Effect of a Shock Micro-Cycle on Biochemical Markers in University Soccer Players
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Ethics
2.3. Variables
2.4. Procedure
2.4.1. Biochemical Measurements
2.4.2. Anthropometric Evaluation
2.4.3. Maximal Oxygen Uptake (VO2max)
2.4.4. Shock Micro-Cycle
- Day 1: Smooth jog, joint movement, muscle activation, active stretching.
- Day 2: Tactical training.
- Day 3: Match 1.
- Day 4: Match 2.
- Day 5: Match 3.
- Day 6: Match 4.
- Day 7: Match 5.
2.5. Statistical Analyses
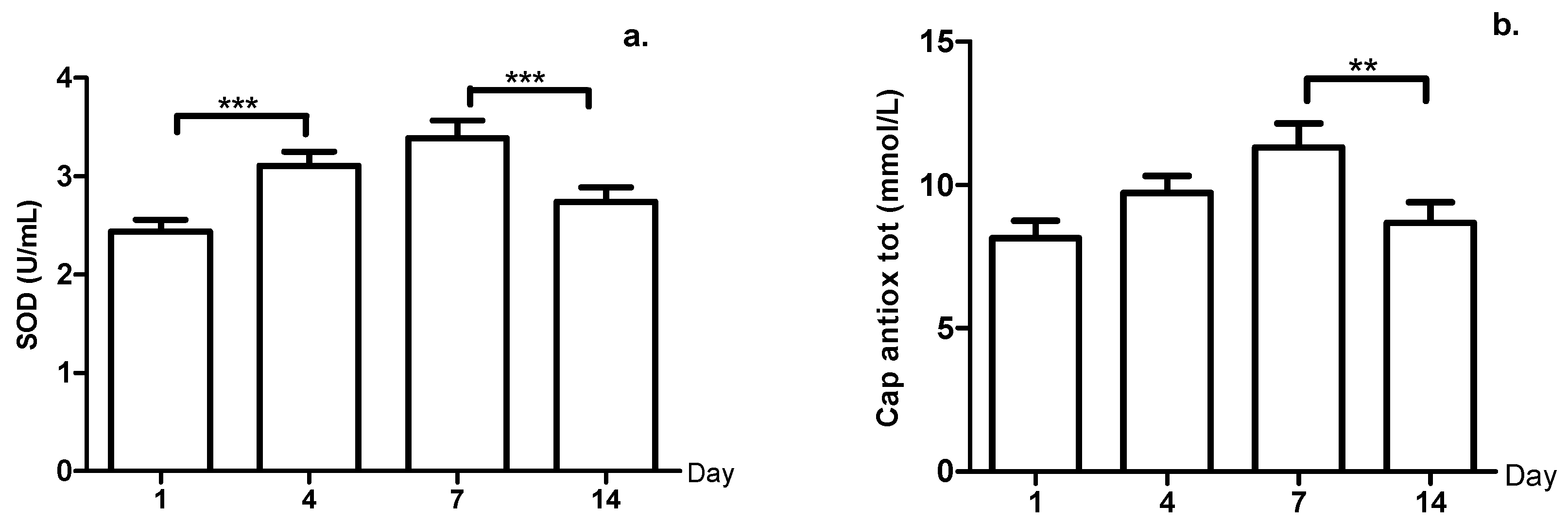
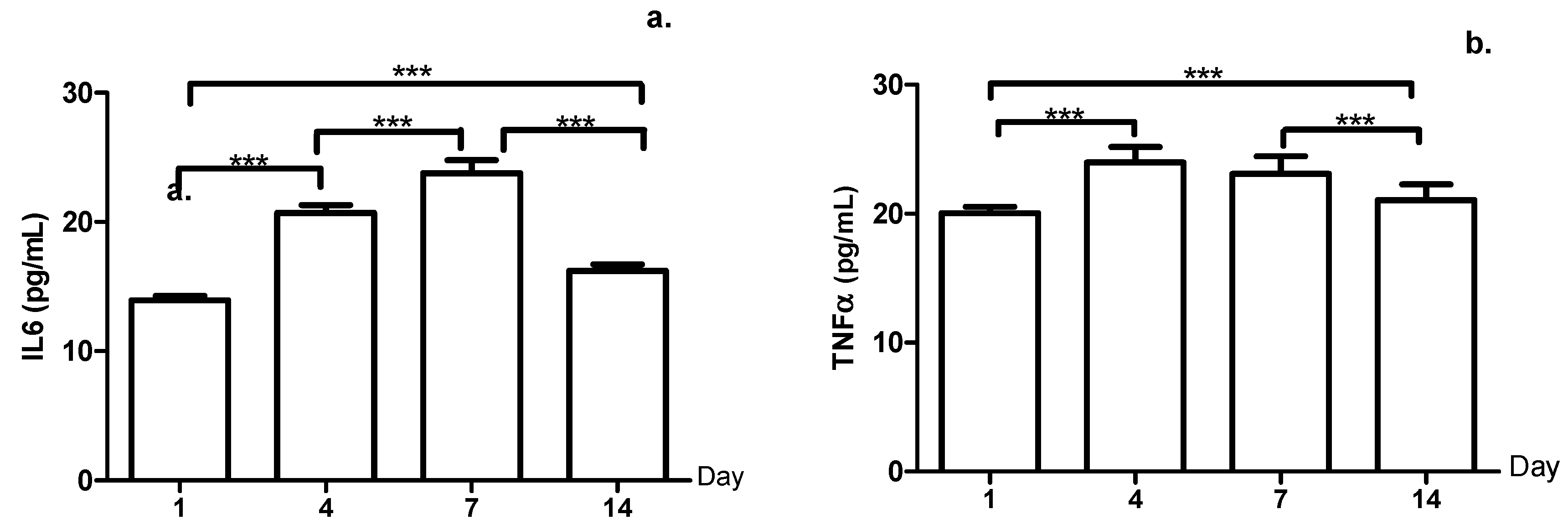
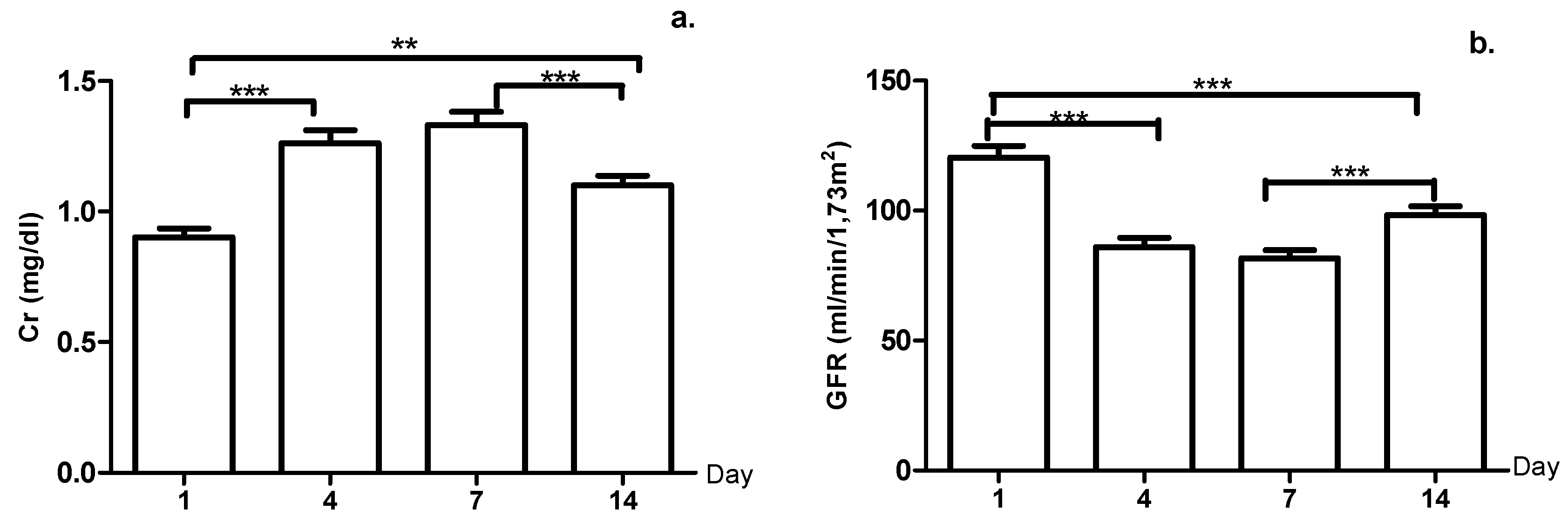
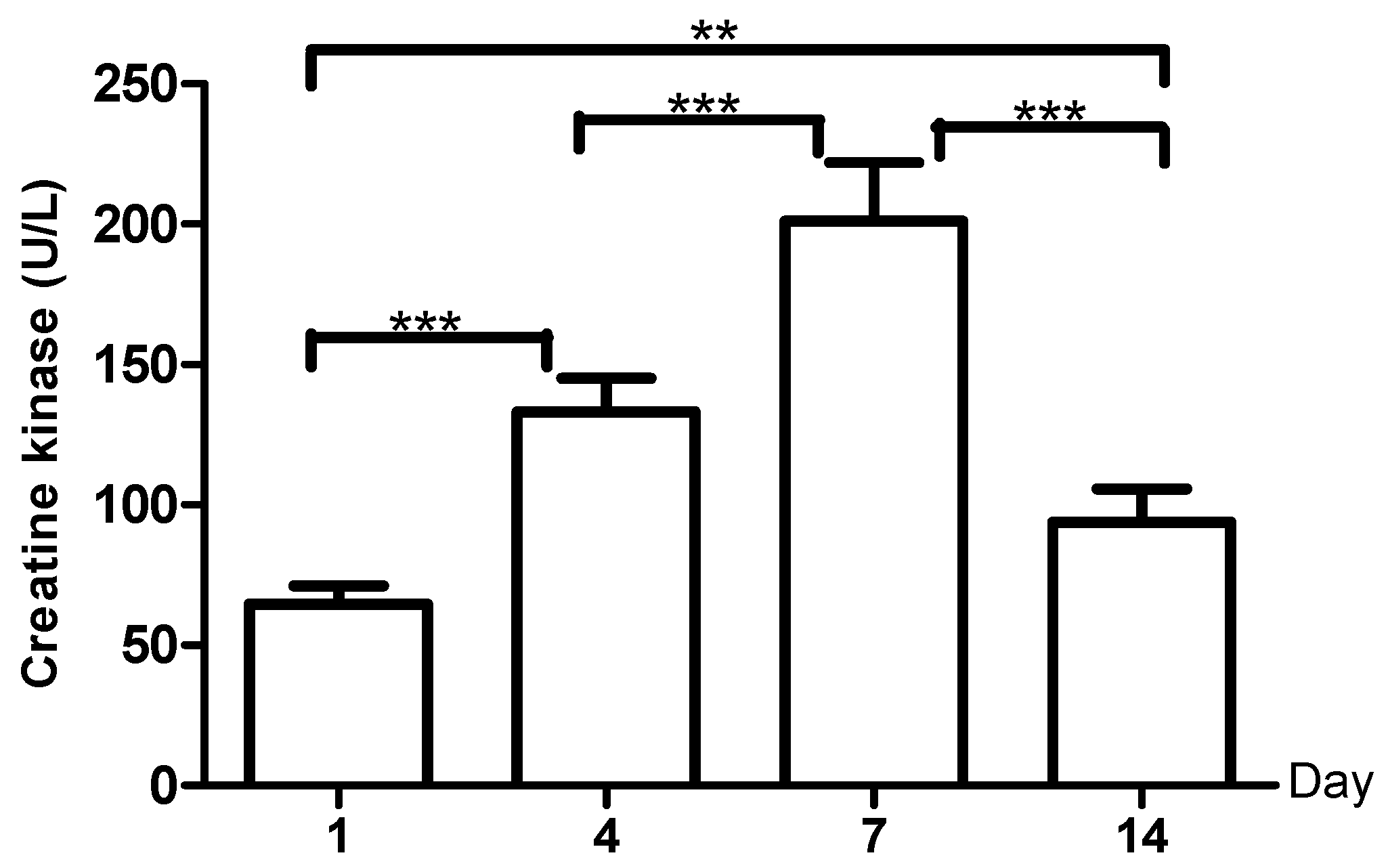
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Padilla, J. Planificación del Entrenamiento Deportivo: Un Enfoque Metodológico de la Estructura Clásica, 1st ed.; Editorial Episteme: Barinas, Venezuela, 2017; 125p. [Google Scholar]
- Pingitore, A.; Lima, G.P.P.; Mastorci, F.; Quinones, A.; Iervasi, G.; Vassalle, C. Exercise and oxidative stress: Potential effects of antioxidant dietary strategies in sports. Nutrition 2015, 31, 916–922. [Google Scholar] [CrossRef] [PubMed]
- Fernández, J.; Da Silva-Grigoletto, M.; Túnez-Fiñana, I. Estrés oxidativo inducido por el ejercicio J.M. Rev. Andal. Med. Del. Deport. 2009, 2, 19–34. [Google Scholar]
- Fernandez, E.; García, C.; de la Espriella, R.; Dueñas, C.; Manzur, F. Biomarcadores cardíacos: Presente y futuro. Rev. Colomb. Cardiol. 2012, 19, 300–311. [Google Scholar] [CrossRef]
- Vendrell, E.; Lanau, P.; Salat, D.; Pérez, V.; Prat, M.S.; de Gracia Roldán, J.; Pujol, J.A. Influencia del medio y del ejercicio físico sobre las inmunoglobulinas séricas durante una carrera de trineos de nieve en montaña. Arch. Med. Deport. 2014, 31, 80–84. [Google Scholar]
