High-Intensity Interval Exercise Performance and Short-Term Metabolic Responses to Overnight-Fasted Acute-Partial Sleep Deprivation
Abstract
:1. Introduction
2. Materials and Methods
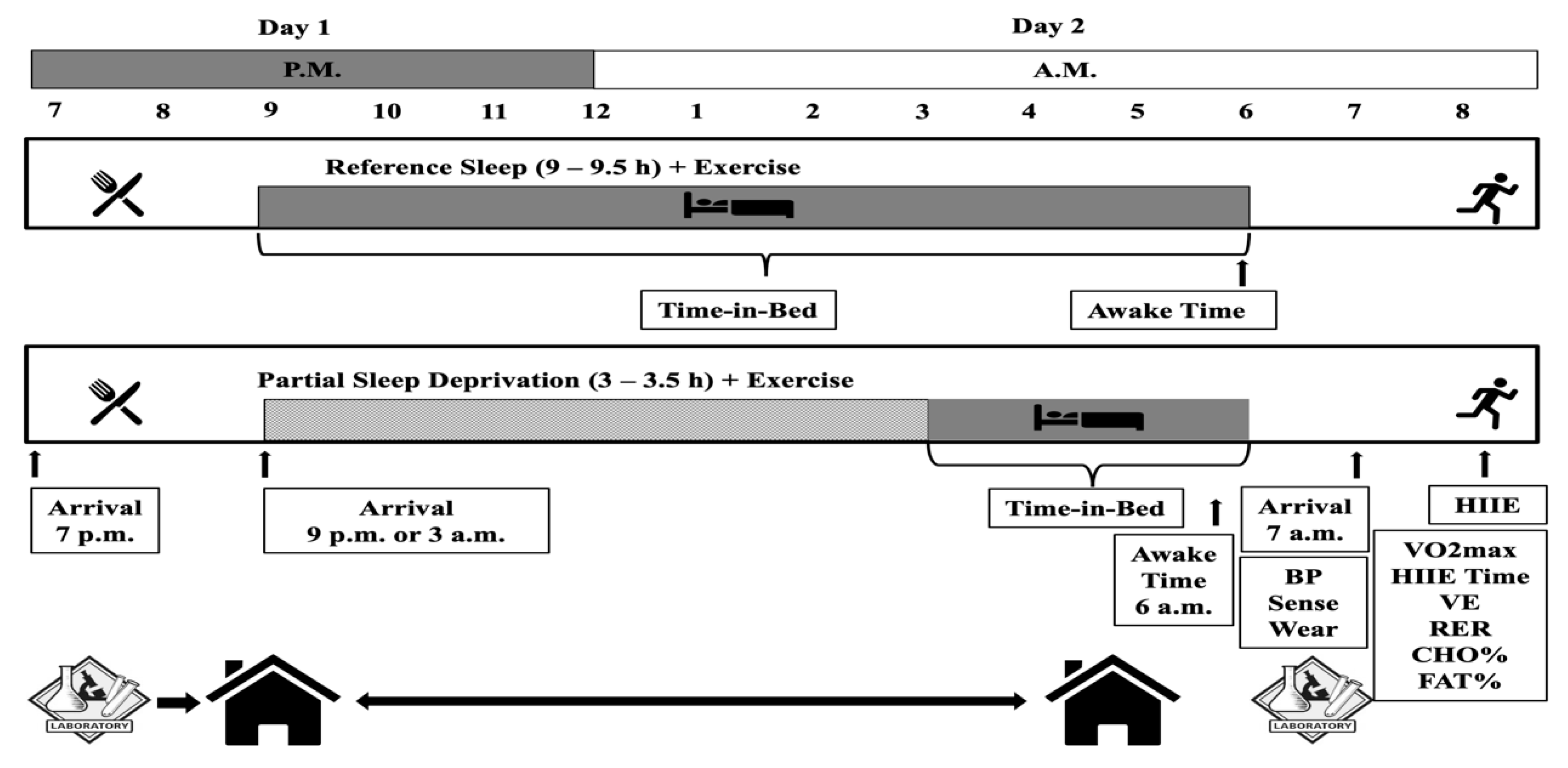
2.1. Study Design and Participants
2.2. Participants
2.3. Preliminary Experimental Procedures
2.3.1. Body Composition and Cardiorespiratory Fitness
2.3.2. Sleep, Physical Activity, and Diet
2.4. Experimental Conditions
2.4.1. Evening Meal
2.4.2. Sleep
2.4.3. High-Intensity Interval Exercise
2.5. Statistical Analyses
3. Results
3.1. Pre-Experimental Data
Caloric Intake, Physical Activity, and Sleep
3.2. Experimental Data
3.2.1. Caloric Intake, Physical Activity, and Sleep
3.2.2. Exercise and Substrate Utilization
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tobaldini, E.; Costantino, G.; Solbiati, M.; Cogliati, C.; Kara, T.; Nobili, L.; Montano, N. Sleep, sleep deprivation, autonomic nervous system and cardiovascular diseases. Neurosci. Biobehav. Rev. 2017, 74, 321–329. [Google Scholar] [CrossRef]
- Tobaldini, E.; Pecis, M.; Montano, N. Effects of acute and chronic sleep deprivation on cardiovascular regulation. Arch. Ital. Biol. 2014, 152, 103–110. [Google Scholar] [CrossRef]
- Antunes, B.M.; Campos, E.Z.; Parmezzani, S.S.; Santos, R.V.; Franchini, E.; Lira, F.S. Sleep quality and duration are associated with performance in maximal incremental test. Physiol. Behav. 2017, 177, 252–256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Papadakis, Z.; Forsse, J.S.; Peterson, M.N. Acute partial sleep deprivation and high-intensity interval exercise effects on postprandial endothelial function. Eur. J. Appl. Physiol. 2020, 120, 2431–2444. [Google Scholar] [CrossRef] [PubMed]
- Papadakis, Z.; Forsse, J.S.; Peterson, M.N. Effects of High-Intensity Interval Exercise and Acute Partial Sleep Deprivation on Cardiac Autonomic Modulation. Res. Q. Exerc. Sport 2020, 1–19. [Google Scholar] [CrossRef]
- Chase, J.D.; Roberson, P.A.; Saunders, M.J.; Hargens, T.A.; Womack, C.J.; Luden, N.D. One night of sleep restriction following heavy exercise impairs 3-km cycling time-trial performance in the morning. Appl. Physiol. Nutr. Metab. Physiol. Appl. Nutr. Metab. 2017, 42, 909–915. [Google Scholar] [CrossRef]
- Backhaus, J.; Junghanns, K.; Broocks, A.; Riemann, D.; Hohagen, F. Test-retest reliability and validity of the Pittsburgh Sleep Quality Index in primary insomnia. J. Psychosom. Res. 2002, 53, 737–740. [Google Scholar] [CrossRef]
- Mollayeva, T.; Thurairajah, P.; Burton, K.; Mollayeva, S.; Shapiro, C.M.; Colantonio, A. The Pittsburgh sleep quality index as a screening tool for sleep dysfunction in clinical and non-clinical samples: A systematic review and meta-analysis. Sleep Med. Rev. 2016, 25, 52–73. [Google Scholar] [CrossRef]
- Brauer, A.A.; Athey, A.B.; Ross, M.J.; Grandner, M.A. Sleep and Health Among Collegiate Student Athletes. Chest 2019, 156, 1234–1245. [Google Scholar] [CrossRef]
