Patulin Contamination of Citrus Fruits from Punjab and Northern Pakistan and Estimation of Associated Dietary Intake
Abstract
1. Introduction
2. Materials and Methods
2.1. Samples
2.2. Reagents and Chemicals
2.3. The Cleanup Procedure
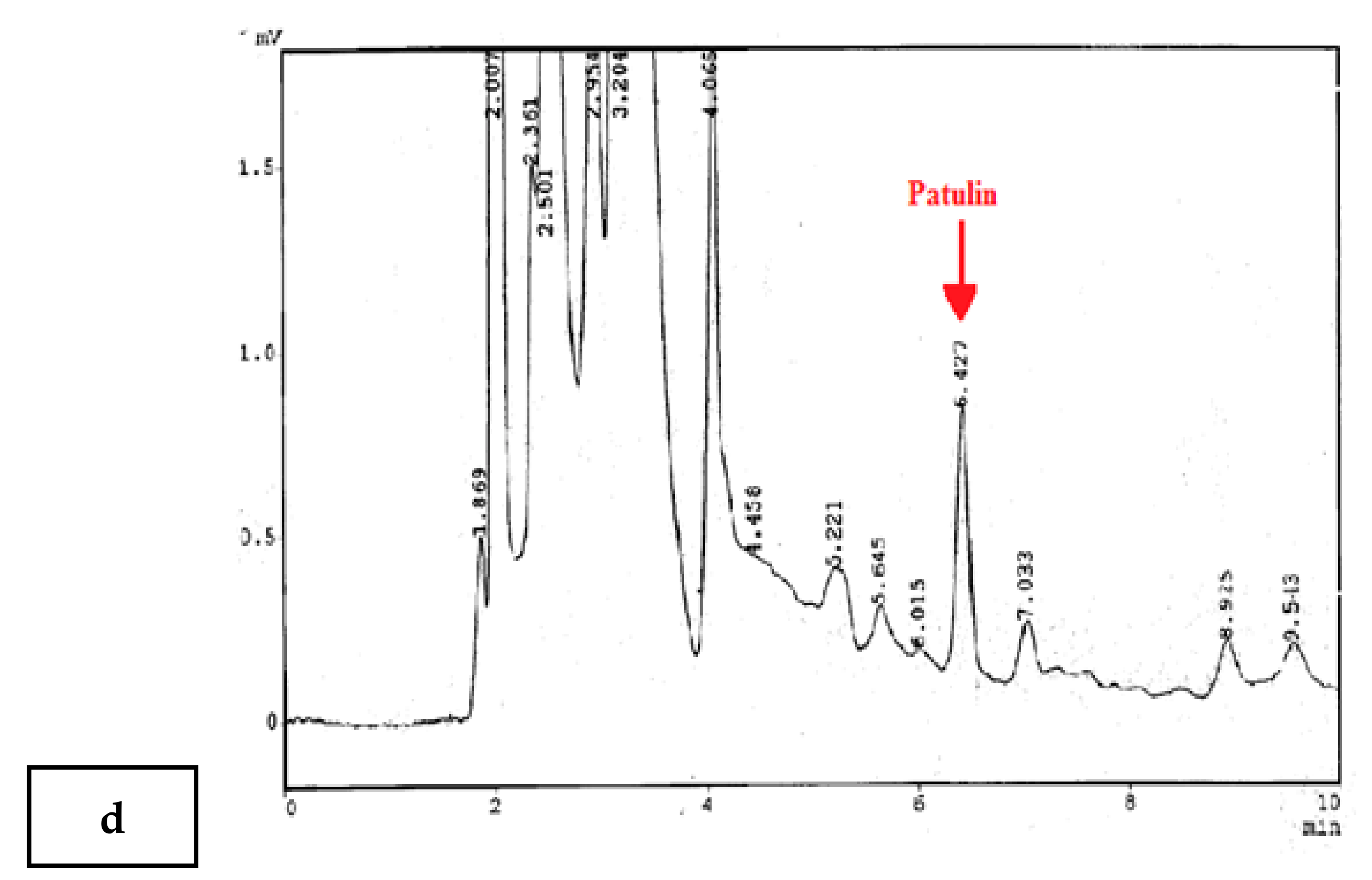
2.4. HPLC Conditions
2.5. Assessment of Daily Intake
2.6. Method Validation
2.7. Statistical Analysis
3. Results and Discussions
3.1. Validation of Method and Quality Control
3.2. Occurrence of PAT in the Citrus Fruit Samples
3.3. Daily Intake Assessment of Patulin
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kahramanoglu, I.; Nisar, M.A.; Chen, C.; Usanmaz, S.; Chen, J.; Wan, C. Light: An Alternative Method for Physical Control of Postharvest Rotting Caused by Fungi of Citrus Fruit. Hindawi J. Food Qual. 2020. [Google Scholar] [CrossRef]
- Abobatta, W.F. Nutritional Benefits of Citrus Fruits. Am. J. Biomed. Sci. Res. 2019, 3, 303–306. [Google Scholar] [CrossRef]
- Government of Pakistan. Fruit, Vegetables and Condiments Statistics of Pakistan 2017–2018; Government of Pakistan, Ministry of National Food Security & Research, Economic Wing: Islamabad, Pakistan, 2019.
- Akhtar, N.; Anjum, T.; Jabeen, R. Isolation and Identification of Storage Fungi from Citrus Sampled from Major Growing areas of Punjab, Pakistan. Int. J. Agric. Biol. 2013, 15, 1283–1288. [Google Scholar]
- Tripathi, P.; Dubey, N.K. Exploitation of natural products as an alternative strategy to control postharvest fungal rotting of fruit and vegetables. Postharvest Biol. Technol. 2004, 32, 235–245. [Google Scholar] [CrossRef]
- Moss, M.O. Fungi, quality and safety issues in fresh fruits and vegetables. J. Appl. Microbiol. 2008, 104, 1239–1243. [Google Scholar] [CrossRef] [PubMed]
- Tola, M.; Kebede, B. Occurrence, importance and control of mycotoxins: A review. Cogent Food Agric. 2016, 2, 1191103. [Google Scholar] [CrossRef]
- Cardona, A.M.; Johnson, N.; Phillips, T.; Hayes, A. Mycotoxins in a changing global environment—A review. Food Chem. Toxicol. 2014, 69, 220–230. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, S.Z.; Usman, S.; Razis, A.F.A.; Ali, N.B.; Saif, T.; Asi, M.R. Assessment of Deoxynivalenol in Wheat, Corn and Its Products and Estimation of Dietary Intake. Int. J. Environ. Res. Public Health 2020, 17, 5602. [Google Scholar] [CrossRef] [PubMed]