- Moreno-Perez, V.; Gallo-Salazar, C.; Del Coso, J.; Ruiz-Pérez, I.; Lopez-Valenciano, A.; Barbado, D.; Cabello-Manrique, D.; Fernandez-Fernandez, J. The influence of a badminton competition with two matches in a day on muscle damage and physical performance in elite junior badminton players. Biol. Sport 2020, 37, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Margaritelis, N.; Paschalis, V.; Theodorou, A.; Kyparos, A.; Nikolaidis, M. Redox basis of exercise physiology. Redox Biol. 2020, 35, 101499. [Google Scholar] [CrossRef]
- Perroni, F.; Migliaccio, S.; Borrione, P.; Vetrano, M.; Amatori, S.; Sisti, D.; Rocchi, M.; Salerno, G.; Vescovo, R.; Cavarretta, E.; et al. Can Haematological and Hormonal Biomarkers Predict Fitness Parameters in Youth Soccer Players? A Pilot Study. Int. J. Environ. Res. Public Health 2020, 17, 6294. [Google Scholar] [CrossRef]
- Bacallao, R.; Badell, A. La creatinina como indicador del tejido muscular esquelético y el estado nutricional. Rev. Cuba. Aliment. Nutr. 2015, 25, 4–23. [Google Scholar]
- Mougios, V. Reference intervals for serum creatine kinase in athletes. Br. J. Sports Med. 2007, 41, 674–678. [Google Scholar] [CrossRef] [PubMed]
- Nowakowska, A.; Kostrzewa-Nowak, D.; Buryta, R.; Nowak, R. Blood Biomarkers of Recovery Efficiency in Soccer Players. Int. J. Environ. Res. Public Health 2019, 16, 3279. [Google Scholar] [CrossRef] [PubMed]
- Nédélec, M.; McCall, A.; Carling, C.; Legall, F.; Berthoin, S.; Dupont, G. Recovery in Soccer. Sport Med. 2012, 42, 997–1015. [Google Scholar]
- Souglis, A.G.; Papapanagiotou, A.; Bogdanis, G.C.; Travlos, A.K.; Apostolidis, N.G.; Geladas, N.D. Comparison of Inflammatory Responses to a Soccer Match Between Elite Male and Female Players. J. Strength Cond. Res. 2015, 29, 1227–1233. [Google Scholar] [CrossRef]
- Nieman, D.C.; Gillitt, N.D.; Knab, A.M.; Shanely, R.A.; Pappan, K.L.; Jin, F.; Lila, M.A. Influence of a Polyphenol-Enriched Protein Powder on Exercise-Induced Inflammation and Oxidative Stress in Athletes: A Randomized Trial Using a Metabolomics Approach. PLoS ONE 2013, 8, e72215. [Google Scholar] [CrossRef]