- Cullen, T.; Thomas, G.; Wadley, A.J.; Myers, T. The effects of a single night of complete and partial sleep deprivation on physical and cognitive performance: A Bayesian analysis. J. Sports Sci. 2019, 37, 2726–2734. [Google Scholar] [CrossRef] [PubMed]
- Esteves, A.M.; Ackel-D’Elia, C.; Tufik, S.; De Mello, M.T. Sleep patterns and acute physical exercise: The effects of gender, sleep disturbances, type and time of physical exercise. J. Sport Med. Phys. Fit. 2014, 54, 809–815. [Google Scholar]
- Souissi, N.; Sesboue, B.; Gauthier, A.; Larue, J.; Davenne, D. Effects of one night’s sleep deprivation on anaerobic performance the following day. Eur. J. Appl. Physiol. 2003, 89, 359–366. [Google Scholar] [CrossRef] [PubMed]
- Souissi, N.; Souissi, M.; Souissi, H.; Chamari, K.; Tabka, Z.; Dogui, M.; Davenne, D. Effect of time of day and partial sleep deprivation on short-term, high-power output. Chronobiol. Int. 2008, 25, 1062–1076. [Google Scholar] [CrossRef] [PubMed]
- Abedelmalek, S.; Chtourou, H.; Aloui, A.; Aouichaoui, C.; Souissi, N.; Tabka, Z. Effect of time of day and partial sleep deprivation on plasma concentrations of IL-6 during a short-term maximal performance. Eur. J. Appl. Physiol. 2013, 113, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Roberson, P.A.; Chase, J.D.; Bigman, M.B.; Saunders, M.J.; Luden, N.D.; Womack, C.J. Time of day, but not sleep restriction, affects markers of hemostasis following heavy exercise. Appl. Physiol. Nutr. Metab. Physiol. Appl. Nutr. Metab. 2019, 44, 148–152. [Google Scholar] [CrossRef]
- Mougin, F.; Bourdin, H.; Simon-Rigaud, M.L.; Didier, J.M.; Toubin, G.; Kantelip, J.P. Effects of a selective sleep deprivation on subsequent anaerobic performance. Int. J. Sports Med. 1996, 17, 115–119. [Google Scholar] [CrossRef] [PubMed]
- Souissi, N.; Chtourou, H.; Aloui, A.; Hammouda, O.; Dogui, M.; Chaouachi, A.; Chamari, K. Effects of time-of-day and partial sleep deprivation on short-term maximal performances of judo competitors. J. Strength Cond. Res. Natl. Strength Cond. Assoc. 2013, 27, 2473–2480. [Google Scholar] [CrossRef]
- Grandner, M.A.; Hale, L.; Moore, M.; Patel, N.P. Mortality associated with short sleep duration: The evidence, the possible mechanisms, and the future. Sleep Med. Rev. 2010, 14, 191–203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mejri, M.A.; Hammouda, O.; Zouaoui, K.; Chaouachi, A.; Chamari, K.; Rayana, M.C.B.; Souissi, N. Effect of two types of partial sleep deprivation on Taekwondo players’ performance during intermittent exercise. Biol. Rhythm Res. 2013, 45, 17–26. [Google Scholar] [CrossRef]
- Omiya, K.; Akashi, Y.J.; Yoneyama, K.; Osada, N.; Tanabe, K.; Miyake, F. Heart-rate response to sympathetic nervous stimulation, exercise, and magnesium concentration in various sleep conditions. Int. J. Sport Nutr. Exerc. Metab 2009, 19, 127–135. [Google Scholar] [CrossRef]
- Khumalo, S.S. The Effect of Partial Sleep Deprivation on Subsequent Aerobic Exercise Performance. 2014. Available online: http://wiredspace.wits.ac.za/bitstream/handle/10539/14560/Khumalo,Sibongile-2013.pdf?sequence=1 (accessed on 2 March 2021).
