A Scoping Review of Selected Studies on Predictor Variables Associated with the Malaria Status among Children under Five Years in Sub-Saharan Africa
Abstract
1. Introduction
2. Materials and Methods
2.1. Criteria for Inclusion and Exclusion of Studies
2.2. Search Strategy
2.3. Sources of Information
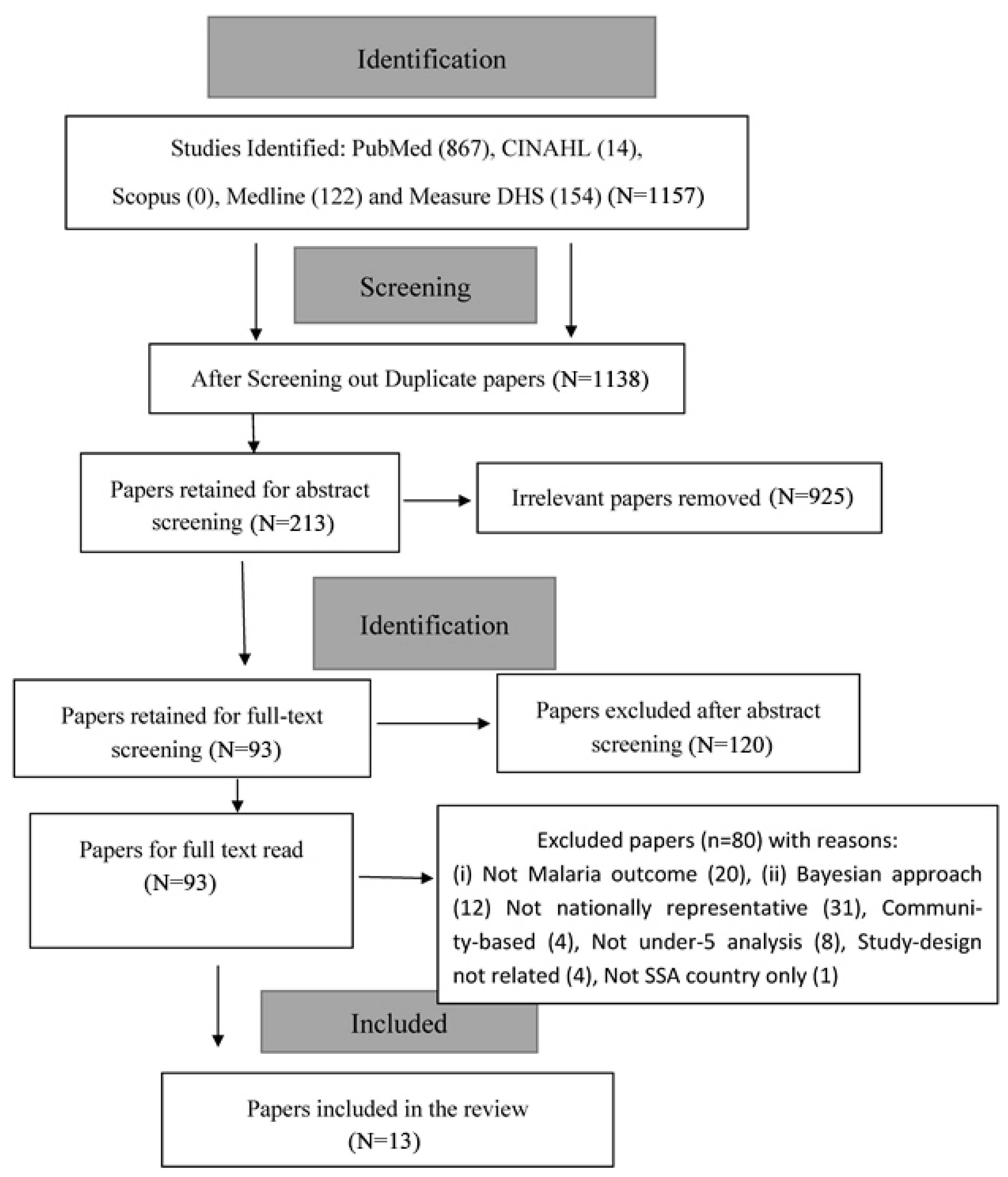
2.4. Study Selection
2.5. Data Selection Process
3. The Results
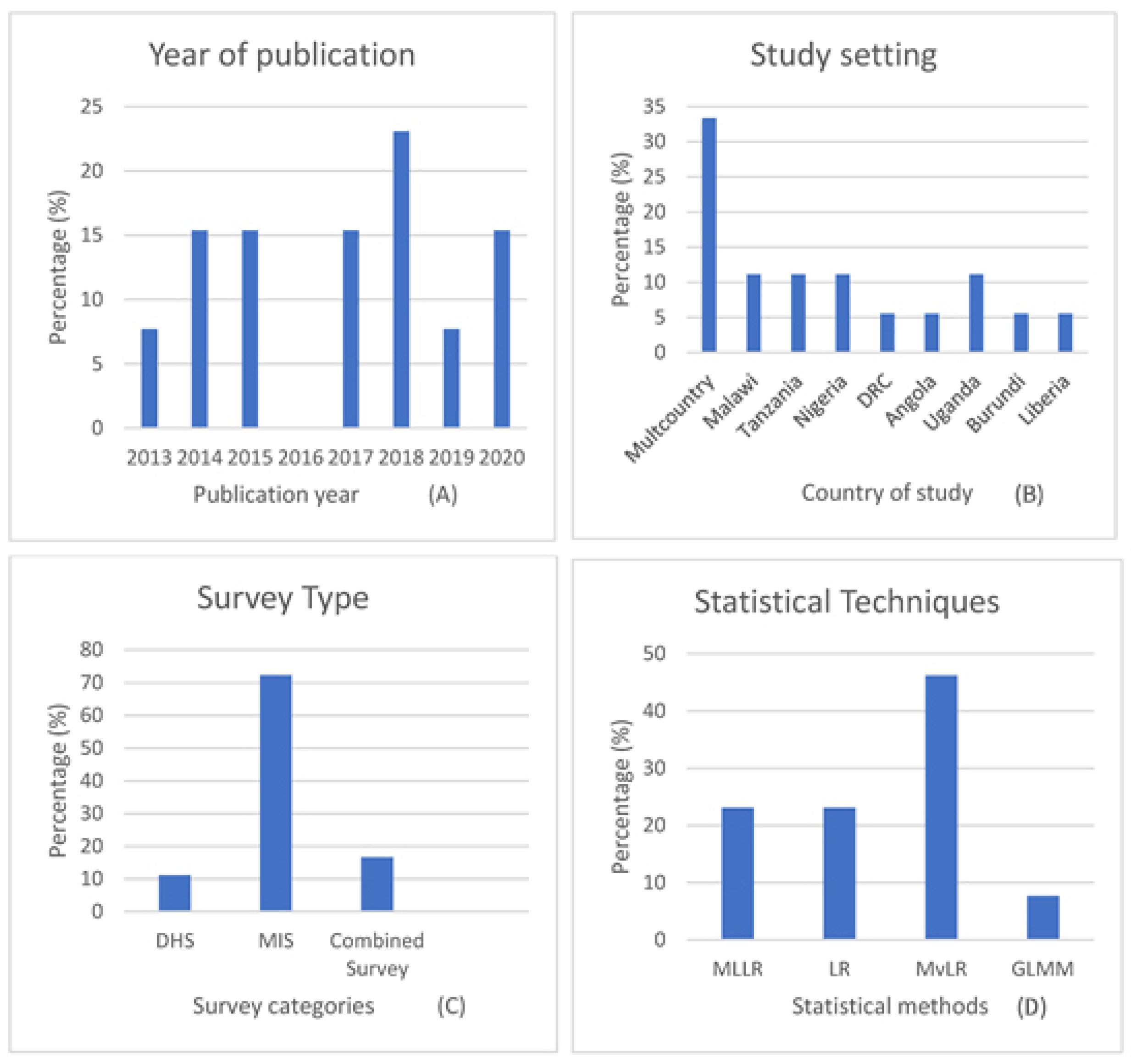
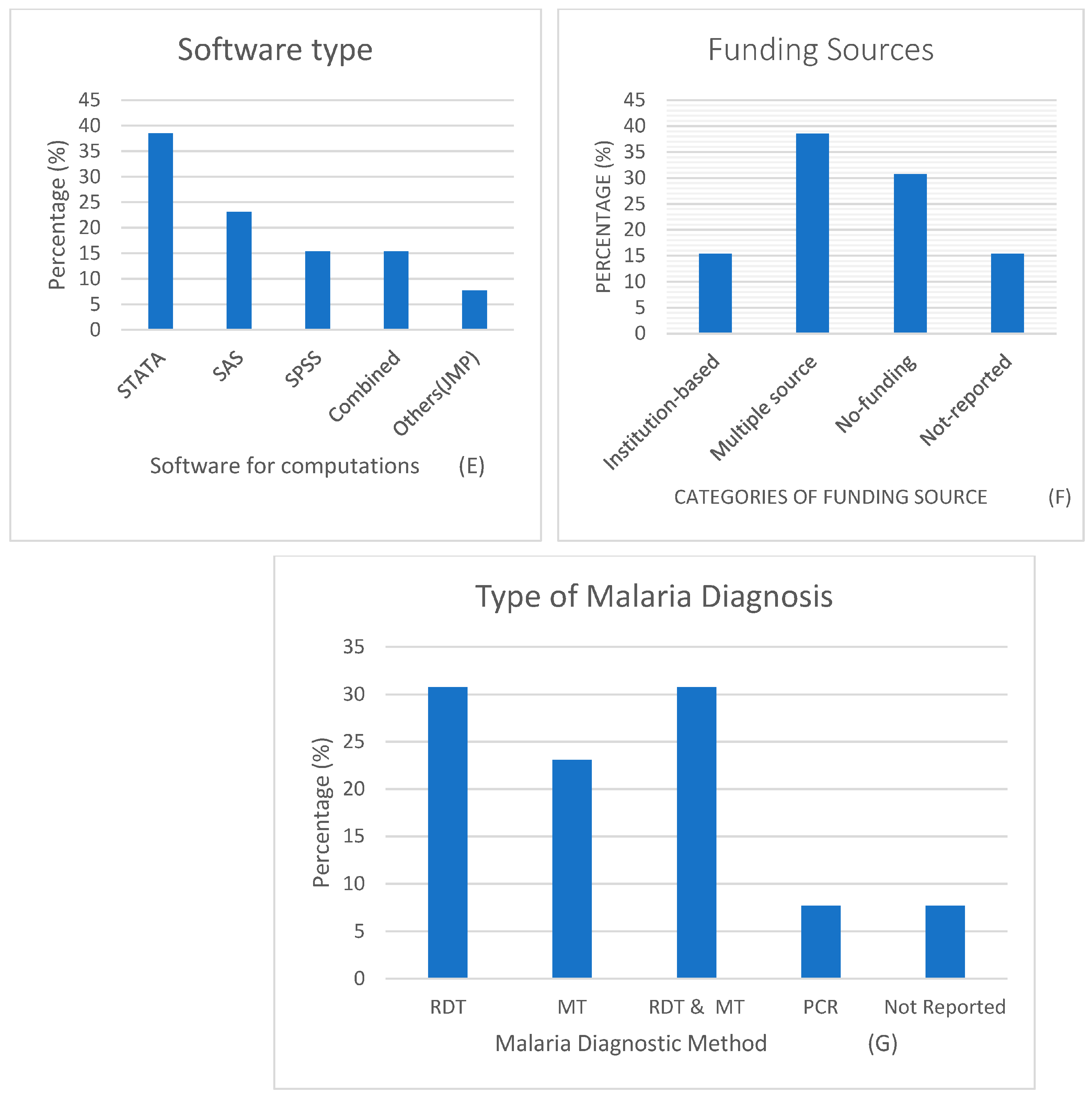
3.1. Description of Study Records
Study characteristics
3.2. Data Synthesis Method
Predictors associated with Malaria Status
Child-Related Variables
Maternal-Related Variables
Household-Related Variables
4. Discussion
5. Strengths and Limitations
6. Future Work
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Bennett, A.; Bisanzio, D.; Yukich, J.O.; Mappin, B.; Fergus, C.A.; Lynch, M.; Cibulskis, R.E.; Bhatt, S.; Weiss, D.J.; Cameron, E.; et al. Population Coverage of Artemisinin-Based Combination Treatment in Children Younger than 5 Years with Fever and Plasmodium Falciparum Infection in Africa, 2003–2015: A Modelling Study Using Data from National Surveys. Lancet Glob. Health 2017, 5, e418–e427. [Google Scholar] [CrossRef]
- Dawaki, S.; Al-Mekhlafi, H.M.; Ithoi, I.; Ibrahim, J.; Atroosh, W.M.; Abdulsalam, A.M.; Sady, H.; Elyana, F.N.; Adamu, A.U.; Yelwa, S.I.; et al. Is Nigeria Winning the Battle against Malaria? Prevalence, Risk Factors and KAP Assessment among Hausa Communities in Kano State. Malar. J. 2016, 15, 351. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. World Malaria Report 2020: 20 Years of Global Progress & Challenges. Available online: https://www.who.int/publications-detail-redirect/9789240015791 (accessed on 3 January 2021).
