Synthesis and Characterization of Na-Zeolites from Textile Waste Ash and Its Application for Removal of Lead (Pb) from Wastewater
Abstract
1. Introduction
2. Materials and Methods
2.1. Pretreatment and Physicochemical Properties of RFA
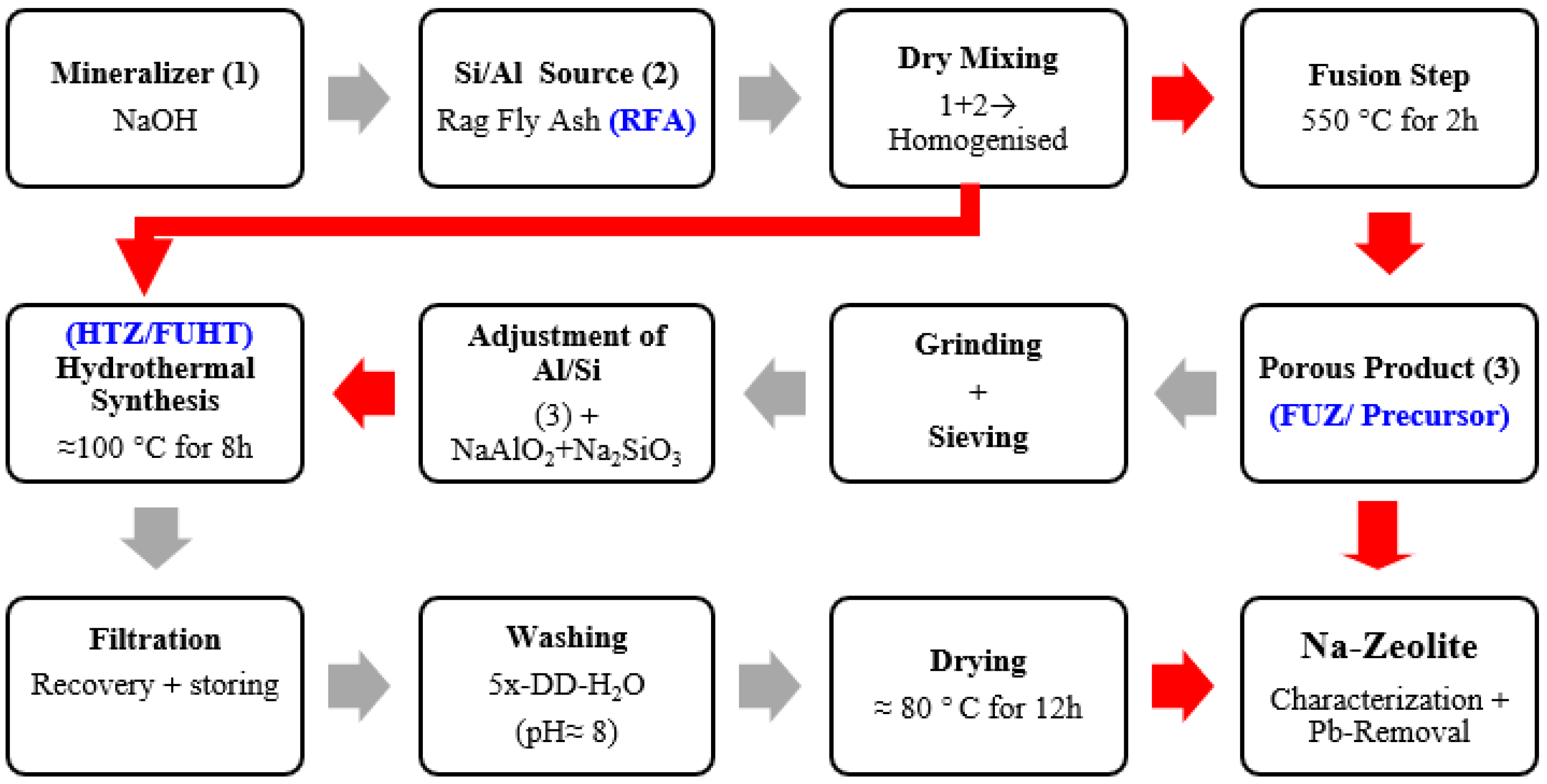
2.2. NaOH-Activated Synthesis of Na-Zeolites
2.3. Characterization of RFA and Na-Zeolites
2.4. Sorption of Toxic Metal (Pb-II) from Wastewater
3. Results and Discussions
3.1. Physicochemical Characteristics of RFA and Na-Zeolites
