Effects of Speciation, Cooking and Changes in Bioaccessibility on Methylmercury Exposure Assessment for Contrasting Diets of Fish and Marine Mammals
Abstract
1. Introduction
2. Materials and Methods
2.1. Population and Diet
2.2. New Proposed Variables
2.3. Probabilistic Risk Assessment
2.4. Impact of RAF on Risk Estimates
2.5. Statistic and Icons
3. Results
3.1. The Impact of the Proposed Variables on Methylmercury Exposure
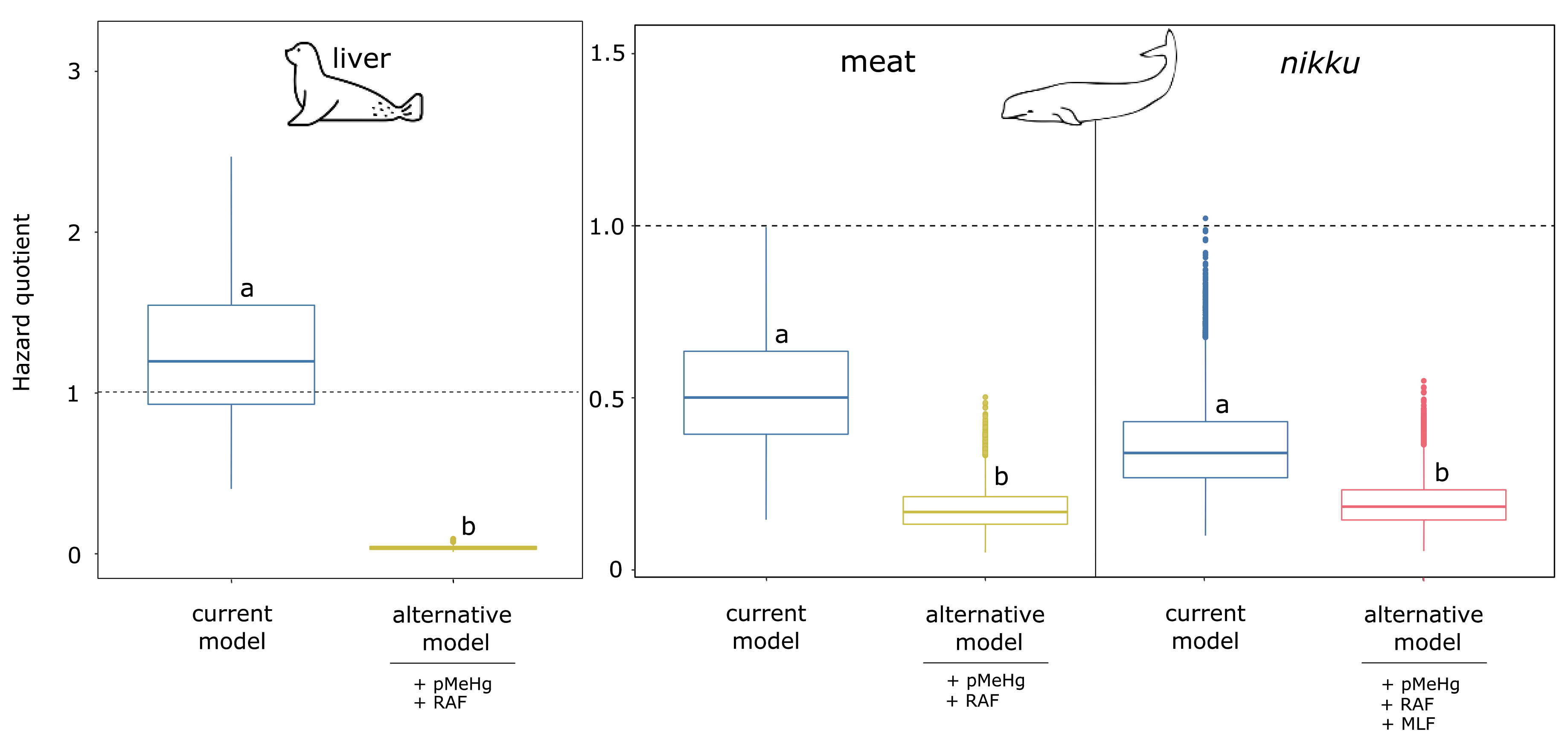
3.2. Risk Characterization
3.2.1. Salmon and Canned Tuna Consumption Scenario
3.2.2. Marine Mammal Consumption Scenario
3.3. The Implication of RAF in Methylmercury Risk Characterization
4. Discussion
4.1. The Proposed Model Modifications Impact Methylmercury Exposure Assessment
4.2. The Assessment of Methylmecury Exposure through Marine Mammals Should Be a Priority
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Joint Expert Committee on Food Additives (JECFA). Safety Evaluation of Certain Food Additives and Contaminants; WHO: Geneva, Switzerland, 2004. [Google Scholar]
- Lemire, M.; Kwan, M.; Laouan-Sidi, A.E.; Muckle, G.; Pirkle, C.; Ayotte, P.; Dewailly, E. Local country food sources of methylmercury, selenium and omega-3 fatty acids in Nunavik, Northern Quebec. Sci. Total Environ. 2015, 509–510, 248–259. [Google Scholar] [CrossRef]
- Amin-Zaki, L.; Elhassani, S.; Majeed, M.A.; Clarkson, T.W.; Doherty, R.A.; Greenwood, M. Intra-uterine Methylmercury Poisoning in Iraq. In Problems of Birth Defects; Springer International Publishing: Berlin, Germany, 1974; pp. 233–241. [Google Scholar]
- Aschner, M.; Aschner, J.L. Mercury neurotoxicity: Mechanisms of blood-brain barrier transport. Neurosci. Biobehav. Rev. 1990, 14, 169–176. [Google Scholar] [CrossRef]
- Kerper, L.E.; Ballatori, N.; Clarkson, T.W. Methylmercury transport across the blood-brain barrier by an amino acid carrier. Am. J. Physiol. Integr. Comp. Physiol. 1992, 262, 761–765. [Google Scholar] [CrossRef]
- Cohen, J.T.; Bellinger, D.C.; Shaywitz, B.A. A quantitative analysis of prenatal methyl mercury exposure and cognitive development. Am. J. Prev. Med. 2005, 29. [Google Scholar] [CrossRef]
- FAO/WHO. Report of the Joint FAO / WHO Expert Consultation on the Risks and Benefits of Fish Consumption; FAO: Rome, Italy, 2010; Volume FIPM. [Google Scholar]
- Adamou, T.Y.; Riva, M.; Muckle, G.; Laouan Sidi, E.A.; Lemire, M.; Ayotte, P. Blood mercury and plasma polychlorinated biphenyls concentrations in pregnant Inuit women from Nunavik: Temporal trends, 1992–2017. Sci. Total Environ. 2020, 743. [Google Scholar] [CrossRef]
- Arbuckle, T.E.; Liang, C.L.; Morisset, A.S.; Fisher, M.; Weiler, H.; Cirtiu, C.M.; Legrand, M.; Davis, K.; Ettinger, A.S.; Fraser, W.D. Maternal and fetal exposure to cadmium, lead, manganese and mercury: The MIREC study. Chemosphere 2016, 163, 270–282. [Google Scholar] [CrossRef]
