Mucosal Melanoma of the Hard Palate: Surgical Treatment and Reconstruction
Abstract
1. Introduction
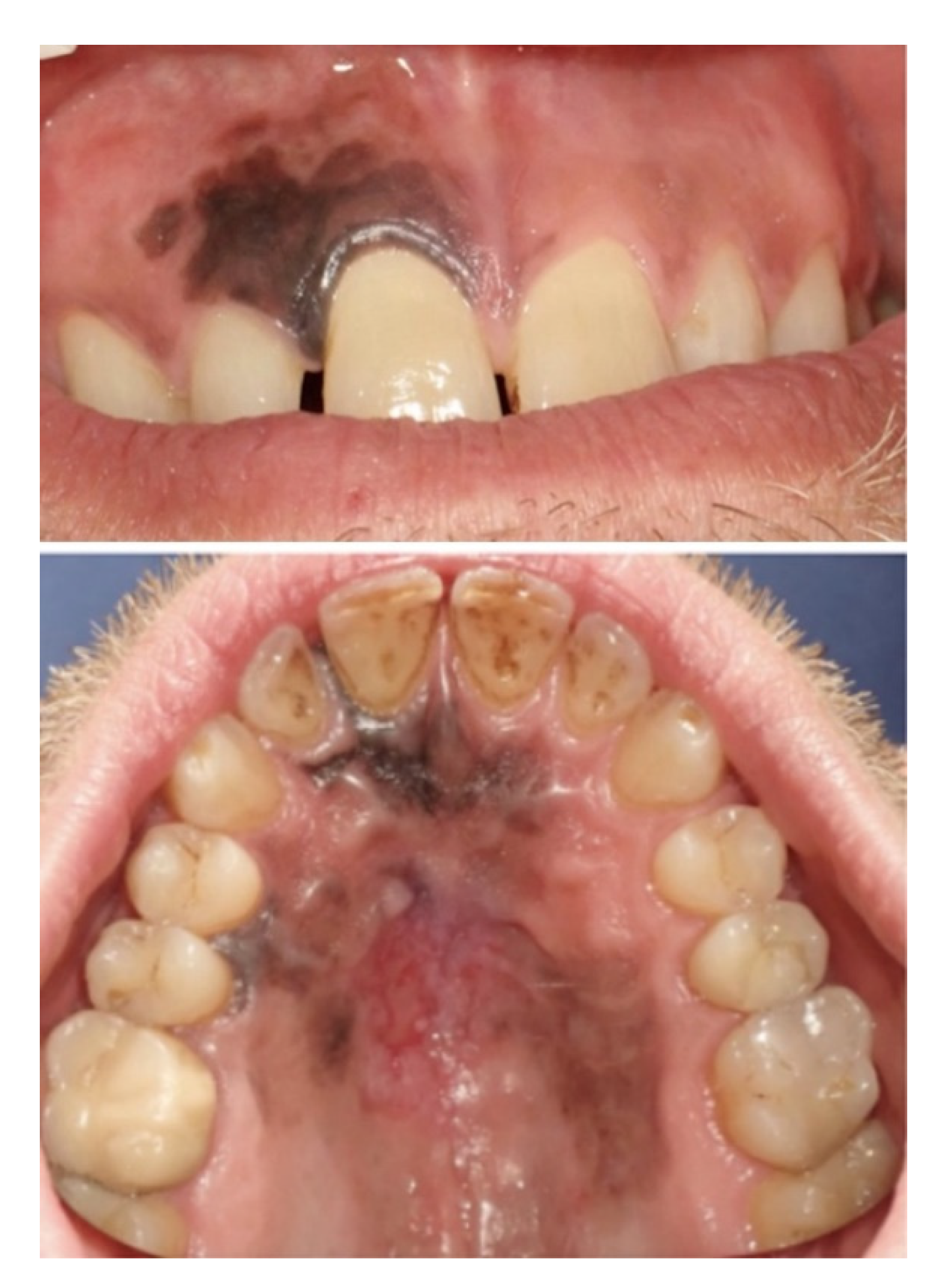
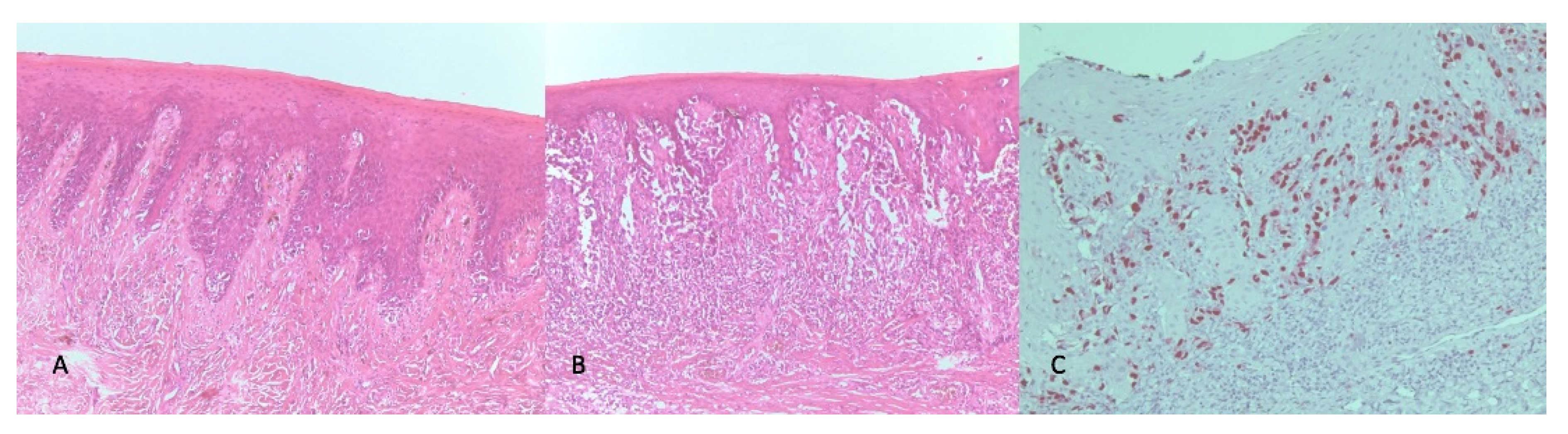
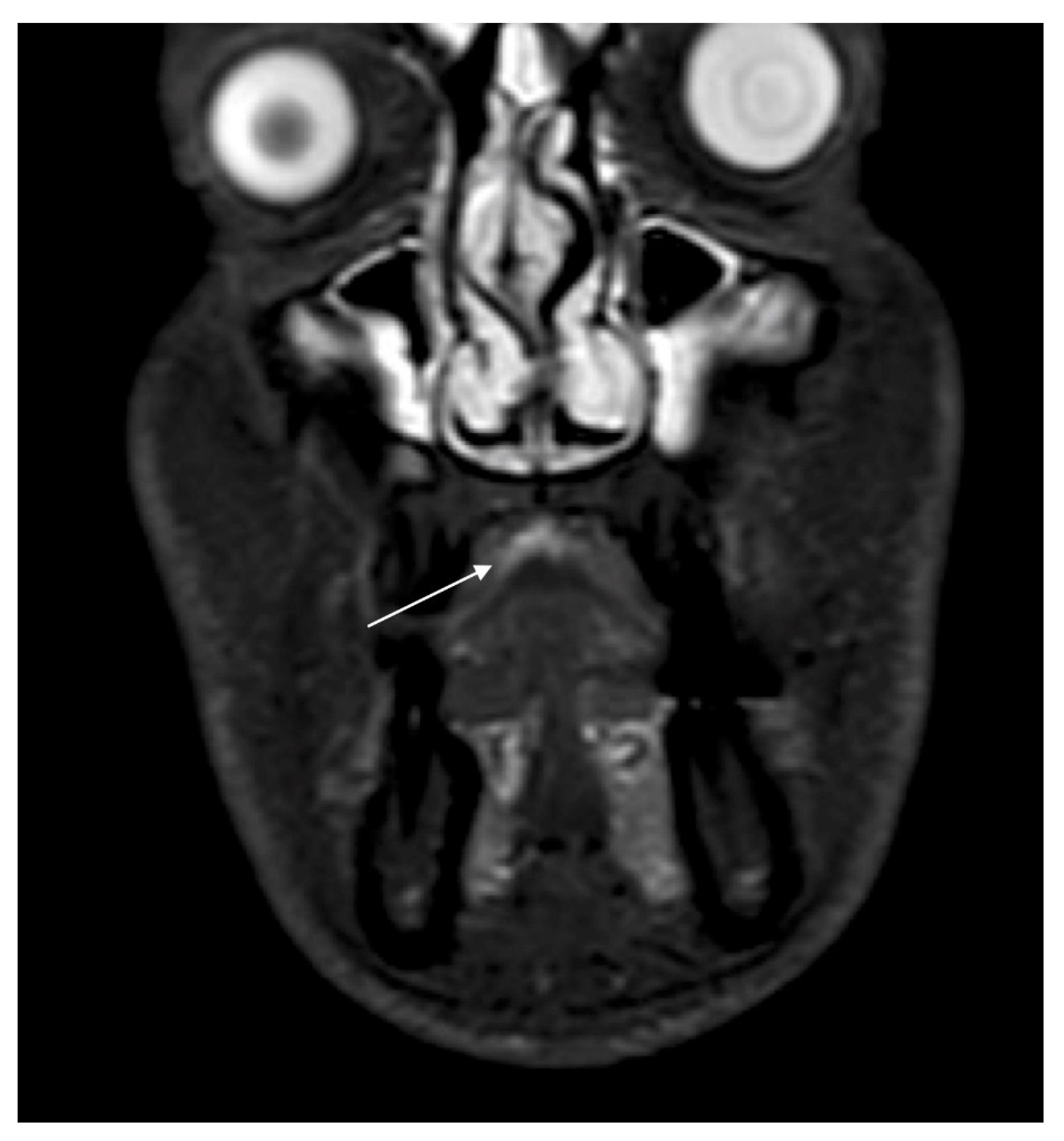
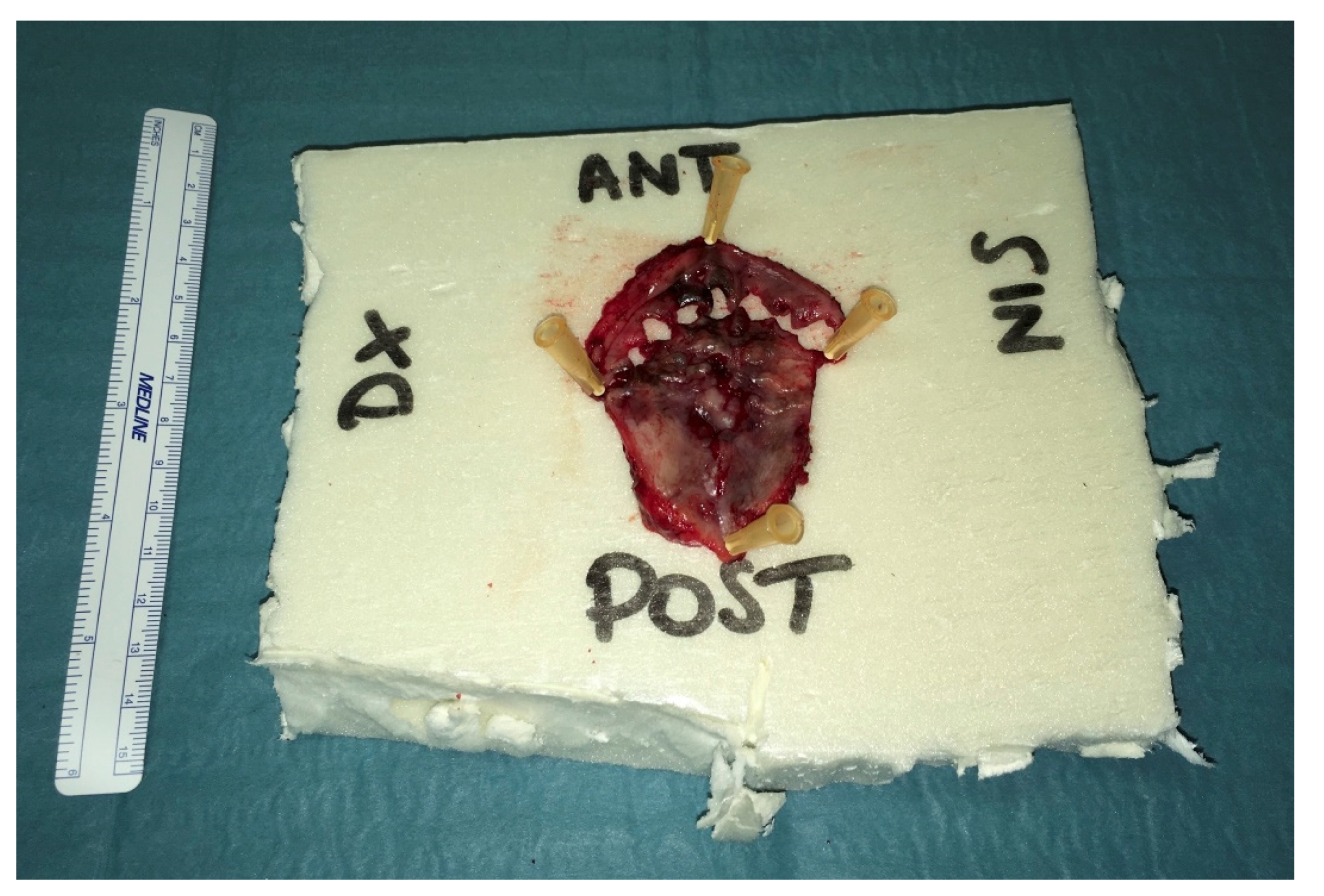
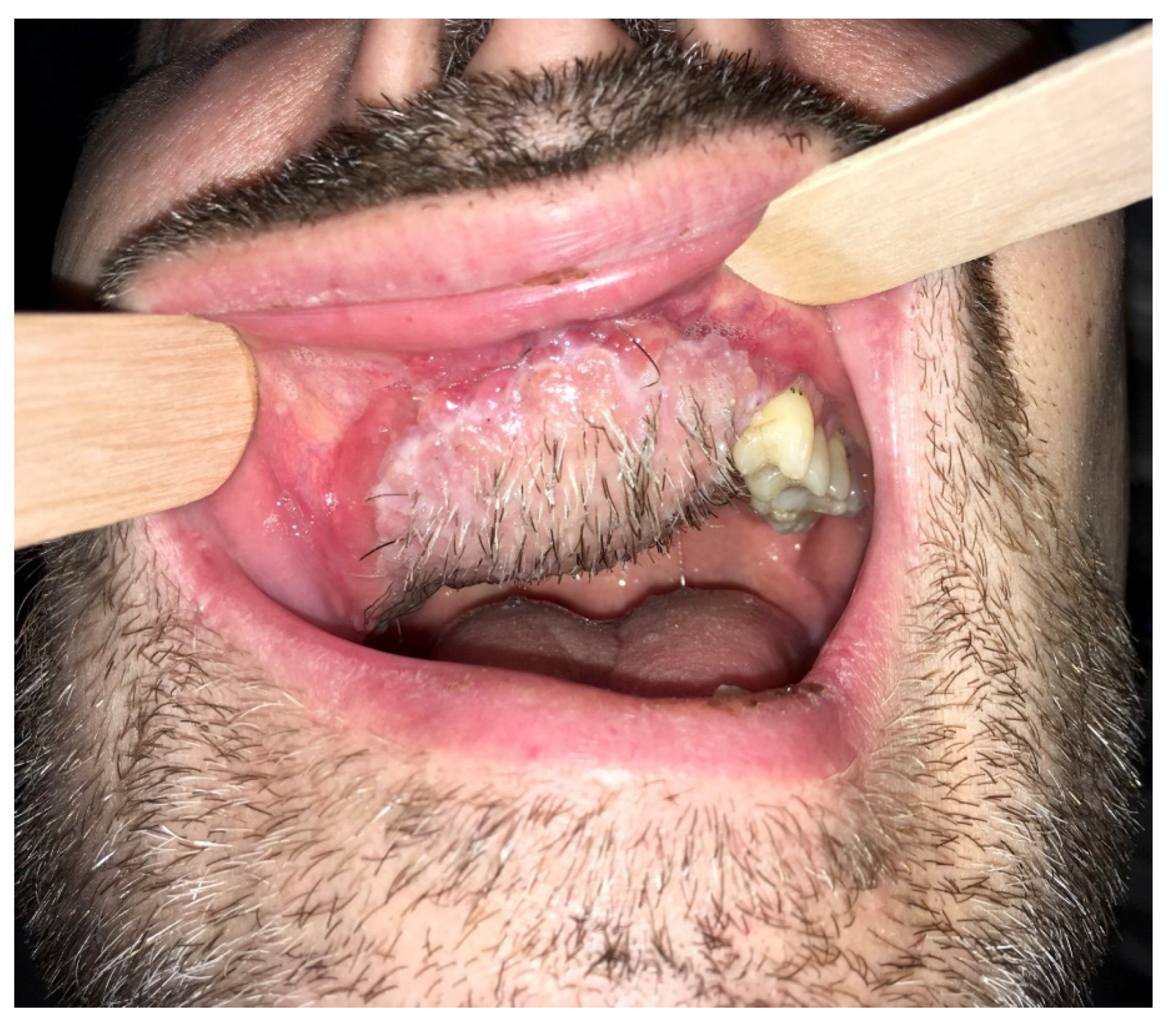
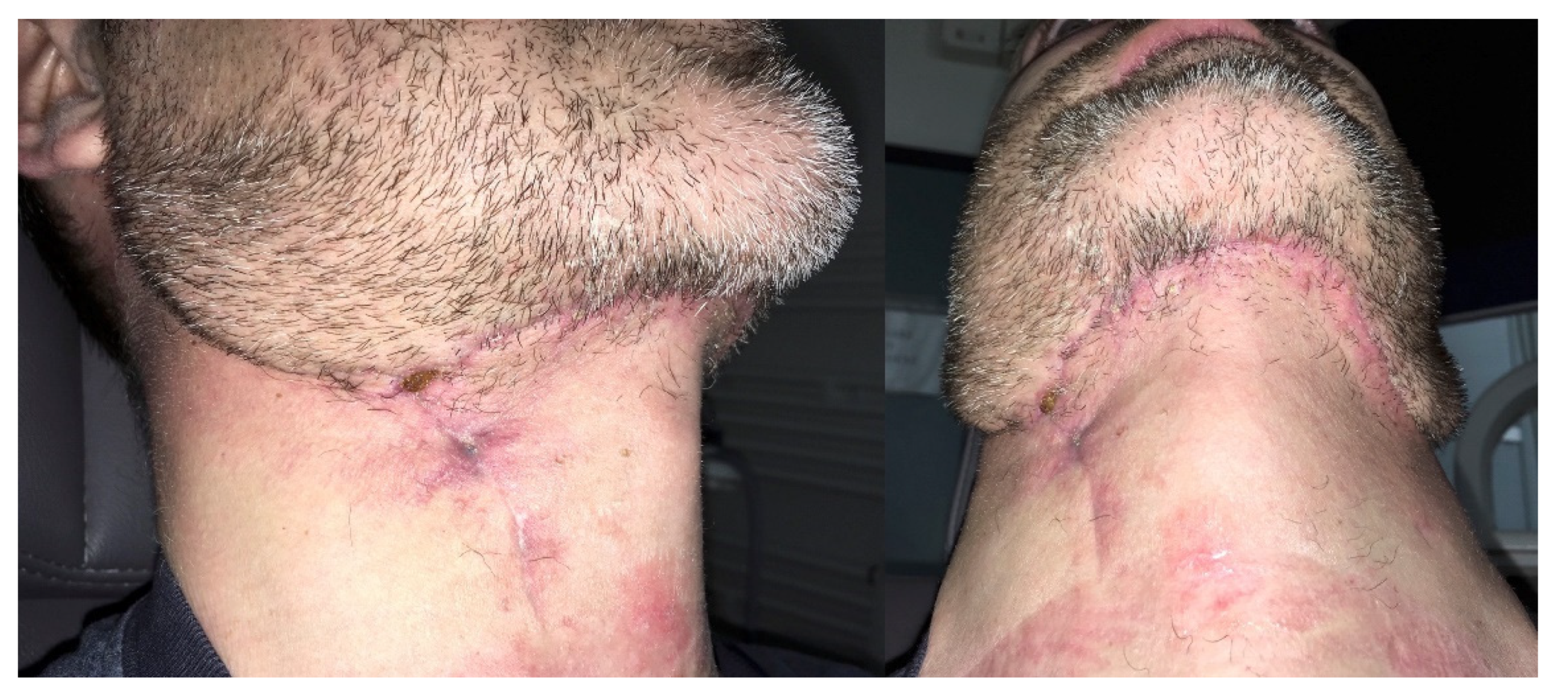
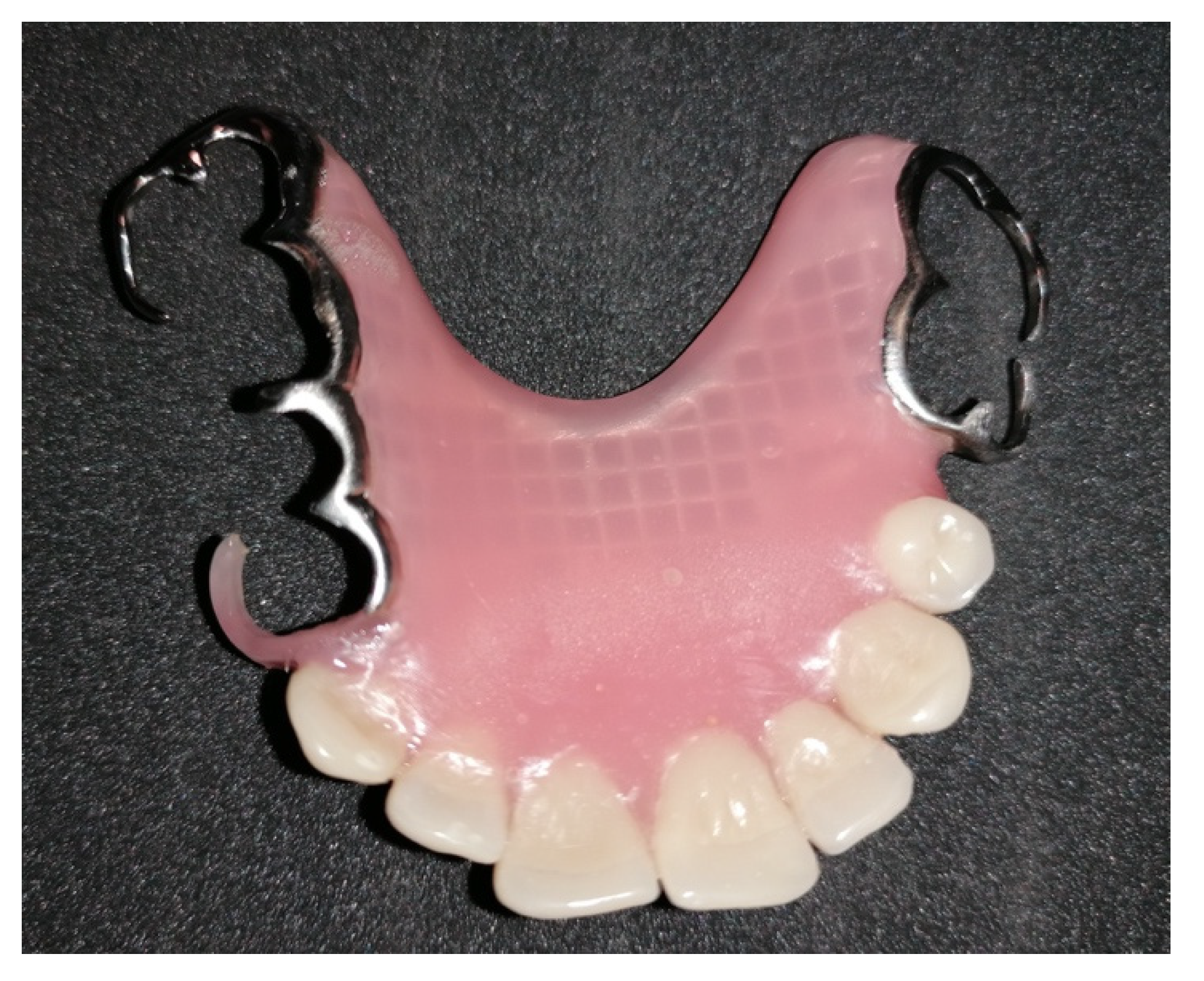
2. Case Report
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ascierto, P.A.; Accorona, R.; Botti, G.; Farina, D.; Fossati, P.; Gatta, G.; Gogas, H.; Lombardi, D.; Maroldi, R.; Nicolai, P.; et al. Mucosal melanoma of the head and neck. Crit. Rev. Oncol. 2017, 112, 136–152. [Google Scholar] [CrossRef] [PubMed]
- Chatzistefanou, I.; Kolokythas, A.; Vahtsevanos, K.; Antoniades, K. Primary mucosal melanoma of the oral cavity: Current therapy and future directions. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2016, 122, 17–27. [Google Scholar] [CrossRef] [PubMed]
- Jarrom, D.; Paleri, V.; Kerawala, C.; Roques, T.; Bhide, S.; Newman, L.; Winter, S.C. Mucosal melanoma of the upper airways tract mucosal melanoma: A systematic review with meta-analyses of treatment. Head Neck 2017, 39, 819–825. [Google Scholar] [CrossRef]
- Gherlone, E.F.; Capparé, P.; Tecco, S.; Polizzi, E.; Pantaleo, G.; Gastaldi, G.; Grusovin, M.G. A Prospective Longitudinal Study on Implant Prosthetic Rehabilitation in Controlled HIV-Positive Patients with 1-Year Follow-Up: The Role of CD4+ Level, Smoking Habits, and Oral Hygiene. Clin. Implant. Dent. Relat. Res. 2015, 18, 955–964. [Google Scholar] [CrossRef] [PubMed]