- Aychiluhm, S.B.; Gelaye, K.A.; Angaw, D.A.; Dagne, G.A.; Tadesse, A.W.; Abera, A.; Dillu, D. Determinants of Malaria among Under-Five Children in Ethiopia: Bayesian Multilevel Analysis. BMC Public Health 2020, 20, 1468. [Google Scholar] [CrossRef] [PubMed]
- World Health Organisation. World Malaria Report 2014. Available online: https://www.who.int/malaria/publications/world_malaria_report_2014/en/ (accessed on 21 February 2019).
- Unicef Malaria in Africa. Available online: https://data.unicef.org/topic/child-health/malaria/ (accessed on 3 January 2021).
- Adinan, J.; Damian, D.J.; Mosha, N.R.; Mboya, I.B.; Mamseri, R.; Msuya, S.E. Individual and Contextual Factors Associated with Appropriate Healthcare Seeking Behavior among Febrile Children in Tanzania. PLoS ONE 2017, 12, e0175446. [Google Scholar] [CrossRef] [PubMed]
- Carlucci, J.G.; Peratikos, M.B.; Cherry, C.B.; Lopez, M.L.; Green, A.F.; Gonzalez-Calvo, L.; Moon, T.D.; Ogumaniha, S.Z. Prevalence and Determinants of Malaria among Children in Zambezia Province, Mozambique. Malar. J. 2017, 16, 108. [Google Scholar] [CrossRef]
- Alene, M.; Yismaw, L.; Berelie, Y.; Kassie, B. Health Care Utilization for Common Childhood Illnesses in Rural Parts of Ethiopia: Evidence from the 2016 Ethiopian Demographic and Health Survey. BMC Public Health 2019, 19, 57. [Google Scholar] [CrossRef]
- Asoba, G.N.; Sumbele, I.U.; Anchang-Kimbi, J.K.; Metuge, S.; Teh, R.N. Influence of Infant Feeding Practices on the Occurrence of Malnutrition, Malaria and Anaemia in Children ≤5 Years in the Mount Cameroon Area: A Cross Sectional Study. PLoS ONE 2019, 14, e0219386. [Google Scholar] [CrossRef] [PubMed]
- Islam, N.; Bonovas, S.; Nikolopoulos, G.K. An Epidemiological Overview of Malaria in Bangladesh. Travel Med. Infect. Dis. 2013, 11, 29–36. [Google Scholar] [CrossRef]
- Kumar, A.; Chery, L.; Biswas, C.; Dubhashi, N.; Dutta, P.; Dua, V.K.; Kacchap, M.; Kakati, S.; Khandeparkar, A.; Kour, D.; et al. Malaria in South Asia: Prevalence and Control. Acta Trop. 2012, 121, 246–255. [Google Scholar] [CrossRef]
- Asia Pacific Leaders Malaria Alliance Bangladesh: New Plan for Malaria Elimination (2017–2021). Available online: https://www.aplma.org/blog/42/bangladesh-new-plan-for-malaria-elimination-2017-2021.html (accessed on 3 January 2021).
- Alemu, A.; Tsegaye, W.; Golassa, L.; Abebe, G. Urban Malaria and Associated Risk Factors in Jimma Town, South-West Ethiopia. Malar. J. 2011, 10, 173. [Google Scholar] [CrossRef] [PubMed]
- Arevalo-Herrera, M.; Quiñones, M.L.; Guerra, C.; Céspedes, N.; Giron, S.; Ahumada, M.; Piñeros, J.G.; Padilla, N.; Terrientes, Z.; Rosas, A.; et al. Malaria in Selected Non-Amazonian Countries of Latin America. Acta Trop. 2012, 121, 303–314. [Google Scholar] [CrossRef]
- National Malaria Elimination Program (NMEP); National Population Commission (NPopC); National Bureau of Statistics (NBS); ICF International. Nigeria Malaria Indicator Survey [MIS8]; National Bureau of Statistics (NBS): Abuja, Nigeria; NMEP, NPopC, and ICF International: Rockville, MD, USA, 2015.