- Kuhnlein, H.V.; Receveur, O. Dietary change and traditional food systems of Indigenous Peoples. Annu. Rev. Nutr. 1996, 16, 417–442. [Google Scholar] [CrossRef] [PubMed]
- Council of Canadian Academies. Aboriginal Food Security in Northern Canada: An Assessment of the State of Knowledge; Council of Canadian Academies: Ottawa, ON, Canada, 2014. [Google Scholar]
- Kuhnlein, H.V.; Appavoo, D.; Morrison, N.; Soueida, R.; Pierrot, P. Use and nutrient composition of traditional sahtú (Hareskin) dene/métis foods. J. Food Compos. Anal. 1994, 7, 144–157. [Google Scholar] [CrossRef]
- Van Oostdam, J.; Gilman, A.; Dewailly, E.; Usher, P.; Wheatley, B. Human health implications of environmental contaminants in Arctic Canada: A review. Sci. Total Environ. 1999, 230, 1–82. [Google Scholar] [CrossRef]
- Chan, H.M.; Fediuk, K.; Hamilton, S.; Rostas, L.; Caughey, A.; Kuhnlein, H.; Egeland, G.; Loring, E.; Man, H.; Fediuk, K.; et al. Food security in Nunavut, Canada: Barriers and recommendations. Int. J. Circumpolar Health 2006, 65, 416–431. [Google Scholar] [CrossRef]
- Health Canada. Human Health Risk Assessment of Mercury in Fish and Health Benefits of Fish Consumption; Health Canada: Ottawa, ON, Canada, 2007. [Google Scholar]
- Rice, G.; Swartout, J.; Mahaffey, K.; Schoeny, R. Issues in risk assessment of chemicals of concern to department of defense and other agencies session: Derivation of U.S EPA’s oral reference dose (RFD) for methylmercury. Drug Chem. Toxicol. 2000, 23, 41–54. [Google Scholar] [CrossRef]
- United States Environmental Protection Agency (US EPA). IRIS Assessment Plan for Methylmercury; EPA: Washington, DC, USA, 2019. [Google Scholar]
- Joint FAO/WHO Expert Committee. Food Additives and Contaminants. Sixty-First Meeting. Summary and Conclusions; WHO: Geneva, Switzerland, 2003. [Google Scholar]
- United States Environmental Protection Agency (US EPA). Mercury Study Report to Congress—Volume I: Executive Summary; EPA: Washington, DC, USA, 1997. [Google Scholar]
- McAuley, C.; Smith, D.; Dersch, A.; Koppe, B.; Mouille-Malbeuf, S.; Sowan, D. Whole fish vs. fish fillet—The risk implications for First Nation subsistence consumers. Cogent Food Agric. 2018, 4, 1–12. [Google Scholar] [CrossRef]
- Magalhães, M.C.; Costa, V.; Menezes, G.M.; Pinho, M.R.; Santos, R.S.; Monteiro, L.R. Intra- and inter-specific variability in total and methylmercury bioaccumulation by eight marine fish species from the Azores. Mar. Pollut. Bull. 2007, 54, 1654–1662. [Google Scholar] [CrossRef]
- Aberg, B.; Ekman, L.; Falk, R.; Greitz, U.; Persson, G.; Snihs, J.O. Metabolism of methyl mercury (203Hg) compounds in man. Arch. Environ. Health 1969, 19, 478–484. [Google Scholar] [CrossRef]