- Della-Torre, E.; Campochiaro, C.; Cassione, E.B.; Albano, L.; Gerevini, S.; Bianchi-Marzoli, S.; Bozzolo, E.; Passerini, G.; Lanzillotta, M.; Terreni, M.; et al. Intrathecal rituximab for IgG4-related hypertrophic pachymeningitis. J. Neurol. Neurosurg. Psychiatry 2018, 89, 441–444. [Google Scholar] [CrossRef]
- Trimarchi, M.; Bellini, C.; Toma, S.; Bussi, M. Back-and-forth endoscopic septoplasty: Analysis of the technique and outcomes. Int. Forum Allergy Rhinol. 2011, 2, 40–44. [Google Scholar] [CrossRef] [PubMed]
- Biafora, M.; Bertazzoni, G.; Trimarchi, M. Maxillary Sinusitis Caused by Dental Implants Extending into the Maxillary Sinus and the Nasal Cavities. J. Prosthodont. 2014, 23, 227–231. [Google Scholar] [CrossRef]
- Mackintosh, A.J. The Antimicrobial Properties of Melanocytes, Melanosomes and Melanin and the Evolution of Black Skin. J. Theor. Biol. 2001, 211, 101–113. [Google Scholar] [CrossRef]
- Plonka, P.M.; Passeron, T.; Brenner, M.; Tobin, D.J.; Shibahara, S.; Thomas, A.; Slominski, A.; Kadekaro, A.L.; Hershkovitz, D.; Peters, E.M.J.; et al. What are melanocytesreallydoing all day long? Exp. Dermatol. 2009, 18, 799–819. [Google Scholar] [CrossRef]
- Juvekar, M.V.; Karle, R.R.; Wankhede, P.; Munde, A. Malignant melanoma of the oral cavity: Report of two cases. Contemp. Clin. Dent. 2014, 5, 227–230. [Google Scholar] [CrossRef]