3.2. Development Process and Crystal Growth Mechanism of Na-Zeolites
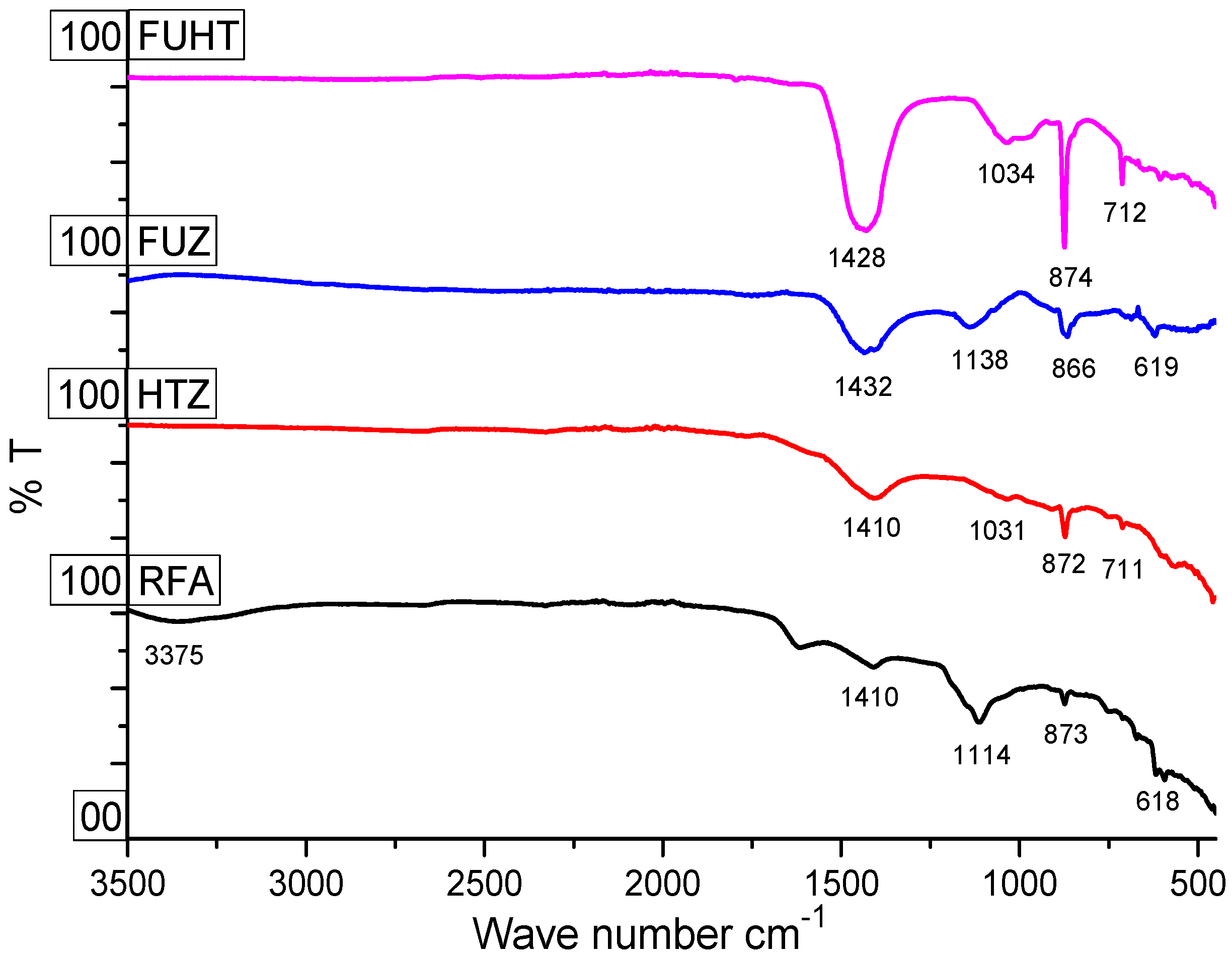
3.3. Surface Chemistry and Functionality of RFA and Na-Zeolites
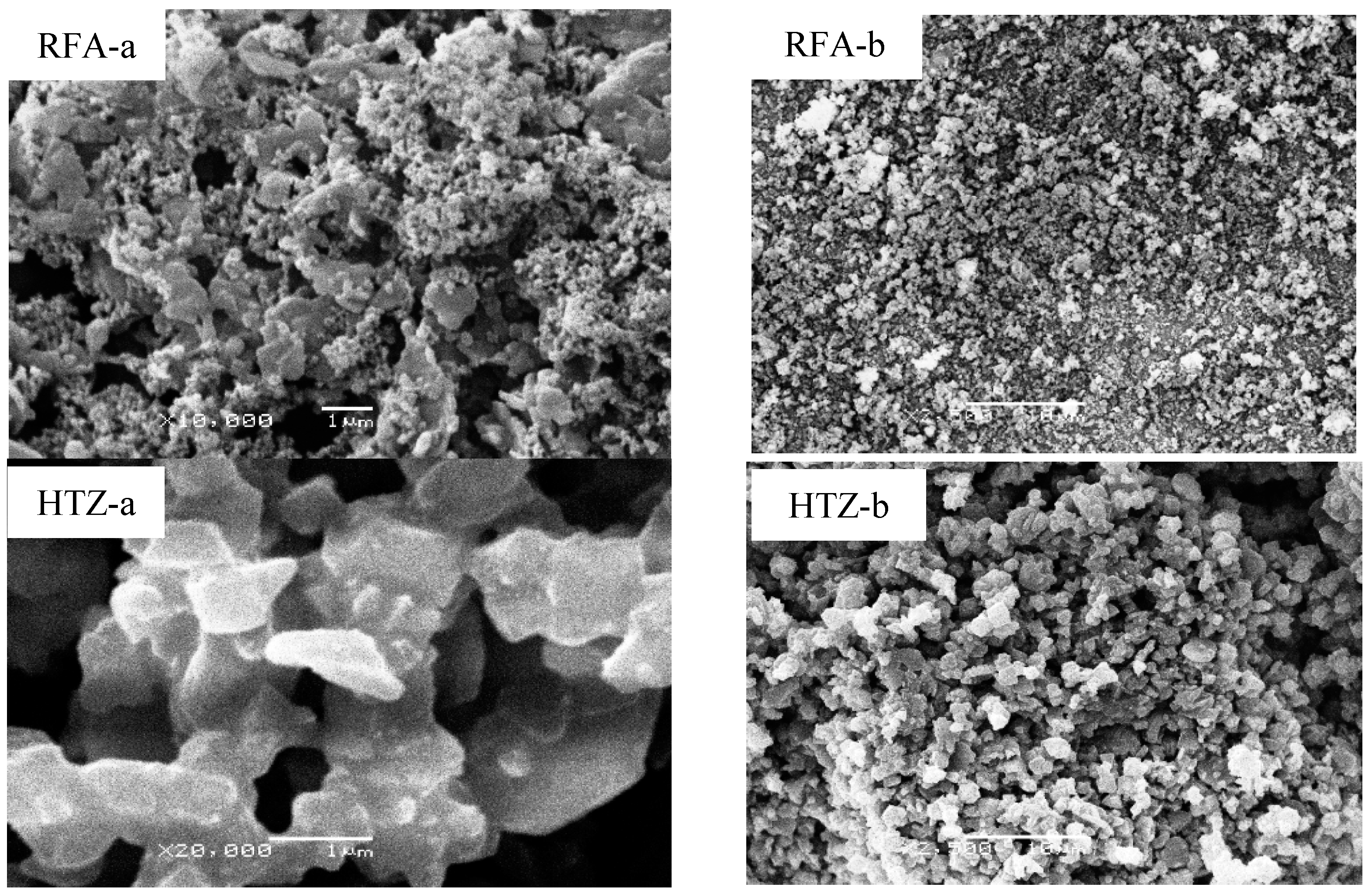
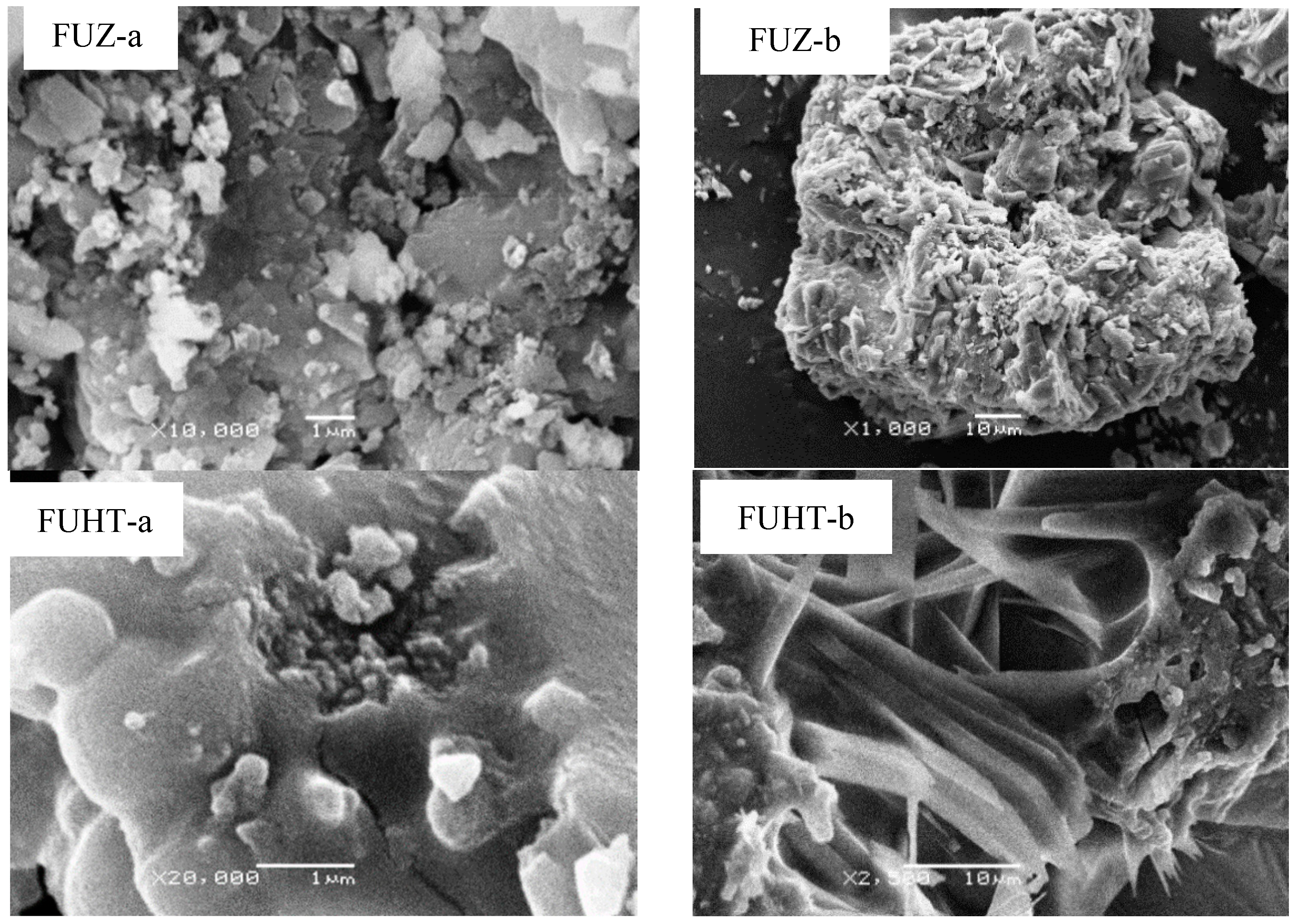
3.4. SEM Micrographs
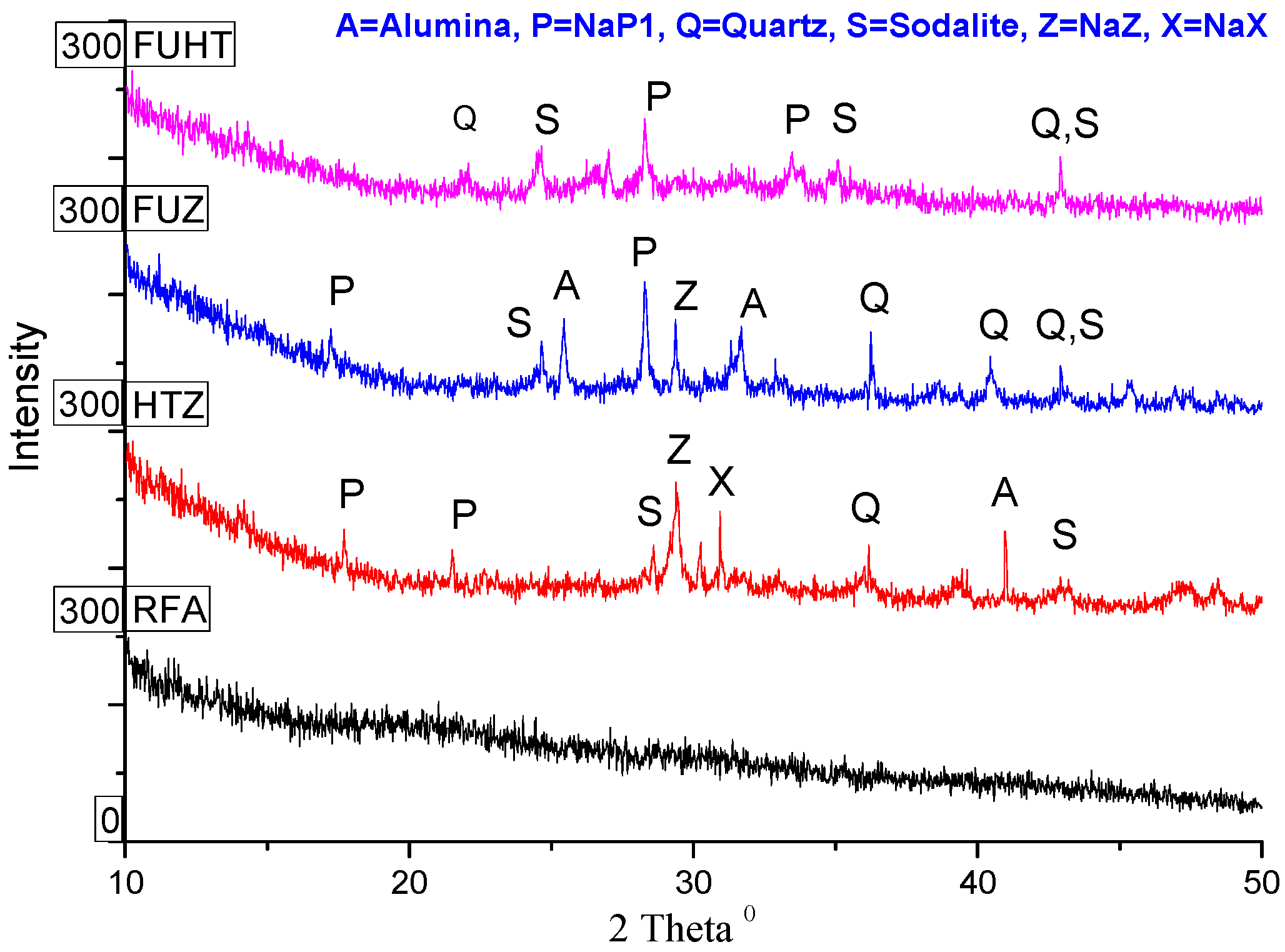
3.5. XRD Analysis
3.6. Applications of Fly-Ash-Based Na-Zeolites for the Removal of Potentially Toxic Metals
3.6.1. Effect of pH on Removal Efficiency of Pb (II) by Na-Zeolites
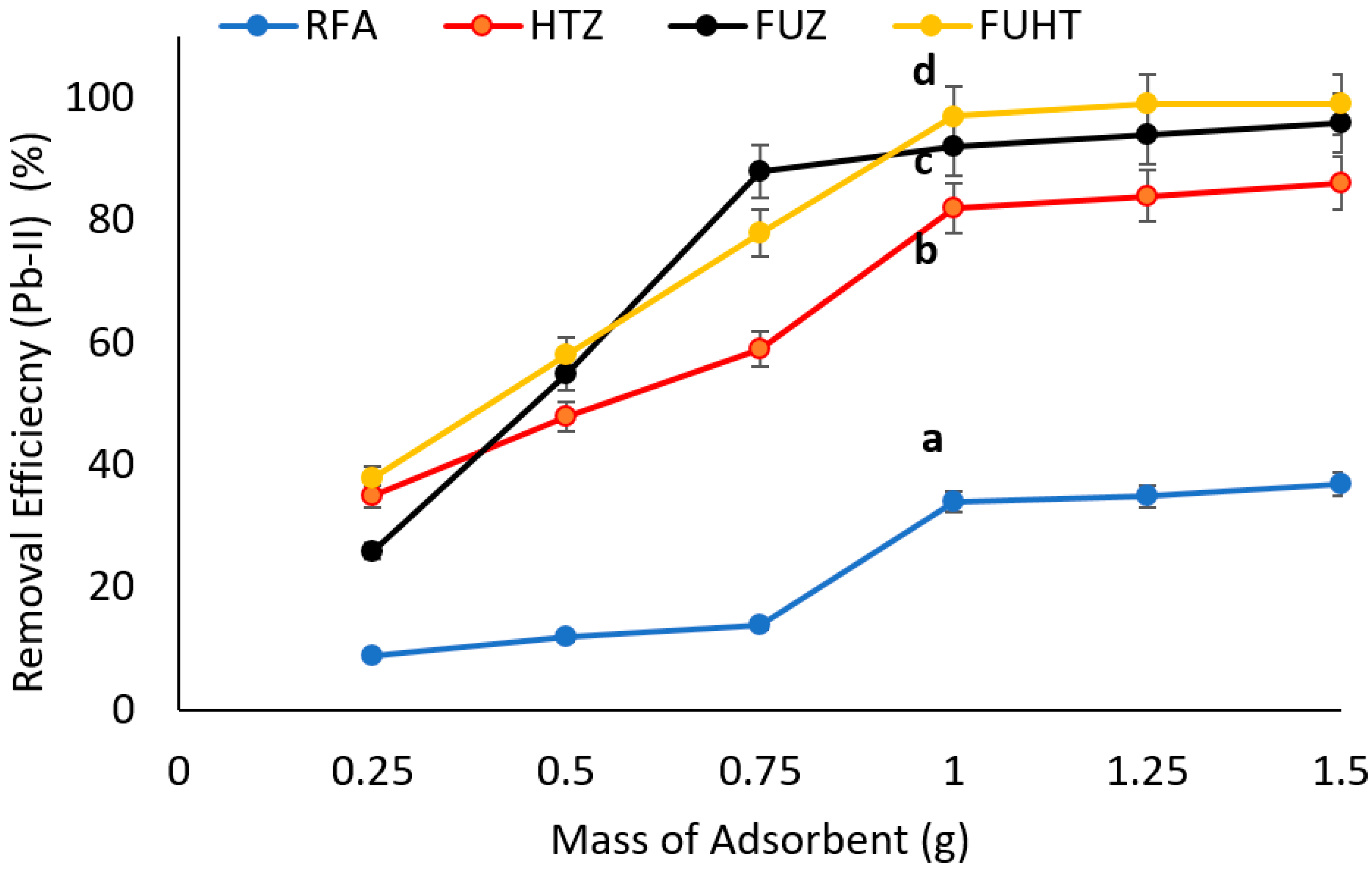