- Bloomfield, J.; Polman, R.; O’Donoghue, P. Physical Demands of Different Positions in FA Premier League Soccer. J. Sports Sci. Med. 2007, 6, 63–70. [Google Scholar] [PubMed]
- Osgnach, C.; Poser, S.; Bernardini, R.; Rinaldo, R.; Enrico, P.; Prampero, D.I. Energy cost and metabolic power in elite soccer: A new match analysis approach. Med. Sci. Sport Exerc. 2010, 42, 170–178. [Google Scholar] [CrossRef] [PubMed]
- Nedelec, M.; McCall, A.; Carling, C.; Legall, F.; Berthoin, S.; Dupont, G. The Influence of Soccer Playing Actions on the Recovery Kinetics After a Soccer Match. J. Strength Cond. Res. 2014, 28, 1517–1523. [Google Scholar] [CrossRef] [PubMed]
- Thorpe, R.; Sunderland, C. Muscle Damage, Endocrine, and Immune Marker Response to a Soccer Match. J. Strength Cond. Res. 2012, 26, 2783–2790. [Google Scholar] [CrossRef] [PubMed]
- Fatouros, I.G.; Chatzinikolaou, A.; I Douroudos, I.; Nikolaidis, M.G.; Kyparos, A.; Margonis, K.; Michailidis, Y.; Vantarakis, A.; Taxildaris, K.; Katrabasas, I.; et al. Time-Course of Changes in Oxidative Stress and Antioxidant Status Responses Following a Soccer Game. J. Strength Cond. Res. 2010, 24, 3278–3286. [Google Scholar] [CrossRef] [PubMed]
- Piñeda, A.; González, Y.; Álvarez, P.; Villareal, C. Selección y análisis de ecuaciones antropométricas para el cálculo de la composición corporal en adultos. Rev. Ing. Matemáticas Cienc. Inf. 2017, 4, 47–56. [Google Scholar] [CrossRef]
- Mayorga-Vega, D.; Aguilar-Soto, P.; Viciana, J. Criterion-Related Validity of the 20-M Shuttle Run Test for Estimating Cardiorespiratory Fitness: A Meta-Analysis. J. Sport Sci. Med. 2015, 14, 536–547. [Google Scholar]
- Paradisis, G.P.; Zacharogiannis, E.; Mandila, D.; Smirtiotou, A.; Argeitaki, P.; Cooke, C.B. Multi-Stage 20-m Shuttle Run Fitness Test, Maximal Oxygen Uptake and Velocity at Maximal Oxygen Uptake. J. Hum. Kinet. 2014, 41, 81–87. [Google Scholar] [CrossRef]
- National Institutes of Health. Adult Treatment Panel III. ATP III Guidelines At-A-Glance Quick Desk Reference; NIH Publication: Bethesda, MD, USA, 2001. [Google Scholar]