- Bassey, S.E.; Izah, S.C. Some Determinant Factors of Malaria Prevalence in Nigeria. J. Mosq. Res. 2017, 7, 7. [Google Scholar] [CrossRef]
- Baragatti, M.; Fournet, F.; Henry, M.C.; Assi, S.; Ouedraogo, H.; Rogier, C.; Salem, G. Social and Environmental Malaria Risk Factors in Urban Areas of Ouagadougou, Burkina Faso. Malar. J. 2009, 8, 1–14. [Google Scholar] [CrossRef]
- Oladeide, B.H.; Omoregie, R.; Osakue, E.O.; Onaiwu, T.O. Asymptomatic Malaria among Blood Donors in Benin City Nigeria. Iran. J. Parasitol. 2014, 9, 415–422. [Google Scholar]
- Tela, I.A.; Modibbo, M.H.; Adamu, L.H.; Taura, M.G. Prevalence of Malaria Infection among ABO Blood Groups in Jama’are, Nigeria. RA J. Appl. Res. 2015, 1, 255–262. [Google Scholar]
- Shamseer, L.; Moher, D.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A. Preferred Reporting Items for Systematic Review and Meta-Analysis Protocols (PRISMA-P) 2015: Elaboration and Explanation. BMJ 2015, 349, g7647. [Google Scholar] [CrossRef]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.; Horsley, T.; Weeks, L.; et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef]
- Teh, R.N.; Sumbele, I.U.; Meduke, D.N.; Ojong, S.T.; Kimbi, H.K. Malaria Parasitaemia, Anaemia and Malnutrition in Children Less than 15 Years Residing in Different Altitudes along the Slope of Mount Cameroon: Prevalence, Intensity and Risk Factors. Malar. J. 2018, 17, 336. [Google Scholar] [CrossRef]
- Wanzira, H.; Katamba, H.; Okullo, A.E.; Agaba, B.; Kasule, M.; Rubahika, D. Factors Associated with Malaria Parasitaemia among Children under 5 Years in Uganda: A Secondary Data Analysis of the 2014 Malaria Indicator Survey Dataset. Malar. J. 2017, 16, 191. [Google Scholar] [CrossRef]
- Akinlua, J.T.; Meakin, R.; Umar, A.M.; Freemantle, N. Current Prevalence Pattern of Hypertension in Nigeria: A Systematic Review. PLoS ONE 2015, 10, e0140021. [Google Scholar] [CrossRef]
- Njau, J.D.; Stephenson, R.; Menon, M.; Kachur, S.P.; McFarland, D.A. Exploring the Impact of Targeted Distribution of Free Bed Nets on Households Bed Net Ownership, Socio-Economic Disparities and Childhood Malaria Infection Rates: Analysis of National Malaria Survey Data from Three Sub-Saharan Africa Countries. Malar. J. 2013, 12, 1–5. [Google Scholar] [CrossRef]
- Berendsen, M.L.; van Gijzel, S.W.; Smits, J.; de Mast, Q.; Aaby, P.; Benn, C.S.; Netea, M.G.; van der Ven, A.J. BCG Vaccination Is Associated with Reduced Malaria Prevalence in Children under the Age of 5 Years in Sub-Saharan Africa. BMJ Glob. Health 2019, 4, e001862. [Google Scholar] [CrossRef]
- Chitunhu, S.; Musenge, E. Direct and Indirect Determinants of Childhood Malaria Morbidity in Malawi: A Survey Cross-Sectional Analysis Based on Malaria Indicator Survey Data for 2012. Malar. J. 2015, 14, 1–9. [Google Scholar] [CrossRef]
- Levitz, L.; Janko, M.; Mwandagalirwa, K.; Thwai, K.L.; Likwela, J.L.; Tshefu, A.K.; Emch, M.; Meshnick, S.R. Effect of Individual and Community-Level Bed Net Usage on Malaria Prevalence among under-Fives in the Democratic Republic of Congo. Malar. J. 2018, 17, 39. [Google Scholar] [CrossRef] [PubMed]
- Morakinyo, O.M.; Balogun, F.M.; Fagbamigbe, A.F. Housing Type and Risk of Malaria among Under-Five Children in Nigeria: Evidence from the Malaria Indicator Survey. Malar. J. 2018, 17, 311. [Google Scholar] [CrossRef] [PubMed]
- Njau, J.D.; Stephenson, R.; Menon, M.P.; Kachur, S.P.; McFarland, D.A. Investigating the Important Correlates of Maternal Education and Childhood Malaria Infections. Am. J. Trop. Med. Hyg. 2014, 91, 509–519. [Google Scholar] [CrossRef] [PubMed]
- Semakula, H.M.; Song, G.B.; Zhang, S.S.; Achuu, S.P. Potential of Household Environmental Resources and Practices in Eliminating Residual Malaria Transmission: A Case Study of Tanzania, Burundi, Malawi and Liberia. Afr. Health Sci. 2015, 15, 819–827. [Google Scholar] [CrossRef] [PubMed]
- Siri, J.G. Independent Associations of Maternal Education and Household Wealth with Malaria Risk in Children. Ecol. Soc. 2014, 19, 33. [Google Scholar] [CrossRef]
- Tusting, L.S.; Gething, P.W.; Gibson, H.S.; Greenwood, B.; Knudsen, J.; Lindsay, S.W.; Bhatt, S. Housing and Child Health in Sub-Saharan Africa: A Cross-Sectional Analysis. PLoS Med. 2020, 17, e1003055. [Google Scholar] [CrossRef]
- Ugwu, C.L.; Zewotir, T.T. Using Mixed Effects Logistic Regression Models for Complex Survey Data on Malaria Rapid Diagnostic Test Results. Malar. J. 2018, 17, 1–10. [Google Scholar] [CrossRef]
- Yang, D.; He, Y.; Wu, B.; Deng, Y.; Li, M.L.; Yang, Q.; Huang, L.T.; Cao, Y.M.; Liu, Y. Drinking Water and Sanitation Conditions Are Associated with the Risk of Malaria among Children under Five Years Old in Sub-Saharan Africa: A Logistic Regression Model Analysis of National Survey Data. J. Adv. Res. 2020, 21, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Zgambo, M.; Mbakaya, B.C.; Kalembo, F.W. Prevalence and Factors Associated with Malaria Parasitaemia in Children under the Age of Five Years in Malawi: A Comparison Study of the 2012 and 2014 Malaria Indicator Surveys (MISs). PLoS ONE 2017, 12, e0175537. [Google Scholar] [CrossRef] [PubMed]
- Popay, J.; Roberts, H.; Sowden, A.; Petticrew, M.; Arai, L.; Rodgers, M.; Britten, N.; Roen, K.; Duffy, S. Guidance on the Conduct of Narrative Synthesis in Systematic Reviews: A Product from the ESRC Methods Programme, Version 1. 2006. Available online: https://www.lancaster.ac.uk/media/lancaster-university/content-assets/documents/fhm/dhr/chir/NSsynthesisguidanceVersion1-April2006.pdf (accessed on 20 January 2021).
- Berendsen, M.L.; Smits, J.; Netea, M.G.; van der Ven, A. Non-Specific Effects of Vaccines and Stunting: Timing May Be Essential. EBioMedicine 2016, 8, 341–348. [Google Scholar] [CrossRef]
- Roll Back Malaria. Household Survey Indicators for Malaria Control 2018. Available online: https://endmalaria.org/sites/default/files/Household%20Survey%20Indicators%20for%20Malaria%20Control_FINAL.pdf (accessed on 21 February 2021).
- MEASURE Evaluation Malaria Indicator Survey Tool Implemented in 8 African Countries. Available online: https://www.measureevaluation.org/our-work/malaria/malaria-indicator-survey-tool-implemented-in-8-african-countries (accessed on 21 February 2021).
- Roll Back Malaria Malaria Indicator Surveys—Access to Malaria Indicator Surveys, Datasets. Available online: https://www.malariasurveys.org/ (accessed on 3 January 2021).