- Steenekamp, T.; Davy, J.; Christie, C.J. The effects of partial sleep restriction on biomechanical, physiological, and perceptual responses during an early morning treadmill run. J. Community Health Sci. 2014, 9, 21–33. [Google Scholar]
- Mougin, F.; Simon-Rigaud, M.L.; Davenne, D.; Renaud, A.; Garnier, A.; Kantelip, J.P.; Magnin, P. Effects of sleep disturbances on subsequent physical performance. Eur. J. Appl. Physiol. Occup. Physiol. 1991, 63, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Mougin, F.; Bourdin, H.; Simon-Rigaud, M.L.; Nguyen, N.U.; Kantelip, J.P.; Davenne, D. Hormonal responses to exercise after partial sleep deprivation and after a hypnotic drug-induced sleep. J. Sports Sci. 2001, 19, 89–97. [Google Scholar] [CrossRef]
- Roberts, S.S.H.; Teo, W.P.; Aisbett, B.; Warmington, S.A. Extended Sleep Maintains Endurance Performance Better than Normal or Restricted Sleep. Med. Sci. Sports Exerc. 2019, 51, 2516–2523. [Google Scholar] [CrossRef] [PubMed]
- Fullagar, H.H.; Skorski, S.; Duffield, R.; Hammes, D.; Coutts, A.J.; Meyer, T. Sleep and athletic performance: The effects of sleep loss on exercise performance, and physiological and cognitive responses to exercise. Sports Med. 2015, 45, 161–186. [Google Scholar] [CrossRef] [PubMed]
- Abedelmalek, S.; Souissi, N.; Chtourou, H.; Denguezli, M.; Aouichaoui, C.; Ajina, M.; Aloui, A.; Dogui, M.; Haddouk, S.; Tabka, Z. Effects of partial sleep deprivation on proinflammatory cytokines, growth hormone, and steroid hormone concentrations during repeated brief sprint interval exercise. Chronobiol. Int. 2013, 30, 502–509. [Google Scholar] [CrossRef] [PubMed]
- Azboy, O.; Kaygisiz, Z. Effects of sleep deprivation on cardiorespiratory functions of the runners and volleyball players during rest and exercise. Acta Physiol. Hung. 2009, 96, 29–36. [Google Scholar] [CrossRef]
- Fullagar, H.H.; Duffield, R.; Skorski, S.; Coutts, A.J.; Julian, R.; Meyer, T. Sleep and Recovery in Team Sport: Current Sleep-Related Issues Facing Professional Team-Sport Athletes. Int. J. Sports Physiol. Perform. 2015, 10, 950–957. [Google Scholar] [CrossRef]
- Vitale, K.C.; Owens, R.; Hopkins, S.R.; Malhotra, A. Sleep Hygiene for Optimizing Recovery in Athletes: Review and Recommendations. Int. J. Sports Med. 2019, 40, 535–543. [Google Scholar] [CrossRef]
- Watson, A.M. Sleep and Athletic Performance. Curr. Sports Med. Rep. 2017, 16, 413–418. [Google Scholar] [CrossRef]
- Simpson, N.S.; Gibbs, E.L.; Matheson, G.O. Optimizing sleep to maximize performance: Implications and recommendations for elite athletes. Scand. J. Med. Sci. Spor. 2017, 27, 266–274. [Google Scholar] [CrossRef]
- Bradley, S.M.; Michos, E.D.; Miedema, M.D. Physical Activity, Fitness, and Cardiovascular Health: Insights From Publications in JAMA Network Open. JAMA Netw. Open. 2019, 2, e198343. [Google Scholar] [CrossRef]
- Chattu, V.K.; Manzar, M.D.; Kumary, S.; Burman, D.; Spence, D.W.; Pandi-Perumal, S.R. The Global Problem of Insufficient Sleep and Its Serious Public Health Implications. Healthcare 2018, 7, 1. [Google Scholar] [CrossRef] [Green Version]
- Medic, G.; Wille, M.; Hemels, M.E. Short- and long-term health consequences of sleep disruption. Nat. Sci. Sleep 2017, 9, 151–161. [Google Scholar] [CrossRef] [Green Version]
- Watson, N.F.; Badr, M.S.; Belenky, G.; Bliwise, D.L.; Buxton, O.M.; Buysse, D.; Dinges, D.F.; Gangwisch, J.; Grandner, M.A.; Kushida, C. Recommended amount of sleep for a healthy adult: A joint consensus statement of the American Academy of Sleep Medicine and Sleep Research Society. Sleep 2015, 38, 843–844. [Google Scholar] [CrossRef]
- Wu, M.C.; Yang, Y.C.; Wu, J.S.; Wang, R.H.; Lu, F.H.; Chang, C.J. Short sleep duration associated with a higher prevalence of metabolic syndrome in an apparently healthy population. Prev. Med. 2012, 55, 305–309. [Google Scholar] [CrossRef]
- Van Cauter, E.; Spiegel, K.; Tasali, E.; Leproult, R. Metabolic consequences of sleep and sleep loss. Sleep Med. 2008, 9 (Suppl. 1), S23–S28. [Google Scholar] [CrossRef] [Green Version]
- Steptoe, A.; Peacey, V.; Wardle, J. Sleep duration and health in young adults. Arch. Intern. Med. 2006, 166, 1689–1692. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Youngstedt, S.D.; Kripke, D.F. Long sleep and mortality: Rationale for sleep restriction. Sleep Med. Rev. 2004, 8, 159–174. [Google Scholar] [CrossRef] [PubMed]