3.6.2. Effect of Mass of Adsorbents on Removal Efficiency of Pb (II) by Na-Zeolites
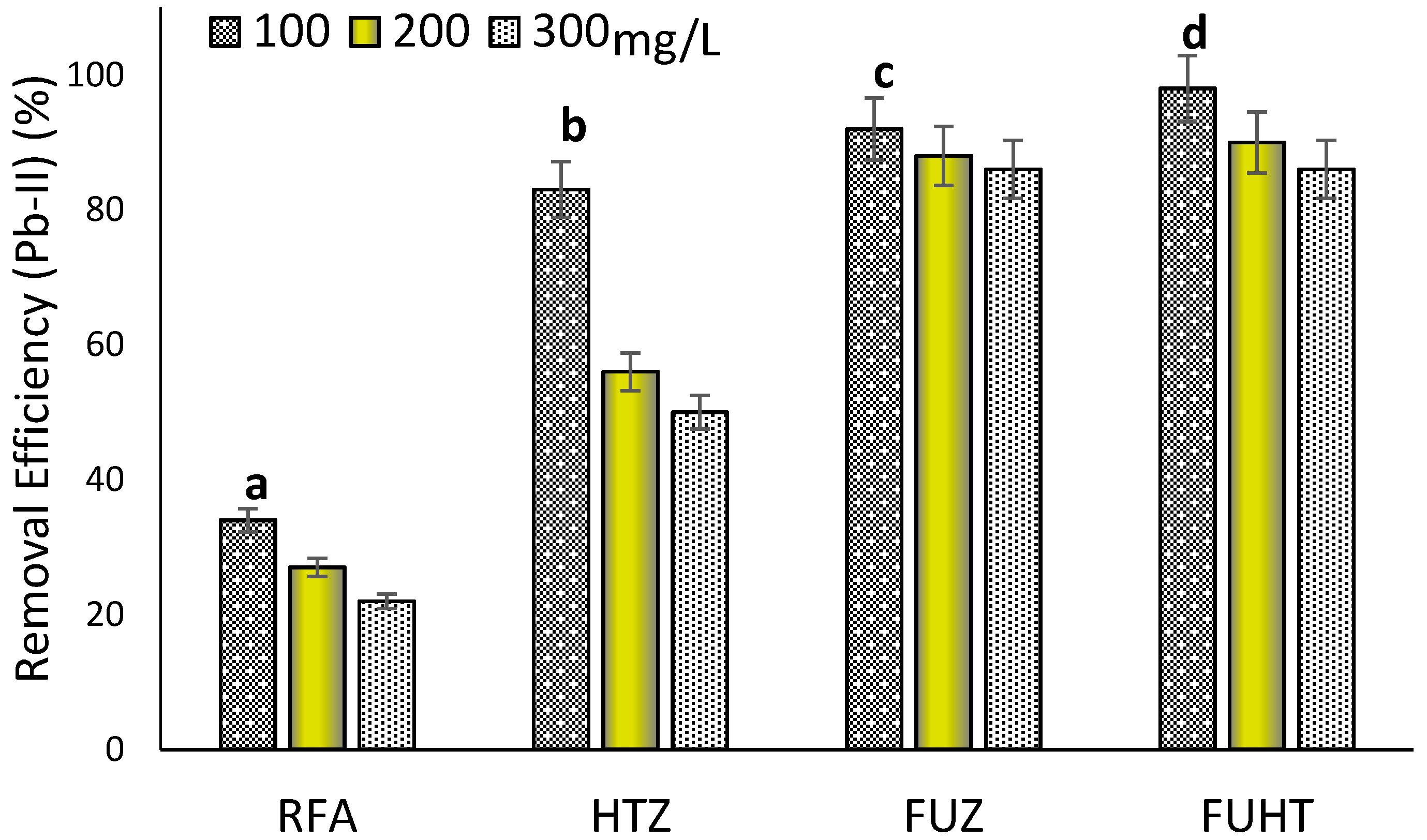
3.6.3. Effect of Metal Concentration on Removal Efficiency of Pb (II) by Na-Zeolites
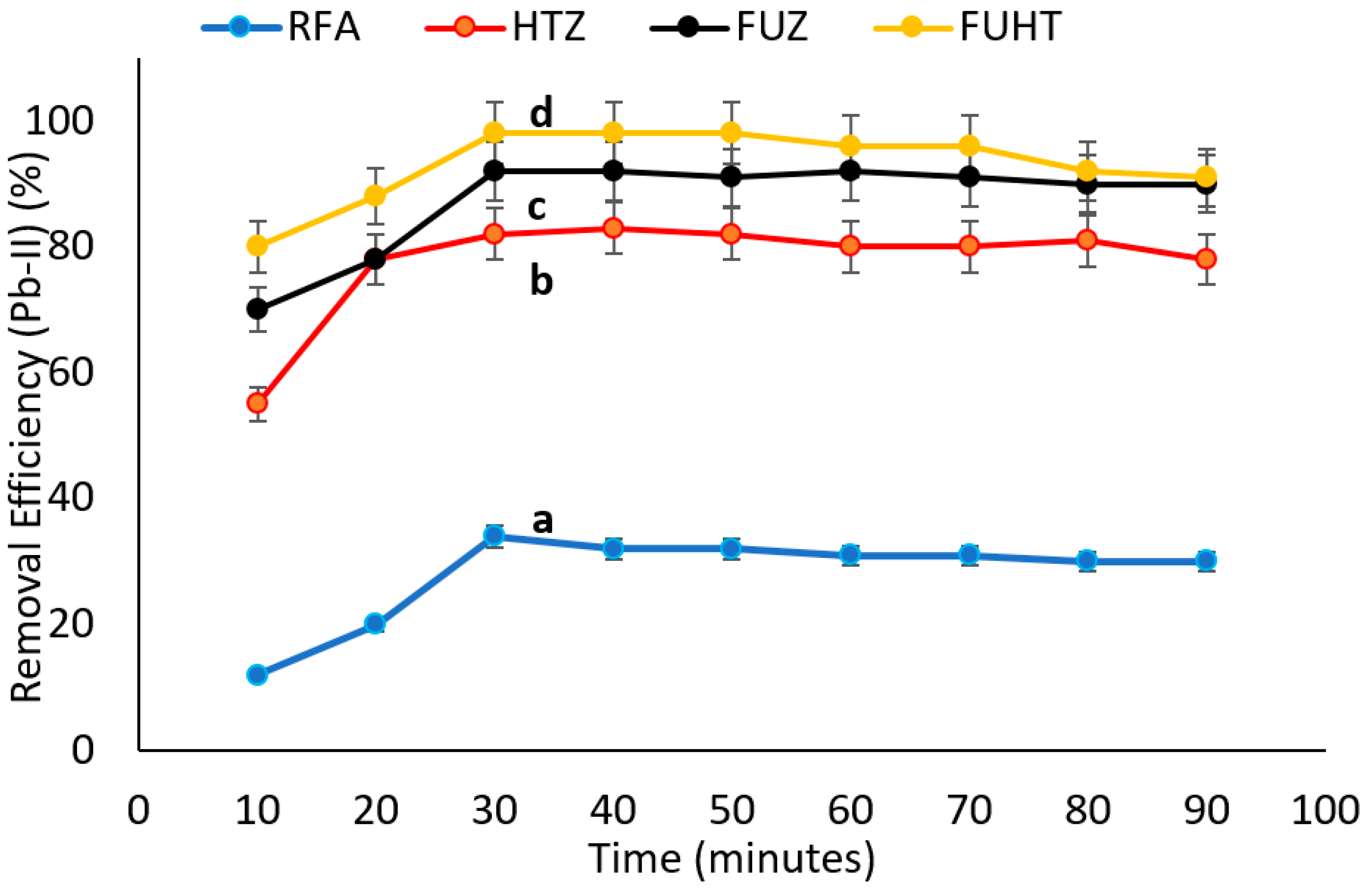
3.6.4. Effect of Time on Removal Efficiency of Pb (II) by Na-Zeolites
3.7. Adsorption Isotherms
3.8. Kinetics Studies
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- The Fiber Year Consulting. The Fiber Year 2015, World Survey on Textiles and Nonwoven. Issue 15; Oerlikon Textile: Pfafficon, Switzerland, 2015. [Google Scholar]
- Thomas, B.; Andreas, A. The Fiber Year 2009/10. World Survey on Textile and Nonwovens Industry (Issue 10); Oerlikon Textile: Pfafficon, Switzerland, 2010. [Google Scholar]
- Qi, L.; Xu, J.; Liu, K. Porous sound-absorbing materials prepared from fly ash. Environ. Sci. Pollut. Res. 2019, 26, 22264–22272. [Google Scholar] [CrossRef] [PubMed]
- United States Environmental Protection Agency (US EPA). Mercury Study Report to Congress; United States Environmental Protection Agency: Washington, DC, USA, 1997; Volume 3.