- Massoda Tonye, S.G.; Kouambeng, C.; Wounang, R.; Vounatsou, P. Challenges of DHS and MIS to Capture the Entire Pattern of Malaria Parasite Risk and Intervention Effects in Countries with Different Ecological Zones: The Case of Cameroon. Malar. J. 2018, 17, 156. [Google Scholar] [CrossRef] [PubMed]
- The DHS Program Malaria Indicators Survey (MIS): Overview. Available online: https://dhsprogram.com/methodology/survey-types/mis.cfm (accessed on 3 January 2021).
- National Population Commission; ICF International. Nigeria Demographic and Health Survey 2018; NPC and ICF: Abuja, Nigeria; Rockville, MD, USA, 2019. [Google Scholar]
- Jeremiah, Z.A.; Uko, E.K. Childhood Asymptomatic Malaria and Nutritional Status among Port Harcourt Children. East Afr. J. Public Health 2007, 4, 55–58. [Google Scholar]
- Mehretie Adinew, Y.; Feleke, S.A.; Mengesha, Z.B.; Workie, S.B. Childhood Mortality: Trends and Determinants in Ethiopia from 1990 to 2015—A Systematic Review. Adv. Public Health 2017, 2017, 7479295. [Google Scholar] [CrossRef]
- Mkhize, M.; Sibanda, M. A Review of Selected Studies on the Factors Associated with the Nutrition Status of Children Under the Age of Five Years in South Africa. Int. J. Environ. Res. Public. Health 2020, 17, 7973. [Google Scholar] [CrossRef]
- Manda, S.; Haushona, N.; Bergquist, R. A Scoping Review of Spatial Analysis Approaches Using Health Survey Data in Sub-Saharan Africa. Int. J. Environ. Res. Public Health 2020, 17, 3070. [Google Scholar] [CrossRef] [PubMed]
- Green, B.N.; Johnson, C.D.; Haldeman, S.; Griffith, E.; Clay, M.B.; Kane, E.J.; Castellote, J.M.; Rajasekaran, S.; Smuck, M.; Hurwitz, E.L.; et al. A Scoping Review of Biopsychosocial Risk Factors and Co-Morbidities for Common Spinal Disorders. PLoS ONE 2018, 13, e0197987. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Global Technical Strategy for Malaria, 2016–2030; WHO: Geneva, Switzerland, 2015; ISBN 978-92-4-156499-1. [Google Scholar]
- ShelterBox Mosquito Nets—Helping Families Protect Themselves. Available online: https://www.shelterbox.org/about/aid/mosquito-nets/ (accessed on 25 July 2020).
- World Health Organisation. Fact Sheet about Malaria. Available online: https://www.who.int/news-room/fact-sheets/detail/malaria (accessed on 25 July 2020).
- Dhiman, S. Are Malaria Elimination Efforts on Right Track? An Analysis of Gains Achieved and Challenges Ahead. Infect. Dis. Poverty 2019, 8, 1–9. [Google Scholar] [CrossRef]
- Sallah, K.; Giorgi, R.; Ba, E.-H.; Piarroux, M.; Piarroux, R.; Cisse, B.; Gaudart, J. Targeting Malaria Hotspots to Reduce Transmission Incidence in Senegal. Int. J. Environ. Res. Public Health 2021, 18, 76. [Google Scholar] [CrossRef] [PubMed]
| S/N | Search Terms |
|---|---|
| 1 | demographic health survey OR AIDS indicator survey OR malaria indicator survey OR multiple indicator cluster surveys OR health survey OR MIS OR DHS |
| 2 | sub-Sahara Africa OR SSA |
| 3 | logistic regression OR multilevel regression OR multinomial logistic OR random-effects OR hierarchical OR fixed effects OR Linear regression |
| 4 | Malaria OR fever OR plasmodium falciparum OR P. malariae OR P. ovale OR P. vivax) |
| 5 | 1 AND 2 AND 3 AND 4 |
| Authors and Dates | Titles | Country | Survey * | Target Population | Prevalence n (%) | Participants (Sample Size) | Malaria Diagnostic Method ** | Methods | Software | Funding Source |
|---|---|---|---|---|---|---|---|---|---|---|
| Berendsen et al., 2019 [27] | BCG vaccination is associated with reduced malaria prevalence in children under the age of 5 years in Sub-Sahara Africa | Multi-country (13 SSA) | DHS | Under 5 years | 12,325 (36) | 34,205 | RDT | Multilevel logistic regression (MLLR) | SPSS, STATA, MLWin | Multiple source |
| Chitunhu et al., 2015 [28] | Direct and indirect determinants of childhood malaria morbidity in Malawi: a survey cross-sectional analysis based on malaria indicator survey data for 2012 | Malawi | MIS | Under 5 years | 367 (27.7) | 1375 | MT | Logistic regression (LR) | STATA | Institution-based |
| Levitz et al., 2018 [29] | Effect of individual and community-level bed net usage on malaria prevalence among under-fives in the Democratic Republic of Congo | Democratic Republic of Congo (DRC) | DHS | Under 5 years | 2191 (37.4) | 5857 | Others (PCR) | Multilevel logistic regression (MLLR) | SAS | Multiple sources |
| Morakinyo et al., 2018 [30] | Housing type and risk of malaria among under-five children in Nigeria: evidence from the malaria indicator survey | Nigeria | MIS | 6–59 months | 6991 | RDT and MT | Logistic regression (LR) | STATA | No funding | |