- Liew, S.C.; Aung, T. Sleep deprivation and its association with diseases- a review. Sleep Med. 2020. [Google Scholar] [CrossRef] [PubMed]
- Tobaldini, E.; Fiorelli, E.M.; Solbiati, M.; Costantino, G.; Nobili, L.; Montano, N. Short sleep duration and cardiometabolic risk: From pathophysiology to clinical evidence. Nat. Rev. Cardiol. 2019, 16, 213–224. [Google Scholar] [CrossRef]
- Crispim, C.A.; Zimberg, I.Z.; dos Reis, B.G.; Diniz, R.M.; Tufik, S.; de Mello, M.T. Relationship between food intake and sleep pattern in healthy individuals. J. Clin. Sleep Med. 2011, 7, 659–664. [Google Scholar] [CrossRef] [Green Version]
- Chennaoui, M.; Arnal, P.J.; Sauvet, F.; Leger, D. Sleep and exercise: A reciprocal issue? Sleep Med. Rev. 2015, 20, 59–72. [Google Scholar] [CrossRef]
- Berryman, P.; Lukes, E.; Ohlmann, K.K.; O’sullivan, M.I. The costs of short sleep. Aaohn J. 2009, 57, 381–385. [Google Scholar] [CrossRef] [PubMed]
- Gibala, M.J. High-intensity interval training: A time-efficient strategy for health promotion? Curr. Sports Med. Rep. 2007, 6, 211–213. [Google Scholar] [CrossRef] [PubMed]
- Gibala, M.J.; Little, J.P.; MacDonald, M.J.; Hawley, J.A. Physiological adaptations to low-volume, high-intensity interval training in health and disease. J. Physiol. 2012, 590, 1077–1084. [Google Scholar] [CrossRef] [PubMed]
- Venn, D.; Strazdins, L. Your money or your time? How both types of scarcity matter to physical activity and healthy eating. Soc. Sci. Med. 2017, 172, 98–106. [Google Scholar] [CrossRef] [PubMed]
- Gillen, J.B.; Gibala, M.J. Is high-intensity interval training a time-efficient exercise strategy to improve health and fitness? Appl. Physiol. Nutr. Metab. Physiol. Appl. Nutr. Metab. 2014, 39, 409–412. [Google Scholar] [CrossRef] [PubMed]
- St-Onge, M.P.; Grandner, M.A.; Brown, D.; Conroy, M.B.; Jean-Louis, G.; Coons, M.; Bhatt, D.L.; American Heart Association Obesity, Behavior Change, Diabetes; Nutrition Committees of the Council on Lifestyle and Cardiometabolic Health; Cardiometabolic, H.; et al. Sleep Duration and Quality: Impact on Lifestyle Behaviors and Cardiometabolic Health: A Scientific Statement From the American Heart Association. Circulation 2016, 134, e367–e386. [Google Scholar] [CrossRef] [Green Version]
- Sloan, A.W. The Royal Society of South Africa. Trans. R. Soc. South. Afr. 2010, 45, vi. [Google Scholar] [CrossRef]
- Zouhal, H.; Saeidi, A.; Salhi, A.; Li, H.; Essop, M.F.; Laher, I.; Rhibi, F.; Amani-Shalamzari, S.; Ben Abderrahman, A. Exercise Training and Fasting: Current Insights. Open Access J. Sports Med. 2020, 11, 1–28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wallis, G.A.; Gonzalez, J.T. Is exercise best served on an empty stomach? Proc. Nutr. Soc. 2019, 78, 110–117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aird, T.P.; Davies, R.W.; Carson, B.P. Effects of fasted vs fed-state exercise on performance and post-exercise metabolism: A systematic review and meta-analysis. Scand. J. Med. Sci. Spor. 2018, 28, 1476–1493. [Google Scholar] [CrossRef]
- Vieira, A.F.; Costa, R.R.; Macedo, R.C.; Coconcelli, L.; Kruel, L.F. Effects of aerobic exercise performed in fasted v. fed state on fat and carbohydrate metabolism in adults: A systematic review and meta-analysis. Br. J. Nutr. 2016, 116, 1153–1164. [Google Scholar] [CrossRef] [Green Version]
- Jeukendrup, A.E. Modulation of carbohydrate and fat utilization by diet, exercise and environment. Biochem. Soc. Trans. 2003, 31, 1270–1273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Bock, K.; Richter, E.A.; Russell, A.P.; Eijnde, B.O.; Derave, W.; Ramaekers, M.; Koninckx, E.; Leger, B.; Verhaeghe, J.; Hespel, P. Exercise in the fasted state facilitates fibre type-specific intramyocellular lipid breakdown and stimulates glycogen resynthesis in humans. J. Physiol. 2005, 564, 649–660. [Google Scholar] [CrossRef] [PubMed]
- Atrooz, F.; Salim, S. Chapter Eight-Sleep deprivation, oxidative stress and inflammation. In Advances in Protein Chemistry and Structural Biology; Donev, R., Ed.; Academic Press: London, UK, 2020; Volume 119, pp. 309–336. [Google Scholar]