- Lim, S.R.; Schoenung, J.M. Human health and ecological toxicity potentials due to heavy metal content in waste electronic devices with flat panel displays. J. Hazard. Mater. 2010, 177, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Mukesh, K.R.; Kumar, P.; Singh, M.; Singh, A. Toxic effect of heavy metals in livestock health. Veterin. World 2008, 1, 28–30. [Google Scholar]
- Pereira, F.V.; Gurgel, L.V.A.; Gil, L.F. Removal of Zn-2þ from aqueous single metal solutions and electroplating wastewater with wood sawdust and sugarcane bagasse modified with EDTA di-Anhydride (EDTAD). J. Hazard. Mater. 2010, 176, 856–863. [Google Scholar] [CrossRef] [PubMed]
- Barbieri, L.; Bonamartin, A.C.; Lancellotti, I. Alkaline and alkaline earth silicate glasses and glass-ceramics from municipal and industrial wastes. J. Eur. Ceram. Soc. 2000, 20, 2477–2483. [Google Scholar] [CrossRef]
- Rawat, M.; Ramanathan, A.L.; Subramanian, V. Quantification and distribution of heavy metals from small scale industrial areas of Kanpur City, India. J. Hazard. Mater. 2009, 172, 1145–1149. [Google Scholar] [CrossRef]
- Attari, M.; Bukhari, S.S.; Kazemian, H.; Rohani, S. A low-cost adsorbent from coal fly ash for mercury removal from industrial wastewater. J. Environ. Chem. Eng. 2017, 5, 391–399. [Google Scholar] [CrossRef]
- USFDA. United States Food and Drug Administration. Q3D Elemental Impurities Guidance for Industry (Report); United States Department of Health and Human Services: Washington, DC, USA, 2020; p. 41, Retrieved on 15 February 2017.
- Emsley, J. Nature’s Building Blocks: An. A-Z Guide to the Elements; Oxford University Press: Oxford, UK, 2011; ISBN 978-0-19-960563-7. [Google Scholar]
- Rivera, J.M.; Rincón, S.; Youssef, C.B.; Zepeda, A. Highly Efficient Adsorption of Aqueous Pb (II) with Mesoporous Metal-Organic Framework-5: An Equilibrium and Kinetic Study. J. Nanomat. 2016. [Google Scholar] [CrossRef]
- Moyo, M.; Chikazaza, L.; Chomunorwa Nyamunda, B.; Guyo, U. Adsorption Batch Studies on the Removal of Pb (II) Using Maize Tassel Based Activated Carbon. J. Chem. 2013. [Google Scholar] [CrossRef]
- Kulkarni, S.J. Studies and Research on Mercury Removal from Water—A Review. Int. J. Res. 2015, 2, 221–225. [Google Scholar]
- Nabi, S.A.; Akhtar, A.; Khan, M.D.A.; Khan, M.A. Synthesis, characterization and electrical conductivity of polyaniline-Sn(IV)tungstophosphate hybrid cation exchanger: Analytical application for removal of heavy metal ions from wastewater. Desalination 2014, 340, 73–83. [Google Scholar] [CrossRef]
- Shahadat, M.; Teng, T.T.; Rafatullah, M.; Arshad, M. Titanium based nanocomposite materials: A review of recent advances and perspectives. Coll. Surf. B Biointerfaces 2015, 126, 121–137. [Google Scholar] [CrossRef]
- Ahmad, R.; Kumar, R.; Haseeb, S. Adsorption of Cu2+ from aqueous solution onto iron oxide coated eggshell powder: Evaluation of equilibrium, isotherms, kinetics, and regeneration capacity. Arab J. Chem. 2012, 5, 353–359. [Google Scholar] [CrossRef]
- Kurniawan, T.A.; Chan, G.Y.S.; Hung, L.W.; Babel, S. Physico–chemical treatment techniques for wastewater laden with heavy metals. Chem. Eng. J. 2006, 118, 83–98. [Google Scholar] [CrossRef]
- Shahadat, M.; Nabi, S.A.; Bushra, R.; Raeissi, A.S.; Umar, K.; Ansari, M.O. Synthesis, characterization, photolytic degradation, electrical conductivity and applications of nanocomposite adsorbent for the treatment of pollutants. RSC Adv. 2012, 2, 7207–7220. [Google Scholar] [CrossRef]
- Khan, A.A. Innamuddin. Application of Hg(II) sensitive polyaniline Sn (IV) phosphate composite cation exchange material in determination of Hg2+ from aqueous solutions and in making ion selective membrane electrode. Saens Actuators B 2006, 120, 10–18. [Google Scholar] [CrossRef]
- Querol, X.; Moreno, N.; Alastuey, A.; Juan, R.; Andres, J.M.; Lopez-Soler, A.; Ayora, C.; Medinaceli, A.; Valero, A. Synthesis of high ion exchange zeolite from coal fly ash. Geol. Acta 2007, 5, 49–57. [Google Scholar]
- Bukhari, S.S.; Rohani, S.; Kazemian, H. Effect of ultrasound energy on the zeolitization of chemical extracts from fused coal fly ash. Ultrason. Sonochem. 2016, 28, 47–53. [Google Scholar] [CrossRef]
- Visa, M.; Chelaru, A.M. Hydrothermally modified fly ash for heavy metals and dyes removal in advanced wastewater treatment. Appl. Surf. Sci. 2014, 303, 14–22. [Google Scholar] [CrossRef]
- Solanki, P.; Gupta, V.; Kulshrestha, R. Synthesis of Zeolite from Fly Ash and Removal of Heavy Metal Ions from Newly Synthesized Zeolite. E J. Chem. 2010, 7, 1200–1205. [Google Scholar] [CrossRef]
- Ojha, K.; Pradhan, N.C.; Samanta, A.N. Zeolite from fly ash: Synthesis and characterization. Bull. Mater. Sci. 2004, 27, 555–564. [Google Scholar] [CrossRef]
- Jha, B.; Singh, D.N. Advanced Structured Materials, Fly Ash Zeolites Innovations, Applications and Directions. Springer: Singapore, 2016. [Google Scholar]
- Shaobin, W.; Zhu, Z.H. Sonochemical treatment of fly ash for dye removal from wastewater. J. Hazard. Mater. (B) 2005, 126, 91–95. [Google Scholar]
- Ryan, J.; Estefan, G.; Rashid, A. Soil and Plant Analysis Laboratory Manual, 2nd ed.; International Center for Agricultural Research in the Dry Areas (ICARDA): Aleppo, Syria, 2001. [Google Scholar]
- Inada, M.; Tsujimoto, H.; Eguchi, Y.; Enomoto, N.; Hojo, J. Microwave-assisted zeolite synthesis from coal fly ash in hydrothermal process. Fuel 2005, 84, 1482–1486. [Google Scholar] [CrossRef]
- Belviso, C.; Cavalcante, F.; Lettino, A.; Fiore, A. Effects of ultrasonic treatment on zeolite synthesized from coal fly ash. Ultrason. Sonochem. 2011, 18, 661–668. [Google Scholar] [CrossRef]
- Treacy, M.M.J.; Higgins, J.B. Collection of Simulated XRD Powder Patterns for Zeolites by Stucture Commision of the International Zeolite Association, 5th ed.; Elsevier: Amsterdam, The Netherlands, 2001. [Google Scholar]
- Chester, A.W.; Derouane, E.G. Zeolite Characterization and Catalysis: A tutorial; Springer: New York, NY, USA, 2009. [Google Scholar]
- Somerset, V.S.; Petrik, L.F.; White, L.R.; Klnka, M.J.; Key, D.; Iwuoha, E.I. Alkaline hydrothermal zeolites synthesized from high SiO2 and Al2O3 Co-disposal fly ash filtrates. Fuel 2005, 84, 2324–2329. [Google Scholar] [CrossRef]