- Salas-Salvadó, J.; Rubio, M.; Moreno, B.; Grupo Colaborativo SEEDO. Consenso SEEDO 2007 para la evaluación del sobrepeso y la obesidad y el establecimiento de criterios de intervención terapéutica. Med. Clin. 2007, 128, 184–196. [Google Scholar] [CrossRef]
- Slimani, M.; Znazen, H.; Hammami, A.; Bragazzi, N.L. Comparison of body fat percentage of male soccer players of different competitive levels, playing positions and age groups: A meta-analysis. J. Sports Med. Phys. Fit. 2017, 58, 857–866. [Google Scholar]
- Leão, C.; Camões, M.; Clemente, F.M.; Nikolaidis, P.T.; Lima, R.; Bezerra, P.; Rosemann, T.; Knechtle, B. Anthropometric Profile of Soccer Players as a Determinant of Position Specificity and Methodological Issues of Body Composition Estimation. Int. J. Environ. Res. Public Health 2019, 16, 2386. [Google Scholar] [CrossRef]
- Cardona, D.M.G.; Muñoz, O.E.S.; Arismendy, C.E.C.; Cortés, B.R. Perfil lipídico, antropométrico y condición física de estudiantes deportistas universitarios. Univ. Salud 2017, 19, 267. [Google Scholar] [CrossRef]
- Gil, J.; Verdoy, P. Caracterización de deportistas universitarios de fútbol y baloncesto: Antropometría y composición corporal. e-balonmano.com. Rev. Cienc. Deport. 2011, 7, 39–51. [Google Scholar]
- Apostolidis, N.; Bogdanis, G.C.; Kostopoulos, N.; Souglis, A.; Papadopoulos, C. Changes in the Lipid Profile of Elite Basketball and Soccer Players After a Match. Res. Sports Med. 2014, 22, 100–110. [Google Scholar] [CrossRef]
- Trefts, E.; Williams, A.S.; Wasserman, D.H. Exercise and the Regulation of Hepatic Metabolism. Prog. Mol. Biol. Transl. Sci. 2015, 135, 203–225. [Google Scholar] [CrossRef]
- Pettersson, J.; Hindorf, U.; Persson, P.; Bengtsson, T.; Malmqvist, U.; Werkström, V.; Ekelund, M. Muscular exercise can cause highly pathological liver function tests in healthy men. Br. J. Clin. Pharmacol. 2007, 65, 253–259. [Google Scholar] [CrossRef] [PubMed]