- Axeix, T.; Hedin, C.A. Epidemiologic study of excessive oral melanin pigmentation with special reference to the influence of tobacco habits. Eur. J. Oral Sci. 1982, 90, 434–442. [Google Scholar] [CrossRef]
- Crespi, R.; Paolo, C.; Georgios, E.R.; Elisabetta, M.; Elisa, B.; Enrico, G. Corticocancellous porcine bone in the healing of human ex-traction sockets: Combining histomorphometry with osteoblast gene expression profiles in vivo. Int. J. Oral Maxillofac. Implants 2011, 26, 866–872. [Google Scholar] [PubMed]
- Lanzillotta, M.; Campochiaro, C.; Trimarchi, M.; Arrigoni, G.; Gerevini, S.; Milani, R.; Bozzolo, E.; Biafora, M.; Venturini, E.; Cicalese, M.P.; et al. Deconstructing IgG4-related disease involvement of midline structures: Comparison to common mimickers. Mod. Rheumatol. 2017, 27, 638–645. [Google Scholar] [CrossRef]
- Morassi, M.L.; Trimarchi, M.; Nicolai, P.; Gregorini, G.; Maroldi, R.; Specks, U.; Facchetti, F. Cocaine, ANCA, and Wegener’s granulomatosis. Pathologica 2001, 93, 581–583. [Google Scholar] [PubMed]
- Trimarchi, M.; Bondi, S.; Della Torre, E.; Terreni, M.; Bussi, M. Palate perforation differentiates cocaine-induced midline destructive lesions from granulomatosis with polyangiitis. Acta Otorhinolaryngol. Ital. 2017, 37, 281–285. [Google Scholar]
- Trimarchi, M.; Bellini, C.; Fabiano, B.; Gerevini, S.; Bussi, M. Multiple mucosal involvement in cicatricial pemphigoid. Acta Otorhinolaryngol. Ital. 2009, 29, 222–225. [Google Scholar] [PubMed]