- Mougin, F.; Davenne, D.; Simon-Rigaud, M.L.; Renaud, A.; Garnier, A.; Magnin, P. Disturbance of sports performance after partial sleep deprivation. C R Seances Soc. Biol. Fil. 1989, 183, 461–466. [Google Scholar] [PubMed]
- Venables, M.C.; Jeukendrup, A.E. Endurance training and obesity: Effect on substrate metabolism and insulin sensitivity. Med. Sci. Sports Exerc. 2008, 40, 495–502. [Google Scholar] [CrossRef] [PubMed]
- Nedeltcheva, A.V.; Kilkus, J.M.; Imperial, J.; Schoeller, D.A.; Penev, P.D. Insufficient sleep undermines dietary efforts to reduce adiposity. Ann. Intern. Med. 2010, 153, 435–441. [Google Scholar] [CrossRef]
- Klingenberg, L.; Sjodin, A.; Holmback, U.; Astrup, A.; Chaput, J.P. Short sleep duration and its association with energy metabolism. Obes. Rev. 2012, 13, 565–577. [Google Scholar] [CrossRef]
- Haram, P.M.; Adams, V.; Kemi, O.J.; Brubakk, A.O.; Hambrecht, R.; Ellingsen, O.; Wisloff, U. Time-course of endothelial adaptation following acute and regular exercise. Eur. J. Cardiovasc. Prev. Rehabil. Off. J. Eur. Soc. Cardiol. Work. Groups Epidemiol. Prev. Card. Rehabil. Exerc. Physiol. 2006, 13, 585–591. [Google Scholar] [CrossRef]
- Magal, M.; Riebe, D. New Preparticipation Health Screening Recommendations. Acsm’s Health Fit. J. 2016, 20, 22–27. [Google Scholar] [CrossRef] [Green Version]
- Buysse, D.J.; Reynolds, C.F., 3rd; Monk, T.H.; Berman, S.R.; Kupfer, D.J. The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Res. 1989, 28, 193–213. [Google Scholar] [CrossRef]
- Sunseri, M.; Liden, C.B.; Farringdon, J.; Pelletier, R.; Safier, S.; Stivoric, J.; Teller, A.; Vishnubhatla, S. The Sensewear Armband as a sleep detection device. Bodymedia Intern. White Pap. 2009. [Google Scholar]
- Sharif, M.M.; Bahammam, A.S. Sleep estimation using BodyMedia’s SenseWear armband in patients with obstructive sleep apnea. Ann. Thorac. Med. 2013, 8, 53–57. [Google Scholar] [CrossRef] [PubMed]
- Shin, M.; Swan, P.; Chow, C.M. The validity of Actiwatch2 and SenseWear armband compared against polysomnography at different ambient temperature conditions. Sleep Sci. 2015, 8, 9–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Wouwe, N.C.; Valk, P.J.; Veenstra, B.J. Sleep monitoring: A comparison between three wearable instruments. Mil. Med. 2011, 176, 811–816. [Google Scholar] [CrossRef] [Green Version]
- Jurado-Fasoli, L.; Mochon-Benguigui, S.; Castillo, M.J.; Amaro-Gahete, F.J. Association between sleep quality and time with energy metabolism in sedentary adults. Sci. Rep. 2020, 10, 4598. [Google Scholar] [CrossRef] [Green Version]
- Hirshkowitz, M.; Whiton, K.; Albert, S.M.; Alessi, C.; Bruni, O.; DonCarlos, L.; Hazen, N.; Herman, J.; Katz, E.S.; Kheirandish-Gozal, L.; et al. National Sleep Foundation’s sleep time duration recommendations: Methodology and results summary. Sleep Health 2015, 1, 40–43. [Google Scholar] [CrossRef]
- Irwin, M.R.; Olmstead, R.; Carroll, J.E. Sleep Disturbance, Sleep Duration, and Inflammation: A Systematic Review and Meta-Analysis of Cohort Studies and Experimental Sleep Deprivation. Biol. Psychiatry 2016, 80, 40–52. [Google Scholar] [CrossRef] [Green Version]
- Almeida, G.J.; Wasko, M.C.; Jeong, K.; Moore, C.G.; Piva, S.R. Physical activity measured by the SenseWear Armband in women with rheumatoid arthritis. Phys. Ther. 2011, 91, 1367–1376. [Google Scholar] [CrossRef] [Green Version]
- American College of Sports Medicine. ACSM’s Guidelines for Exercise Testing and Prescription; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2013. [Google Scholar]
- Soric, M.; Turkalj, M.; Kucic, D.; Marusic, I.; Plavec, D.; Misigoj-Durakovic, M. Validation of a multi-sensor activity monitor for assessing sleep in children and adolescents. Sleep Med. 2013, 14, 201–205. [Google Scholar] [CrossRef]
- Littlefield, L.A.; Papadakis, Z.; Rogers, K.M.; Moncada-Jimenez, J.; Taylor, J.K.; Grandjean, P.W. The effect of exercise intensity and excess postexercise oxygen consumption on postprandial blood lipids in physically inactive men. Appl. Physiol. Nutr. Metab. Physiol. Appl. Nutr. Metab. 2017, 42, 986–993. [Google Scholar] [CrossRef]
- Papadakis, Z.; Forsse, J.S.; Bane, A.A.; Taylor, K.J.; Qian, L.; Morales Marroquin, F.E.; Grandjean, P.W. High-density Lipoprotein Antioxidant Responses To High-intensity Interval And Steady-state Moderate-intensity Exercise. Med. Sci. Sports Exerc. 2015, 47, 871. [Google Scholar] [CrossRef] [Green Version]