- Sjogren, M. Transaminase Levels and Vigorous Ex. Adv. Hepatol. 2007, 3, 913–914. [Google Scholar]
- Chinedu, I.A.; Chima, I.S.; Emmanuel, O.; Nwabunwanne, O.V.; Chinedu, M.S. Effects of regular exercise on the liver function tests of male subjects in college of health sciences, Nnamdi Azikiwe. Int. J. Curr. Res. Med. Sci. 2018, 4, 73–79. [Google Scholar]
- Yusof, A.; Leithauser, R.M.; Roth, H.J.; Finkernagel, H.; Wilson, M.T.; Beneke, R. Exercise-induced hemolysis is caused by protein modification and most evident during the early phase of an ultraendurance race. J. Appl. Physiol. 2007, 102, 582–586. [Google Scholar] [CrossRef]
- Domínguez, R.; Garnacho-Castaño, M.; Maté-Muñoz, J. Efecto de la hepcidina en el metabolismo del hierro en deportistas. Nutr. Hosp. 2014, 30, 1218–1231. [Google Scholar]
- Lippi, G.; Sanchis-Gomar, F. Epidemiological, biological and clinical update on exercise-induced hemolysis. Ann. Transl. Med. 2019, 7, 270. [Google Scholar] [CrossRef]
- Domínguez, R. Hemólisis en el deporte: Una revisión bibliográfica. Rev. Entren. Deport. 2013, 27, 1–8. [Google Scholar]
- Sim, M.; Dawson, B.; Landers, G.; Trinder, D.; Peeling, P. Iron Regulation in Athletes: Exploring the Menstrual Cycle and Effects of Different Exercise Modalities on Hepcidin Production. Int. J. Sport Nutr. Exerc. Metab. 2014, 24, 177–187. [Google Scholar] [CrossRef]
- Pussieldi, G.; Veneroso, C.; De Paz, J.; Teixeira, M. Respuesta inflamatoria y antiinflamatoria tras el esfuerzo agudo en natación. Rev. Int. Med. Cienc. Act. Fís. Deporte. 2018, 18, 413–421. [Google Scholar]
- Ghafourian, M.; Ashtary-Larky, D.; Chinipardaz, R.; Eskandary, N.; Mehavaran, M. Inflammatory Biomarkers’ Response to Two Different Intensities of a Single Bout Exercise Among Soccer Players. Iran. Red Crescent Med J. 2016, 18, e21498. [Google Scholar] [CrossRef] [PubMed]