- Sun, C.-Z.; Chen, Y.-F.; Jiang, Y.-E.; Hu, Z.-D.; Yang, A.-K.; Song, M. Treatment and prognosis of oral mucosal melanoma. Oral Oncol. 2012, 48, 647–652. [Google Scholar] [CrossRef]
- Penel, N.; Mallet, Y.; Mirabel, X.; Van, J.T.; Lefebvre, J.-L. Primary Mucosal Melanoma of Head and Neck: Prognostic Value of Clear Margins. Laryngoscope 2006, 116, 993–995. [Google Scholar] [CrossRef]
- Lyu, J.; Wu, Y.; Li, C.; Wang, R.; Song, H.; Ren, G.; Guo, W. Mutation scanning of BRAF, NRAS, KIT, and GNAQ/GNA11 in oral mucosal melanoma: A study of 57 cases. J. Oral Pathol. Med. 2016, 45, 295–301. [Google Scholar] [CrossRef]
- Tanaka, N.; Amagasa, T.; Iwaki, H.; Shioda, S.; Takeda, M.; Ohashi, K.; Reck, S.F. Oral malignant melanoma in Japan. Oral Surgery Oral Med. Oral Pathol. 1994, 78, 81–90. [Google Scholar] [CrossRef]
- Dupin, E.; Le Douarin, N.M. Development of melanocyte precursors from the vertebrate neural crest. Oncogene 2003, 22, 3016–3023. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.G.; Prasad, M.L.; Escrig, M.; Singh, B.; Shaha, A.R.; Kraus, D.H.; Boyle, J.O.; Huvos, A.G.; Busam, K.; Shah, J.P. Primary mucosal malignant melanoma of the head and neck. Head Neck 2002, 24, 247–257. [Google Scholar] [CrossRef]
- Wu, Y.; Zhong, Y.; Li, C.; Song, H.; Guo, W.; Ren, G. Neck dissection for oral mucosal melanoma: Caution of nodular lesion. Oral Oncol. 2014, 50, 319–324. [Google Scholar] [CrossRef]