- Souza, G.S. Concepts in Exercise Dynamics For Fitness And Health Essentials. Int. J. Physiol. Nutr. Phys. Educ. 2019, 4, 62–67. [Google Scholar]
- Forsse, J.S.; Papadakis, Z.; Bane, A.A.; Taylor, J.K.; Qian, L.; Marroquín, F.E.M.; Grandjean, P.W. 3-Nitrotyrosine and Soluble Vascular and Intracellular Adhesion Molecule Responses to High-Intensity Interval and Steady-State Moderate-Intensity Exercise. Int. J. Exerc. Sci. 2015, 2, 73. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.Q.; Ji, L.L.; Fretwell, V.S.; Nunez, G. Effect of exercise on postprandial lipemia in men with hypertriglyceridemia. Eur. J. Appl. Physiol. 2006, 98, 575–582. [Google Scholar] [CrossRef] [PubMed]
- Plaisance, E.P.; Mestek, M.L.; Mahurin, A.J.; Taylor, J.K.; Moncada-Jimenez, J.; Grandjean, P.W. Postprandial triglyceride responses to aerobic exercise and extended-release niacin. Am. J. Clin. Nutr. 2008, 88, 30–37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mestek, M.L.; Plaisance, E.P.; Ratcliff, L.A.; Taylor, J.K.; Wee, S.O.; Grandjean, P.W. Aerobic exercise and postprandial lipemia in men with the metabolic syndrome. Med. Sci. Sports Exerc. 2008, 40, 2105–2111. [Google Scholar] [CrossRef] [PubMed]
- Mestek, M.L.; Garner, J.C.; Plaisance, E.P.; Taylor, J.K.; Alhassan, S.; Grandjean, P.W. Blood lipid responses after continuous and accumulated aerobic exercise. Int. J. Sport Nutr. Exerc. Metab. 2006, 16, 245–254. [Google Scholar] [CrossRef]
- Ghasemi, A.; Zahediasl, S. Normality tests for statistical analysis: A guide for non-statisticians. Int. J. Endocrinol. Metab. 2012, 10, 486–489. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harris, R.A.; Padilla, J.; Hanlon, K.P.; Rink, L.D.; Wallace, J.P. Reproducibility of the flow-mediated dilation response to acute exercise in overweight men. Ultrasound Med. Biol. 2007, 33, 1579–1585. [Google Scholar] [CrossRef]
- Vgontzas, A.N.; Zoumakis, E.; Bixler, E.O.; Lin, H.M.; Follett, H.; Kales, A.; Chrousos, G.P. Adverse effects of modest sleep restriction on sleepiness, performance, and inflammatory cytokines. J. Clin. Endocrinol. Metab. 2004, 89, 2119–2126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Julious, S.A. Sample size of 12 per group rule of thumb for a pilot study. Pharm. Stat. 2005, 4, 287–291. [Google Scholar] [CrossRef]
- Hertzog, M.A. Considerations in determining sample size for pilot studies. Res. Nurs. Health 2008, 31, 180–191. [Google Scholar] [CrossRef]
- Oliver, S.J.; Costa, R.J.; Laing, S.J.; Bilzon, J.L.; Walsh, N.P. One night of sleep deprivation decreases treadmill endurance performance. Eur. J. Appl. Physiol. 2009, 107, 155–161. [Google Scholar] [CrossRef]
- Plyley, M.J.; Shephard, R.J.; Davis, G.M.; Goode, R.C. Sleep deprivation and cardiorespiratory function. Influence of intermittent submaximal exercise. Eur. J. Appl. Physiol. Occup. Physiol. 1987, 56, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Meerlo, P.; Sgoifo, A.; Suchecki, D. Restricted and disrupted sleep: Effects on autonomic function, neuroendocrine stress systems and stress responsivity. Sleep Med. Rev. 2008, 12, 197–210. [Google Scholar] [CrossRef]
- Dettoni, J.L.; Consolim-Colombo, F.M.; Drager, L.F.; Rubira, M.C.; Souza, S.B.; Irigoyen, M.C.; Mostarda, C.; Borile, S.; Krieger, E.M.; Moreno, H., Jr.; et al. Cardiovascular effects of partial sleep deprivation in healthy volunteers. J. Appl. Physiol. 2012, 113, 232–236. [Google Scholar] [CrossRef]
- Fullagar, H.H.; Skorski, S.; Duffield, R.; Julian, R.; Bartlett, J.; Meyer, T. Impaired sleep and recovery after night matches in elite football players. J. Sports Sci. 2016, 34, 1333–1339. [Google Scholar] [CrossRef]
- Ekkekakis, P.; Parfitt, G.; Petruzzello, S.J. The pleasure and displeasure people feel when they exercise at different intensities: Decennial update and progress towards a tripartite rationale for exercise intensity prescription. Sports Med. 2011, 41, 641–671. [Google Scholar] [CrossRef] [PubMed]
- Krustrup, P.; Mohr, M.; Amstrup, T.; Rysgaard, T.; Johansen, J.; Steensberg, A.; Pedersen, P.K.; Bangsbo, J. The yo-yo intermittent recovery test: Physiological response, reliability, and validity. Med. Sci. Sports Exerc. 2003, 35, 697–705. [Google Scholar] [CrossRef]