| Njau et al., 2013 [26] | Exploring the impact of targeted distribution of free bed nets on households bed net ownership, socioeconomic disparities and childhood malaria infection rates: analysis of national malaria survey data from three sub-Saharan Africa countries | Angola, Tanzania and Uganda | MIS | Under 5 years | 214 (20) 895 (39) 782 (18) | 1125 3109 1954 | RDT and MT | Multilevel logistic regression (MLLR) | STATA | Multiple source |
| Njau et al., 2014 [31] | Investigating the Important Correlates of Maternal Education and Childhood Malaria Infections | Angola, Tanzania and Uganda (Pooled) | MIS | Under 5 years | - | 1390 5975 2997 | RDT | Multivariate logistic regression (MvLR) | STATA | Not reported |
| Semakula et al., 2015 [32] | Potential of household environmental resources and practices in eliminating residual malaria transmission: a case study of Tanzania, Burundi, Malawi and Liberia | Tanzania, Burundi, Malawi and Liberia | MIS | Under 5 years | - | 7695 3750 2115 3187 | RDT | Multivariate logistic regression (MvLR) | JMP 10 | Multiple source |
| Siri 2014 [33] | Independent Associations of Maternal Education and Household Wealth with Malaria Risk in Children | Multi-country (pooled) | Under 5 years | - | 24,043 | - | Multivariate logistic regression (MvLR) | SAS | Institution-based | |
| Tusting et al., 2020 [34] | Housing and child health in sub-Saharan Africa: A cross-sectional analysis | Multi-country (pooled) | Multiple surveys | Under 5 years | 40,178 (21) | 188,651 | RDT and MT | Conditional logistic regression (LR) | STATA and R | Multiple source |
| Ugwu and Zewotir, 2018 [35] | Using mixed effects logistic regression models for complex survey data on malaria rapid diagnostic test results | Nigeria | MIS | 6–59 months | - | 5236 | RDT | Generalized linear mixed model (GLMM) | SAS | No funding |
| Wanzira et al., 2017 [24] | Factors associated with malaria parasitaemia among children under 5 years in Uganda: a secondary data analysis of the 2014 Malaria Indicator Survey dataset | Uganda | MIS | Under 5 years | 938 (19.04) | 4930 | MT | Multivariate logistic regression (MvLR) | STATA | no funding |
| Yang et al., 2020 [36] | Drinking water and sanitation conditions are associated with the risk of malaria among children under five-year-old in sub-Saharan Africa: A logistic regression model analysis of national survey data | Multi-country (pooled) | Multiple surveys | Under 5 years | 40,217 (18.8) | 213,920 | RDT and MT | Multivariate logistic regression (MvLR) | SPSS | not reported |
| Zgambo et al., 2017 [37] | Prevalence and factors associated with malaria parasitaemia in children under the age of five years in Malawi: A comparison study of the 2012 and 2014 Malaria Indicator Surveys (MISs) | Malawi | MIS | Under 5 years | 636 (33) | 1928 | MT | Multivariate logistic regression (MvLR) | SPSS | no funding |
| S/N | Variables | Significance Levels | Number of Studies | Association Effect (95% CI) |
|---|---|---|---|---|
| 1 | Age of the child | S: | 9 | Increased significant factors (ISF) OR: 1.05 (1.04–1.06) [27] OR: 1.03 (1.02, 1.04) [28] 7–23: OR: 2.29 (1.21–4.34), 24–59: OR: 5.67 (3.01–10.70) [30] OR: 1.85 (1.33–2.56) [32] OR: 2.10 (1.59–2.80) [32] 6–11: OR: 2.22 (1.88, 2.62); 12–23: OR: 3.70 (3.12, 4.37) 24–35: OR: 5.00 (4.25, 5.87) [33] 13–24: OR: 1.7039 (1.34–2.16); 25–36: OR: 2.624 (2.06–3.33); 37–48: OR: 3.591 (2.82–4.55); 49–59: OR: 4.97 (3.888–6.38) [35] 7–12: OR: 1.62 (1.04–2.52); 13–24: OR: 2.20 (1.47–3.29); 25–36: OR: 3.47 (2.32–5.20); 37–48: OR: 3.69 (2.47–5.50); 49–59: OR: 4.01 (2.57–6.45) [24] 24–35: OR: 1.5 (1.0–2.5) ≥48: OR: 2.2 (1.4–3.5) [37] decreased significant factors (DSF) 36 month+ OR: 0.80 (0.72, 0.88) [33] |
| NS: | 2 [32] | |||
| 2 | Vaccination status | S: | 1 | DSF: OR: 0.88 (0.82 to 0.94) [27] |
| NS: | - | |||
| 3 | Preceding birth interval | S: | 1 | ISF: OR: 1.00 (1.00 to 1.00) [27] |
| NS: | - | |||
| 4 | Birth order | S: | 3 [27,28,31] | ISF: OR: 1.03 (1.01–1.06) [27] Second: OR: 1.43 (1.04, 1.96) [28] β: 0.045 [31] |
| NS: | - | |||
| 5 | Breastfeeding status | S: | 1 | DSF: currently: 0.85 (0.73–0.99) [27] |
| NS: | - | |||
| 6 | Fever in the last 2 weeks | S: | 1 | ISF: OR: 1.967 (1.71–2.26) [35] |
| NS: | - | |||
| 7 | Anemic | S: | 2 | ISF: OR: 2.982 (2.54–3.49) [35] DSF: OR: 0.95 (0.94, 0.96) [28] |
| NS: | - | |||
| 8 | Place of delivery | S: | 1 | DSF: public: 0.85 (0.78 to 0.92); private: 0.78 (0.70 to 0.87) [27] |
| NS: | - | |||
| 9 | Child slept under a mosquito bed net | S: | 4 | ISF: OR: 1.21 (1.08–1.36) [30] OR: 1.47 (1.16–1.89) [32] DSF: OR: 0.77 (0.60, 0.99) [28] OR:0.65 (0.56–0.77) [32] |
| NS: | 5 [27,32,33,37] |
| S/N | Variables | Significance Levels | Number of Country Studies | Association Effect (95% CI) |
|---|---|---|---|---|
