Relationships between Socioeconomic Status, Handgrip Strength, and Non-Alcoholic Fatty Liver Disease in Middle-Aged Adults
Abstract
1. Introduction
2. Materials and Methods
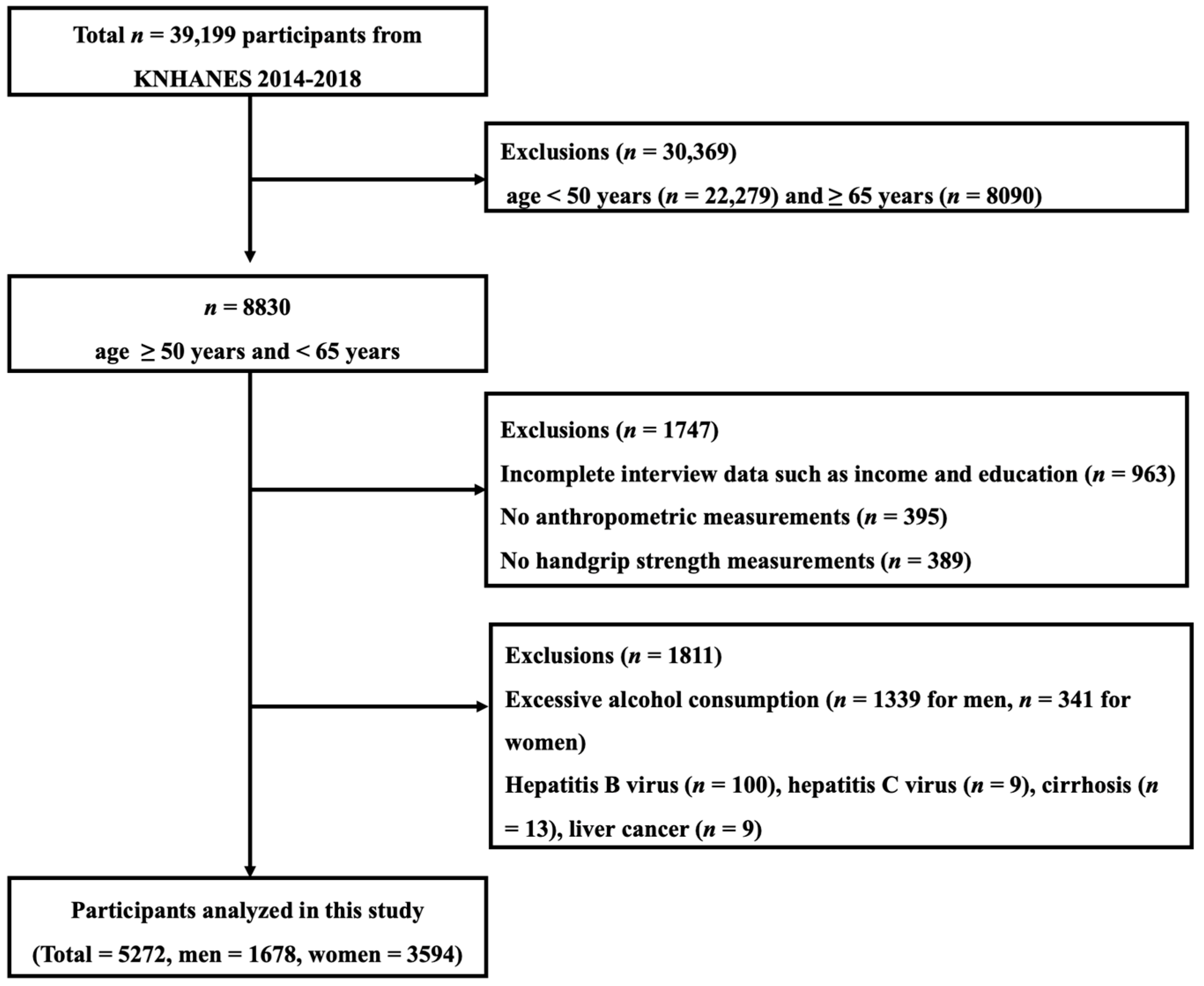
2.1. Participants
2.2. Socioeconomic Status
2.3. Covariates
2.4. Definition of NAFLD
2.5. Relative Handgrip Strength
2.6. Statistics
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Younossi, Z.M.; Koenig, A.B.; Abdelatif, D.; Fazel, Y.; Henry, L.; Wymer, M. Global epidemiology of nonalcoholic fatty liver disease—meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016, 64, 73–84. [Google Scholar] [CrossRef]
- Cotter, T.G.; Rinella, M. NAFLD 2020: The State of the Disease. Gastroenterology 2020, 158, 1851–1864. [Google Scholar] [CrossRef] [PubMed]
- Rabiee, B.; Roozafzai, F.; Hemasi, G.R.; Poustchi, H.; Keyvani, H.; Khonsari, M.R.; Ajdarkosh, H.; Maadi, M.; Saeedian, F.S.; Zamani, F. The prevalence of non-alcoholic fatty liver disease and diabetes mellitus in an Iranian population. Middle East J. Dig. Dis. 2017, 9, 86. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rinella, M.; Charlton, M. The globalization of nonalcoholic fatty liver disease: Prevalence and impact on world health. Hepatology 2016, 64, 19–22. [Google Scholar] [CrossRef]
- Kneeman, J.M.; Misdraji, J.; Corey, K.E. Secondary causes of nonalcoholic fatty liver disease. Ther. Adv. Gastroenterol. 2012, 5, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Jia, G.; Li, X.; Wang, L.; Li, Q.; Yang, L.; Li, N.; Di, F. Relationship of socioeconomic status and non-alcoholic fatty liver disease in patients with type 2 diabetes mellitus. Zhonghua Gan Zang Bing Za Zhi Zhonghua Ganzangbing Zazhi Chin. J. Hepatol. 2015, 23, 760–764. [Google Scholar]
- Orkin, S.; Brokamp, C.; Yodoshi, T.; Trout, A.T.; Liu, C.; Meryum, S.; Taylor, S.; Wolfe, C.; Sheridan, R.; Seth, A. Community Socioeconomic Deprivation and Nonalcoholic Fatty Liver Disease Severity. J. Pediatric Gastroenterol. Nutr. 2020, 70, 364–370. [Google Scholar] [CrossRef]
- Lim, E.X.; Forde, C.G.; Cheon, B.K. Low subjective socioeconomic status alters taste-based perceptual sensitivity to the energy density of beverages. Physiol. Behav. 2020, 223, 112989. [Google Scholar] [CrossRef]
- McMaughan, D.J.; Oloruntoba, O.; Smith, M.L. Socioeconomic Status and Access to Healthcare: Interrelated Drivers for Healthy Aging. Front. Public Health 2020, 8, 231. [Google Scholar] [CrossRef]
- Murray, T.C.; Rodgers, W.M.; Fraser, S.N. Exploring the relationship between socioeconomic status, control beliefs and exercise behavior: A multiple mediator model. J. Behav. Med. 2012, 35, 63–73. [Google Scholar] [CrossRef]