- Marin, S.; Ramos, A.J.; Sancho, C.G.; Sanchis, V. Mycotoxins: Occurrence, toxicology, and exposure assessment. Chem. Toxicol. 2013, 60, 218–237. [Google Scholar] [CrossRef] [PubMed]
- Wu, F. Measuring the economic impacts of Fusarium toxins in animal feeds. Anim. Feed Sci. Technol. 2007, 137, 363–374. [Google Scholar] [CrossRef]
- Luo, Y.; Liu, X.; Li, J. Updating techniques on controlling mycotoxins—A review. Food Control 2018, 89, 123–132. [Google Scholar] [CrossRef]
- Li, X.; Li, H.; Li, X.; Zhang, Q. Determination of trace patulin in apple-based food matrices. Food Chem. 2017, 233, 290–301. [Google Scholar] [CrossRef] [PubMed]
- Adeyeye, S.A.O. Fungal mycotoxins in foods: A review. Cogent Food Agric. 2016, 2, 1–11. [Google Scholar] [CrossRef]
- Yun, N.J. Occurrence, Control and Determination of Patulin Contamination in Fruits and Fruit Products. Sci. Agric. Sin. 2017, 50, 3591–3607. [Google Scholar]
- Gaspar, E.M.S.M.; Lucena, A.F.F. Improved HPLC methodology for food control—furfurals and patulin as markers of quality. Food Chem. 2009, 114, 1576–1582. [Google Scholar] [CrossRef]
- Moake, M.M.; Zakour, O.I.P.; Worobo, R.W. Comprehensive review of patulin control methods in food. Compr. Rev. Food Sci. Food Saf. 2005, 4, 8–21. [Google Scholar] [CrossRef]
- International Agency for Research on Cancer (IARC). Some traditional herbal medicines, some mycotoxins, naphthalene and styrene. WHO IARC Monogr. Eval. Carcinog. Risks Hum. 2002, 82, 1–556. [Google Scholar]
- World Health Organization. Evaluation of certain food additives and contaminants—44th report of the Joint food and agriculture organization/world health organization Expert committee on food additives. Tech. Rep. Ser. 1995, 859, 36–38. [Google Scholar]
- Codex Alimentarius Commission. Code of practice for the prevention and reduction of patulin contamination in apple juice and apple juice ingredients in other beverages. Codex Aliment. Comm. Recomm. Code Pract. 2003, 50, 1–6. [Google Scholar]
- Commission Regulations. Commission regulation (EC) No 1881/2006 of 19 December 2006 setting maximum levels for certain contaminants in foodstuffs. Off. J. Eur. Union 2006, 364, 5–24. [Google Scholar]