- Williams, D.B. Practical Analytical Electron Microscopy in Materials Science; Electron Optics Publishing Group: Mahwah, NJ, USA, 1984. [Google Scholar]
- Nguyen, T.C.; Loganathan, P.; Nguyen, T.V.; Kandasamy, J.; Naidu, R.; Vigneswaran, S. Adsorptive removal of five heavy metals from water using blast furnace slag and fly ash. Environ. Sci. Pollut. Res. 2018, 25, 20430–20438. [Google Scholar] [CrossRef]
- Nevin, K.; Singh, D.N. Utilization of fly ash for synthesizing zeolites and determination of its application. J. Environ. Chem Eng. 2016, 4, 1460–1472. [Google Scholar]
- Chunfeng, W.; Jiansheng, L.; Sun, X.; Wang, L.; Xiuyun, S. Evaluation of zeolites synthesized from fly ash as potential adsorbents for wastewater containing heavy metals. J. Environ. Sci. 2009, 21, 127–136. [Google Scholar]
- Down, R.D.; Lehr, J.H. Conductivity analyzers and their application. In Environmental Instrumentation and Analysis Handbook; Wiley: Hoboken, NJ, USA, 2004; pp. 491–510. [Google Scholar]
- Molina, A.; Poole, C. A comparative study using two methods to produce zeolites from fly ash. Min. Eng. 2004, 17, 167–173. [Google Scholar] [CrossRef]
- Querol, X.; Moreno, N.; Umaña, J.T.; Alastuey, A.; Hernández, E.; Lopez-Soler, A.; Plana, F. Synthesis of zeolites from coal fly ash: An overview. Inter. J. Coal Geol. 2020, 50, 413–423. [Google Scholar] [CrossRef]
- Zhao, X.S.; Lu, G.Q.; Zhu, H.Y. Effects of ageing and seeding on the formation of zeolite Y from coal fly ash. J. Porous Mat. 1997, 4, 245–251. [Google Scholar] [CrossRef]
- Rayalu, S.S.; Udhoji, J.S.; Munshi, K.N.; Hasan, M.Z. Highly crystalline zeolite—A from fly ash of bituminous and lignite coal combustion. J. Hazard. Mater. 2001, 88, 107–121. [Google Scholar] [CrossRef]
- Franus, W. Characterization of X-type Zeolite Prepared from Coal Fly Ash. Pol. J. Env. Stud. 2012, 21, 337–343. [Google Scholar]
- Hower, J.C. Petrographic examination of coal-combustion fly ash. Int. J. Coal Geol. 2012, 92, 90–97. [Google Scholar] [CrossRef]
- Steenari, B.M.; Schelander, S.; Lindqvist, O. Chemical and leaching characteristics of ash from combustion of coal, peat and wood in a 12 MW CFB—a comparative study. Fuel 1999, 78, 249–258. [Google Scholar] [CrossRef]
- Tong, L.; Tang, Y.; Wang, F.; Hu, B.; Shi, P.; Hu, Q. Investigation of controlling factors on toxic metal leaching behavior in municipal solid wastes incineration fly ash. Environ. Sci. Pollut. Res. 2019, 26, 29316–29326. [Google Scholar] [CrossRef]
- Maroto-Valer, M.M.; Taulbee, D.N.; Hower, J.C. Novel separation of the differing forms of unburned carbon present in fly ash using density gradient centrifugation. Energy Fuels 1999, 13, 947–953. [Google Scholar] [CrossRef]
- Bukhari, S.S.; Behin, J.; Kazemian, H.; Rohani, S. Conversion of coal fly ash to zeolite utilizing microwave and ultrasound energies. A review. Fuel 2015, 140, 250–266. [Google Scholar] [CrossRef]
- Ahmaruzzaman, M. A review on the utilization of fly ash. Prog. Energy Combustion Sci. 2010, 36, 327–363. [Google Scholar] [CrossRef]
- Maruyama, N.; Yamamoto, H.; Shibata, J. Zeolite synthesis from coal fly ash by hydrothermal reaction using various alkali sources. J. Chem. Tech. Bio. Tech. 2002, 77, 280–286. [Google Scholar] [CrossRef]
- Channabasavaraj, W.; Reddy, R. A review on characterization and application of fly ash zeolites. Int. J. Dev. Res. 2017, 7, 14294–14300. [Google Scholar]
- Wajima, T.; Sugawara, K. Material conversion from various incinerated ashes using alkali fusion method. Int. J. Soc. Mater. Eng. Resour. 2010, 17, 47–52. [Google Scholar] [CrossRef][Green Version]
- Shigemoto, M.; Hayashi, H.; Miyaura, K. Selective formation of Na-X zeolite from fly ash by fusion with sodium hydroxide prior to hydrothermal reaction. J. Mater. Sci. 1993, 28, 4781–4786. [Google Scholar] [CrossRef]
- Sakthivel, T.; Reid, D.; Goldstein, I.; Hench, L.; Seal, S. Hydrophobic high surface area zeolites derived from fly ash for oil spill remediation. Environ. Sci. Technol. 2013, 47, 5843–5850. [Google Scholar] [CrossRef]
- Fernández-Jiménez, A.; Palomo, A. Composition and microstructure of alkali activated fly ash binder: Effect of the activator. Cem. Conc. Res. 2005, 35, 1984–1992. [Google Scholar] [CrossRef]
- Fernández-Jiménez, A.; Palomo, A.; Criado, M. Microstructure development of alkali-activated fly ash cement: A descriptive model. Cem. Conc. Res. 2005, 32, 1204–1209. [Google Scholar] [CrossRef]
- Juan, R.; Hernández, S.; Andrés, J.M.; Ruiz, C. Synthesis of granular zeolitic materials with high cation exchange capacity from agglomerated coal fly ash. Fuel 2007, 86, 1811–1821. [Google Scholar] [CrossRef]
- Jansen, J.C. The preparation of molecular sieves. In Introduction to Zeolite Science and Practice; van Bekkum, H., Flanigen, E.M., Jansen, J.C., Eds.; Elsevier: Amsterdam, Netherlands, 1991; p. 754. [Google Scholar]
- Baerlocher, C.; McCusker, L.B.; Olson, D.H. Atlas of Zeolite Framework Types; Structure Commission of the International Zeolite Association; Elsevier: Zurich, Switzerland, 2007. [Google Scholar]
- Mumpton, F.A. Using zeolites in agriculture. In Innovative Biological Technologies for Lesser Developed Countries; Workshop Proceedings, Office of Technology Assessment, US Congress: Washington, DC, USA, 1985; pp. 125–158. [Google Scholar]
- Shams, K.; Mirmohammadi, S.J. Preparation of 5A zeolite monolith granular extrudes using kaolin: Investigation of the effect of binder on sieving/adsorption properties using a mixture of linear and branched paraffin hydrocarbons. Micro. Meso. Mater. 2007, 106, 268–277. [Google Scholar] [CrossRef]
- Lee, Y.R.; Soe, J.T.; Zhang, S.; Ahn, J.W.; Park, M.B.; Ahn, W.S. Synthesis of nanoporous materials via recycling coal fly ash and other solid wastes: A mini review. Chem. Eng. J. 2017, 317, 821–843. [Google Scholar] [CrossRef]
- Hui, K.S.; Chao, C.Y.H.; Kot, S.C. Removal of mixed heavy metal ions in wastewater by zeolite 4A and residual products from recycled coal fly ash. J. Hazard. Mater. 2005, 127, 89–101. [Google Scholar] [CrossRef]
- Murayama, N.; Yamamoto, H.; Shibata, J. Mechanism of zeolite synthesis from coal fly ash by alkali hydrothermal reaction. Int. J. Miner. Process. 2002, 64, 1–17. [Google Scholar] [CrossRef]