- Zarean, E.; Goujani, R.; Rahimian, G.; Ahamdi, A. Prevalence and risk factors of non-alcoholic fatty liver disease in southwest Iran: A population-based case-control study. Clin. Exp. Hepatol. 2019, 5, 224. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Guo, G.; Xia, L.; Yang, X.; Zhang, B.; Liu, F.; Ma, J.; Hu, Z.; Li, Y.; Li, W. Relative handgrip strength is inversely associated with metabolic profile and metabolic disease in the general population in China. Front. Physiol. 2018, 9, 59. [Google Scholar] [CrossRef]
- Lee, W.-J.; Peng, L.-N.; Chiou, S.-T.; Chen, L.-K. Relative handgrip strength is a simple indicator of cardiometabolic risk among middle-aged and older people: A nationwide population-based study in Taiwan. PLoS ONE 2016, 11, e0160876. [Google Scholar] [CrossRef] [PubMed]
- Lawman, H.G.; Troiano, R.P.; Perna, F.M.; Wang, C.-Y.; Fryar, C.D.; Ogden, C.L. Associations of relative handgrip strength and cardiovascular disease biomarkers in US adults, 2011–2012. Am. J. Prev. Med. 2016, 50, 677–683. [Google Scholar] [CrossRef]
- Cheng, Y.J.; Gregg, E.W.; De Rekeneire, N.; Williams, D.E.; Imperatore, G.; Caspersen, C.J.; Kahn, H.S. Muscle-strengthening activity and its association with insulin sensitivity. Diabetes Care 2007, 30, 2264–2270. [Google Scholar] [CrossRef]
- Blakeley, C.E.; Van Rompay, M.I.; Schultz, N.S.; Sacheck, J.M. Relationship between muscle strength and dyslipidemia, serum 25 (OH) D, and weight status among diverse schoolchildren: A cross-sectional analysis. BMC Pediatrics 2018, 18, 23. [Google Scholar] [CrossRef]
- Jackson, A.W.; Lee, D.C.; Sui, X.; Morrow, J.R., Jr.; Church, T.S.; Maslow, A.L.; Blair, S.N. Muscular strength is inversely related to prevalence and incidence of obesity in adult men. Obesity 2010, 18, 1988–1995. [Google Scholar] [CrossRef]
- Meng, G.; Wu, H.; Fang, L.; Li, C.; Yu, F.; Zhang, Q.; Liu, L.; Du, H.; Shi, H.; Xia, Y. Relationship between grip strength and newly diagnosed nonalcoholic fatty liver disease in a large-scale adult population. Sci. Rep. 2016, 6, 1–7. [Google Scholar] [CrossRef]
- Park, S.H.; Kim, D.J.; Plank, L.D. Association of grip strength with non-alcoholic fatty liver disease: Investigation of the roles of insulin resistance and inflammation as mediators. Eur. J. Clin. Nutr. 2020, 1–9. [Google Scholar] [CrossRef]
- Carney, C.; Benzeval, M. Social patterning in grip strength and in its association with age; a cross sectional analysis using the UK Household Longitudinal Study (UKHLS). BMC Public Health 2018, 18, 385. [Google Scholar] [CrossRef] [PubMed]
- Roland, J., Jr.; Thorpe, E.S.; Alan Zonderman, M.K.E. Association between race, household income and grip strength in middle-and older-aged adults. Ethn. Dis. 2016, 26, 493. [Google Scholar]