- Alves, R.N.; Maulvault, A.L.; Barbosa, V.L.; Fernandez-Tejedor, M.; Tediosi, A.; Kotterman, M.; van den Heuvel, F.H.M.; Robbens, J.; Fernandes, J.O.; Romme Rasmussen, R.; et al. Oral bioaccessibility of toxic and essential elements in raw and cooked commercial seafood species available in European markets. Food Chem. 2018, 267, 15–27. [Google Scholar] [CrossRef]
- Anacleto, P.; Barbosa, V.; Alves, R.N.; Maulvault, A.L.; Bronze, M.R.; Marques, A. Green tea infusion reduces mercury bioaccessibility and dietary exposure from raw and cooked fish. Food Chem. Toxicol. 2020, 145, 135577. [Google Scholar] [CrossRef] [PubMed]
- Girard, C.; Charette, T.; Leclerc, M.; Shapiro, B.J.; Amyot, M. Cooking and co-ingested polyphenols reduce in vitro methylmercury bioaccessibility from fish and may alter exposure in humans. Sci. Total Environ. 2017, 616–617, 863–874. [Google Scholar] [CrossRef] [PubMed]
- Torres-Escribano, S.; Velez, D.; Montoro, R.; Vélez, D.; Montoro, R. Mercury and methylmercury bioaccessibility in swordfish. Food Addit. Contam. Part a-Chemistry Anal. Control Expo. Risk Assess. 2010, 27, 327–337. [Google Scholar] [CrossRef] [PubMed]
- Bradley, M.; Barst, B.; Basu, N. A review of mercury bioavailability in humans and fish. Int. J. Environ. Res. Public Health 2017, 14, 169. [Google Scholar] [CrossRef]
- Health Canada. Federal Contaminated Site Risk Assessment in Canada Part V: Guidance on Human Health Detailed Quantitative Risk Assessment for Chemicals (DQRA Chem); Health Canada: Ottawa, ON, Canada, 2010. [Google Scholar]
- United States Environmental Protection Agency (US EPA). Risk Assessment Guidance for Superfund: Volume III-Part A, Process for Conducting Probabilistic Risk Assessment; EPA: Washington, DC, USA, 2001. [Google Scholar]
- European Food Safety Authority. Scientific Opinion on the Risk for Public Health Related to the Presence of Mercury and Methylmercury in Food; European Food Safety Authority: Parma, Italy, 2012. [Google Scholar]
- Hinck, J.E.; Schmitt, C.J.; Chojnacki, K.A.; Tillitt, D.E. Environmental contaminants in freshwater fish and their risk to piscivorous wildlife based on a national monitoring program. Environ. Monit. Assess. 2009, 152, 469–494. [Google Scholar] [CrossRef]
- Hoekstra, P.F.; O’Hara, T.M.; Backus, S.M.; Hanns, C.; Muir, D.C.G. Concentrations of persistent organochlorine contaminants in bowhead whale tissues and other biota from northern Alaska: Implications for human exposure from a subsistence diet. Environ. Res. 2005, 98, 329–340. [Google Scholar] [CrossRef] [PubMed]
- Health Canada Mercury in Fish. Available online: https://www.canada.ca/en/health-canada/services/food-nutrition/food-safety/chemical-contaminants/environmental-contaminants/mercury/mercury-fish.html (accessed on 10 February 2020).