- Gordina, N.E.; Prokof’ev, V.Y.; Kul’pina, Y.N.; Petuhova, N.V.; Gazahova, S.I.; Hmylova, O.E. Effect of ultrasound on the synthesis of low-modulus zeolites from a metakaolin. Ultrason. Sonochem. 2016, 33, 210–219. [Google Scholar] [CrossRef]
- Sitarz, M.; Mozgawa, W.; Handke, M. Rings in the structure of silicate glasses. J. Mol. Struct. 1999, 281, 511–512. [Google Scholar] [CrossRef]
- Raghavendra, S.C.; Khasim, S.; Revanasiddappa, M.; Prasad, M.A.; Kulkarni, A.B. Synthesis, characterization and low frequency a.c. conduction of polyaniline/fly ash composites. Bull. Mater. Sci. 2003, 26, 733–739. [Google Scholar] [CrossRef]
- Jha, B.; Singh, D.N. Synthesis of higher grade fly ash zeolites X from fly ash via three-step fusion. Mater. Perform. Charact. 2013, 2, 1–12. [Google Scholar] [CrossRef]
- Belviso, C.; Cavalcante, F.; Ragone, P.; Fiore, S. Immobilization of Zn and Pb in polluted soil by in situ crystallization zeolites from fly ash. Water Air Soil Pollut. 2012, 223, 5357–5364. [Google Scholar] [CrossRef]
- Lanzafame, P.; Temi, D.M.; Perathoner, S.; Spadaro, A.N.; Centi, G. Direct conversion of cellulose to glucose and valuable intermediates in mild reaction conditions over solid acid catalysts. Catal. Today. 2012, 179, 178–184. [Google Scholar] [CrossRef]
- Gualtieri, M. Synthesis and Characterization of Zeolite Films and Membranes. Ph.D. Thesis, Luleå University of Technology, Luleå, Sweden, 2006; p. 95. [Google Scholar]
- Kokotailo, G.T.; Fyfe, C.A. Zeolite structure analysis with powder x-ray diffraction and solid-state NMR techniques. Rigaku J. 1995, 12, 3–10. [Google Scholar]
- Querol, X.; Umana, J.C.; Plana, F.; Alastuey, A.; López-Soler, A.; Medinaceli, A.; Valero, A.; Domingo, M.J.; Rojo, E.G. Synthesis of zeolites from fly ash at pilot plant scale. Examples of potential applications. Fuel 2001, 80, 857–865. [Google Scholar] [CrossRef]
- Belviso, C. Ultrasonic vs hydrothermal method: Different approaches to convert fly ash into zeolite. How they affect the stability of synthetic products over time? Ultrason. Sonochem. 2018, 43, 9–14. [Google Scholar] [CrossRef]
- Breck, D.W. Zeolites Molecular Sieves, Chemistry and Use; Wiley: New York, NY, USA, 1974; pp. 771–779. [Google Scholar]
- Nascimento, M.; Soares, P.S.M.; Souza, D.P.V. Adsorption of heavy metal cations using coal fly ash modified by hydrothermal method. Fuel 2009, 88, 1714–1719. [Google Scholar] [CrossRef]
- Lee, M.G.; Yi, G.; Ahn, B.J.; Roddick, F. Conversion of coal fly ash into zeolite and heavy metal removal characteristics of the products. Korean J. Chem. Eng. 2000, 17, 325–331. [Google Scholar] [CrossRef]
- Qiu, W.; Zheng, Y. Removal of lead, copper, nickel, cobalt, and zinc from water by a cancrinite-type zeolite synthesized from fly ash. Chem. Eng. J. 2009, 14, 483–488. [Google Scholar] [CrossRef]
- Derkowski, A.; Franus, W.; Beran, E.; Czímerová, A. Properties and potential applications of zeolitic materials produced from fly ash using simple method of synthesis. Powder Tech. 2006, 166, 47–54. [Google Scholar] [CrossRef]
- Jha, V.K.; Nagae, M.; Matsuda, M.; Miyake, M. Zeolite formation from coal fly ash and heavy metal ion removal characteristics of thus-obtained Zeolite X in multi-metal systems. J. Environ. Manag. 2009, 90, 2507–2514. [Google Scholar] [CrossRef] [PubMed]
- Kalin, M.; Fyson, A.; Wheeler, W.N. The chemistry of conventional and alternative treatment systems for the neutralization of acid mine drainage. Sci. Total Environ. 2006, 366, 395–408. [Google Scholar] [CrossRef] [PubMed]
- Chaizasith, S.P.; Chaizasith, C. Septhum. Removal of cadmium and nickel from aqueous solutions by adsorption onto treated fly ash from Thailand. J. Sci. Tech. 2006, 11, 13–19. [Google Scholar]
- Ikhsan, J.; Johnson, I.B.B.; Wells, J.D. A comparative study of the adsorption of transition metals on kaolinite. J. Colloid Interface Sci. 1999, 217, 403–410. [Google Scholar] [CrossRef]
- Soni, R.; Shukla, D.P. Synthesis of fly ash based zeolite-reduced graphene oxide composite and its evaluation as an adsorbent for arsenic removal. Chemosphere 2019, 219, 504–509. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.M.; Bomani, J.O.; Matsunaga, H.; Yokoyama, T. Preparation of porous resin loaded with crystalline hydrous zirconium oxide and its application to the removal of arsenic. React. Funct. Polym. 2000, 43, 165–172. [Google Scholar] [CrossRef]
- Lenoble, V.; Laclautre, C.; Deluchat, V.; Serpaud, B.; Bollinger, J.C. Arsenic removal by adsorption on iron (III) phosphate. J. Hazard. Mater. 2005, 123, 262–268. [Google Scholar] [CrossRef]
- Katsoyiannis, I.A.; Zouboulis, A.I. Removal of arsenic from contaminated water sources by sorption onto iron-oxide-coated polymeric materials. Water Res. 2002, 36, 5141–5155. [Google Scholar] [CrossRef]
- Li, X.; Zhang, D.; Sheng, F.; Qing, H. Adsorption characteristics of copper (II), zinc (II) and mercury (II) by four kinds of immobilized fungi residues. Ecotoxicol. Environ. Saf. 2018, 147, 357–366. [Google Scholar] [CrossRef]
- Tan, G.; Yuan, H.; Liu, Y.; Xiao, D. Removal of lead from aqueous solution with native and chemically modified corncob. J. Hazard. Mater. 2010, 174, 740–745. [Google Scholar] [CrossRef] [PubMed]
- Bozbas, S.K.; Boz, Y. Low-cost biosorbent: Anadara inaequivalvis shells for removal of Pb (II) and Cu(II) from aqueous solution. Proc. Saf. Environ. Protectec. 2016, 103, 144–152. [Google Scholar] [CrossRef]
- Joseph, L.; Jun, B.-M.; Flora, J.R.; Park, C.M.; Yoon, Y. Removal of heavy metals from water sources in the developing world using low-cost materials: A review. Chemosphere 2019, 229, 142–159. [Google Scholar] [CrossRef]
- Gode, A.; Pehlivan, E. A comparative study of two chelating ion exchange resins for the removal of chromium (III) from aqueous solution. J. Hazard. Mater. B 2003, 100, 231–243. [Google Scholar] [CrossRef]
- Apiratikul, R.; Pavasant, P. Sorption of Cu2+, Cd2+, and Pb2+ using modified zeolite from coal fly ash. Chem. Eng. J. 2008, 144, 245–258. [Google Scholar] [CrossRef]
- Chutia, P.; Kato, S.; Kojima, T.; Satokawa, S. Arsenic adsorption from aqueous solution on synthetic zeolites. J. Hazard. Mater. 2009, 162, 440–447. [Google Scholar] [CrossRef]
- Shaobin, W.; Saudi, M.; Zhu, H. Coal ashconversion into effective adsorbens for removal of heavy metals and dyes from wastewater. J. Hazard. Mater. 2006, 133, 243–251. [Google Scholar]