- Stumpf, C.; Simon, M.; Wilhelm, M.; Zimmermann, S.; Rost, C.; Achenbach, S.; Brem, M.H. Left atrial remodeling, early repolarization pattern, and inflammatory cytokines in professional soccer players. J. Cardiol. 2016, 68, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Jamurtas, A.Z.; Fatouros, I.G.; Deli, C.K.; Georgakouli, K.; Poulios, A.; Draganidis, D.; Papanikolaou, K.; Tsimeas, P.; Chatzinikolaou, A.; Avloniti, A.; et al. The Effects of Acute Low-Volume HIIT and Aerobic Exercise on Leukocyte Count and Redox Status. J. Sports Sci. Med. 2018, 17, 501–508. [Google Scholar] [PubMed]
- Knez, W.; Jenkins, D.; Coombes, J. The Effect of an Increased Training Volume on Oxidative Stress. Int. J. Sports Med. 2013, 35, 8–13. [Google Scholar] [CrossRef]
- Peternelj, T.; Coombes, J.S. Antioxidant Supplementation during Exercise Training: Beneficial or Detrimental? Sport Med. 2011, 41, 1043–1069. [Google Scholar] [CrossRef]
- Silva, J.R.; Rebelo, A.; Marques, F.; Pereira, L.; Seabra, A.; Ascensão, A. Biochemical impact of socce: An analysis of hormonal, muscle damage, and redox markers during the season. Appl. Physiol. Nutr. Metab. 2014, 39, 432–438. [Google Scholar] [CrossRef]
- Inal, M.; Akyüz, F.; Turgut, A.; Getsfrid, W.M. Effect of aerobic and anaerobic metabolism on free radical generation swimmers. Med. Sci. Sports Exerc. 2001, 33, 564–567. [Google Scholar] [CrossRef]
- Gaeta, L.; Tozzi, G.; Pastore, A.; Federici, G.; Bertini, E.; Piemonte, F. Determination of superoxide dismutase and glutathione peroxidase activities in blood of healthy pediatric subjects. Clin. Chim. Acta 2002, 322, 117–120. [Google Scholar] [CrossRef]
- Zivkovic, V.; Lazarevic, P.; Djuric, D.; Čubrilo, D.; Macura, M.; Vuletic, M.; Barudzic, N.; Nešić, M.; Jakovljevic, V. Alteration in basal redox state of young male soccer players after a six-month training programme. Acta Physiol. Hung. 2013, 100, 64–76. [Google Scholar] [CrossRef] [PubMed]
- Viada Pupo, E.; Gómez Robles, L.; Campaña Marrero, I.R. Estrés oxidativo. Correo Científico Médico 2017, 21, 171–186. [Google Scholar]