- Mohd Hairi, F.; Mackenbach, J.P.; Andersen-Ranberg, K.; Avendano, M. Does socio-economic status predict grip strength in older Europeans? Results from the SHARE study in non-institutionalised men and women aged 50+. J. Epidemiol. Community Health 2010, 64, 829–837. [Google Scholar] [CrossRef] [PubMed]
- Freeman, A.; Tyrovolas, S.; Koyanagi, A.; Chatterji, S.; Leonardi, M.; Ayuso-Mateos, J.L.; Tobiasz-Adamczyk, B.; Koskinen, S.; Rummel-Kluge, C.; Haro, J.M. The role of socio-economic status in depression: Results from the COURAGE (aging survey in Europe). BMC Public Health 2016, 16, 1098. [Google Scholar] [CrossRef]
- Lee, Y.-H.; Bang, H.; Park, Y.M.; Bae, J.C.; Lee, B.-W.; Kang, E.S.; Cha, B.S.; Lee, H.C.; Balkau, B.; Lee, W.-Y. Non–Laboratory-Based Self-Assessment Screening Score for Non-Alcoholic Fatty Liver Disease: Development, Validation and Comparison with Other Scores. PLoS ONE 2014, 9, e107584. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.-H.; Jung, K.S.; Kim, S.U.; Yoon, H.-J.; Yun, Y.J.; Lee, B.-W.; Kang, E.S.; Han, K.-H.; Lee, H.C.; Cha, B.-S. Sarcopaenia is associated with NAFLD independently of obesity and insulin resistance: Nationwide surveys (KNHANES 2008–2011). J. Hepatol. 2015, 63, 486–493. [Google Scholar] [CrossRef]
- Lee, J.-H.; Kim, D.; Kim, H.J.; Lee, C.-H.; Yang, J.I.; Kim, W.; Kim, Y.J.; Yoon, J.-H.; Cho, S.-H.; Sung, M.-W. Hepatic steatosis index: A simple screening tool reflecting nonalcoholic fatty liver disease. Dig. Liver Dis. 2010, 42, 503–508. [Google Scholar] [CrossRef] [PubMed]
- Byrne, C.D.; Targher, G. NAFLD: A multisystem disease. J. Hepatol. 2015, 62, S47–S64. [Google Scholar] [CrossRef]
- Duseja, A.; Chalasani, N. Epidemiology and risk factors of nonalcoholic fatty liver disease (NAFLD). Hepatol. Int. 2013, 7 (Suppl. 2), 755–764. [Google Scholar] [CrossRef]
- Shafiei, S.; Yazdani, S.; Jadidfard, M.P.; Zafarmand, A.H. Measurement components of socioeconomic status in health-related studies in Iran. BMC Res. Notes 2019, 12, 70. [Google Scholar] [CrossRef] [PubMed]
- Galobardes, B.; Shaw, M.; Lawlor, D.A.; Lynch, J.W.; Davey Smith, G. Indicators of socioeconomic position (part 1). J. Epidemiol. Community Health 2006, 60, 7–12. [Google Scholar] [CrossRef]
- Darin-Mattsson, A.; Fors, S.; Kåreholt, I. Different indicators of socioeconomic status and their relative importance as determinants of health in old age. Int. J. Equity Health 2017, 16, 173. [Google Scholar] [CrossRef] [PubMed]
- Pamuk, E.; Makuc, D.; Heck, K.; Reuben, C.; Lochner, K. Socioeconomic status and health chartbook. HealthUnited States 1998, 1998. [Google Scholar]