- Kim, S.S.; Han, M.H.; Kim, J.E.; Lee, C.H.; Chung, H.W.; Lee, J.S.; Chang, K.-H. Malignant melanoma of the sinonasal cavity: Explanation of magnetic resonance signal intensities with histopathologic characteristics. Am. J. Otolaryngol. 2000, 21, 366–378. [Google Scholar] [CrossRef] [PubMed]
- Bachar, G.; Loh, K.S.; O’Sullivan, B.; Goldstein, D.; Wood, S.; Brown, D.; Irish, J. Mucosal melanomas of the head and neck: The Princess Margaret Hospital experience. Head Neck 2008, 30, 1325–1331. [Google Scholar] [CrossRef] [PubMed]
- Michel, J.; Perret-Court, A.; Fakhry, N.; Braustein, D.; Monestier, S.; Richard, M.-A.; Grob, J.-J.; Giovanni, A.; Dessi, P. Sinonasal mucosal melanomas: The prognostic value of tumor classifications. Head Neck 2013, 36, 311–316. [Google Scholar] [CrossRef] [PubMed]
- Krengli, M.; Masini, L.; Kaanders, J.H.; Maingon, P.; Oei, S.B.; Zouhair, A.; Ozyar, E.; Roelandts, M.; Amichetti, M.; Bosset, M.; et al. Radiotherapy in the treatment of mucosal melanoma of the upper aerodigestive tract: Analysis of 74 cases. A Rare Cancer Network study. Int. J. Radiat. Oncol. 2006, 65, 751–759. [Google Scholar] [CrossRef] [PubMed]
- Medina, J.E.; Ferlito, A.; Pellitteri, P.K.; Shaha, A.R.; Khafif, A.; Devaney, K.O.; Fisher, S.R.; O’Brien, C.J.; Byers, R.M.; Robbins, K.T.; et al. Current management of mucosal melanoma of the head and neck. J. Surg. Oncol. 2003, 83, 116–122. [Google Scholar] [CrossRef]
- Musha, A.; Saitoh, J.-I.; Shirai, K.; Yokoo, S.; Ohno, T.; Nakano, T. Oral mucosal melanoma treated with carbon ion radiotherapy: A case report. J. Med Case Rep. 2016, 10, 284. [Google Scholar] [CrossRef][Green Version]