- Richardson, G.M.; Stantec Consulting Ltd. Canadian Exposure Factors Handbook; Toxicology Centre, University of Saskatchewan: Saskatoon, SK, Canada, 2013. [Google Scholar]
- Palaniyandi, S. Determining Mercury and Selenium In Vitro Bioaccessibility in Country Foods Collected from Nunavik, Québec; University of Waterloo: Waterloo, ON, Canada, 2016. [Google Scholar]
- Laird, B.D.; Goncharov, A.B.; Egeland, G.M.; Chan, H.M. Dietary advice on inuit traditional food use needs to balance benefits and risks of mercury, selenium, and n3 fatty acids. J. Nutr. 2013, 143, 923–930. [Google Scholar] [CrossRef] [PubMed]
- Afonso, C.; Costa, S.; Cardoso, C.; Oliveira, R.; Lourenço, H.M.; Viula, A.; Batista, I. Benefits and risks associated with consumption of raw, cooked, and canned tuna (Thunnus spp.) based on the bioaccessibility of selenium and methylmercury. Environ. Res. 2015, 143 Part B, 130–137. [Google Scholar] [CrossRef]
- Liao, W.; Zhao, W.; Wu, Y.; Rong, N.; Liu, X.; Li, K.; Wang, G. Multiple metal(loid)s bioaccessibility from cooked seafood and health risk assessment. Environ. Geochem. Health 2020, 9. [Google Scholar] [CrossRef]
- Yassine, R. Mercury Bioavailability in Traditional Food and the Effect of Selenium. University of Ottawa: Ottawa, ON, Canada, 2017. [Google Scholar]
- Laird, B.D.; Shade, C.; Gantner, N.; Chan, H.M.; Siciliano, S.D. Bioaccessibility of mercury from traditional northern country foods measured using an in vitro gastrointestinal model is independent of mercury concentration. Sci. Total Environ. 2009, 407, 6003–6008. [Google Scholar] [CrossRef]
- Burger, J.; Dixon, C.; Boring, S.; Gochfield, M. Effect of deep-frying fish on risk from mercury. J. Toxicol. Environ. Health Part A 2003, 66, 817–828. [Google Scholar] [CrossRef] [PubMed]
- Costa, S.; Afonso, C.; Cardoso, C.; Batista, I.; Chaveiro, N.; Nunes, M.L.; Bandarra, N.M. Fatty acids, mercury, and methylmercury bioaccessibility in salmon (Salmo salar) using an in vitro model: Effect of culinary treatment. Food Chem. 2015, 185, 268–276. [Google Scholar] [CrossRef]
- Morgan, J.N.; Berry, M.R.; Graves, R.L. Effects of commonly used cooking practices on total mercury concentration in fish and their impact on exposure assessments. J. Expo. Anal. Environ. 1997, 7, 119–133. [Google Scholar]
- Moses, S.K.; Whiting, A.V.; Bratton, F.R.; Taylor, R.J.; O’Hara, T.M. Inorganic nutrients and contaminants in subsistence species of Alaska: Linking wildlife and human health. Int. J. Circumpolar Health 2009, 68, 53–74. [Google Scholar] [CrossRef]
- Vincent, C. Retorting Machinery for the Manufacture of Heat-sterilised Fish Products. In Fish Canning Handbook; Wiley-Blackwell: Worcester, UK, 2010; pp. 179–209. [Google Scholar]
- Costa, F.D.N.; Korn, M.G.A.; Brito, G.B.; Ferlin, S.; Fostier, A.H. Preliminary results of mercury levels in raw and cooked seafood and their public health impact. Food Chem. 2016, 192, 837–841. [Google Scholar] [CrossRef]
- Costa, S.; Afonso, C.; Bandarra, N.M.; Gueifão, S.; Castanheira, I.; Carvalho, M.L.; Cardoso, C.; Nunes, M.L. The emerging farmed fish species meagre (Argyrosomus regius): How culinary treatment affects nutrients and contaminants concentration and associated benefit-risk balance. Food Chem. Toxicol. 2013, 60, 277–285. [Google Scholar] [CrossRef]