- Iqbal, S.Z.; Malik, S.; Asi, M.R.; Selamat, J.; Malik, N. Natural occurrence of patulin in different fruits, juices and smoothies and evaluation of dietary intake in Punjab, Pakistan. Food Control 2018, 84, 370–374. [Google Scholar] [CrossRef]
- Zhang, H.; Sun, J.; Zhang, Y.; Lu, M.; Sun, L.; Li, W.; Hu, X.; Wang, B. Retention of deoxynivalenol and its derivatives during storage of wheat grain and flour. Food Control 2016, 65, 177–181. [Google Scholar] [CrossRef]
- European Commission. Commission Regulation (EC) No 401/2006 of 23 February 2006 laying down the methods of sampling and analysis for the official control of the levels of mycotoxins in foodstuffs. Off. J. Eur. Union 2006, 70, 19–23. [Google Scholar]
- Li, X.; Li, H.; Ma, W.; Guo, Z.; Li, X.; Li, X.; Zhang, Q. Determination of patulin in apple juice by single-drop liquid-liquid-liquid microextraction coupled with liquid chromatography-mass spectrometry. Food Chem. 2018, 257, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Sengul, U. Comparing determination methods of detection and quantification limits for aflatoxin analysis in Hazelnut. J. Food Drug Anal. 2016, 24, 56–62. [Google Scholar] [CrossRef]
- Hussain, S.; Asi, M.R.; Iqbal, M.; Khalid, N.; Hassan, S.W.; Arino, A. Patulin Mycotoxin in Mango and Orange Fruits, Juices, Pulps, and Jams Marketed in Pakistan. Toxins 2020, 12, 52. [Google Scholar] [CrossRef]
- Majerus, P.; Kapp, K. Assessment of Dietary Intake of Patulin by the Population of EU Member States; Reports on Tasks for Scientific Cooperation, Task 3.2.8; SCOOP Report: Brussels, Belgium, 2002. [Google Scholar]
- Funes, G.J.; Resnik, S.L. Determination of patulin in solid and semisolid apple and pear products marketed in Argentina. Food Control 2009, 20, 277–280. [Google Scholar] [CrossRef]
- Cho, M.S.; Kim, K.; Seo, E.; Kassim, N.; Mtenga, A.B.; Shim, W.B.; Lee, S.H.; Chung, D.H. Occurrence of Patulin in Various Fruit Juices from South Korea: An Exposure Assessment. Food Sci. Biotechnol. 2010, 19, 1–5. [Google Scholar] [CrossRef]
- Spadaro, D.; Ciavorella, A.; Frati, S.; Garibaldi, A.; Gullino, M.L. Incidence and level of patulin contamination in pure and mixed apple juices marketed in Italy. Food Control 2007, 18, 1098–1102. [Google Scholar] [CrossRef]