- Pampel, F.C.; Krueger, P.M.; Denney, J.T. Socioeconomic Disparities in Health Behaviors. Annu. Rev. Sociol. 2010, 36, 349–370. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Geng, L. Effects of Socioeconomic Status on Physical and Psychological Health: Lifestyle as a Mediator. Int. J. Environ. Res. Public Health 2019, 16, 281. [Google Scholar] [CrossRef]
- Zhan, Y.; Yu, J.; Chen, R.; Gao, J.; Ding, R.; Fu, Y.; Zhang, L.; Hu, D. Socioeconomic status and metabolic syndrome in the general population of China: A cross-sectional study. BMC Public Health 2012, 12, 921. [Google Scholar] [CrossRef] [PubMed]
- Goodman, E.; Daniels, S.R.; Dolan, L.M. Socioeconomic disparities in insulin resistance: Results from the Princeton School District Study. Psychosom. Med. 2007, 69, 61–67. [Google Scholar] [CrossRef]
- Mizgier, M.L.; Casas, M.; Contreras-Ferrat, A.; Llanos, P.; Galgani, J. Potential role of skeletal muscle glucose metabolism on the regulation of insulin secretion. Obes. Rev. 2014, 15, 587–597. [Google Scholar] [CrossRef]
- Bohannon, R.W. Grip strength: An indispensable biomarker for older adults. Clin. Interv. Aging 2019, 14, 1681. [Google Scholar] [CrossRef]
- Bohannon, R.W.; Magasi, S.R.; Bubela, D.J.; Wang, Y.C.; Gershon, R.C. Grip and knee extension muscle strength reflect a common construct among adults. Muscle Nerve 2012, 46, 555–558. [Google Scholar] [CrossRef]
- Kuh, D.; Bassey, E.J.; Butterworth, S.; Hardy, R.; Wadsworth, M.E. Grip strength, postural control, and functional leg power in a representative cohort of British men and women: Associations with physical activity, health status, and socioeconomic conditions. J. Gerontol. Ser. A Biol. Sci. Med Sci. 2005, 60, 224–231. [Google Scholar] [CrossRef]
- Lee, I.; Cho, J.; Park, J.; Kang, H. Association of hand-grip strength and non-alcoholic fatty liver disease index in older adults. J. Exerc. Nutr. Biochem. 2018, 22, 62. [Google Scholar] [CrossRef] [PubMed]
- Leopold, L.; Engelhartdt, H. Education and physical health trajectories in old age. Evidence from the Survey of Health, Ageing and Retirement in Europe (SHARE). Int. J. Public Health 2013, 58, 23–31. [Google Scholar] [CrossRef] [PubMed]
| Variables | Total (n = 5272) | Men (n = 1678) | Women (n = 3594) | p Value |
|---|---|---|---|---|
| Anthropometrics | ||||
| Age (years) | 57.1 ± 4.2 | 57.3 ± 4.2 | 57.0 ± 4.2 | 0.081 |
| BMI (kg/m2) | 24.1 ± 3.2 | 24.4 ± 2.8 | 24.0 ± 3.3 | <0.001 |
| WC (cm) | 82.2 ± 8.9 | 86.4 ± 7.8 | 80.3 ± 8.7 | <0.001 |
| Absolute HGS (kg) | 29.7 ± 9.1 | 40.6 ± 6.5 | 24.6 ± 4.4 | <0.001 |
| Relative HGS (kg/BMI) | 47.8 ± 11.8 | 1.68 ± 0.30 | 1.04 ± 0.22 | <0.001 |
| Socioeconomic status | ||||
