Y.; Hu, Z.X.; Shang, L.X.; Leaw, C.P.; Lim, P.T.; Tang, Y.Z. Contact micropredation may play a more important role than exotoxicity does in the lethal effects of Karlodinium australe blooms: Evidence from laboratory bioassays. Harmful Algae 2020, 99, 101926. [Google Scholar] [CrossRef]
- Sheng, J.; Malkiel, E.; Katz, J.; Adolf, J.E.; Place, A.R. A dinoflagellate exploits toxins to immobilize prey prior to ingestion. Proc. Natl. Acad. Sci. USA 2010, 107, 2082–2087. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vogelbein, W.K.; Lovko, V.J.; Shields, J.D.; Reece, K.S.; Mason, P.L.; Haas, L.W.; Walker, C.C. Pfiesteria shumwayae kills fish by micropredation not exotoxin secretion. Nature 2002, 418, 967–970. [Google Scholar] [CrossRef]
- Tang, Y.Z.; Gobler, C.J. Cochlodinium polykrikoides blooms and clonal isolates from the northwest Atlantic coast cause rapid mortality in larvae of multiple bivalve species. Mar. Biol. 2009, 156, 2601–2611. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, X.; Yang, Y.; Yang, Y.; Zhong, P.; Xu, N. Toxic Characteristics and Action Mode of the Mixotrophic Dinoflagellate Akashiwo sanguinea on Co-Occurring Phytoplankton and Zooplankton. Int. J. Environ. Res. Public Health 2022, 19, 404. https://doi.org/10.3390/ijerph19010404
Wu X, Yang Y, Yang Y, Zhong P, Xu N. Toxic Characteristics and Action Mode of the Mixotrophic Dinoflagellate Akashiwo sanguinea on Co-Occurring Phytoplankton and Zooplankton. International Journal of Environmental Research and Public Health. 2022; 19(1):404. https://doi.org/10.3390/ijerph19010404
Chicago/Turabian StyleWu, Xiaoer, Ying Yang, Yeyin Yang, Ping Zhong, and Ning Xu. 2022. "Toxic Characteristics and Action Mode of the Mixotrophic Dinoflagellate Akashiwo sanguinea on Co-Occurring Phytoplankton and Zooplankton" International Journal of Environmental Research and Public Health 19, no. 1: 404. https://doi.org/10.3390/ijerph19010404
APA StyleWu, X., Yang, Y., Yang, Y., Zhong, P., & Xu, N. (2022). Toxic Characteristics and Action Mode of the Mixotrophic Dinoflagellate Akashiwo sanguinea on Co-Occurring Phytoplankton and Zooplankton. International Journal of Environmental Research and Public Health, 19(1), 404. https://doi.org/10.3390/ijerph19010404