- Maulvault, A.L.; Machado, R.; Afonso, C.; Lourenço, H.M.; Nunes, M.L.; Coelho, I.; Langerholc, T.; Marques, A. Bioaccessibility of Hg, Cd and As in cooked black scabbard fish and edible crab. Food Chem. Toxicol. 2011, 49, 2808–2815. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Afonso, C.; Costa, S.; Cardoso, C.; Bandarra, N.M.; Batista, I.; Coelho, I.; Castanheira, I.; Nunes, M.L. Evaluation of the risk/benefit associated to the consumption of raw and cooked farmed meagre based on the bioaccessibility of selenium, eicosapentaenoic acid and docosahexaenoic acid, total mercury, and methylmercury determined by an in vitro digestion mo. Food Chem. 2015, 170, 249–256. [Google Scholar] [CrossRef]
- Afonso, C.; Costa, S.; Cardoso, C.; Coelho, I.; Castanheira, I.; Lourenço, H.; Gonçalves, S.; Oliveira, R.; Carvalho, M.L.; Martins, M.F.; et al. Bioaccessibility in risk-benefit analysis of raw and cooked seabream consumption. J. Food Compos. Anal. 2018, 68, 118–127. [Google Scholar] [CrossRef]
- Cano-Sancho, G.; Perelló, G.; Maulvault, A.L.; Marques, A.; Nadal, M.; Domingo, J.L. Oral bioaccessibility of arsenic, mercury and methylmercury in marine species commercialized in Catalonia (Spain) and health risks for the consumers. Food Chem. Toxicol. 2015. [Google Scholar] [CrossRef] [PubMed]
- Matos, J.; Lourenço, H.M.; Brito, P.; Maulvault, A.L.; Martins, L.L.; Afonso, C. Influence of bioaccessibility of total mercury, methyl-mercury and selenium on the risk/benefit associated to the consumption of raw and cooked blue shark (Prionace glauca). Environ. Res. 2015, 143, 123–129. [Google Scholar] [CrossRef]
- Siedlikowski, M.; Bradley, M.; Kubow, S.; Goodrich, J.M.; Franzblau, A.; Basu, N. Bioaccessibility and bioavailability of methylmercury from seafood commonly consumed in North America: In vitro and epidemiological studies. Environ. Res. 2016, 149, 266–273. [Google Scholar] [CrossRef]
- Wang, H.S.; Xu, W.F.; Chen, Z.J.; Cheng, Z.; Ge, L.C.; Man, Y.B.; Giesy, J.P.; Du, J.; Wong, C.K.C.; Wong, M.H. In vitro estimation of exposure of Hong Kong residents to mercury and methylmercury via consumption of market fishes. J. Hazard. Mater. 2013, 248–249, 387–393. [Google Scholar] [CrossRef]
- Lescord, G.L.; Johnston, T.A.; Branfireun, B.A.; Gunn, J.M. Percent methylmercury in the muscle tissue of freshwater fish varies with body size, age, and among species. Environ. Toxicol. Chem. 2018, 37, 2682–2691. [Google Scholar] [CrossRef]
- Lemes, M.; Wang, F.; Stern, G.A.; Ostertag, S.K.; Chan, H.M. Methylmercury and selenium speciation in different tissues of beluga whales (Delphinapterus leucas) from the western Canadian Arctic. Environ. Toxicol. Chem. 2011, 30, 2732–2738. [Google Scholar] [CrossRef]
- Wagemann, R.; Trebacz, E.; Boila, G.; Lockhart, W.L. Methylmercury and total mercury in tissues of arctic marine mammals. Sci. Total Environ. 1998, 218, 19–31. [Google Scholar] [CrossRef]
- Charette, T.; Bueno Dalto, D.; Rosabal, M.; Matte, J.J.; Amyot, M. Assessment of in vitro bioaccessibility and in vivo oral bioavailability as complementary tools to better understand the effect of cooking on methylmercury, arsenic, and selenium in tuna. Toxics 2021, 9, 27. [Google Scholar] [CrossRef] [PubMed]