- Zoppi, C.C.; Hohl, R.; Silva, F.C.; Lazarim, F.L.; Neto, J.A.; Stancanneli, M.; Macedo, D.V. Vitamin C and E Supplementation Effects in Professional Soccer Players Under Regular Training. J. Int. Soc. Sports Nutr. 2006, 3, 37–44. [Google Scholar] [CrossRef]
- Ekun, O.A.; Emiabata, A.F.; Abiodun, O.C.; Ogidi, N.O.; O Adefolaju, F.; Ekun, O.O. Effects of football sporting activity on renal and liver functions among young undergraduate students of a Nigerian tertiary institution. BMJ Open Sport Exerc. Med. 2017, 3, e000223. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, D.; Sluik, D.; Rokling-Andersen, M.; Anderssen, S.; Drevon, C. Association of 1-y changes in diet pattern with cardiovascular disease risk factors and adipokines: Results from the 1-y randomized Oslo Diet and Exercise Study. Am. J. Clin. Nutr. 2009, 89, 509–517. [Google Scholar] [CrossRef]
- Baird, M.; Graham, S.; Baker, J.; Bickerstaff, G. Creatine-kinase- and exercise-related muscle damage implications for muscle performance and recovery. J. Nutr. Metab. 2012, 2012, 13. [Google Scholar] [CrossRef] [PubMed]
- Fisher-Wellman, K.; Bell, H.K.; Bloomer, R.J. Oxidative Stress and Antioxidant Defense Mechanisms Linked to Exercise During Cardiopulmonary and Metabolic Disorders. Oxidative Med. Cell. Longev. 2009, 2, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Wilk, M.; Michalczyk, M.; Gołaś, A.; Krzysztofik, M.; Maszczyk, A.; Zając, A. Endocrine responses following exhaustive strength exercise with and without the use of protein and protein-carbohydrate supplements. Biol. Sport 2018, 35, 399–405. [Google Scholar] [CrossRef] [PubMed]
| Variables | n = 22 |
|---|---|
| BMI (kg/m2) | 23.66 ± 2.14 |
| Body fat (%) | 8.90 ± 0.93 |
| Muscle percentage | 46.84 ± 3.8 |
| VO2max (mL/kg/min) | 51.66 ± 2.24 |
| VARIABLES | D1 | D14 | p-Value |
|---|---|---|---|
| TC (mg/dL) | 153.80 ± 28.20 | 163.52 ± 32.3 | 0.495 |
| HDL (mg/dL) | 47.38 ± 7.10 | 48.55 ± 7.90 | 0.622 |
| LDL (mg/dL) | 88.58 ± 20.47 | 93.01 ± 21.56 | 0.666 |
| VLDL (mg/dL) | 16.66 ± 5.09 | 19.86 ± 4.67 | 0.101 |