- Bangsbo, J.; Iaia, F.M.; Krustrup, P. The Yo-Yo intermittent recovery test: A useful tool for evaluation of physical performance in intermittent sports. Sports Med. 2008, 38, 37–51. [Google Scholar] [CrossRef] [PubMed]
- Zoccoli, G.; Walker, A.M.; Lenzi, P.; Franzini, C. The cerebral circulation during sleep: Regulation mechanisms and functional implications. Sleep Med. Rev. 2002, 6, 443–455. [Google Scholar] [CrossRef] [PubMed]
- Walker, M.P.; Stickgold, R. It’s practice, with sleep, that makes perfect: Implications of sleep-dependent learning and plasticity for skill performance. Clin. Sports Med. 2005, 24, 301–317. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Skein, M.; Duffield, R.; Edge, J.; Short, M.J.; Mundel, T. Intermittent-sprint performance and muscle glycogen after 30 h of sleep deprivation. Med. Sci. Sports Exerc. 2011, 43, 1301–1311. [Google Scholar] [CrossRef] [PubMed]
- Jafari, B. Cerebrovascular Regulation and Sleep Apnea. Curr. Sleep Med. Rep. 2018, 4, 196–201. [Google Scholar] [CrossRef]
- Myllymaki, T.; Rusko, H.; Syvaoja, H.; Juuti, T.; Kinnunen, M.L.; Kyrolainen, H. Effects of exercise intensity and duration on nocturnal heart rate variability and sleep quality. Eur. J. Appl. Physiol. 2012, 112, 801–809. [Google Scholar] [CrossRef]
- Ohayon, M.; Wickwire, E.M.; Hirshkowitz, M.; Albert, S.M.; Avidan, A.; Daly, F.J.; Dauvilliers, Y.; Ferri, R.; Fung, C.; Gozal, D.; et al. National Sleep Foundation’s sleep quality recommendations: First report. Sleep Health 2017, 3, 6–19. [Google Scholar] [CrossRef] [Green Version]
- Vlahoyiannis, A.; Aphamis, G.; Andreou, E.; Samoutis, G.; Sakkas, G.K.; Giannaki, C.D. Effects of High vs. Low Glycemic Index of Post-Exercise Meals on Sleep and Exercise Performance: A Randomized, Double-Blind, Counterbalanced Polysomnographic Study. Nutrients 2018, 10, 1795. [Google Scholar] [CrossRef] [Green Version]
- Faul, F.; Erdfelder, E.; Lang, A.G.; Buchner, A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef]
- McNeish, D. Challenging Conventional Wisdom for Multivariate Statistical Models With Small Samples. Rev Educ Res 2017, 87, 1117–1151. [Google Scholar] [CrossRef]
- Keramidas, M.E.; Gadefors, M.; Nilsson, L.O.; Eiken, O. Physiological and psychological determinants of whole-body endurance exercise following short-term sustained operations with partial sleep deprivation. Eur. J. Appl. Physiol. 2018, 118, 1373–1384. [Google Scholar] [CrossRef] [Green Version]
- King, A.C.; Pruitt, L.A.; Woo, S.; Castro, C.M.; Ahn, D.K.; Vitiello, M.V.; Woodward, S.H.; Bliwise, D.L. Effects of moderate-intensity exercise on polysomnographic and subjective sleep quality in older adults with mild to moderate sleep complaints. J. Gerontol. A Biol. Sci. Med. Sci. 2008, 63, 997–1004. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonato, M.; Agnello, L.; Galasso, L.; Montaruli, A.; Roveda, E.; Merati, G.; La Torre, A.; Vitale, J.A. Acute Modification of Cardiac Autonomic Function of High-Intensity Interval Training in Collegiate Male Soccer Players with Different Chronotype: A Cross-Over Study. J. Sports Sci. Med. 2017, 16, 286–294. [Google Scholar] [PubMed]
- Rossi, A.; Formenti, D.; Vitale, J.A.; Calogiuri, G.; Weydahl, A. The Effect of Chronotype on Psychophysiological Responses during Aerobic Self-Paced Exercises. Percept. Mot. Skills 2015, 121, 840–855. [Google Scholar] [CrossRef]
- Vitale, J.A.; Bonato, M.; Galasso, L.; La Torre, A.; Merati, G.; Montaruli, A.; Roveda, E.; Carandente, F. Sleep quality and high intensity interval training at two different times of day: A crossover study on the influence of the chronotype in male collegiate soccer players. Chronobiol. Int. 2017, 34, 260–268. [Google Scholar] [CrossRef] [PubMed]
- Chtourou, H.; Souissi, N. The effect of training at a specific time of day: A review. J. Strength Cond. Res./Natl. Strength Cond. Assoc. 2012, 26, 1984–2005. [Google Scholar] [CrossRef]
- Reilly, T.; Garrett, R. Effects of time of day on self-paced performances of prolonged exercise. J. Sports Med. Phys. Fitness 1995, 35, 99–102. [Google Scholar]