- Moukas, A.; Panagiotopoulou, V.; Markaki, P. Determination of patulin in fruit juices using HPLC-DAD and GC-MSD techniques. Food Chem. 2008, 109, 860–867. [Google Scholar] [CrossRef] [PubMed]
- Zouaoui, N.; Sbaii, N.; Bacha, H.; Abid-Essef, S. Occurrence of patulin in various fruit juice marketed in Tunisia. Food Control 2015, 51, 356–360. [Google Scholar] [CrossRef]
- Murillo-Arbizu, M.; Amézqueta, S.; González-Peñas, E.; Certain, A.L.D. Occurrence of patulin and its dietary intake through apple juice consumption by the Spanish population. Food Chem. 2009, 113, 420–423. [Google Scholar] [CrossRef]
- Saxena, N.; Dwivedi, P.D.; Ansari, K.M.; Das, M. Patulin in apple juices: Incidence and likely intake in an Indian population. Food Addit. Contam. Part B 2008, 1, 140–146. [Google Scholar] [CrossRef] [PubMed]
- Rahimi, E.; Jeiran, M.R. Patulin and its dietary intake by fruit juice consumption in Iran. Food Addit. Contam. Part B 2015, 8, 40–43. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Zhou, Z.; Yuan, Y.; Yue, T. Survey of patulin in apple juice concentrates in Shaanxi (China) and its dietary intake. Food Control 2013, 34, 570–573. [Google Scholar] [CrossRef]
- Piemontese, L.; Solfrizzo, M.; Visconti, A. Occurrence of patulin in conventional and organic fruit products in Italy and subsequent exposure assessment. Food Addit. Contam. 2005, 22, 437–442. [Google Scholar] [CrossRef] [PubMed]

| Patulin Fortified Level (µg/kg) | Recovery | RSD | Precision | Retention Time | Coefficient of Determination | LOD | LOQ | |
|---|---|---|---|---|---|---|---|---|
| (%) | (%) | Repeatability | Reproducibility | (min) | R2 | (µg/kg) | (µg/kg) | |
| 50 | 85.5 | 12.5 | 14 | 12 | 6.437 ± 0.050 | 0.9947 | 0.04 | 0.12 |
| 100 | 89.7 | 10.6 | 10 | 11 | ||||
| 200 | 91.5 | 14.5 | 14 | 18 | ||||
| Citrus Fruits Type | Toba Tek Singh | Sargodha | Multan | Jhang | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total/Positive (Positive %) | Mean µg/kg | Range µg/kg | Total/Positive (Positive %) | Mean µg/kg | Range µg/kg | Total/Positive (Positive %) | Mean µg/kg | Range µg/kg | Total/Positive (Positive %) | Mean µg/kg | Range µg/kg | |
| Kinnow | 45/22 (49) | 89.9 ± 5.36 | 0.12–130 | 50/26 (52) | 90.9 ± 6.32 | 0.12–140 | 40/22 (55) | 104.5 ± 4.8 | 0.12- 127 | 50/30 (60) | 91.7 ± 5.6 | 0.12- 115 |