| 1 | Maternal age | S: | 1 | DSF: OR: 0.99 (0.98 to 0.99) [27] |
| NS: | 2 [31,33] | |||
| 2 | Maternal education status | S: | 6 | ISF: no Education: OR: 2.0454 (1.36–3.07); primary: OR: 1.5311 (1.03–2.28); secondary+: OR: 1.547 (1.07–2.23) [35] DSF: primary: OR: 0.91 (0.86 to 0.96); secondary+: OR: 0.73 (0.67 to 0.78) [27]. primary: OR: 0.53 (0.37, 0.76) [28] PS: β: −0.032; above primary: β: −0.047 [31] OR: 0.993 (0.990–0.996) [33] Primary: OR: 0.75 (0.59–0.96); secondary: OR: 0.61 (0.43–0.86); Tet: OR: 0.11 (0.02–0.53) [24] |
| NS: | 1 [37] | |||
| 3 | Maternal body mass index | S: | 1 | DSF: OR: 0.97 (0.96–0.98) [27] |
| NS: | - | |||
| 4 | Maternal ante-natal care | S: | 1 | DSF: β: −0.029 [31] |
| NS: | - | |||
| 5 | Number of births in 5 years | S: | 1 | ISF: OR: 1.08 (1.03–1.13) [27] |
| NS: | - | |||
| 6 | Maternal knowledge of malaria fever | S: | 2 | ISF β: 0.013 [31] DSF: yes: OR: 0.78 (0.62–0.99) [36] |
| NS: | - | |||
| 7 | Number of children ever born | S: | 1 | ISF β: 0.003 [31] |
| NS: | - | |||
| 8 | Mother has access to phone | S: | 1 | DSF: β: −0.030 [31] |
| NS: | - |
| S/N | Variables | Significance Levels | Number of Country Studies | Association Effect (95% CI) |
|---|---|---|---|---|
| 1 | Household wealth status | S: | 11 | ISF: international wealth index square: 1.00 (1.00 to 1.00) [27] poor: 5.51 (3.83–7.93) poorer: 5.15 (3.72–7.13) middle: 3.51 (2.64–4.65) richer: 1.89 (1.46–2.45) [30] poorest: OR: 3.5498 (1.508–8.35); poorer: OR: 5.6013 (2.69–11.63); middle: OR: 2.4569 (1.46–4.12); richer: OR: 1.8258 (1.24–2.67) [35]; poorest: OR: 4.7 (1.3–16.2) [37] DSF: OR: 0.95 (0.93–0.98) [28] ME: −0.034 (−0.1543– 0.0773) [26]; ME: −0.070 (−0.0943–0.0267) [26]; ME: −0.116 (−0.1876–−0.0583) [26] poor: β: −0.019 (0.017); less poor: β: −0.033 (0.018); middle: β: −0.065 (0.018); rich: β: −0.123 (0.019) [31] OR: 0.990 (0.987–0.992) [33] poorer: 0.70 (0.50–0.99); middle: 0.75 (0.50–1.12) 0.157; richer: OR: 0.40 (0.27–0.61); richest: OR: 0.17 (0.08–0.36) [24] |
| NS: | - | |||
| 2 | Place of residence | S: | 13 | ISF: rural: OR: 1.91 (1.63–2.25) [27] rural: OR: 1.83 (1.18–2.83) [28], rural: OR: 1.59 (1.33–1.89) [30], ME: 0.002 (0.0781–0.1228) ME: 0.055, CI: (0.0005–0.1097) [26] rural: β: 0.024 [31] rural: OR: 4.57 (1.86–11.25) [35] DSF: urban: OR: 0.94 (0.61–1.42) [32] OR: 0.26 (0.13–0.49) [32] urban: OR: 0.39 (0.25–0.60) [32] urban: OR: 0.72 (0.570.92) [32] urban: OR: 0.59 (0.50–0.71) [33] |
| NS: | 2 [24,37] | |||
| 3 | Household had bed net | S: | 4 | DSF: ME: −0.055 (−0.1187–0.008) [26]; ME: −0.034 (−0.1233–0.0387) [26] ME: −0.098 (−0.0419–0.1494) [26] β: −0.076 [31] |
| NS: | 1 [37] | |||
| 4 | Age of household head | S: | 4 | ISF: ME: 0.006 (−0.0004–0.0016) [26]; ME: 0.001 (−0.0005–0.0029) [26], OR: 1.019 (1.007–1.031) [35] DSF: ME: −0.009 (0.0012–0.0032) [26] |
| NS: | - | |||
| 5 | Insecticide residual spray | S: | 2 | DSF: OR: 0.37 (1.08–1.36) [30] OR: 0.23 (0.08–0.61) [24] |
| NS: | 2 [35,37] | |||
| 6 | Household size | S: | 7 | ISF: OR: 1.03 (1.01–1.04) [27] ME: 0.015 (0.0021–0.0285) [26] ME: 0.004 (−0.0059–0.0050) [26] ME: 0.005 (−0.0163–0.0055) [26] β: 0.009 [31] OR: 1.46 (1.24–1.73) [33], OR: 1.108 (1.03–1.17) [35] |
| NS: | - | |||
| 7 | Number of under-5 in household | S: | 3 | ISF: ME: 0.049 (0.0331–0.6565) [26] DSF: ME: −0.025 (−0.1787–−0.0181) [26] ME: −0.044 (−0.0742–−0.0156) [26] |
| NS: | 1 [31] | |||
| 8 | Source of water outside | S: | 1 | DSF: OR: 0.97 (0.96, 0.99) [28] |
| NS: | 1 [35] | |||
| 9 | Improved water source | S: | 5 | ISF: borehole: OR: 1.50 (1.10–1.88); unprotected well: OR: 1.56 (1.29–1.88); protected well: OR: 2.19 (1.53–3.10); river/lakes: OR: 2.45 (1.81–3.31) [32] borehole: OR: 1.75 (0.61–0.93); protected well: OR: 1.44 (0.25–0.78) [32] borehole: OR: 1.19 (0.36–3.60); protected well: OR: 1.36 (1.041.78); unprotected spring: OR: 1.65 (1.012.71) 0.047; river/lakes: OR: 1.55 (1.12–2.16) [32] unprotected: OR: 1.17 (1.07, 1.27) [36] DSF: piped (yard): OR: 0.13 (0.03–0.32); public pipe: OR: 0.70 (0.51–0.95); private taps: OR: 0.62 (0.39–0.95) protected spring: OR: 0.78 (1.06–2.83) [32]. piped (yard): OR: 0.05 (0.00–0.58); public pipes: OR: 0.52 (1.25–1.84); private tap: OR: 0.23 (0.04–0.75) [32]; piped (yard): OR: 0.23 (0.12–0.43); public: OR: 0.33 (0.23–0.47) [32] public: OR: 0.27 (0.13–0.51) [32] piped: 0.52 (0.45–0.59) [36] |
| NS: | 2 [27,35] | |||
| 10 | Improved toilet facility | S: | 7 | ISF: open toilet: OR: 1.35 (1.11–1.63) no toilet: OR: 3.57 (2.35–5.42); pit: OR: 1.30 (1.07–1.58) [32] no toilet: OR: 1.66 (1.20–2.30) [32] no toilet: OR: 1.24 (0.821.28 [32] no toilet: 1.635 (1.209–2.21) [35] no toilet: OR: 1.35 (1.24, 1.47) [36] DSF: medium-quality: OR: 0.85 (0.78 to 0.92) [27]; flush toilet: 0.40 (0.18–0.78) [32] flush toilet: OR: 0.04 (0.02–8.01) [32] flush toilet: 0.53 (0.390.73) [32]; flush toilet: OR: 0.51 (0.43, 0.61) [36] |