| Household income (KRW 10,000/month) | 449.7 ± 330.8 | 473.3 ± 322.5 | 438.6 ± 334.2 | <0.001 |
| Education (years) | 11.4 ± 3.8 | 12.5 ± 3.8 | 10.9 ± 3.7 | <0.001 |
| Sociodemographic status | ||||
| Marital status, n (%) | <0.001 | |||
| Married | 4487 (85.1) | 1501 (89.5) | 2986 (83.1) | |
| Widow/divorced | 686 (13.0) | 123 (7.3) | 563 (15.6) | |
| Unmarried | 99 (1.9) | 54 (3.2) | 45 (1.3) | |
| Region, n (%) | 0.009 | |||
| Urban | 4290 (81.4) | 1331 (79.3) | 2959 (82.3) | |
| Rural | 982 (18.6) | 347 (20.7) | 635 (17.7) | |
| Type of housing, n (%) | 0.263 | |||
| Apartment | 2762 (52.4) | 898 (53.5) | 1864 (51.9) | |
| General house | 2510 (47.6) | 780 (46.5) | 1730 (48.1) | |
| Health-related factors | ||||
| Smoking, n (%) | 1494 (28.3) | 1304 (77.7) | 190 (5.3) | <0.001 |
| Alcohol consumption, n (%) | 138 (2.6) | 65 (3.9) | 73 (2.0) | <0.001 |
| Regular exercise, n (%) | 1115 (21.1) | 444 (26.5) | 671 (18.7) | <0.001 |
| Hypertension, n (%) | 1304 (24.7) | 461 (27.5) | 843 (23.5) | 0.002 |
| Diabetes, n (%) | 512 (9.7) | 217 (12.9) | 295 (8.2) | <0.001 |
| Menopause, n (%) | 3202 (60.7) | 0 (0.0) | 3202 (89.1) | <0.001 |
| Variables | Low SES (n = 1247) | Middle SES (n = 2646) | High SES (n = 1379) | p for Linear Trends |
|---|---|---|---|---|
| Socioeconomic status | ||||
| Household income (KRW 10,000/month) | 157.7 ± 134.9 | 404.3 ± 237.3 | 800.6 ± 299.6 | <0.001 |
| Education (years) | 7.6 ± 2.9 | 11.3 ± 2.7 | 15.1 ± 2.7 | <0.001 |
| SES index | 15.3 ± 6.5 | 41.0 ± 11.2 | 73.0 ± 12.6 | <0.001 |
| Anthropometrics | ||||
| Women, n (%) | 817 (65.5) | 1780 (67.3) | 997 (72.3) | <0.001 |
| Age (years) | 59.0 ± 3.8 | 57.0 ± 4.1 | 55.6 ± 3.9 | <0.001 |
| BMI (kg/m2) | 24.6 ± 3.4 | 24.2 ± 3.1 | 23.4 ± 3.0 | <0.001 |
| WC (cm) | 84.0 ± 9.2 | 82.5 ± 8.8 | 80.0 ± 8.5 | <0.001 |
| Absolute HGS (kg) | 29.1 ± 9.2 | 30.2 ± 9.3 | 29.3 ± 8.4 | 0.685 |
| Relative HGS (kg/BMI) | 1.20 ± 0.41 | 1.26 ± 0.40 | 1.26 ± 0.35 | <0.001 |
| Sociodemographic status | ||||
| Marital status, n (%) | <0.001 | |||
| Married | 886 (71.1) | 2294 (86.7) | 1307 (94.7) | |
| Widow/divorced | 308 (24.6) | 315 (11.9) | 63 (4.6) | |
| Unmarried | 53 (4.3) | 37 (1.4) | 9 (0.7) | |
| Region, n (%) | <0.001 | |||
| Urban | 911 (73.1) | 2142 (81.0) | 1237 (89.7) | |
| Rural | 336 (26.9) | 504 (19.0) | 142 (10.3) | |
| Type of housing, n (%) | <0.001 | |||
| Apartment | 430 (34.5) | 1295 (48.9) | 1037 (75.2) | |
| General house | 817 (65.5) | 1,351 (51.1) | 342 (24.8) | |
| Health-related factors | ||||
| Smoking, n (%) | 421 (33.8) | 771 (29.1) | 302 (21.9) | <0.001 |
| Alcohol consumption, n (%) | 42 (3.4) | 61 (2.3) | 35 (2.5) | 0.201 |