- Grichnik, J.M. Melanoma, Nevogenesis, and Stem Cell Biology. J. Investig. Dermatol. 2008, 128, 2365–2380. [Google Scholar] [CrossRef]
- Keswell, D.; Davids, L.M.; Kidson, S.H. Migration of human melanocytes into keratinocyte monolayers in vitro. J. Dermatol. Sci. 2012, 66, 160–163. [Google Scholar] [CrossRef]
- Mihajlovic, M.; Vlajkovic, S.; Jovanovic, P.; Stefanovic, V. Primary mucosal melanomas: A comprehensive review. Int. J. Clin. Exp. Pathol. 2012, 5, 739–753. [Google Scholar]
- Crespi, R.; Capparè, P.; Gherlone, E. Sinus floor elevation by osteotome: Hand mallet versus electric mallet. A prospective clinical study. Int. J. Oral Maxillofac. Implant. 2012, 27, 1140–1150. [Google Scholar]
- Hu, S.; Fan, C.; Bs, B.P.; Rosenberg, J.D. Submental island flap vs free tissue transfer in oral cavity reconstruction: Systematic review and meta-analysis. Head Neck 2020, 42, 2155–2164. [Google Scholar] [CrossRef]
- Patel, U.A. The submental flap for head and neck reconstruction: Comparison of outcomes to the radial forearm free flap. Laryngoscope 2019, 130. [Google Scholar] [CrossRef]
- Jørgensen, M.G.; Tabatabaeifar, S.; Toyserkani, N.M.; Sørensen, J.A. Submental Island Flap versus Free Flap Reconstruction for Complex Head and Neck Defects. Otolaryngol. Neck Surg. 2019, 161, 946–953. [Google Scholar] [CrossRef] [PubMed]
- Martin, D.; Baudet, J.; Mondie, J.M.; Peri, G. The submental island skin flap. A surgical protocol. Prospects of use. Ann. Chir. Plast. Esthet. 1990, 35, 480–484. [Google Scholar] [PubMed]