| Orange | 35/18 (52) | 140.4 ± 6.70 | 0.12–220 | 40/19 (48) | 150.6 ± 7.21 | 0.12–225 | 44/25 (54) | 142.6 ± 4.36 | 0.12–245 | 55/32 (58) | 150.3 ± 7.3 | 0.12–221 |
| Grapefruit | 55/22 (40) | 150.9 ± 7.6 | 0.12–190 | 45/23 (51) | 145.7 ± 8.1 | 0.12–198 | 50/26 (52) | 125.8 ±7.5 | 0.12–178 | 35/10 (29) | 115.9 ± 4.3 | 0.12–216 |
| Bitter orange | 40/16 (40) | 190.6 ± 9.7 | 0.12–230 | 35/12 (34) | 195.2 ± 10.4 | 0.12–232 | 30/15 (50) | 198.4 ± 6.1 | 0.12–210 | 25/5 (20) | 175.2 ± 4.7 | 0.12–198 |
| Mosambi | 45/19 (42) | 170.6 ±11.7 | 0.12–210 | 40/25 (63) | 165.3 ± 10.9 | 0.12–200 | 50/28 (56) | 194.3 ± 12.5 | 0.12–295 | 52/30 (58) | 165.6 ± 10.3 | 0.12–201 |
| Red blood | 30/10 (67) | 89.9 ± 13.7 | 0.12–150 | 50/30 (60) | 76.1 ± 12.8 | 0.12–153 | 45/22 (49) | 75.4 ± 10.6 | 0.12–135 | 46/25 (54) | 77.4 ± 8.4 | 0.12–111 |
| Pineapple | 20/10 (50) | 45.7 ± 15.7 | 0.12–120 | 30/15 (50) | 50.2 ± 15.2 | 0.12–121 | 25/9 (36) | 54.1 ± 14.3 | 0.12–110 | 20/9 (45) | 65.7 ± 11.5 | 0.12–113 |
| Sweet orange | 30/17 (57) | 55.7 ± 9.8 | 0.12–140 | 40/22 (55) | 65.4 ± 10.1 | 0.12–155 | 45/26 (58) | 45.2 ± 7.5 | 0.12–1150 | 25/6 (24) | 55.2 ± 8.5 | 0.04–99 |
| Rough lime | 48/27 (56) | 78.6 ± 10.7 | 0.12–150 | 45/27 (60) | 65.3 ± 9.3 | 0.12–168 | 30/11 (37) | 88.3 ± 9.6 | 0.12–146 | 30/12 (40) | 97.4 ± 11.3 | 0.12–123 |
| Sweet lime | 50/28 (56) | 99.7 ± 15.8 | 0.12–205 | 55/33 (60) | 104.6 ± 14.9 | 0.12–210 | 30/8 (27) | 103.1 ± 12.5 | 0.12–225 | 22/5 (23) | 122.6 ± 8.9 | 0.12–197 |
| Kagzi lime | 43/22 (51) | 110.6 ± 11.7 | 0.12–220 | 45/32 (71) | 122.5 ± 10.4 | 0.12–234 | 55/30 (55) | 125.4 ± 15.3 | 0.12–215 | 53/35 (66) | 130.1 ± 14.3 | 0.12–231 |
| Lemon | 40/26 (65) | 85.5 ± 16.7 | 0.12–130 | 50/30 (60) | 92.3 ± 15.9 | 0.12–145 | 54/36 (67) | 106.7 ± 15.3 | 0.12–155 | 50/32 (64) | 125.5 ± 9.6 | 0.12–147 |
| Total | 481/247 (51.4) | 0.12–230 | 525/294 (56) | 0.12–234 | 498/329 (66) | 0.12–1150 | 463/231 (50) | 0.12–231 | ||||
| Total | 1967/1101 (56.0) | |||||||||||
| Citrus Fruits Type | Mirpur | Peshawar | Swat | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Total/Positive (Positive %) | Mean µg/kg | Range µg/kg | Total/Positive (Positive %) | Mean µg/kg | Range µg/kg | Total/Positive (Positive %) | Mean µg/kg | Range µg/kg | |
| Kinnow | 45/24 (53.3) | 75.3 ± 4.1 | 0.12- 178 | 52/31 (59.7) | 80.2 ± 9.6 | 0.12- 167 | 25/10 (40) | 80.5 ± 7.3 | 0.12- 320 |