| Regular exercise, n (%) | 139 (11.1) | 541 (20.4) | 435 (31.5) | <0.001 |
| Hypertension, n (%) | 428 (34.3) | 636 (24.0) | 240 (17.4) | <0.001 |
| Diabetes, n (%) | 172 (13.8) | 257 (9.7) | 83 (6.0) | <0.001 |
| Menopause, n (%) | 778 (62.4) | 1,607 (60.7) | 817 (59.2) | 0.100 |
| Blood markers | ||||
| FBG (mg/dL) | 106.2 ± 28.0 | 102.2 ± 23.9 | 99.2 ± 18.0 | <0.001 |
| HDL-C (mg/dL) | 49.1 ± 11.8 | 50.6 ± 12.3 | 52.5 ± 12.8 | <0.001 |
| TG (mg/dL) | 149.5 ± 105.7 | 133.8 ± 85.2 | 126.8 ± 89.4 | <0.001 |
| AST (IU/L) | 24.5 ± 12.0 | 23.4 ± 8.8 | 22.9 ± 8.5 | <0.001 |
| ALT (IU/L) | 23.5 ± 15.5 | 22.7 ± 14.3 | 22.0 ± 13.8 | 0.011 |
| Uric acid (mg/dL) | 5.79 ± 0.85 | 5.84 ± 0.87 | 5.82 ± 0.84 | 0.492 |
| NAFLD index | ||||
| HSI | 33.8 ± 4.8 | 33.3 ± 4.5 | 32.5 ± 4.4 | <0.001 |
| CNS | 58.4 ± 31.4 | 52.3 ± 31.3 | 44.2 ± 31.1 | <0.001 |
| Variables | Low HGS (n = 1317) | Middle HGS (n = 2638) | High HGS (n = 1317) | p for Linear Trends |
|---|---|---|---|---|
| Handgrip strength | ||||
| Absolute HGS (kg) | 24.5 ± 7.7 | 29.9 ± 8.2 | 34.4 ± 9.3 | <0.001 |
| Relative HGS (kg/BMI) | 0.94 ± 0.28 | 1.24 ± 0.31 | 1.56 ± 0.37 | <0.001 |
| Socioeconomic status | ||||
| Household income (KRW 10,000/month) | 408.3 ± 33.41 | 451.7 ± 326.0 | 486.9 ± 332.8 | <0.001 |
| Education (years) | 10.7 ± 4.1 | 11.5 ± 3.8 | 12.0 ± 3.4 | <0.001 |
| SES index | 38.6 ± 23.9 | 43.7 ± 22.8 | 47.1 ± 21.9 | <0.001 |
| Anthropometrics | ||||
| Women, n (%) | 898 (68.2) | 1798 (68.2) | 898 (68.2) | 1.000 |
| Age (years) | 57.9 ± 4.2 | 57.2 ± 4.1 | 56.1 ± 4.1 | <0.001 |
| BMI (kg/m2) | 26.2 ± 3.4 | 24.1 ± 2.7 | 22.1 ± 2.3 | <0.001 |
| WC (cm) | 87.4 ± 9.2 | 82.0 ± 7.9 | 77.4 ± 7.5 | <0.001 |
| Sociodemographic status | ||||
| Marital status, n (%) | <0.001 | |||
| Married | 1063 (80.7) | 2248 (85.2) | 1176 (89.3) | |
| Widow/divorced | 215 (16.3) | 343 (13.0) | 128 (9.7) | |
| Unmarried | 39 (3.0) | 47 (1.8) | 13 (1.0) | |
| Region, n (%) | 0.109 | |||
| Urban | 1050 (79.7) | 2158 (81.8) | 1082 (82.2) | |
| Rural | 267 (20.3) | 480 (18.2) | 235 (17.8) | |
| Type of housing, n (%) | 0.001 | |||
| Apartment | 652 (49.5) | 1370 (51.9) | 740 (56.2) | |
| General house | 665 (50.5) | 1268 (48.1) | 577 (43.8) | |
| Health-related factors | ||||
| Smoking, n (%) | 374 (28.4) | 746 (28.3) | 374 (28.4) | 0.995 |
| Alcohol consumption, n (%) | 29 (2.2) | 66 (2.5) | 43 (3.3) | 0.088 |
| Regular exercise, n (%) | 207 (15.7) | 578 (21.9) | 330 (25.1) | <0.001 |
| Hypertension, n (%) | 431 (32.7) | 661 (25.1) | 212 (16.1) | <0.001 |
| Diabetes, n (%) | 183 (13.9) | 248 (9.4) | 81 (6.2) | <0.001 |
| Menopause, n (%) | 813 (61.7) | 1631 (61.8) | 758 (57.6) | 0.028 |
| Blood markers | ||||