| NS: | - | |||
| 11 | Sex of household head | S: | 1 | DSF: male: ME: −0.029 (−0.0637–0.0049) [26] |
| NS: | 4 [26,31,32] | |||
| 12 | Use biomass for cooking | S: | 2 | ISF: firewood: OR: 1.80 (1.23–2.68) [32] firewood: OR: 1.44 (0.98–2.16) [32] DSF: charcoal: OR: 0.58(0.38–0.85) [32] |
| NS: | - | |||
| 13 | Under 5 years child slept under bed net | S: | 2 | ISF: yes: OR: 1.33 (1.04–1.71) [24] DSF: OR: 0.83 (0.78–0.88) [34] |
| NS: | 1 [35] | |||
| 14 | Household ownership of livestock | S: | 4 | ISF: goat: OR: 1.32 (1.09–1.60) [32] goat: 1.26 (1.07–1.48) OR: 1.17 (0.98–1.38) [32] DSF: cattle: OR: 0.55 (0.45–0.67) pigs: OR: 0.18 (0.09–0.33) [32] cattle: OR: 0.51 (0.40–0.65) [32] cattle: OR: 0.54 (0.35–0.83) cattle: OR: 0.74 (0.55 1.00) [32] |
| NS: | - | |||
| 15 | Improve building materials | S: | 2 | ISF: nothing improved: OR: 1.05 (1.02–1.12) [30]; OR: 0.88 (0.83–0.93) [34] |
| NS: | 1 [34] | |||
| 16 | Household head education status | S: | 2 | ISF: ME: 0.027 (−0.0023–0.0567) [26] DSF: primary school+: β: −0.009 (0.004) [31] |
| NS: | 1 [26] | |||
| 18 | Household connected electricity | S: | 1 | ISF: no: OR: 1.14 (0.88–1.48) [35] |
| NS: | - | |||
| 19 | Roofing material | S: | 1 | DSF: palm leaf: OR: 0.7171 [35] |
| NS: | - |
| S/N | Variables | Significance Levels | Number of Country Studies | Association Effect (95% CI) |
|---|---|---|---|---|
| 1 | Community wealth status | S: | 1 | ISF: cluster level: OR: 0.984 (0.979, 0.988) [33] |
| NS: | - | |||
| 2 | Community distance to health facilities | S: | 2 | ISF: ME: 0.084 (0.0560–0.1128) [26] ME: 0.102 (0.0525–0.1521) [26] |
| NS: | - | |||
| 3 | Cluster altitude | S: | 1 | ISF: OR 1.0003 (0.991–1.1003) [35] |
| NS: | 1 [28] | |||
| 4 | Community insecticide net use | S: | 1 | ISF: OR: 0.43 (0.27, 0.70) [29] |
| NS: | - | |||
| 5 | Regional variations | S: | 3 [24,28,30] | |
| NS: | 1 [37] | |||
| 6 | Malaria endemicity | S: | 4 | ISF: ME: 0.010 (−0.0778–0.0572) [26] ME: 0.095 (0.0357–0.1561) [26] ME: 0.288 (−0.5526–−0.0247) [26] high: β: 0.093 [31] |
| NS: | - | |||
| 7 | Free bed net in community | S: | 3 | ISF: ME: 0.251 (0.0226–0.4801) [26] DSF: ME: −0.015 (−0.0134–0.0405) [26] ME: −0.082 (0.1479–0.0494) [26] |
| NS: | - | |||
| Country-specific | S: | 1 | ISF: Liberia: OR: 1.09 (0.95–1.24); Uganda: OR: 40.15 (29.74–54.20); Malawi: OR: 16.68 (12.38, 22.48); Senegal: OR: 1.01 (0.77, 1.32); Nigeria: OR: 31.91 (23.86, 42.67) [33] DSF: Rwanda: OR: 0.15 (0.10, 0.21); Tanzania: OR: 0.82 (0.63, 1.07); Madagascar: OR:0.73 (0.57, 0.94) [33] | |
| NS: | - |
| S/N | Variables | Significance Levels | Number of Country Studies | Association Effect (95% CI) |
|---|---|---|---|---|
| 1 | Free bed net/wealth status | S: | 1 | DSF: ME: −0.046 (−0.0668–0.1772) [26] |
| NS: | 2 [26] | |||
| 2 | Wealth/place of residence | S: | 1 | DSF: poorest/rural: OR: 0.3567 (0.13–0.96); poorer/rural: OR: 0.2770 (0.11–0.66); middle/rural OR: 0.4477 (0.22–0.91); richer/rural: OR: 0.4174 (0.22–0.78) [35] |
| NS: | - | |||
| 3 | Number in household/age of household head | S: | 1 | DSF: OR: 0.9984 (0.997–0.999) [35] |
| NS: | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Obasohan, P.E.; Walters, S.J.; Jacques, R.; Khatab, K. A Scoping Review of Selected Studies on Predictor Variables Associated with the Malaria Status among Children under Five Years in Sub-Saharan Africa. Int. J. Environ. Res. Public Health 2021, 18, 2119. https://doi.org/10.3390/ijerph18042119
Obasohan PE, Walters SJ, Jacques R, Khatab K. A Scoping Review of Selected Studies on Predictor Variables Associated with the Malaria Status among Children under Five Years in Sub-Saharan Africa. International Journal of Environmental Research and Public Health. 2021; 18(4):2119. https://doi.org/10.3390/ijerph18042119
Chicago/Turabian StyleObasohan, Phillips Edomwonyi, Stephen J. Walters, Richard Jacques, and Khaled Khatab. 2021. "A Scoping Review of Selected Studies on Predictor Variables Associated with the Malaria Status among Children under Five Years in Sub-Saharan Africa" International Journal of Environmental Research and Public Health 18, no. 4: 2119. https://doi.org/10.3390/ijerph18042119
APA StyleObasohan, P. E., Walters, S. J., Jacques, R., & Khatab, K. (2021). A Scoping Review of Selected Studies on Predictor Variables Associated with the Malaria Status among Children under Five Years in Sub-Saharan Africa. International Journal of Environmental Research and Public Health, 18(4), 2119. https://doi.org/10.3390/ijerph18042119