- Brodkorb, A.; Egger, L.; Alminger, M.; Alvito, P.; Assunção, R.; Ballance, S.; Bohn, T.; Bourlieu-Lacanal, C.; Boutrou, R.; Carrière, F.; et al. INFOGEST static in vitro simulation of gastrointestinal food digestion. Nat. Protoc. 2019, 14, 991–1014. [Google Scholar] [CrossRef]
- Charette, T.; Rosabal, M.; Amyot, M. Mapping metal (Hg, As, Se), lipid and protein levels within fish muscular system in two fish species (Striped Bass and Northern Pike). Chemosphere 2021, 265, 129036. [Google Scholar] [CrossRef]
- Health Canada. Part V: Guidance on Human Health Detailed Quantitative Risk Assessement for Chemicals (DQRA Chem); Health Canada: Ottawa, ON, Canada, 2010. [Google Scholar]
- Gaudin Laberge, V.; Receveur, O.; Walz, L.; Girard, F.; Potvin, L. A mixed methods inquiry into the determinants of traditionnal food consumption among three Cree communities of Eeyou Istchee from an ecological perspective. Int. J. Circumpolar Health 2014. [Google Scholar] [CrossRef]
- Legrand, M.; Feeley, M.; Tikhonov, C.; Schoen, D.; Li-Muller, A. Methylmercury blood guidance values for Canada. Can. J. Public Health 2010, 101, 28–31. [Google Scholar] [CrossRef]
- Watzl, B.; Gelencsér, E.; Hoekstra, J.; Kulling, S.; Lydeking-Olsen, E.; Rowland, I.; Schilter, B.; van Klaveren, J.; Chiodini, A. Application of the BRAFO-tiered approach for benefit-risk assessment to case studies on natural foods. Food Chem. Toxicol. 2012, 50, S699–S709. [Google Scholar] [CrossRef]
- Van Oostdam, J.; Donaldson, S.G.; Feeley, M.; Arnold, D.; Ayotte, P.; Bondy, G.; Chan, L.; Dewaily, É.; Furgal, C.M.; Kuhnlein, H.; et al. Human health implications of environmental contaminants in Arctic Canada: A review. Sci. Total Environ. 2005, 351–352, 165–246. [Google Scholar] [CrossRef]


| Input Variables | Point Estimate | Probability Distribution | Units | References | |||
|---|---|---|---|---|---|---|---|
| CTE a (SD) | RMaE b | Distribution Type | Parameters min;max | ||||
| CR | Scenario 1—Fish flesh | ||||||
| Medium consumer | 22 | g/day | [15] | ||||
| High consumer | 40 | g/day | [15] | ||||
| Scenario 2—Marine mammals | |||||||
| Beluga meat (pre-drying) | 3.4 | g/day | [2] | ||||
| Beluga nikku | 2.3 | g/day | [2] | ||||
| Seal liver | 1.0 | g/day | [2] | ||||
| BW | Scenario 1—Fish flesh | ||||||
| General population | 76.5 (15.8) | 53.3 | lognormal | [40;120] *** | kg | [34] | |
| Sensitive population | 69.8 (16.3) | 46.5 | lognormal | [35;115] *** | kg | [34] | |
| Scenario 2—Marine mammals | |||||||
| Indigenous population | 68.4 (20) * | 43.9 | lognormal | [38.2–129.4] | kg | [2] | |
| [THg] | Scenario 1—Fish flesh | ||||||
| Salmon | 0.03 (0.03) * | 0.07 | lognormal | [0–0.12] | ug/g (ww) | [15] | |
| Albacore canned tuna | 0.35 (0.1) ** | 0.5 | lognormal | [0.2–0.6] | ug/g (ww) | [15] | |
| Scenario 2—Marine mammals | |||||||
| Beluga meat (pre-drying) | 2.0 (0.5) † | 3.0 | lognormal | [0.8–4.0] | ug/g (ww) | [35] | |
| Beluga nikku | 5.0 (1.2) | 7.2 | lognormal | [2,3,4,5,6,7,8,9,10] *** | ug/g (ww) | [35] | |
| Ringed seal liver | 19 (11.1) | 23.7 | lognormal | [10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25] *** | ug/g (ww) | [35] | |
| pMeHg | Scenario 1—Fish flesh | ||||||
| Health authorities | 100 | % | [15] | ||||
| Literature mid data | 90 | % | [30] | ||||
| Scenario 2—Marine mammals | |||||||