| Orange | 40/16 (40) | 99.4 ± 8.5 | 0.12–156 | 44/22 (50) | 144.2 ± 9.2 | 0.12–167 | 25/5 (20) | 163.3 ± 10.1 | 0.12–204 |
| Grapefruit | 20/6 (30) | 76.2 ± 11.4 | 0.12–187 | 25/3 (12) | 88.4 ± 6.3 | 0.12–168 | 10/1 (10) | 109.6 ± 5.8 | 0.12–121 |
| Bitter orange | 10/1 (10) | 102.8 ± 9.4 | 0.12–199 | 16/1 (6.2) | 133.4 ± 10.5 | 0.12–186 | 05/1 (20) | 156.3 ± 6.3 | 0.12–209 |
| Mosambi | 33/8 (24.2) | 111.4 ± 11.7 | 0.12–150 | 35/7 (20) | 125.8 ± 15.2 | 0.12–221 | 40/23 (57.5) | 145.2 ± 12.1 | 0.12–178 |
| Red blood | 30/6 (20) | 72.1 ± 9.4 | 0.12–187 | 40/18 (45) | 67.3 ± 3.8 | 0.12–196 | 20/6 (30) | 56.7 ± 5.2 | 0.12–120 |
| Pineapple | 15/3 (20) | 45.2 ± 11.8 | 0.12–165 | 15/2 (13.3) | 36.4 ± 8.2 | 0.12–178 | 20/5 (25) | 53.1 ± 13.7 | 0.12–101 |
| Sweet orange | 15/2 (13.3) | 56.3± 8.8 | 0.12–133 | 14/1 (7.1) | 65.2 ± 6.2 | 0.12–130 | 20/4 (20) | 67.4 ± 12.4 | 0.12–102 |
| Rough lime | 30/10 (3.3) | 88.9 ± 13.6 | 0.12–156 | 25/5 (20) | 95.3 ± 12.7 | 0.12–168 | 35/13 (37.1) | 86.9 ± 4.5 | 0.12–132 |
| Sweet lime | 20/4 (20) | 56.8 ± 10.7 | 0.12–203 | 22/8 (36.3) | 44.1 ± 5.3 | 0.12–199 | 27/10 (37) | 35.8 ± 4.2 | 0.12–210 |
| Kagzi lime | 45/25 (55.5) | 109.6 ± 8.7 | 0.12–225 | 40/21 (52.5) | 125.4 ± 13.9 | 0.12–208 | 40/20 (50) | 156.3 ± 11.4 | 0.12–234 |
| Lemon | 40/21 (52.5) | 114.5 ± 15.8 | 0.12–221 | 33/14 (42.4) | 125.2 ± 7.9 | 0.12–199 | 32/15 (47) | 111.9 ± 13.4 | 0.12–178 |
| Total | 343/126 (36.7) | 0.12–225 | 361/133 (36.8) | 0.12–221 | 299/113 (37.8) | 0.12–320 | |||
| Total | 1003/372 (37.1) | ||||||||
| Citrus Fruits Type | Toba Tek Singh | Sargodha | Multan | Jhang | Mirpur | Peshawar | Swat | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n ≥ 50 µg/kg | N (%) ≥ 50 µg/kg | n ≥ 50 µg/kg | N (%) ≥ 50 µg/kg | n ≥ 50 µg/kg | N (%) ≥ 50 µg/kg | n ≥ 50 µg/kg | N (%) ≥ 50 µg/kg | n ≥ 50 µg/kg | N (%) ≥ 50 µg/kg | n ≥ 50 µg/kg | N (%) ≥ 50 µg/kg | n ≥ 50 µg/kg | N (%) ≥ 50 µg/kg | |
| Kinnow | 12 | 26.7 | 14 | 28.0 | 10 | 25.0 | 18 | 36.0 | 13 | 28.9 | 14 | 26.9 | 5 | 20.0 |
| Orange | 11 | 31.4 | 10 | 25.0 | 12 | 27.3 | 16 | 29.1 | 9 | 22.5 | 10 | 22.7 | 0 | 0.0 |
| Grapefruit | 10 | 18.2 | 12 | 26.7 | 20 | 40.0 | 5 | 14.3 | 1 | 5.0 | 0 | 0.0 | 0 | 0.0 |