| TG (mg/dL) | 78.62 ± 21.30 | 94.42 ± 21.49 | 0.051 |
| AI | 2.71 ± 0.84 | 2.90 ± 0.83 | 0.503 |
| VARIABLES | D1 | D4 | D7 | D14 | p-Value | |
|---|---|---|---|---|---|---|
| GPT (U/L) | 16.26 ± 3.2 | 17.49 ±2.30 | 21.27 ± 3.60 | 17.74 ± 3.50 | D1 vs. D4 | 0.563 |
| D4 vs. D7 | 0.076 | |||||
| D7 vs. D14 | 0.400 | |||||
| D1 vs. D14 | 0.435 | |||||
| GOT (U/L) | 19.50 ± 2.78 | 21.06 ± 3.10 | 24.37 ± 4.26 | 22.55 ±3.15 | D1 vs. D4 | 0.586 |
| D4 vs. D7 | 0.086 | |||||
| D7 vs. D14 | 0.290 | |||||
| D1 vs. D14 | 0.296 | |||||
| Hemolysis (%) | 80.24 ± 3.90 | 81.74 ±3.60 | 81.95 ± 2.54 | 81.25 ± 3.10 | D1 vs. D4 | 0.204 |
| D4 vs. D7 | 0.833 | |||||
| D7 vs. D14 | 0.429 | |||||
| D1 vs. D14 | 0.107 | |||||
| CAT (U/mL) | 28.64 ± 3.50 | 29.39 ±0.30 | 29.31 ± 0.26 | 29.4 ± 0.15 | D1 vs. D4 | 0.341 |
| D4 vs. D7 | 0.403 | |||||
| D7 vs. D14 | 0.217 | |||||
| D1 vs. D14 | 0.345 | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-Cardona, D.; Landázuri, P.; Sánchez-Muñoz, O. Effect of a Shock Micro-Cycle on Biochemical Markers in University Soccer Players. Int. J. Environ. Res. Public Health 2021, 18, 3581. https://doi.org/10.3390/ijerph18073581
García-Cardona D, Landázuri P, Sánchez-Muñoz O. Effect of a Shock Micro-Cycle on Biochemical Markers in University Soccer Players. International Journal of Environmental Research and Public Health. 2021; 18(7):3581. https://doi.org/10.3390/ijerph18073581
Chicago/Turabian StyleGarcía-Cardona, Diana, Patricia Landázuri, and Oscar Sánchez-Muñoz. 2021. "Effect of a Shock Micro-Cycle on Biochemical Markers in University Soccer Players" International Journal of Environmental Research and Public Health 18, no. 7: 3581. https://doi.org/10.3390/ijerph18073581
APA StyleGarcía-Cardona, D., Landázuri, P., & Sánchez-Muñoz, O. (2021). Effect of a Shock Micro-Cycle on Biochemical Markers in University Soccer Players. International Journal of Environmental Research and Public Health, 18(7), 3581. https://doi.org/10.3390/ijerph18073581