| Beluga meat | 65 | % | [2] | ||||
| Ringed seal liver | 11 | % | [2] | ||||
| RAF | Scenario 1—Fish flesh | ||||||
| Health authorities | 100 | % | [15] | ||||
| Cooked—In vitro bioaccessibility | 40 | % | [24,25,37,38] | ||||
| Scenario 2—Marine mammals | |||||||
| Beluga meat | 51 | % | [35] | ||||
| Beluga nikku | 33 | % | [35,39] | ||||
| Seal liver | 27 | % | [35,39,40] | ||||
| MLF | Scenario 1—Fish flesh | ||||||
| Salmon—all cooking methods | 1.35 | unitless | [41,42,43,44] | ||||
| Albacore canned tuna | 1 | unitless | |||||
| Scenario 2—Marine mammals | |||||||
| Beluga meat (raw) | 1 | unitless | [2] | ||||
| Beluga nikku (Air-dried) | 2.5 | unitless | [2] | ||||
| Ringed seal liver (raw) | 1 | unitless | [2] | ||||
| Scenario 1—Fish Flesh | Albacore Canned Tuna | ||||
| Medium Consumption Rate | |||||
| current model | pMeHg | RAF | alternative model | ||
| Canadian general population | 0.100 | 0.090 * | 0.040 * | 0.036 * | |
| Sensitive Canadian population | 0.110 (4%) | 0.010 (1%) * | 0.044 * | 0.040 * | |
| Scenario 1—Fish flesh | Albacore Canned Tuna | ||||
| High consumption rate | |||||
| current model | pMeHg | RAF | alternative model | ||
| Canadian general population | 0.182 (0.1%) | 0.164 * | 0.073 * | 0.065 * | |
| Sensitive Canadian population | 0.200 (50%) | 0.180 (38%) * | 0.08 (0.2 %) * | 0.072 (0.1 %) * | |
| Scenario 2—Marine mammals | Seal liver | ||||
| current model | pMeHg | RAF | alternative model | ||
| Indigenous population | 0.242 (2.7%) | 0.027 * | 0.065 * | 0.007 * | |
| Scenario 2—Marine mammals | Beluga meat | ||||
| current model | pMeHg | RAF | alternative model | ||
| Indigenous population | 0.100 | 0.065 * | 0.051 * | 0.033 * | |
| Scenario 2—Marine mammals | Beluga nikku | ||||
| current model | pMeHg | RAF | MLF | alternative model | |
| Indigenous population | 0.068 | 0.044 * | 0.056 * | 0.169 (0.1%) * | 0.036 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Charette, T.; Kaminski, G.; Rosabal, M.; Amyot, M. Effects of Speciation, Cooking and Changes in Bioaccessibility on Methylmercury Exposure Assessment for Contrasting Diets of Fish and Marine Mammals. Int. J. Environ. Res. Public Health 2021, 18, 2565. https://doi.org/10.3390/ijerph18052565
Charette T, Kaminski G, Rosabal M, Amyot M. Effects of Speciation, Cooking and Changes in Bioaccessibility on Methylmercury Exposure Assessment for Contrasting Diets of Fish and Marine Mammals. International Journal of Environmental Research and Public Health. 2021; 18(5):2565. https://doi.org/10.3390/ijerph18052565
Chicago/Turabian StyleCharette, Tania, Gregory Kaminski, Maikel Rosabal, and Marc Amyot. 2021. "Effects of Speciation, Cooking and Changes in Bioaccessibility on Methylmercury Exposure Assessment for Contrasting Diets of Fish and Marine Mammals" International Journal of Environmental Research and Public Health 18, no. 5: 2565. https://doi.org/10.3390/ijerph18052565
APA StyleCharette, T., Kaminski, G., Rosabal, M., & Amyot, M. (2021). Effects of Speciation, Cooking and Changes in Bioaccessibility on Methylmercury Exposure Assessment for Contrasting Diets of Fish and Marine Mammals. International Journal of Environmental Research and Public Health, 18(5), 2565. https://doi.org/10.3390/ijerph18052565