- Sterne, G.; Januszkiewicz, J.; Hall, P.; Bardsley, A. The submental island flap. Br. J. Plast. Surg. 1996, 49, 85–89. [Google Scholar] [CrossRef]
- Martin, D.; Legaillard, P.; Bakhach, J.; Hu, W.; Baudet, J. Reverse flow YV pedicle extension: A method of doubling the arc of rotation of a flap under certain conditions. Ann. Chir. Plast. Esthétique 1994, 39, 403–414. [Google Scholar]
- Kudva, A.; Aramanadka, C.; D’Souza, C.; Lakshmi, R.; Karegowda, L.H. Anatomic Variation of Submental Artery: A Case of Submental Artery Coursing Through a Developmental Defect of Mylohyoid Muscle. J. Maxillofac. Oral Surg. 2020, 1–4. [Google Scholar] [CrossRef]
- Hayden, R.E.; Nagel, T.H.; Donald, C.B. Hybrid submental flaps for reconstruction in the head and neck: Part pedicled, part free. Laryngoscope 2013, 124, 637–641. [Google Scholar] [CrossRef]
- Gupta, V.; Cohan, D.M.; Arshad, H.; Kuriakose, M.A.; Hicks, W.L. Palatal reconstruction. Curr. Opin. Otolaryngol. Head Neck Surg. 2012, 20, 225–230. [Google Scholar] [CrossRef] [PubMed]
- Hammouda, Y.; Halily, S.; Oukessou, Y.; Rouadi, S.; Abada, R.; Roubal, M.; Mahtar, M. Malignant tumors of the hard palate: Report of 4 cases and review of the literature. Int. J. Surg. Case Rep. 2021, 78, 228–234. [Google Scholar] [CrossRef] [PubMed]
- Okay, D.J.; Genden, E.; Buchbinder, D.; Urken, M. Prosthodontic guidelines for surgical reconstruction of the maxilla: A classification system of defects. J. Prosthet. Dent. 2001, 86, 352–363. [Google Scholar] [CrossRef]