| Bitter orange | 9 | 22.5 | 8 | 22.9 | 10 | 33.3 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| Mosambi | 12 | 26.7 | 15 | 37.5 | 16 | 32.0 | 14 | 26.9 | 2 | 6.1 | 0 | 0.0 | 8 | 20.0 |
| Red blood | 7 | 23.3 | 18 | 36.0 | 11 | 24.4 | 12 | 26.1 | 0 | 0.0 | 7 | 17.5 | 0 | 0.0 |
| Pineapple | 7 | 35.0 | 7 | 23.3 | 4 | 16.0 | 4 | 20.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| Sweet orange | 11 | 36.7 | 10 | 25.0 | 6 | 13.3 | 2 | 8.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| Rough lime | 13 | 27.1 | 13 | 28.9 | 5 | 16.7 | 4 | 13.3 | 2 | 6.7 | 0 | 0.0 | 7 | 20.0 |
| Sweet lime | 14 | 28.0 | 16 | 29.1 | 4 | 13.3 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 5 | 18.5 |
| Kagzi lime | 12 | 27.9 | 18 | 40.0 | 13 | 23.6 | 14 | 26.4 | 6 | 13.3 | 10 | 25.0 | 13 | 32.5 |
| Lemon | 14 | 35.0 | 15 | 30.0 | 15 | 27.8 | 14 | 28.0 | 9 | 22.5 | 8 | 24.2 | 11 | 34.4 |
| Total | 132 | 27.4 | 156 | 29.7 | 126 | 25.3 | 103 | 22.2 | 42 | 12.2 | 49 | 13.6 | 49 | 16.4 |
| Total | 657 (22.1) a | |||||||||||||
| Citrus Fruits Type | Toba Tek Singh | Sargodha | Multan | Jhang | |||||
|---|---|---|---|---|---|---|---|---|---|
| Consumption | Mean | Patulin Intake | Mean | Patulin Intake | Mean | Patulin Intake | Mean | Patulin Intake | |
| g/day | (µg/g) | µg/kg bw/day | (µg/g) | µg/kg bw/day | (µg/g) | µg/kg bw/day | (µg/g) | µg/kg bw/day | |
| Kinnow | 200 | 79.9 | 0.23 | 80.9 | 0.23 | 84.5 | 0.24 | 81.7 | 0.23 |
| Orange | 250 | 140.4 | 0.51 | 150.6 | 0.54 | 142.6 | 0.51 | 150.3 | 0.54 |
| Grapefruit | 100 | 150.9 | 0.22 | 145.7 | 0.21 | 125.8 | 0.18 | 115.9 | 0.17 |
| Bitter orange | 300 | 190.6 | 0.82 | 195.2 | 0.84 | 198.4 | 0.85 | 175.2 | 0.75 |
| Mosambi | 400 | 170.6 | 0.97 | 165.3 | 0.94 | 194.3 | 1.11 | 165.6 | 0.95 |
| Red blood | 250 | 89.9 | 0.32 | 76.1 | 0.27 | 75.4 | 0.27 | 77.4 | 0.28 |
| Pineapple | 150 | 45.7 | 0.10 | 50.2 | 0.11 | 54.1 | 0.12 | 65.7 | 0.14 |
| Sweet orange | 1000 | 55.7 | 0.80 | 65.4 | 0.93 | 45.2 | 0.65 | 55.2 | 0.79 |
| Rough lime | 400 | 78.6 | 0.45 | 65.3 | 0.37 | 88.3 | 0.50 | 97.4 | 0.56 |
| Sweet lime | 200 | 99.7 | 0.28 | 104.6 | 0.30 | 103.1 | 0.29 | 122.6 | 0.35 |
| Kagzi lime | 100 | 110.6 | 0.16 | 122.5 | 0.18 | 125.4 | 0.18 | 130.1 | 0.19 |
| Lemon | 100 | 85.5 | 0.12 | 92.3 | 0.13 | 106.7 | 0.15 | 125.5 | 0.18 |
| Citrus Fruits Type | Mirpur | Peshawar | Swat | ||||