- Malik, A. Preparation and modification of various adsorbents from rice husk and their use for the removal of toxic dyes and heavy metal ions from aqueous media. Ph.D. Thesis, Department of Chemistry, Faculty of Chemical and Life Sciences, Abdul Wali Khan University Mardan, HEC, Mardan, Pakistan, 2019. [Google Scholar]
- Baskan, M.B.; Pala, A. Removal of arsenic from drinking water using modified natural zeolite. Desalination 2011, 281, 396–403. [Google Scholar] [CrossRef]
- Yang, X.; Xia, L.; Song, S. Arsenic adsorption from water using graphene-based materials as adsorbents: A critical review. Surf. Rev. Lett. 2017, 24, 1730001. [Google Scholar] [CrossRef]
- Nevine, K.A. Removal of reactive dye from aqueous solutions by adsorption onto activated carbons prepared from sugarcane bagasse pith. Desalination 2008, 223, 152–161. [Google Scholar]
- Gurusamy, A.; Ruey, S.D. Use of cellulose-based wastes for adsorption of dyes from aqueous solutions. J. Hazard. Mater. 2002, 92, 263–274. [Google Scholar]
- Malik, D.S.; Jain, C.K.; Anuj, K.Y. Removal of heavy metals from emerging cellulosic low cost adsorbents: A review. Appl. Water Sci. 2016, 7, 212–224. [Google Scholar] [CrossRef]
- Foo, K.; Hameed, B. Insights into the modeling of adsorption isotherm systems. Chem. Eng. J. 2010, 156, 2–10. [Google Scholar] [CrossRef]
- Wang, H.; Zhou, A.; Peng, F.; Yu, H.; Yang, J. Mechanism study on adsorption of acidified multiwalled carbon nanotubes to Pb (II). J. Colloid Interface Sci. 2007, 316, 277–283. [Google Scholar] [CrossRef] [PubMed]
- Abdelwahab, O.; Amin, N.; EL-Ashtoukhy, E.Z. Removal of zinc ions from aqueous solution using a cation exchange resin. Chem. Engin Res. Des. 2003, 91, 165–173. [Google Scholar] [CrossRef]
- Sari, A.; Tuzen, M. Kinetic and equilibrium studies of biosorption of Pb (II) and Cd (II) from aqueous solution by macrofungus (Amanita rubescens) biomass. J. Hazard. Mater. 2009, 164, 1004–1011. [Google Scholar] [CrossRef]
- Zhang, R.; Zhang, J.; Zhang, X.; Dou, C.; Han, R. Adsorption of Congo red from aqueous solutions using cationic surfactant modified wheat straw in batch mode: Kinetic and equilibrium study. J. Taiwan Inst. Chem. Eng. 2014, 45, 2578–2583. [Google Scholar] [CrossRef]

| Sample | Treatment | pH | EC (ᶙS/cm) | LOI (%) | CEC (meq/100 g) | SG (25 °C) |
|---|---|---|---|---|---|---|
| DI H2O | N/A | 7.0 ± 0.2 b | 20 ± 1 a | N/A | 0 | 1.00 ± 0.12 a |
| RFA | N/A | 6.5 ± 0.2 a | 110 ± 5 b | 2.2 ± 0.2 c | 78 ± 4 a | 2.06 ± 0.13 b |
| HTZ | Hydrothermal | 12.5 ± 0.4 d | 148 ± 4 c | 1.2 ± 0.1 b | 150 ± 6 b | 2.71 ± 0.12 d |
| FUZ | Alkaline Fusion | 11.1 ± 0.5 c | 210 ± 8 d | 0.01 ± 0.0 a | 440 ± 18 c | 2.22 ± 0.14 c |
| FUHT | Fusion + Hydrothermal | 13.0 ± 0.4 d | 215 ± 6 d | 1.1 ± 0.1 b | 435 ± 19 c | 2.27 ± 0.11 c |
| Metallic Oxides | SiO2 | Al2O3 | TiO2 | Fe2O3 | Na2O | K2O | CaO | MgO | Others |
| Composition (% wt) | 60 ± 3 f | 23 ± 3 e | 1.8 ± 0.1 a | 4.2 ± 0.2 | 1.6 ± 0.1 b | 1.0 ± 0.1 a | 2.6 ± 0.1 c | 0.8 ± 0.1 a | 5.0 ± 0.3 d |
| Leached metals concentrations | |||||||||
| Leached metals | Si | Al | Ti | Fe | Na | K | Ca | Mg | Pb |
| Concentration (ppm) | 3.5 ± 0.2 c | 38 ± 1 g | 0.6 ± 0.1 b | 0.4 ± 0.1 b | 40 ± 2 f | 20 ± 1 e | 6.5 ± 0.3 d | 30 ± 2 f | 0.005 ± 0.0 a |
| S. # | Fly-Ash-Based Na-Zeolites | Source of Fly Ash | Potentially Toxic Metals Removal Series |
|---|---|---|---|
| 1 | ANA, PHI | Brazil | Pb2+ > Cu2+ ≈ Zn2+ ≥ Mn2+ |
| 2 | CAN | Canada | Pb2+ > Cu2+ > Ni2+ |
| 3 | FAU | UK | Fe2+ > As5+ > Pb2+ > Zn2+ > Cu2+ > Ni2+ > Cr2+ |
| 4 | Na-P1 | Brazil | As5+ > Mn2+ > Fe2+ > Cu2+ > Ni2+ > Zn2+ |
| 5 | Na-P1 | Spain | Fe2+ ≥ Cu2+ ≥ Pd2+ ≥ Cd2+ > Zn2+ > Mn2+ > Sr2+ |
| 6 | Na-P1 | South Korea | Pb2+ > Cu2+ > Cd2+ > Zn2+ |
| 7 | Na-P1 | Netherland | Ba2+ > Cu2+ > Cd2+ ≈ Zn2+ > Co2+ > Ni2+ |
| 8 | SOD | India | Pb2+ > Cd2+ > Zn2+ |
| 9 | Na-X | Thailand | Pb > Cu > Cd |
| 10 | Na-X, FAU | Japan | Pb > Cu > Cd > Ni |
| Model | Parameters | RFA | HTZ | FUZ | FUHT |
|---|---|---|---|---|---|
| Langmuir Isotherm | Kl (L/mg) | 0.142 | 0.367 | 0.682 | 0.703 |
| Qm (mg/g) | 34.3 | 107.6 | 179.7 | 173.8 | |
| R2 | 0.916 | 0.962 | 0.963 | 0.968 | |
| Freundlich Isotherm | Kf (L/mg) | 26.28 | 34.67 | 51.96 | 50.02 |
| n | 2.264 | 2.757 | 3.278 | 4.862 | |
| R2 | 0.948 | 0.982 | 0.983 | 0.991 |
| Adsorbent | Pseudo First-Order Model | Pseudo Second-Order Model | ||||
|---|---|---|---|---|---|---|
| K1 (min−1) | Qe (mg/g) | R2 | K2 (g/mg.min) | Qe (mg/g) | R2 | |
| RFA | 1.21 × 10−2 | 17.2 | 0.916 | 8.17 × 10−4 | 34.3 | 0.922 |
| HTZ | 1.54 × 10−2 | 46.8 | 0.973 | 4.17 × 10−3 | 130 | 0.981 |
| FUZ | 8.62 × 10−3 | 54.6 | 0.981 | 4.54 × 10−3 | 180 | 0.991 |
| FUHT | 7.65 × 10−3 | 59.7 | 0.980 | 3.57 × 10−3 | 178 | 0.994 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hussain, T.; Hussain, A.I.; Chatha, S.A.S.; Ali, A.; Rizwan, M.; Ali, S.; Ahamd, P.; Wijaya, L.; Alyemeni, M.N. Synthesis and Characterization of Na-Zeolites from Textile Waste Ash and Its Application for Removal of Lead (Pb) from Wastewater. Int. J. Environ. Res. Public Health 2021, 18, 3373. https://doi.org/10.3390/ijerph18073373
Hussain T, Hussain AI, Chatha SAS, Ali A, Rizwan M, Ali S, Ahamd P, Wijaya L, Alyemeni MN. Synthesis and Characterization of Na-Zeolites from Textile Waste Ash and Its Application for Removal of Lead (Pb) from Wastewater. International Journal of Environmental Research and Public Health. 2021; 18(7):3373. https://doi.org/10.3390/ijerph18073373
Chicago/Turabian StyleHussain, Tabassum, Abdullah Ijaz Hussain, Shahzad Ali Shahid Chatha, Adnan Ali, Muhammad Rizwan, Shafaqat Ali, Parvaiz Ahamd, Leonard Wijaya, and Mohammed Nasser Alyemeni. 2021. "Synthesis and Characterization of Na-Zeolites from Textile Waste Ash and Its Application for Removal of Lead (Pb) from Wastewater" International Journal of Environmental Research and Public Health 18, no. 7: 3373. https://doi.org/10.3390/ijerph18073373
APA StyleHussain, T., Hussain, A. I., Chatha, S. A. S., Ali, A., Rizwan, M., Ali, S., Ahamd, P., Wijaya, L., & Alyemeni, M. N. (2021). Synthesis and Characterization of Na-Zeolites from Textile Waste Ash and Its Application for Removal of Lead (Pb) from Wastewater. International Journal of Environmental Research and Public Health, 18(7), 3373. https://doi.org/10.3390/ijerph18073373