- Beier, U.S.; Salinas, T.; Puelacher, W. Resection of a primary oral malignant melanoma and rehabilitative management using nasolabial flap: A case report. Oral Maxillofac. Surg. 2011, 16, 141–145. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bondi, S.; Vinciguerra, A.; Lissoni, A.; Rizzo, N.; Barbieri, D.; Indelicato, P.; Abati, S. Mucosal Melanoma of the Hard Palate: Surgical Treatment and Reconstruction. Int. J. Environ. Res. Public Health 2021, 18, 3341. https://doi.org/10.3390/ijerph18073341
Bondi S, Vinciguerra A, Lissoni A, Rizzo N, Barbieri D, Indelicato P, Abati S. Mucosal Melanoma of the Hard Palate: Surgical Treatment and Reconstruction. International Journal of Environmental Research and Public Health. 2021; 18(7):3341. https://doi.org/10.3390/ijerph18073341
Chicago/Turabian StyleBondi, Stefano, Alessandro Vinciguerra, Alessandra Lissoni, Nathalie Rizzo, Diego Barbieri, Pietro Indelicato, and Silvio Abati. 2021. "Mucosal Melanoma of the Hard Palate: Surgical Treatment and Reconstruction" International Journal of Environmental Research and Public Health 18, no. 7: 3341. https://doi.org/10.3390/ijerph18073341
APA StyleBondi, S., Vinciguerra, A., Lissoni, A., Rizzo, N., Barbieri, D., Indelicato, P., & Abati, S. (2021). Mucosal Melanoma of the Hard Palate: Surgical Treatment and Reconstruction. International Journal of Environmental Research and Public Health, 18(7), 3341. https://doi.org/10.3390/ijerph18073341