|---|---|---|---|---|---|---|---|
| Consumption | Mean | Patulin Intake | Mean | Patulin Intake | Mean | Patulin Intake | |
| g/day | (µg/g) | µg/kg bw/day | (µg/g) | µg/kg bw/day | (µg/g) | µg/kg bw/day | |
| Kinnow | 240 | 95.3 | 0.33 | 101.2 | 0.35 | 90.5 | 0.31 |
| Orange | 270 | 99.4 | 0.38 | 144.2 | 0.56 | 163.3 | 0.63 |
| Grapefruit | 200 | 76.2 | 0.22 | 88.4 | 0.25 | 109.6 | 0.31 |
| Bitter orange | 350 | 102.8 | 0.51 | 133.4 | 0.67 | 156.3 | 0.78 |
| Mosambi | 300 | 111.4 | 0.48 | 125.8 | 0.54 | 145.2 | 0.62 |
| Red blood | 350 | 72.1 | 0.36 | 67.3 | 0.34 | 56.7 | 0.28 |
| Pineapple | 250 | 45.2 | 0.16 | 36.4 | 0.13 | 53.1 | 0.19 |
| Sweet orange | 2000 | 56.3 | 1.61 | 65.2 | 1.86 | 67.4 | 1.93 |
| Rough lime | 300 | 88.9 | 0.38 | 95.3 | 0.41 | 86.9 | 0.37 |
| Sweet lime | 400 | 56.8 | 0.32 | 44.1 | 0.25 | 35.8 | 0.20 |
| Kagzi lime | 200 | 109.6 | 0.31 | 125.4 | 0.36 | 156.3 | 0.45 |
| Lemon | 300 | 134.5 | 0.58 | 145.2 | 0.62 | 131.9 | 0.57 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aslam, K.; Iqbal, S.Z.; Razis, A.F.A.; Usman, S.; Ali, N.B. Patulin Contamination of Citrus Fruits from Punjab and Northern Pakistan and Estimation of Associated Dietary Intake. Int. J. Environ. Res. Public Health 2021, 18, 2270. https://doi.org/10.3390/ijerph18052270
Aslam K, Iqbal SZ, Razis AFA, Usman S, Ali NB. Patulin Contamination of Citrus Fruits from Punjab and Northern Pakistan and Estimation of Associated Dietary Intake. International Journal of Environmental Research and Public Health. 2021; 18(5):2270. https://doi.org/10.3390/ijerph18052270
Chicago/Turabian StyleAslam, Kinza, Shahzad Zafar Iqbal, Ahmad Faizal Abdull Razis, Sunusi Usman, and Nada Basheir Ali. 2021. "Patulin Contamination of Citrus Fruits from Punjab and Northern Pakistan and Estimation of Associated Dietary Intake" International Journal of Environmental Research and Public Health 18, no. 5: 2270. https://doi.org/10.3390/ijerph18052270
APA StyleAslam, K., Iqbal, S. Z., Razis, A. F. A., Usman, S., & Ali, N. B. (2021). Patulin Contamination of Citrus Fruits from Punjab and Northern Pakistan and Estimation of Associated Dietary Intake. International Journal of Environmental Research and Public Health, 18(5), 2270. https://doi.org/10.3390/ijerph18052270