- Chtourou, H.; Chaouachi, A.; Driss, T.; Dogui, M.; Behm, D.G.; Chamari, K.; Souissi, N. The effect of training at the same time of day and tapering period on the diurnal variation of short exercise performances. J. Strength Cond. Res./Natl. Strength Cond. Assoc. 2012, 26, 697–708. [Google Scholar] [CrossRef] [PubMed]
- Vitale, J.A.; Calogiuri, G.; Weydahl, A. Influence of chronotype on responses to a standardized, self-paced walking task in the morning vs afternoon: A pilot study. Percept. Mot. Skills 2013, 116, 1020–1028. [Google Scholar] [CrossRef] [PubMed]
- Vitale, J.A.; La Torre, A.; Baldassarre, R.; Piacentini, M.F.; Bonato, M. Ratings of Perceived Exertion and Self-reported Mood State in Response to High Intensity Interval Training. A Crossover Study on the Effect of Chronotype. Front. Psychol. 2017, 8, 1232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Knutson, K.L.; Spiegel, K.; Penev, P.; Van Cauter, E. The metabolic consequences of sleep deprivation. Sleep Med. Rev. 2007, 11, 163–178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Variable | Mean ± SD | Min | Max |
|---|---|---|---|
| Age (year) | 31.1 ± 5.3 | 24 | 40 |
| Height (cm) | 179.3 ± 6.8 | 167.6 | 188.0 |
| Weight (kg) | 83.3 ± 11.4 | 70.7 | 105.7 |
| BMI (kg/m2) | 25.8 ± 2.7 | 21.1 | 29.9 |
| BF (%) | 21.0 ± 6.5 | 11.4 | 35.3 |
| VO2 (mL/kg/min) | 49.2 ± 8.5 | 36.0 | 66.0 |
| Time to complete VO2 test (min) | 10.1 ± 1.9 | 6.5 | 13.4 |
| VE (L/min) | 68.9 ± 17.3 | 32 | 95 |
| RER | 0.97 ± 0.5 | 0.89 | 1.03 |
| PSQI | 3.7 ± 0.9 | 2 | 5 |
| 7 Days Caloric intake (kcal) | 2372 ± 576 | 1421 | 3440 |
| 7 Days Activity levels (METs) | 1.49 ± 0.15 | 1.3 | 1.7 |
| 7 Days SL (h:mm:ss) | 8:25:44 ± 1:22:50 | 6:33:00 | 11:15:00 |
| 7 Days SD (h:mm:ss) | 6:43:28 ± 1:22:50 | 5:03:00 | 9:11:00 |
| 7 Days SLE (%) | 81 ± 11 | 55.19 | 91.1 |
| Variable | RSE | SSE |
|---|---|---|
| Caloric intake (kcal) | 1989 ± 749 | 2106 ± 870 |
| Activity levels (METs) | 1.4 ± 0.15 | 1.5 ± 0.15 |
| Vitamin A (μg) | 782.53 ± 618.17 | 795 ± 603.30 |
| Vitamin C (μg) | 156.73 ± 378.91 | 191.1 ± 376.41 |
| Vitamin E (μg) | 11.8 ± 7.86 | 14.53 ± 12.62 |
| SL (h:mm:ss) | 8:11:04 ± 1:01:48 | 3:18:09 ± 0:52:02 *; p < 0.05 |
| SD (h:mm:ss) | 6:57:09 ± 0:47:38 | 2:39:30 ± 0:40:11 *; p < 0.05 |
| SLE (%) | 86 ± 8 | 81 ± 12 |
| Variable | RSE | SSE | Within-Subjects Effects |
|---|---|---|---|
| 90% VO2reserve (mL/kg/min) | 41.6 ± 7.3 | 41.2 ± 7.3 | F1,14 = 0.30, p = 0.6, η2 = 0.021 |
| 40% VO2reserve (mL/kg/min) | 20.4 ± 3.2 | 20.3 ± 3.1 | F1,14 = 0.03, p = 0.87, η2 = 0.002 |
| VO2max (mL/kg/min) | 45.8 ± 8.1 | 45.39 ± 8.1 | F1,14 = 0.22, p = 0.6, η2 = 0.016 |
| Time to complete VO2 test (min) | 24.31 ± 2.6 | 24.44 ± 2 | F1,14 = 0.09, p = 0.77, η2 = 0.006 |
| VE (L/min) | 72.8 ± 9.39 | 71.6 ± 10.84 | F1,14 = 0.41, p = 0.53, η2 = 0.029 |
| RER | 0.97 ± 0.037 | 0.96 ± 0.041 | F1,14 = 0.41, p = 0.53, η2 = 0.029 |
| CHO (%) | 88.91 ± 12.63 | 86.65 ± 13.94 | F1,14 = 0.41, p = 0.53, η2 = 0.029 |
| FAT (%) | 11.08 ± 12.63 | 13.34 ± 13.94 | F1,14 = 0.41, p = 0.53, η2 = 0.029 |
| MAP (mmHg) | 98.6 ± 6.3 | 72 ± 9.8 | F1,14 = 222.97, p < 0.001, η2 = 0.941 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Papadakis, Z.; Forsse, J.S.; Stamatis, A. High-Intensity Interval Exercise Performance and Short-Term Metabolic Responses to Overnight-Fasted Acute-Partial Sleep Deprivation. Int. J. Environ. Res. Public Health 2021, 18, 3655. https://doi.org/10.3390/ijerph18073655
Papadakis Z, Forsse JS, Stamatis A. High-Intensity Interval Exercise Performance and Short-Term Metabolic Responses to Overnight-Fasted Acute-Partial Sleep Deprivation. International Journal of Environmental Research and Public Health. 2021; 18(7):3655. https://doi.org/10.3390/ijerph18073655
Chicago/Turabian StylePapadakis, Zacharias, Jeffrey S. Forsse, and Andreas Stamatis. 2021. "High-Intensity Interval Exercise Performance and Short-Term Metabolic Responses to Overnight-Fasted Acute-Partial Sleep Deprivation" International Journal of Environmental Research and Public Health 18, no. 7: 3655. https://doi.org/10.3390/ijerph18073655
APA StylePapadakis, Z., Forsse, J. S., & Stamatis, A. (2021). High-Intensity Interval Exercise Performance and Short-Term Metabolic Responses to Overnight-Fasted Acute-Partial Sleep Deprivation. International Journal of Environmental Research and Public Health, 18(7), 3655. https://doi.org/10.3390/ijerph18073655