| FBG (mg/dL) | 106.9 ± 30.3 | 102.2 ± 21.9 | 98.2 ± 18.3 | <0.001 |
| HDL-C (mg/dL) | 48.5 ± 11.9 | 50.7 ± 12.2 | 52.9 ± 12.9 | <0.001 |
| TG (mg/dL) | 149.1 ± 94.8 | 138.2 ± 98.4 | 117.2 ± 69.9 | <0.001 |
| AST (IU/L) | 24.7 ± 10.4 | 23.3 ± 9.8 | 22.6 ± 8.3 | <0.001 |
| ALT (IU/L) | 25.6 ± 16.5 | 22.7 ± 14.4 | 19.9 ± 11.6 | <0.001 |
| Uric acid (mg/dL) | 5.73 ± 0.80 | 5.83 ± 0.87 | 5.90 ± 0.88 | <0.001 |
| NAFLD index | ||||
| HSI | 35.9 ± 4.8 | 33.2 ± 4.2 | 30.5 ± 3.6 | <0.001 |
| CNS | 69.4 ± 28.4 | 52.3 ± 30.2 | 32.5 ± 26.5 | <0.001 |
| OR (95% CI) | p Value | |
|---|---|---|
| SES categories | ||
| High SES | 1 (reference) | |
| Middle SES | 1.340 (1.144–1.570) | <0.001 |
| Low SES | 1.703 (1.424–2.037) | <0.001 |
| Each one score increase | 1.008 (1.006–1.011) | <0.001 |
| Relative HGS categories | ||
| High HGS | 1 (reference) | |
| Middle HGS | 4.300 (3.402–5.435) | <0.001 |
| Low HGS | 12.161 (9.548–15.488) | <0.001 |
| Each 0.1 kg/BMI increase | 1.157 (1.135–1.179) | <0.001 |
| Crude Model | Model 1 | |||
|---|---|---|---|---|
| OR (95% CI) | p Value | OR (95% CI) | p Value | |
| High SES plus | ||||
| High HGS | 1 (reference) | 1 (reference) | ||
| Low HGS | 9.286 (5.868–14.694) | <0.001 | 2.277 (1.307–3.967) | 0.004 |
| Low SES plus | ||||
| High HGS | 1.364 (0.748–2.490) | 0.311 | 0.811 (0.391–1.682) | 0.573 |
| Low HGS | 13.499 (8.755–20.812) | <0.001 | 2.479 (1.351–4.549) | 0.003 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cho, J.; Lee, I.; Park, D.-H.; Kwak, H.-B.; Min, K. Relationships between Socioeconomic Status, Handgrip Strength, and Non-Alcoholic Fatty Liver Disease in Middle-Aged Adults. Int. J. Environ. Res. Public Health 2021, 18, 1892. https://doi.org/10.3390/ijerph18041892
Cho J, Lee I, Park D-H, Kwak H-B, Min K. Relationships between Socioeconomic Status, Handgrip Strength, and Non-Alcoholic Fatty Liver Disease in Middle-Aged Adults. International Journal of Environmental Research and Public Health. 2021; 18(4):1892. https://doi.org/10.3390/ijerph18041892
Chicago/Turabian StyleCho, Jinkyung, Inhwan Lee, Dong-Ho Park, Hyo-Bum Kwak, and Kisuk Min. 2021. "Relationships between Socioeconomic Status, Handgrip Strength, and Non-Alcoholic Fatty Liver Disease in Middle-Aged Adults" International Journal of Environmental Research and Public Health 18, no. 4: 1892. https://doi.org/10.3390/ijerph18041892
APA StyleCho, J., Lee, I., Park, D.-H., Kwak, H.-B., & Min, K. (2021). Relationships between Socioeconomic Status, Handgrip Strength, and Non-Alcoholic Fatty Liver Disease in Middle-Aged Adults. International Journal of Environmental Research and Public Health, 18(4), 1892. https://doi.org/10.3390/ijerph18041892







